Magnesium and Zinc Oxide Nanoparticles from Datura alba Improve Cognitive Impairment and Blood Brain Barrier Leakage
Abstract
:1. Introduction
2. Results
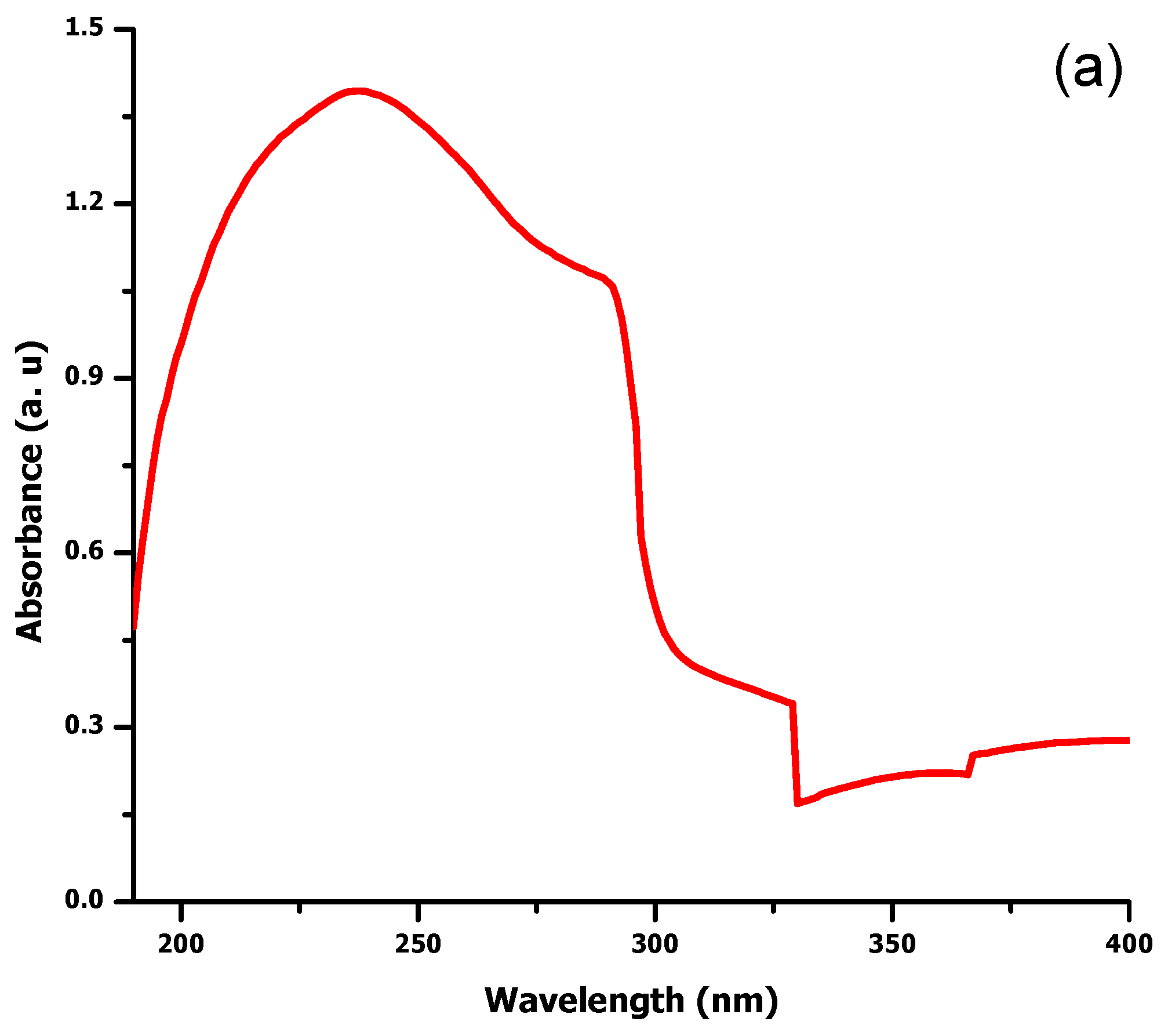
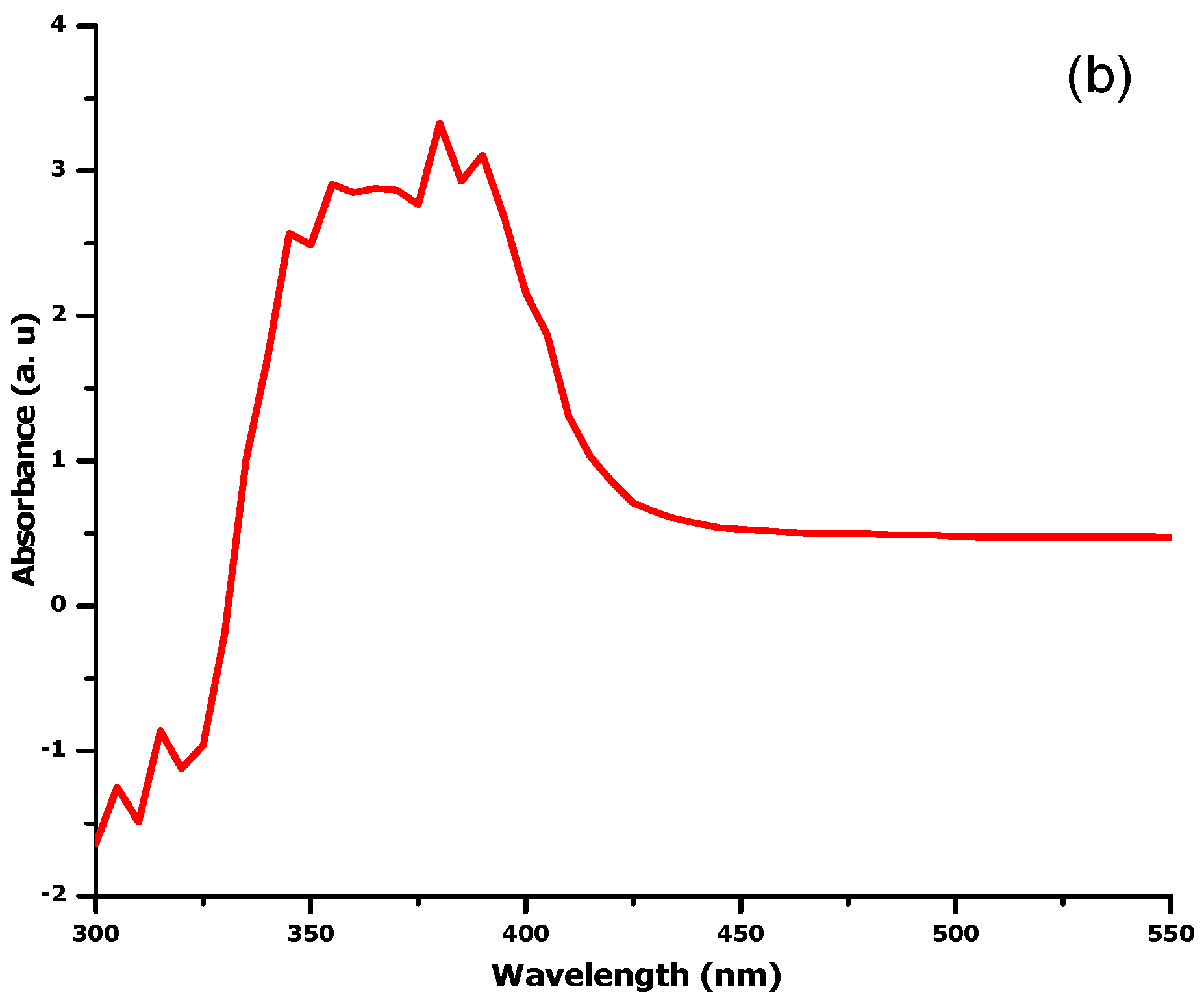
2.1. UV Visible Spectroscopy
2.2. Functional Group Analysis by FTIR Spectroscopy
2.3. X-ray Diffraction Analysis
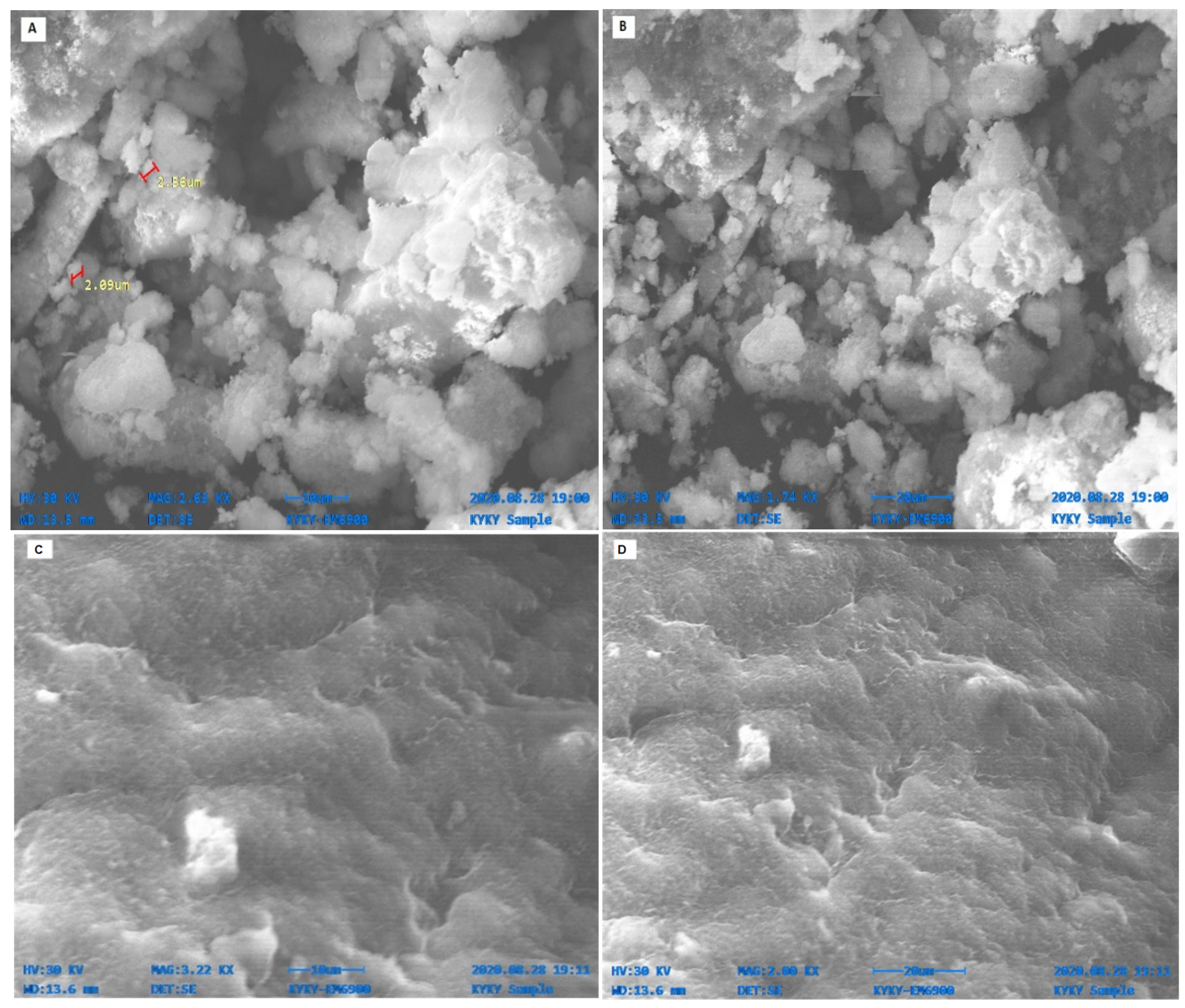
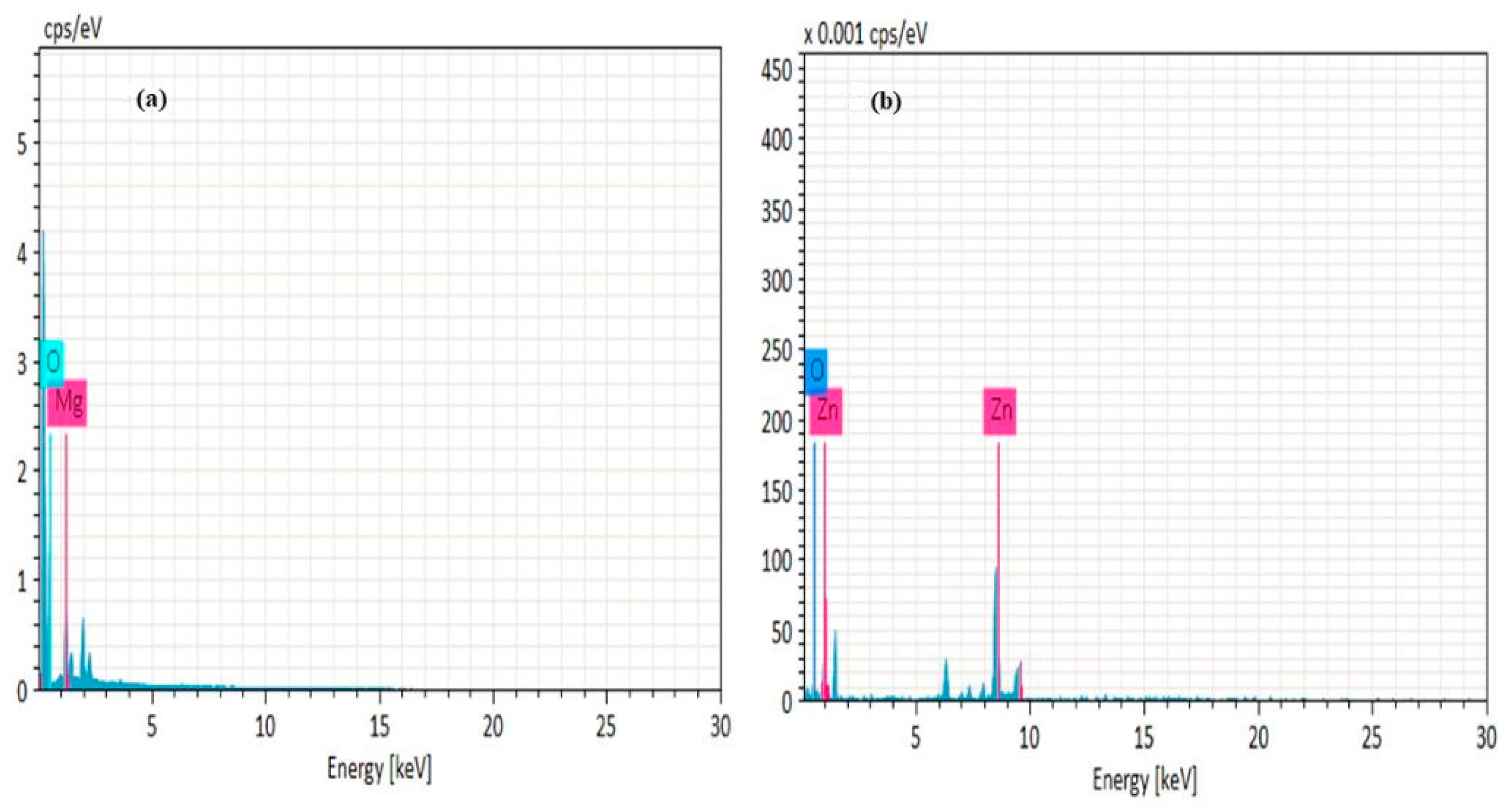
2.4. SEM and EDX Analysis
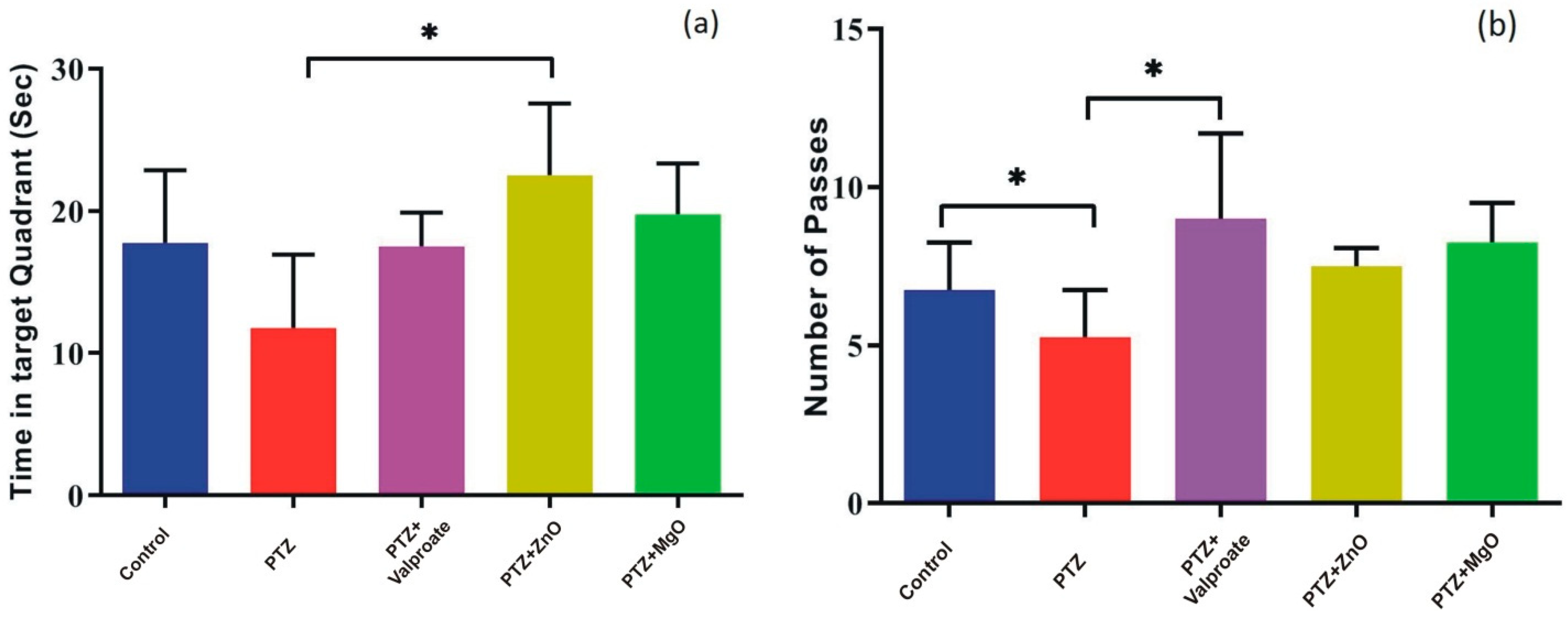
2.5. Impact of MgO and ZnO NPs on Learning and Spatial Memory
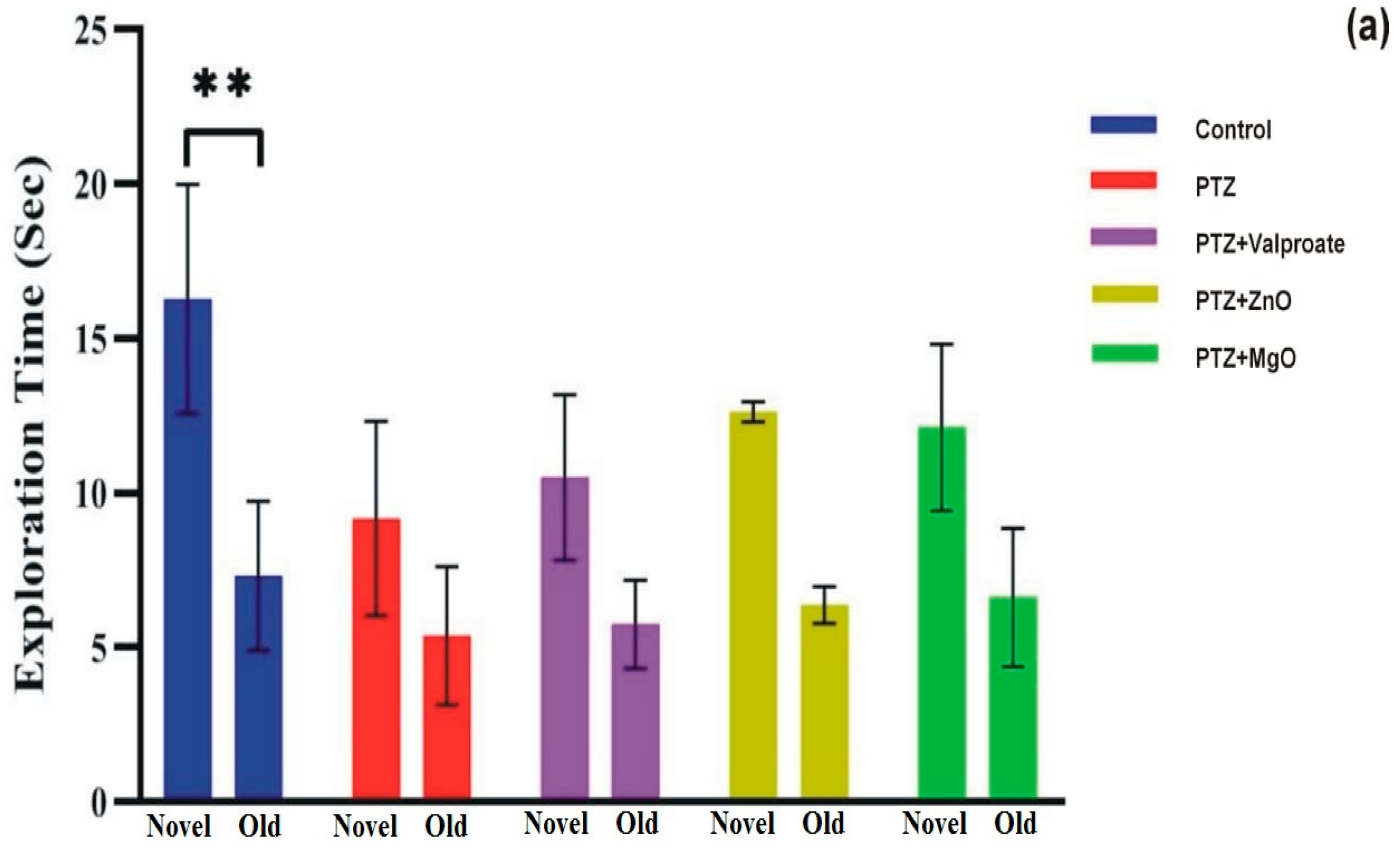
2.6. Effect of MgO and ZnO NPs on Working Memory
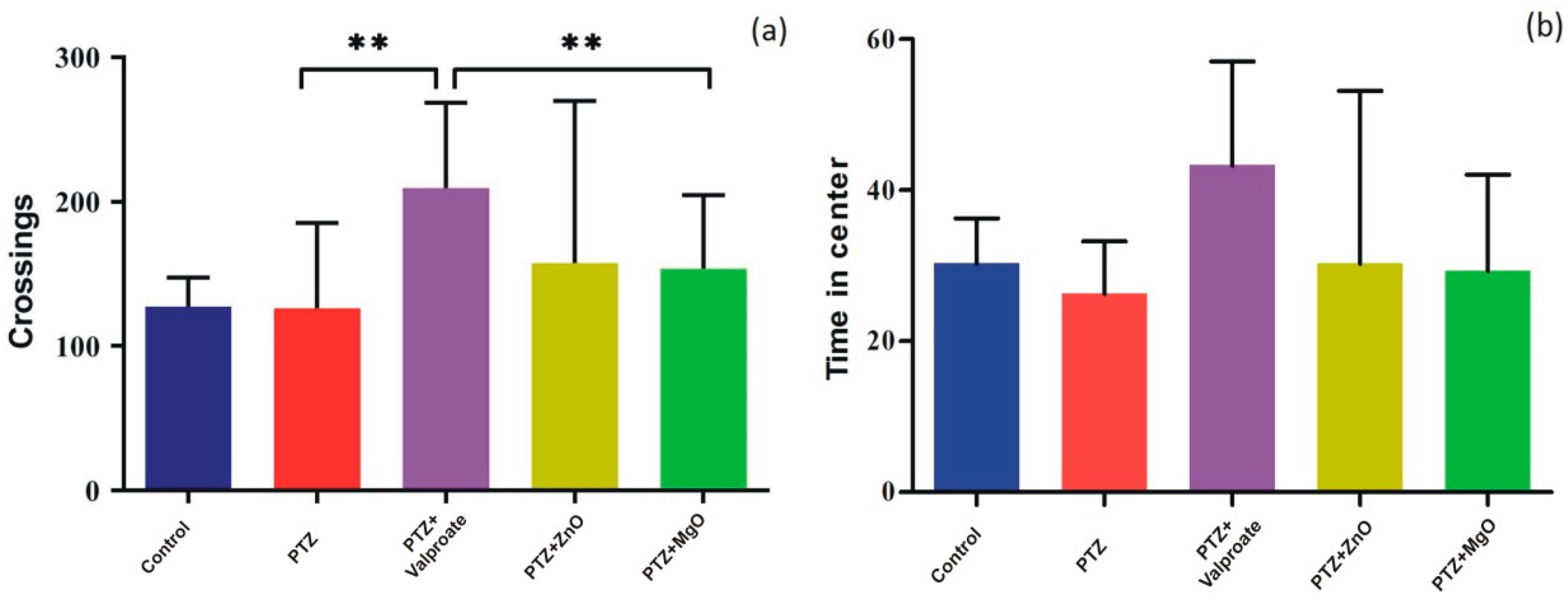
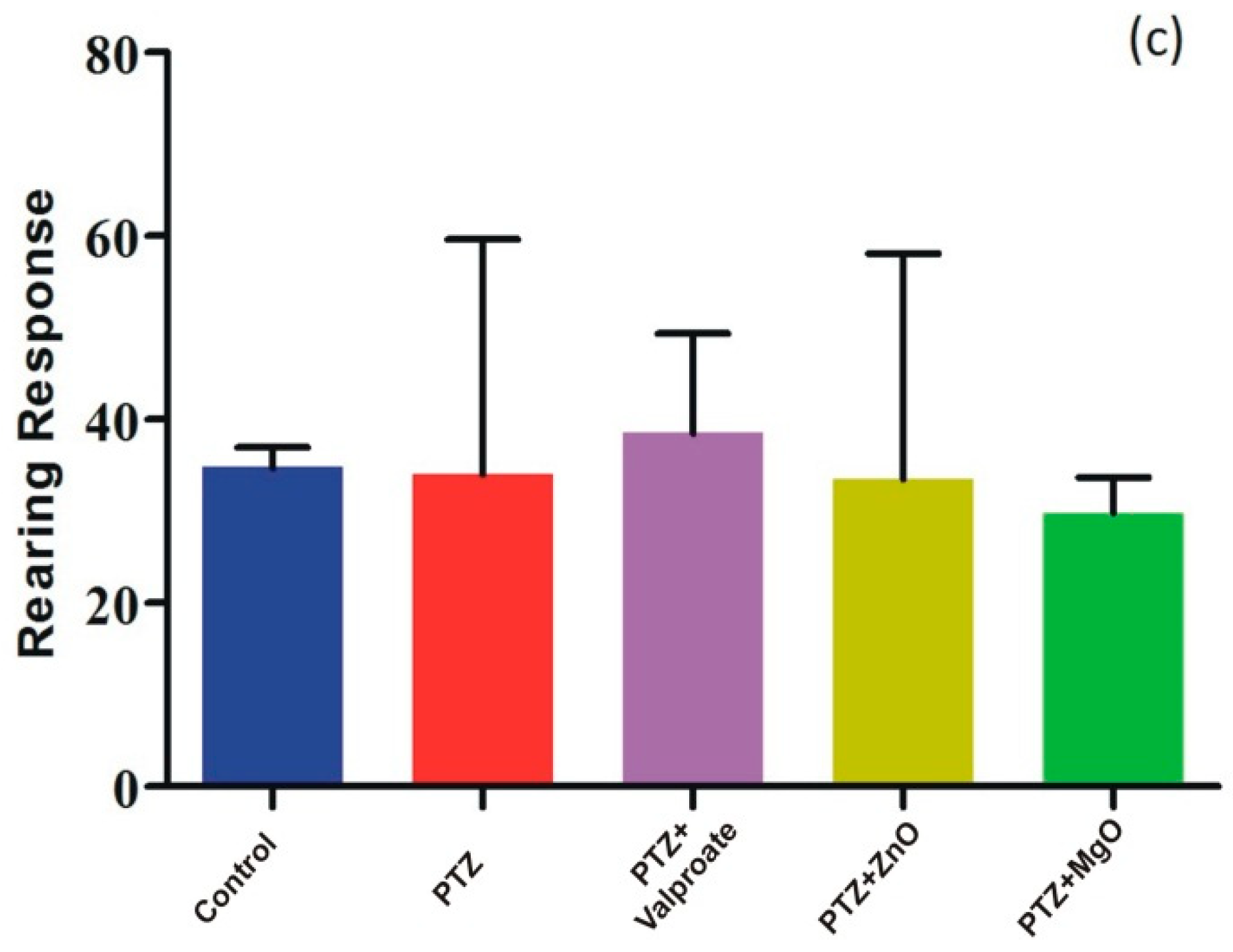
2.7. Effect of ZnO and MgO NPs on Exploratory Behavior and Locomotion
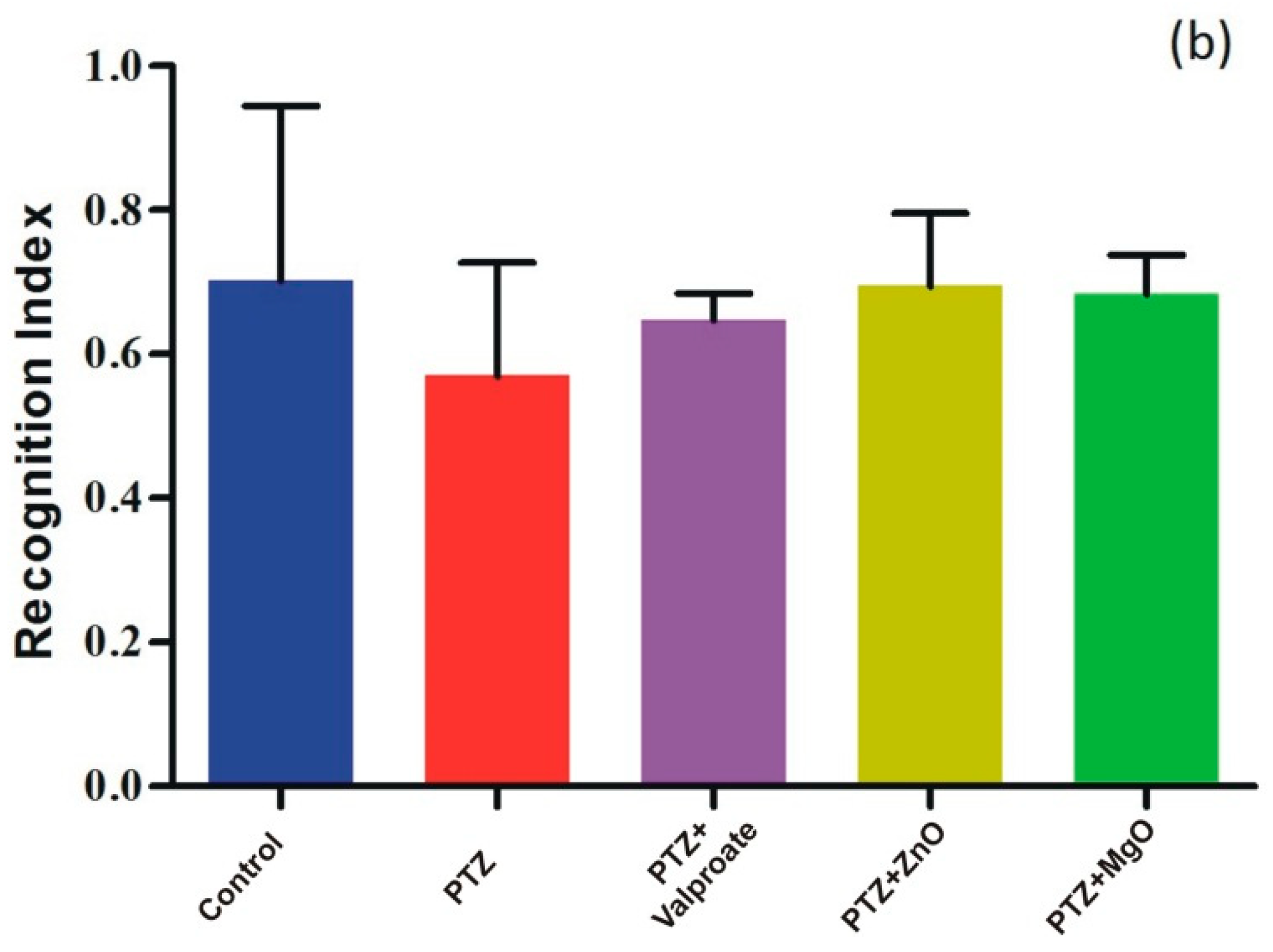
2.8. Cognition Improvement Assessments of ZnO and MgO NPs
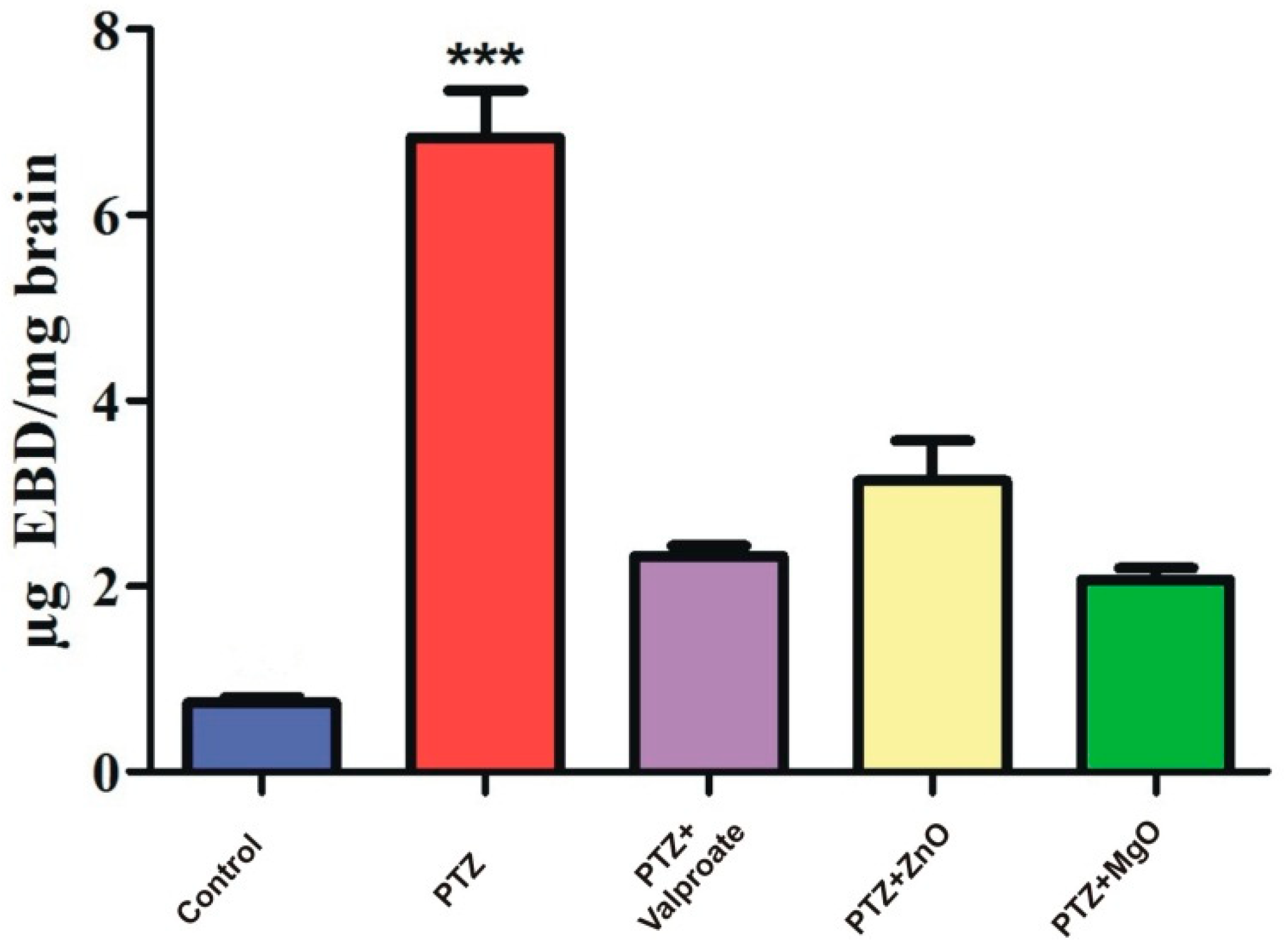
2.9. Assessment of BBB Integrity
2.10. Impact of ZnO and MgO NPs on Body Weight
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Extract Preparation
4.3. MgO NPs Synthesis
4.4. ZnO NPs Synthesis
4.5. Biosynthesized NPs Characterization
4.6. Animals and Condition
4.7. PTZ Kindling Model: In Vivo Epileptogenesis
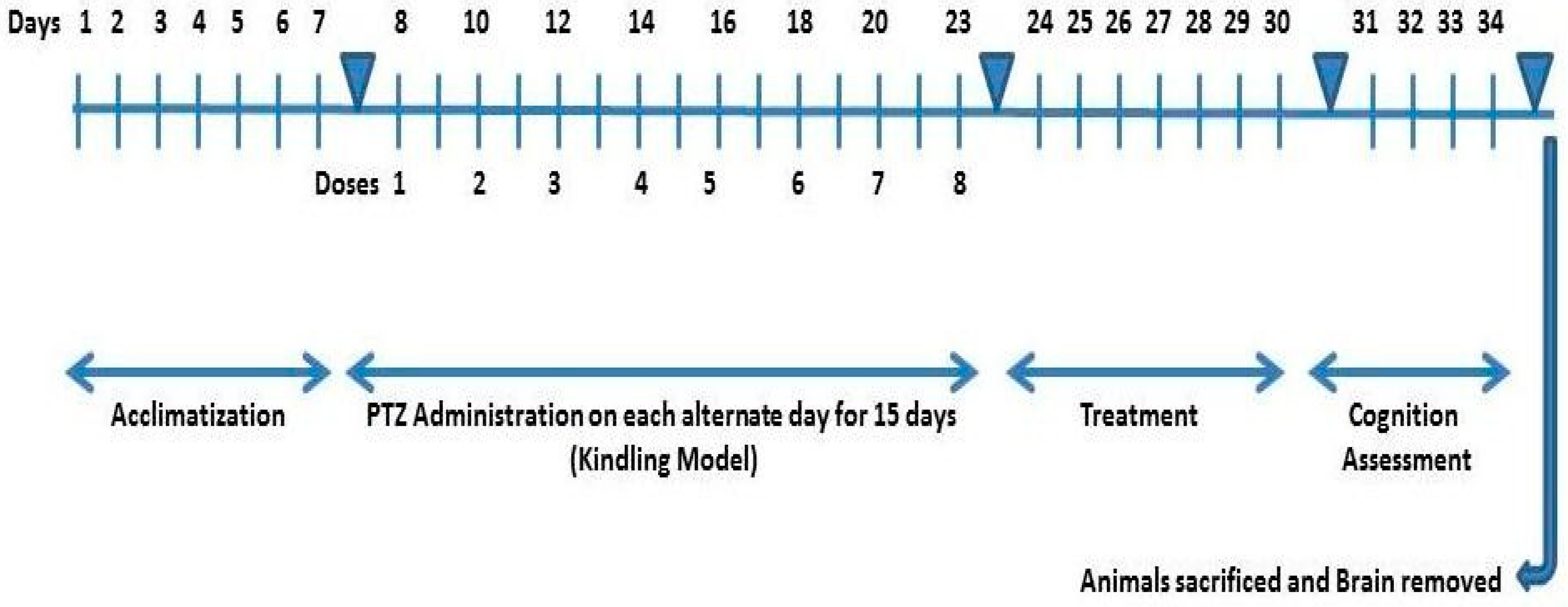
4.8. Study Design
4.9. Neurobehavioral Studies
4.9.1. Morris Water Maze Task
4.9.2. Y-Maze Test
4.9.3. Open Field Test
4.9.4. Novel Object Recognition Test
4.9.5. Evan’s Blue Dye (EBD) Leakage Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Pearson-Smith, J.N.; Patel, M. Metabollic dysfunction and oxidative stress in epilepsy. Int. J. Mol. Sci. 2017, 18, 2365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyed, R.; Ahmadiana, B.; Maryam, G.; Mahdi, P.; Farzin, S. Arbutin attenuates cognitive impairment and inflammatory response in pentylenetetrazol-induced kindling model of epilepsy. Neuropharmacology 2019, 146, 117–127. [Google Scholar]
- Löscher, W.; Potschka, H.; Sisodiya, S.M.; Vezzani, A. Drug resistance in epilepsy: Clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol. Rev. 2020, 72, 606–638. [Google Scholar] [CrossRef] [PubMed]
- Wolfgang, L.; Pavel, K. The Pharmacology and Clinical Efficacy of Antiseizure Medications: From Bromide Salts to Cenobamate and Beyond. CNS Drugs 2021, 35, 935–963. [Google Scholar]
- He, L.-Y.; Hu, M.-B.; Li, R.-L.; Zhao, R.; Fan, L.-H.; He, L.; Lu, F.; Ye, X.; Huang, Y.-L.; Wu, C.-J. Natural Medicines for the Treatment of Epilepsy: Bioactive Components, Pharmacology and Mechanism. Front. Pharmacol. 2021, 12, 604–640. [Google Scholar] [CrossRef] [PubMed]
- Christopher, J.Y.; Leigh-Ana, R.; Karenna, J.G.; Sameer, C.D.; Bo, Z.; Lahin, K.L.; Achint, K.S.; Alexander, R.; Mustafa, S. Factors influencing the acute pentylenetetrazole-induced seizure paradigm and a literature review. Ann. Clin. Transl. Neurol. 2021, 8, 1388–1397. [Google Scholar]
- Razack, S.A.; Suresh, A.; Sriram, S.; Ramakrishnan, G.; Sadanandham, S.; Veerasamy, M. Green Synthesis of Iron Oxide Nanoparticles Using Hibiscus Rosa-Sinensis for Fortifying Wheat Biscuits. SN Appl. Sci. 2020, 2, 898. [Google Scholar] [CrossRef] [Green Version]
- Rabanel, J.M.; Piec, P.A.; Landri, S.; Patten, S.A.; Ramassamy, C. Transport of PEGylated-PLA nanoparticles across a blood brain barrier model, entry into neuronal cells and in vivo brain bioavailability. J. Control. Release 2020, 328, 679–695. [Google Scholar] [CrossRef]
- Fang, S.T.; Liu, X.; Kong, N.N.; Liu, S.J.; Xia, C.H. Two new withanolides from the halophyte Datura stramonium L. Nat. Prod. Res. (Former. Nat. Prod. Lett.) 2013, 27, 1965–1970. [Google Scholar] [CrossRef] [PubMed]
- Wali, M.; Muhammad, H.; Muzamil, S.; Muhammad, A.U.; Iqra, H. Optical, morphological, and biological analysis of zinc oxide nanoparticles (ZnO NPs) using Papaver somniferum L. R. Soc. Chem. 2019, 9, 29541–29548. [Google Scholar]
- Xu, J.; Zhang, M.; Cao, P.; Adhikari, B. Effect of ZnO nanoparticles combined radio frequency pasteurization on the protein structure and water state of chicken thigh meat. LWT 2020, 134, 110–168. [Google Scholar] [CrossRef]
- Krall, R.F.; Tzounopoulos, T.; Aizenman, E. The Function and Regulation of Zinc in the Brain. Neuroscience 2021, 457, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Zeiler, F.A.; Matuszczak, M.; Teitelbaum, J.; Gillman, L.M.; Kazina, C.J. Magnesium sulfate for non-eclamptic status epilepticus. Seizure 2015, 32, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Hou, H.; Wang, L.; Fu, T.; Papasergi, M.; Yule, D.I.; Xia, H. Magnesium acts as a second messenger in the regulation of NMDA receptor mediated CREB signaling in neurons. Mol. Neurobiol. 2020, 57, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
- Azam, Z.; Ayaz, A.; Younas, M.; Qureshi, Z.; Arshad, B.; Zaman, W.; Saqib, S. Microbial synthesized cadmium oxide nanoparticles induce oxidative stress and protein leakage in bacterial cells. Microb. Pathog. 2020, 144, 104188. [Google Scholar] [CrossRef] [PubMed]
- Saqib, S.; Zaman, W.; Ullah, F.; Majeed, I.; Ayaz, A.; Hussain Munis, M.F. Organometallic assembling of chitosan-Iron oxide nanoparticles with their antifungal evaluation against Rhizopus oryzae. Appl. Organomet. Chem. 2019, 33, e5190. [Google Scholar] [CrossRef]
- Liaqat, F.; Hanif, U.; Bahadur, S.; Faheem, M.; Rasool, S.; Gulzar, S.; Munir, M. Comparative evaluation of the toxicological effect of silver salt (AgNO3) and silver nanoparticles on Cyprinus carpio synthesized by chemicals and marine algae using scanning electron microscopy. Microsc. Res. Tech. 2021, 84, 1531–1541. [Google Scholar] [CrossRef]
- Asghar, M.; Habib, S.; Zaman, W.; Hussain, S.; Ali, H.; Saqib, S. Synthesis and characterization of microbial mediated cadmium oxide nanoparticles. Microsc. Res. Tech. 2020, 83, 1574–1584. [Google Scholar] [CrossRef]
- Saqib, S.; Nazeer, A.; Ali, M.; Zaman, W.; Younas, M.; Shahzad, A.; Sunera; Nisar, M. Catalytic potential of endophytes facilitates synthesis of biometallic zinc oxide nanoparticles for agricultural application. BioMetals 2022, 35, 1–16. [Google Scholar] [CrossRef]
- Yukihiro, O.; Shizuka, I.; Ryo, T.; Tadao, S.; Masashi, S. Antiepileptogenic and anticonvulsive actions of levetiracetam in a pentylenetetrazole kindling model. Epilepsy Res. 2010, 89, 360–364. [Google Scholar]
- Weisong, L.; Huixian, L.; Haidong, W.; Yang, L.; Shan, L.; Juan, Z.; Haixia, L.; Xinlin, C.; Yong, L.; Pengbo, Z. 17b-Estradiol Treatment Attenuates Neurogenesis Damage and Improves Behavior Performance after Ketamine Exposure in Neonatal Rats. Front. Cell. Neurosci. 2019, 13, 251. [Google Scholar]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Pre-Clin. Models 2019, 1916, 105–107. [Google Scholar]
- Eva, Z.; Julius, S.; Iva, K.; Jan, K.; Jana, M. Behavioral effects of antiepileptic drugs in rats: Are the effects on mood and behavior detectable in open-field test? Seizure 2017, 52, 35–40. [Google Scholar]
- Mariana, P.S.D.; Amanda, D.G.; Fabricia, P. Using evans blue dye to determine Blood Brain Barrier integrity in rodents. Curr. Protoc. Immunol. 2019, 126, e83. [Google Scholar]
- Santhoshkumar, J.; Venkat, K.S.; Rajeshkumar, S. Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resour.-Effic. Technol. 2017, 3, 459–465. [Google Scholar] [CrossRef]
- Solabomi, O.O.; Feng, Z.; Yasmine, A.; Muchen, Z.; Yangli, W.; Guochang, S.; Wen, Q.; Bin, L. Biosynthesis and characterization of magnesium oxide and manganese dioxide nanoparticles using Matricaria chamomilla L. extract and its inhibitory effect on Acidovorax oryzae strain rs-2. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2230–2239. [Google Scholar]
- Govindasamy, S.; Marimuthu, T.; Chandrasekaran, M. Green synthesis of ZnO nanoparticles using Tecoma castanifolia leaf extract: Characterization and evaluation of its antioxidant, bactericidal and anticancer activities. Microchem. J. 2019, 145, 578–587. [Google Scholar]
- Joghee, S.; Ganeshan, P.; Vincent, A.; Mahalingam, S.; Sun, I.H. Green synthesis and characterization of hexagonal shaped MgO nanoparticles using insulin plant (Costus pictus D. Don) leave extract and its antimicrobial as well as anticancer activity. Adv. Powder Technol. 2018, 29, 1685–1694. [Google Scholar]
- Jejenija, O.; Onwudiwe, D.C.; Eno, E.E. Green synthesis of ZnO nanoparticles using aqueous Brassica oleracea L. var. italica and the photocatalytic activity. Green Chem. Lett. Rev. Tylor Fr. 2019, 12, 444–457. [Google Scholar]
- Ranathunge, T.A.; Karunaratne, D.G.G.P.; Rajapakse, R.M.G.; Davita, L.W. Doxorubicin Loaded Magnesium Oxide Nanoflakes as pH Dependent Carriers for Simultaneous Treatment of Cancer and Hypomagnesemia. Nanomaterials 2019, 9, 208. [Google Scholar] [CrossRef] [Green Version]
- Jhansi, K.; Jayarambabu, N.; Paul, R.K.; Manohar, R.N.; Padma, S.R.; Venkateswara, K.R.; Ramesh, K.V.; Rajendar, V. Biosynthesis of MgO nanoparticles using mushroom extract: Effect on peanut (Arachis hypogaea L.) seed germination. Biotechnology 2017, 7, 263. [Google Scholar] [CrossRef] [PubMed]
- Shabnam, F.; Mina, J.; Hassan, K.F. Green synthesis of zinc oxide nanoparticles: A comparison. Green Chem. Lett. Rev. 2018, 12, 19–24. [Google Scholar]
- Essien, E.R.; Atasie, V.N.; Okeafor, A.O.; Nwude, D.O. Biogenic synthesis of magnesium oxide nanoparticles using Manihot esculenta (Crantz) leaf extract. Int. Nano Lett. 2019, 123, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Chikkanna, M.M.; Neelagund, S.E.; Rajashekarappa, K.K. Green synthesis of Zinc oxide nanoparticles (ZnO NPs) and their biological activity. SN Appl. Sci. 2019, 1, 117. [Google Scholar] [CrossRef] [Green Version]
- Kesmati, M.; Konani, M.; Torabi, M.; Khajehpour, L. Magnesium oxide nanoparticles reduce anxiety induced by morphine withdrawal in adult male mice. Physiol. Pharmacol. 2016, 20, 197–205. [Google Scholar]
- Xie, Y.; Wang, Y.; Zhang, T.; Ren, G.; Yang, Z. Effects of nanoparticle zinc oxide on spatial cognition and synaptic plasticity in mice with depressive-like behaviors. J. Biomed. Sci. 2012, 19, 14. [Google Scholar] [CrossRef] [Green Version]
- Zeinab, S.N.; Mahnaz, K.; Mahdi, P.B. Effect of Magnesium Oxide Nanoparticles on Atropine-Induced Memory Impairment in Adult Male Mice. Avicenna J. Neuro Psycho Physiol. 2015, 2, 106–112. [Google Scholar]
- Tian, L.; Lin, B.; Wu, L.; Li, K.; Liu, H.; Yan, J.; Liu, X.; Xi, Z. Neurotoxicity induced by zinc oxide nanoparticles: Age-related differences and interaction. Sci. Rep. 2015, 5, 16117. [Google Scholar] [CrossRef] [Green Version]
- Farzaneh, Z.; Mahnaz, K.; Lotfollah, K.; Mozhgan, T. Effects of magnesium oxide nanoparticles on memory impairment induced by postpartum depression model. Physiol. Pharmacol. 2020, 24, 63–73. [Google Scholar]
- Andreas, M.G.; Magali, R.; Craig, C.G. Brain-Delivery of zinc-ions as potential treatment for Neurological Diseases. Natl. Inst. Health 2011, 1, 13–23. [Google Scholar]
- Javeria, Z.; Shahid, I.; Kiran, Z.; Zulha, J.; Muhammad, A.; Atif, A.; Muhammad, N.A.; Furhan, I. Effect of Variable Doses of Zinc Oxide Nanoparticles on Male Albino Mice Behavior. Neurochem. Res. 2007, 42, 439–445. [Google Scholar]
- Hatice, Y.; Fatma, B.S.; Goksel, D.; Ibrahim, E.Y.; Baris, O. The effects of zinc treatment on the blood brain barrier permeability and brain elements levels during convulsion. Biol. Trace Elem. Res. 2013, 151, 256–262. [Google Scholar]




| Treatment Groups | Dose mg/kg | Initial Weight (g) | Gained Weight (g) |
|---|---|---|---|
| PTZ | 40 | 31.01 ± 0.4079 | 34.31 ± 1.610 |
| Control | Saline | 28.80 ± 1.110 | 35.01 ± 1.230 ** |
| Valproate | 200 | 31.01 ± 0.4078 | 35.26 ± 0.4787 *** |
| MgO | 10 | 32.01 ± 1.236 | 36.80 ± 0.9470 |
| ZnO | 10 | 22.51 ± 6.560 | 33.26 ± 1.501 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, H.; Ullah, I.; Rehman, G.; Hamayun, M.; Ali, S.; Rahman, A.; Lee, I.-J. Magnesium and Zinc Oxide Nanoparticles from Datura alba Improve Cognitive Impairment and Blood Brain Barrier Leakage. Molecules 2022, 27, 4753. https://doi.org/10.3390/molecules27154753
Ullah H, Ullah I, Rehman G, Hamayun M, Ali S, Rahman A, Lee I-J. Magnesium and Zinc Oxide Nanoparticles from Datura alba Improve Cognitive Impairment and Blood Brain Barrier Leakage. Molecules. 2022; 27(15):4753. https://doi.org/10.3390/molecules27154753
Chicago/Turabian StyleUllah, Habib, Ikram Ullah, Gauhar Rehman, Muhammad Hamayun, Sajid Ali, Abdur Rahman, and In-Jung Lee. 2022. "Magnesium and Zinc Oxide Nanoparticles from Datura alba Improve Cognitive Impairment and Blood Brain Barrier Leakage" Molecules 27, no. 15: 4753. https://doi.org/10.3390/molecules27154753
APA StyleUllah, H., Ullah, I., Rehman, G., Hamayun, M., Ali, S., Rahman, A., & Lee, I.-J. (2022). Magnesium and Zinc Oxide Nanoparticles from Datura alba Improve Cognitive Impairment and Blood Brain Barrier Leakage. Molecules, 27(15), 4753. https://doi.org/10.3390/molecules27154753










