Phytochemical Evaluation and Anti-Inflammatory Potential of Miconia albicans (Sw.) Triana Extracts
Abstract
:1. Introduction
2. Results and Discussion
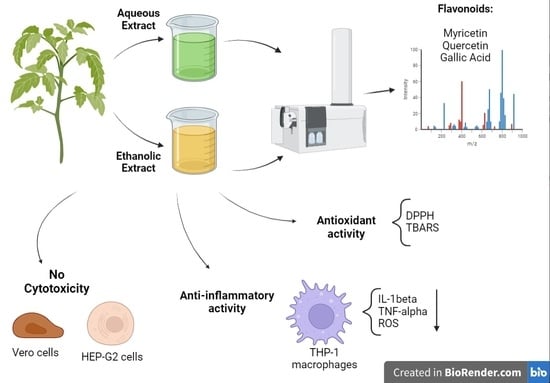
2.1. Chemical Evaluation of M. albicans Solvent Fractions
| Peak | Rt | MS 1 | MS 2 | Tentative Identification | Ref |
|---|---|---|---|---|---|
| 1 | 2.5 | 193.0718 | 172.097, 147.066, 139.039, 129.054 | Quinic acid | [13] |
| 2 | 4.5 | 171.0297 | 125.0240, 107.0128, 81.0334 | Gallic acid | [13] |
| 3 | 7.2 | 202.1462 | 146.0830, 100.0771 | N.I. | |
| 4 | 7.4 | 355.1039 | 289.0727, 259.0602, 235.0617, 205.0507, 165.0557, 99.0632, 73.0467 | Chlorogenic acid | [13] |
| 5 | 8.4 | 291.0874 | 207.0666, 165.0553, 147.0450, 139.0400, 123.0449 | Catechin | [13] |
| 6 | 8.9 | 387.2042 | 225.1505, 207.1401, 95.0866 | Glycoside | |
| 7 | 9.6 | 617.1177 | 315.0743, 303.0529, 171.0305, 153.0200 | Quercetin-galloyl-hexoside | [13] |
| 8 | 9.7 | 611.1636 | 465.1056, 303.0527, 147.0666 | Quercetin-rhamnosyl-hexoside | [13] |
| 9 | 9.8 | 611.1604 | 465.1056, 303.0527, 147.066 | Quercetin-rhamnosyl-hexoside | [13] |
| 10 | 10.0 | 465.1058 | 319.0489, 245.0444, 217.0511, 153.0200, | Myricetin-rhamnoside | |
| 11 | 10.1 | 539.2146 | 315.0735, 207.1397, 171.0300, 153.0194, 127.0389 | Hydrolysable tannin | [17] |
| 12 | 10.3 | 611.1634 | 449.109, 345.0618, 303.0533, 147.0667 | Quercetin-hexosyl-rhamnoside | [13] |
| 13 | 10.7 | 435.0421 | 303.0522, 225.0771, 147.0663, 97.0288 | Quercetin-pentoside | [13] |
| 14 | 10.9 | 581.1516 | 449.1080, 303.0521, 225.0770, 147.0662 | Quercetin-pentosyl-rhamnoside | [13] |
| 15 | 11.0 | 465.1044 | 319.0472, 129.0552 | Myricetin-rhamnoside | [18] |
| 16 | 11.1 | 449.1123 | 303.0527, 229.0510, 153.0187 | Quercetin rhamnoside | [13] |
| 17 | 14.4 | 303.0511 | 229.0517, 153.0186, 68.9959 | Quercetin | |
| 18 | 15.0 | 629.1528 | 423.0567, 277.0364 | N.I. | |
| 19 | 15.4 | 615.3907 | 537.2719, 453.3377, 407.3329 | Triterpenoid hexoside | |
| 20 | 17.9 | 345.0629 | 330.0387, 287.0217, 199.0388, 157.0864, 99.0443 | Trihydroxy-methoxy-methylenedioxy-flavone | [16] |
| 21 | 18.5 | 329.0310 | N.I. |
| Peak | Compound | Res a | But a | EtAc a | Ch a |
|---|---|---|---|---|---|
| 1 | Quinic acid | +++ | ++ | - | - |
| 2 | Gallic acid | - | ++ | +++ | - |
| 3 | N.I. | ++ | - | ++ | - |
| 4 | Chlorogenic acid | ++ | + | - | - |
| 5 | Catechin | + | + | - | - |
| 6 | Glycoside | + | + | + | - |
| 7 | Quercetin-galloyl-hexoside | - | ++ | + | - |
| 8 | Quercetin-rhamnosyl-hexoside | - | + | - | - |
| 9 | Quercetin-rhamnosyl-hexoside | - | + | - | - |
| 10 | Myricetin-rhamnoside | - | + | + | - |
| 11 | Hydrolysable tannin | - | + | + | - |
| 12 | Quercetin-hexosyl-rhamnoside | + | +++ | ++ | - |
| 13 | Quercetin-pentoside | + | ++ | ++ | - |
| 14 | Quercetin-pentosyl-rhamnoside | - | +++ | + | - |
| 15 | Myricetin-rhamnoside | - | ++ | +++ | - |
| 16 | Quercetin rhamnoside | - | + | +++ | - |
| 17 | Quercetin | - | + | + | + |
| 18 | N.I. | - | - | ++ | + |
| 19 | Triterpenoid hexoside | - | - | - | + |
| 20 | Trihydroxy-methoxy-methylenedioxy-flavone | - | + | - | ++ |
| 21 | N.I. | - | + | - | +++ |
2.2. Cytotoxicity Evaluation of the Extracts and Solvent Fractions
2.3. Redox Potential of M. Albicans Extracts
2.4. Evaluation of M. albicans Extracts on THP-1 Macrophages
3. Material and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Miconia albicans Extracts
3.4. Fractionation of Extracts
3.5. Determination of Phenolic Compounds
3.6. Phytochemical Analysis
3.7. Scavenging Activity of M. albicans Extracts Measured by DPPH Assay
3.8. Antioxidant Activity of M. albicans Extract Measured by TBARS Assay
3.9. Cell Culture
3.10. THP-1 Macrophage Differentiation
3.11. Cytotoxicity of M. albicans Extracts on THP-1, Vero, and Hep-G2 Cells
3.12. Evaluation of Oxidative Stress after Treatment with M. Albicans Extracts
3.13. Evaluation of Cytokines Released after Treatment with M. Albicans Extracts
3.14. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Sayed, M.E.; Asmaa, K.F.; Samar, E.S. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019, 234, 116786. [Google Scholar] [CrossRef]
- Prieto-Alhambra, D.; Judge, A.; Javaid, M.K.; Cooper, C.; Diez-Perez, A.; Arden, N.K. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: Influences of age, gender and osteoarthritis affecting other joints. Ann. Rheum. Dis. 2014, 73, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Wallace, I.J.; Worthington, S.; Felson, D.T.; Jurmain, R.D.; Wren, K.T.; Maijanen, H.; Woods, R.J.; Lieberman, D.E. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc. Natl. Acad. Sci. USA 2017, 1, 9332–9336. [Google Scholar] [CrossRef]
- Thomas, E.; Peat, G.; Croft, P. Defining and mapping the person with osteoarthritis for population studies and public health. Rheumatology 2014, 53, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Nees, T.A.; Schiltenwolf, M. Pharmacological treatment of osteoarthritis-related pain. Schmerz 2019, 33, 30–48. [Google Scholar] [CrossRef]
- Sakurai, Y.; Fujita, M.; Kawasaki, S.; Sanaki, T.; Yoshioka, T.; Higashino, K.; Tofukuji, S.; Voneda, S.; Takahashi, T.; Koda, K.; et al. Contribution of synovial macrophages to rat advanced osteoarthritis pain resistant to cyclooxygenase inhibitors. Pain 2019, 160, 895–907. [Google Scholar] [CrossRef]
- Nasir, N.N.; Sekar, M.; Fuloria, S.; Gan, S.H.; Rani, N.N.I.M.; Ravi, S.; Begum, M.Y.; Chidambaram, K.; Sathasivam, K.V.; Jeyabalan, S.; et al. Kirenol: A Potential Natural Lead Molecule for a New Drug Design, Development, and Therapy for Inflammation. Molecules 2022, 27, 734. [Google Scholar] [CrossRef]
- Quintans-Júnior, L.J.; Gandhi, S.R.; Passos, F.R.S.; Heimfarth, L.; Pereira, E.W.M.; Monteiro, B.S.; Santos, K.S.; Duarte, M.C.; Abreu, L.S.; Nascimento, Y.M.; et al. Dereplication and quantification of the ethanol extract of Miconia albicans (Melastomaceae) by HPLC-DAD-ESI-/MS/MS, and assessment of its anti-hyperalgesic and anti-inflammatory profiles in a mice arthritis-like model: Evidence for involvement of TNF-α, IL-1β and IL-6. J. Ethnopharmacol. 2020, 258, 112938. [Google Scholar] [CrossRef]
- Cerqueira, T.M.G.; de Carvalho Correia, A.C.; dos Santos, R.V.; Lemos, R.P.L.; da Silva, S.A.S.; Barreto, E. The Use of Medicinal Plants in Maceió, Northeastern Brazil: An Ethnobotanical Survey. Medicines 2020, 7, 7. [Google Scholar] [CrossRef]
- Falzon, C.C.; Balabanova, A. Phytotherapy: An Introduction to Herbal Medicine. Prim. Care Clin. Off. Prac. 2017, 44, 217–227. [Google Scholar] [CrossRef]
- Tomé, L.U.; Ferreira, H.D.; Alves, V.F.; de Oliveira, L.G.; Borges, L.L.; de Sá, S.; de Paula, J.R.; Fiuza, T.S. Morphological Analysis, Phytochemical Screening, Evaluation of the Antimicrobial Activity of the Crude Extract and Fractions of Miconia albicans (Sw.) Triana Leaves. Front. J. Social Technol. Environ. Sci. 2019, 8, 372–391. [Google Scholar] [CrossRef]
- Lima, T.C.; Matos, S.S.; Carvalho, T.F.; Silveira-Filho, A.J.; Couto, L.P.S.M.; Quintans-Júnior, L.J.; Quintansm, J.S.S.; Silva, A.M.O. Evidence for the involvement of IL-1β and TNF-α in anti-inflammatory effect and antioxidative stress profile of the standardized dried extract from Miconia albicans Sw. (Triana) Leaves (Melastomataceae). Acta Odontol. Scand. 2020, 259, 112908. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.M.; Dartora, N.; Scoparo, C.T.; Gorin, P.A.J.; Iacomini, M.; Sassaki, G.L. Differentiation of flavonol glucoside and galactoside isomers combining chemical isopropylidenation with liquid chromatography-mass spectrometry analysis. J. Chromatogr. A 2016, 1447, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, S.; Kadoma, Y. Relationship between phenol-induced cytotoxicity and experimental inhibition rate constant or a theoretical parameter. Mini-Rev. Med. Chem. 2012, 12, 477–490. [Google Scholar] [CrossRef]
- Pieroni, L.G.; de Rezende, F.M.; Ximenes, V.F.; Dokkedal, A.L. Antioxidant Activity and Total Phenols from the Methanolic Extract of Miconia albicans (Sw.) Triana Leaves. Molecules 2011, 16, 9439–9450. [Google Scholar] [CrossRef]
- Azizah, M.; Pripdeevech, P.; Thonongkongkae, T.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. UHPLC-ESI-QTOF-MS/MS-Based molecular networking guided isolation and dereplication of antibacterial and antifungal constituents of ventilago denticulate. Antibiotics 2020, 9, 606. [Google Scholar] [CrossRef]
- Mancarz, G.F.; Laba, L.C.; Silva, E.C.P.; Machado, P.M.R.; Souza, L.M.; Mello, R.G. Liquidambar styraciflua I: A new potential source for therapeutic uses. J. Pharm. Biomed. Anal. 2019, 174, 422–431. [Google Scholar] [CrossRef]
- Jaiswal, R.; Jayasinghe, L.; Kuhnert, N. Identification and characterization of proanthocyanidins of 16 members of the rhododendron genus (Ericaceae) by tandem LC-MS. J. Mass Spectrom. 2012, 47, 502–515. [Google Scholar] [CrossRef]
- Charlier, E.; Deroyer, C.; Ciregia, F.; Malaise, O.; Neuville, S.; Plener, Z.; Malaise, M.; de Seny, D. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem. Pharmacol. 2019, 165, 49–65. [Google Scholar] [CrossRef]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2019, 14, 709–721. [Google Scholar] [CrossRef] [Green Version]
- Abramson, S.B. Osteoarthritis and nitric oxide. Osteoarthr. Cartil. 2008, 16, S15–S20. [Google Scholar] [CrossRef]
- Siebert, S.; Tsoukas, A.; Robertson, J.; McInnes, I. Cytokines as therapeutic targets in rheumatoid arthritis and other inflammatory diseases. Pharmacol. Rev. 2015, 67, 280–309. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D. M1 and m2 macrophages: Oracles of health and disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.M.; Maria-Ferreira, D.; dal Lin, F.T.; Kimura, A.; de Santana-Filho, A.P.; Werner, M.F.P.; Souza, L.M. Phytochemical analysis and anti-inflammatory evaluation of compounds from an aqueous extract of Croton cajucara Benth. J. Pharm. Biomed. Anal. 2017, 145, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Kujula, T.; Loponen, J.; Klika, K.; Piihlaja, K. Phenolics and betacyanins in red beetroot (Beta v ulgaris) root: Distribution and effect of cold storage on the content of total phenolics and three individual compounds. J. Agric. Food Chem. 2000, 48, 5338–5342. [Google Scholar] [CrossRef] [PubMed]
- Kamdem, J.P.; Adekunle, A.; Boligon, A.; Vargas, C.; Elekofehinti, O.; Hassam, W.; Ibrahim, M.; Waczuk, E.; Meinerz, D.; Linde, A.M. Antioxidant activity, genotoxicity and cytotoxicity evaluation of lemon balm (Melissa officinalis L.) Ethanolic extract: Its potential role in neuroprotection. Ind. Crops Prod. 2013, 51, 26–34. [Google Scholar] [CrossRef]
- Pinho, A.I.; Oliveira, C.S.; Lovato, F.L.; Waczuk, E.P.; Piccoli, B.C.; Boligon, A.A.; Leite, N.F.; Coutinho, H.D.M.; Posser, T.; Da Rocha, J.B.T.; et al. Antioxidant and mercury chelating activity of Psidium guajava var. pomifera L. leaves hydroalcoholic extract. J. Toxicol. Environ. Health Part A 2017, 80, 1301–1313. [Google Scholar] [CrossRef]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. Advanced protocols in oxidative stress II. Methods Mol. Biol. 2010, 594, 57–72. [Google Scholar] [CrossRef]
- Drehmer, D.L.; de Aguiar, A.M.; Brandt, A.P.; Petiz, L.; Cadena, S.M.S.C.; Rebelatto, C.K.; Brofman, P.R.S.; Filipak Neto, F.; Dallagiovanna, B.; Abud, A.P.R. Metabolic switches during the first steps of adipogenic stem cells differentiation. Stem Cell Res. 2016, 17, 413–421. [Google Scholar] [CrossRef] [Green Version]










| Fraction | Yield (%) |
|---|---|
| Aq | 6.0 a |
| Aq-Ch | 0.9 b |
| Aq-EtAc | 4.6 b |
| Aq-But | 19.7 b |
| Aq-Res | 69.9 b |
| Et | 6.6 a |
| Et-Ch | 22.8 c |
| Et-EtAc | 10.1 c |
| Et-But | 27.8 c |
| Et-Res | 52.5 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzano, M.I.; Centa, A.; Veiga, A.d.A.; da Costa, N.S.; Bonatto, S.J.R.; de Souza, L.M.; Smiderle, F.R. Phytochemical Evaluation and Anti-Inflammatory Potential of Miconia albicans (Sw.) Triana Extracts. Molecules 2022, 27, 5954. https://doi.org/10.3390/molecules27185954
Manzano MI, Centa A, Veiga AdA, da Costa NS, Bonatto SJR, de Souza LM, Smiderle FR. Phytochemical Evaluation and Anti-Inflammatory Potential of Miconia albicans (Sw.) Triana Extracts. Molecules. 2022; 27(18):5954. https://doi.org/10.3390/molecules27185954
Chicago/Turabian StyleManzano, Mariana Inocencio, Ariana Centa, Alan de Almeida Veiga, Nayara Souza da Costa, Sandro J. R. Bonatto, Lauro M. de Souza, and Fhernanda Ribeiro Smiderle. 2022. "Phytochemical Evaluation and Anti-Inflammatory Potential of Miconia albicans (Sw.) Triana Extracts" Molecules 27, no. 18: 5954. https://doi.org/10.3390/molecules27185954
APA StyleManzano, M. I., Centa, A., Veiga, A. d. A., da Costa, N. S., Bonatto, S. J. R., de Souza, L. M., & Smiderle, F. R. (2022). Phytochemical Evaluation and Anti-Inflammatory Potential of Miconia albicans (Sw.) Triana Extracts. Molecules, 27(18), 5954. https://doi.org/10.3390/molecules27185954