Analysis of Citrus Bioflavonoid Content and Dipeptidyl Peptidase-4 Inhibitory Potential of Commercially Available Supplements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Supplements
2.2. Chemicals and Reagents
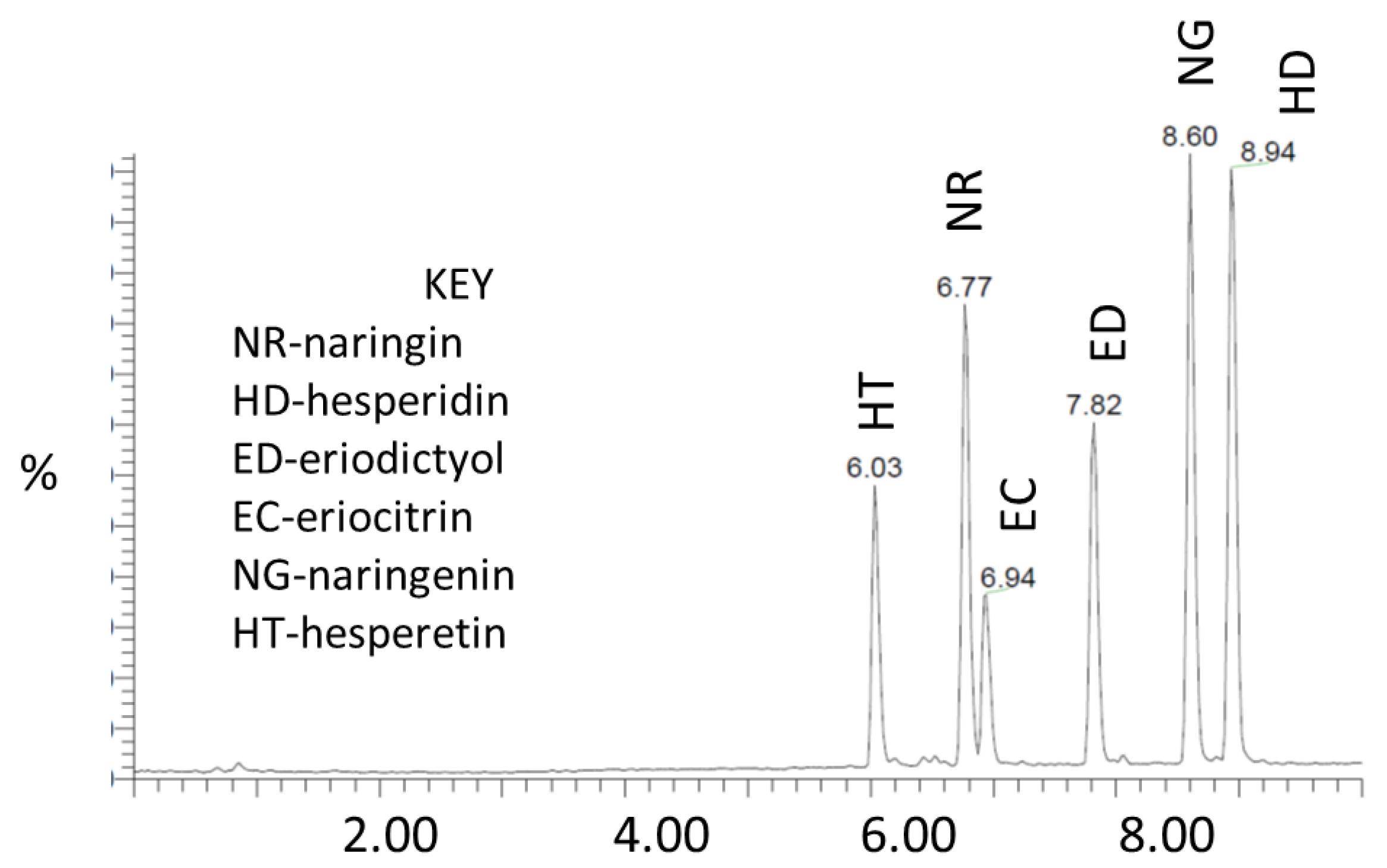
2.3. UPLC–MS/MS Analysis
2.4. Rutin Equivalence
- IC50
- ub>50 (rutin) = Concentration of rutin to inhibit DPP-4 by 50%.
- IC50
- IC50 (fl) = Concentration of bioflavonoid to inhibit DPP-4 by 50%.
- IC50
- Mass (fl) = Mass of flavonoid in each tablet × daily dosing (number of tablets per day).
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rees, A.; Dodd, G.; Spencer, J. The effects of flavonoids on cardiovascular health: A review of human intervention trials and implications for cerebrovascular function. Nutrients 2018, 10, 1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, W.; Song, X.; Liu, Z. Impact of dipeptidyl-peptidase 4 inhibitors on cardiovascular diseases. Vascul. Pharmacol. 2018, 109, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Jacobson, G.A.; Burgess, J.R.; Jelinek, H.F.; Nichols, D.S.; Narkowicz, C.K.; Al-Aubaidy, H.A. Citrus bioflavonoids dipeptidyl peptidase-4 inhibition compared with gliptin antidiabetic medications. Biochem. Biophys. Res. Commun. 2018, 503, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid composition of citrus juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J. Flavonoids in grapefruit and commercial grapefruit juices: Concentration, distribution, and potential health benefits. Proc. Fla. State Hort. Soc. 2007, 120, 288–294. [Google Scholar]
- Hanley, M.J.; Cancalon, P.; Widmer, W.W.; Greenblatt, D.J. The effect of grapefruit juice on drug disposition. Expert Opin. Drug Metab. Toxicol. 2011, 7, 267–286. [Google Scholar] [CrossRef]
- Dahan, A.; Altman, H. Food—drug interaction: Grapefruit juice augments drug bioavailability—Mechanism, extent and relevance. Eur. J. Clin. Nutr. 2004, 58, 1. [Google Scholar] [CrossRef] [Green Version]
- Liao, J.; Qu, B.; Liu, D.; Zheng, N. New method to enhance the extraction yield of rutin from Sophora japonica using a novel ultrasonic extraction system by determining optimum ultrasonic frequency. Ultrason. Sonochem. 2015, 27, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Kreft, I.; Fabjan, N.; Yasumoto, K. Rutin content in buckwheat (Fagopyrum esculentum Moench) food materials and products. Food Chem. 2006, 98, 508–512. [Google Scholar] [CrossRef]
- Humphreys, F. The occurrence and industrial production of Rutin in Southeastern Australia. Econ. Bot 1964, 18, 195–253. [Google Scholar] [CrossRef]
- Williamson, G.; Kay, C.D.; Crozier, A. The bioavailability, transport, and bioactivity of dietary flavonoids: A review from a historical perspective. Compr. Rev. Food Sci. F 2018, 17, 1054–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aschoff, J.K.; Riedl, K.M.; Cooperstone, J.L.; Högel, J.; Bosy-Westphal, A.; Schwartz, S.J.; Carle, R.; Schweiggert, R.M. Urinary excretion of citrus flavanones and their major catabolites after consumption of fresh oranges and pasteurized orange juice: A randomized cross-over study. Mol. Nutr. Food Res. 2016, 60, 2602–2610. [Google Scholar] [CrossRef] [PubMed]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Supplements | Origin | Type | Sample Access Date | Citrus Bioflavonoids | +Rutin (mg) | +Hesperidin (mg) | +Vit C (mg) |
|---|---|---|---|---|---|---|---|
| CBF-1 | USA | Tablet | 10 June 2019 | 1000 mg complex | 0 | 0 | 0 |
| CBF-2 | AUS | Tablet | 10 June 2019 | 100 mg extract | 0 | 0 | 1000 |
| CBF-3 | USA | Tablet | 11 June 2019 | 500 mg complex | 75 | 75 | 0 |
| CBF-4 | AUS | Tablet | 11 June 2019 | 500 mg extract | 100 | 0 | 500 |
| CBF-5 | AUS | Tablet | 12 June 2019 | 500 mg extract | 0 | 0 | 500 |
| CBF-6 | USA | Capsule | 12 June 2019 | 750 mg complex | 0 | 0 | 0 |
| CBF-7 | USA | Tablet | 13 June 2019 | 550 mg complex | 500 | 0 | 0 |
| CBF-8 | USA | Tablet | 13 June 2019 | 900 mg complex | 100 | 37.5 | 0 |
| CBF-9 | CAN | Capsule | 14 June 2019 | 500 mg extract | 0 | 0 | 150 |
| CBF-10 | USA | Capsule | 14 June 2019 | 700 mg complex | 245 | 0 | 500 |
| Analytes | Rt (min) | Precursor (m/z) | Products (m/z) | Cone V | Col V | LLoQ (pg/mL) |
|---|---|---|---|---|---|---|
| Rutin (94%) | 3.65 | 609.4 | 300.2 | 49 | 36 | 220 |
| Eriocitrin (98%) | 3.67 | 595.4 | 287.2 | 49 | 22 | 130 |
| Naringin (98%) | 4.53 | 579.4 | 271.2 | 49 | 32 | 280 |
| Hesperidin (98%) | 4.71 | 609.4 | 301.2 | 49 | 24 | 30 |
| Eriodictyol (98%) | 5.87 | 287.2 | 151.1 | 49 | 15 | 70 |
| Naringenin (98%) | 6.91 | 271.2 | 151.1 | 49 | 18 | 100 |
| Hesperitin (98%) | 7.15 | 301.2 | 164.1 | 49 | 25 | 430 |
| Supplements | RT % | EC % | ED % | NR % | NG % | HD % | HT % | Total % w/w |
|---|---|---|---|---|---|---|---|---|
| CBF-1 | 0.93 | 0.18 | 1.99 | 0.00 | 1.47 | 93.8 | 1.65 | 15.7 |
| CBF-2 | 0.4 | 0.4 | 0.27 | 0.00 | 0.09 | 98.3 | 0.54 | 1.9 |
| CBF-3 | 3.32 | 0.83 | 0.21 | 0.00 | 0.06 | 95.2 | 0.37 | 33.3 |
| CBF-4 | 31.1 | 0.04 | 0.04 | 0.00 | 0.13 | 67.9 | 0.72 | 22.1 |
| CBF-5 | 0.32 | 1.66 | 0.01 | 0.00 | 0.1 | 97.4 | 0.47 | 6.1 |
| CBF-6 | 0.00 | 0.00 | 0.4 | 84.1 | 1.27 | 14.25 | 0.00 | 0.8 |
| CBF-7 | 79.7 | 0.00 | 0.09 | 0.26 | 0.00 | 19.9 | 0.00 | 32.1 |
| CBF-8 | 25.4 | 0.00 | 0.01 | 0.00 | 0.02 | 74.5 | 0.11 | 21.5 |
| CBF-9 | 0.32 | 0.39 | 0.00 | 0.00 | 0.18 | 98.8 | 0.31 | 24.4 |
| CBF-10 | 0.04 | 0.00 | 0.00 | 0.00 | 0.02 | 99.7 | 0.23 | 11.7 |
| Supplements | Daily Dose (mg) | Daily Flavonoid Dose (mg) | Rutin Equivalence (mg) |
|---|---|---|---|
| CBF-1 | 1442 (1 tablet) | 226 | 30.6 |
| CBF-2 | 4598 (3 tablets) | 88 | 11.2 |
| CBF-3 | 1329 (2 tablets) | 443 | 68 |
| CBF-4 | 2532 (2 tablets) | 560 | 222 |
| CBF-5 | 2864 (2 tablets) | 174 | 22.1 |
| CBF-6 | 2468 (2 tablets) | 19 | 1.9 |
| CBF-7 | 1513 (1 tablet) | 486 | 400 |
| CBF-8 | 1303 (1 tablet) | 280 | 96.9 |
| CBF-9 | 1915 (3 tablets) | 467 | 59.4 |
| CBF-10 | 2747 (3 tablets) | 321 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, A.; Al-Aubaidy, H.A.; Narkowicz, C.K.; Jelinek, H.F.; Nichols, D.S.; Burgess, J.R.; Jacobson, G.A. Analysis of Citrus Bioflavonoid Content and Dipeptidyl Peptidase-4 Inhibitory Potential of Commercially Available Supplements. Molecules 2022, 27, 4741. https://doi.org/10.3390/molecules27154741
Gupta A, Al-Aubaidy HA, Narkowicz CK, Jelinek HF, Nichols DS, Burgess JR, Jacobson GA. Analysis of Citrus Bioflavonoid Content and Dipeptidyl Peptidase-4 Inhibitory Potential of Commercially Available Supplements. Molecules. 2022; 27(15):4741. https://doi.org/10.3390/molecules27154741
Chicago/Turabian StyleGupta, Ankit, Hayder A. Al-Aubaidy, Christian K. Narkowicz, Herbert F. Jelinek, David S. Nichols, John R. Burgess, and Glenn A. Jacobson. 2022. "Analysis of Citrus Bioflavonoid Content and Dipeptidyl Peptidase-4 Inhibitory Potential of Commercially Available Supplements" Molecules 27, no. 15: 4741. https://doi.org/10.3390/molecules27154741
APA StyleGupta, A., Al-Aubaidy, H. A., Narkowicz, C. K., Jelinek, H. F., Nichols, D. S., Burgess, J. R., & Jacobson, G. A. (2022). Analysis of Citrus Bioflavonoid Content and Dipeptidyl Peptidase-4 Inhibitory Potential of Commercially Available Supplements. Molecules, 27(15), 4741. https://doi.org/10.3390/molecules27154741









