Detection of Bisphenol A and Four Analogues in Atmospheric Emissions in Petrochemical Complexes Producing Polypropylene in South America
Abstract
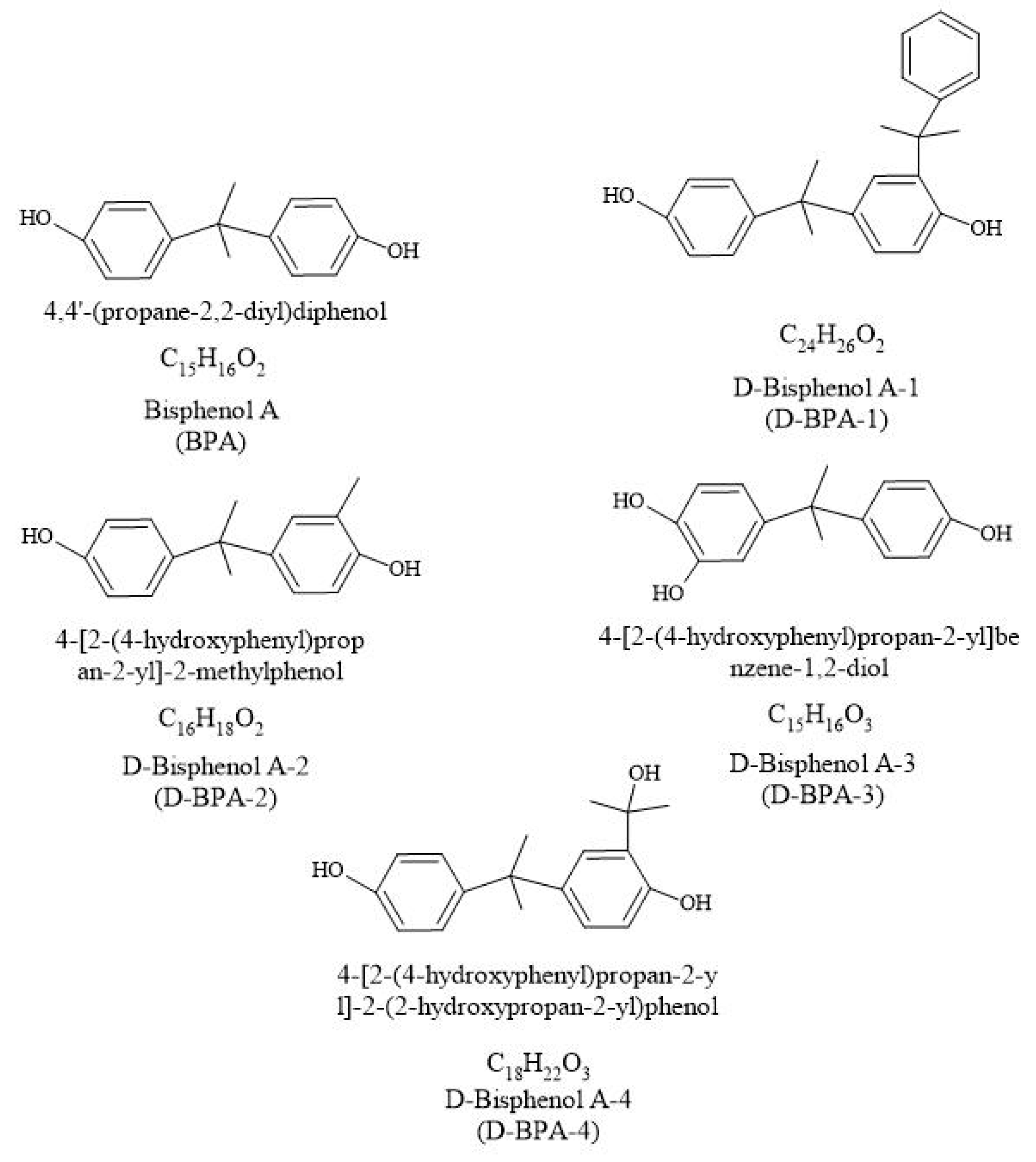
:1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Sample Collection
2.3. Instrumental Analysis
2.4. Treatment of Samples Based on SPE
2.4.1. Pretreatment
2.4.2. Preconcentration and Cleaning
2.5. Quality Assurance and Quality Control (QA/QC)
3. Results
Occurrence and Concentrations of BPs in Atmospheric Emissions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.-L.; Wu, Y.; Widelka, M. Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity—A Review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef]
- Moon, M.K. Concern about the Safety of Bisphenol A Substitutes. Diabetes Metab. J. 2019, 43, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Morin, N.; Arp, H.P.H.; Hale, S.E. Bisphenol A in Solid Waste Materials, Leachate Water, and Air Particles from Norwegian Waste-Handling Facilities: Presence and Partitioning Behavior. Environ. Sci. Technol. 2015, 49, 7675–7683. [Google Scholar] [CrossRef]
- Mihaich, E.M.; Friederich, U.; Caspers, N.; Hall, A.T.; Klecka, G.M.; Dimond, S.S.; Staples, C.A.; Ortego, L.S.; Hentges, S.G. Acute and chronic toxicity testing of bisphenol A with aquatic invertebrates and plants. Ecotoxicol. Environ. Saf. 2009, 72, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Belfroid, A.; van Velzen, M.; van der Horst, B.; Vethaak, D. Occurrence of bisphenol A in surface water and uptake in fish: Evaluation of field measurements. Chemosphere 2002, 49, 97–103. [Google Scholar] [CrossRef]
- Gao, H.; Yang, B.-J.; Li, N.; Feng, L.-M.; Shi, X.-Y.; Zhao, W.-H.; Liu, S.-J. Bisphenol A and hormone-associated cancers: Current progress and perspectives. Medicine 2015, 94, e211. [Google Scholar] [CrossRef] [PubMed]
- Catenza, C.J.; Farooq, A.; Shubear, N.S.; Donkor, K.K. A targeted review on fate, occurrence, risk and health implications of bisphenol analogues. Chemosphere 2021, 268, 129273. [Google Scholar] [CrossRef]
- Cimmino, I.; Fiory, F.; Perruolo, G.; Miele, C.; Beguinot, F.; Formisano, P.; Oriente, F. Potential Mechanisms of Bisphenol A (BPA) Contributing to Human Disease. Int. J. Mol. Sci. 2020, 21, 5761. [Google Scholar] [CrossRef]
- Gao, X.; Wang, H.-S. Impact of bisphenol a on the cardiovascular system-epidemiological and experimental evidence and molecular mechanisms. Int. J. Environ. Res. Public. Health 2014, 11, 8399–8413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, C.; Kannan, K. Concentrations and Profiles of Bisphenol A and Other Bisphenol Analogues in Foodstuffs from the United States and Their Implications for Human Exposure. J. Agric. Food Chem. 2013, 61, 4655–4662. [Google Scholar] [CrossRef]
- Qiu, W.; Zhan, H.; Hu, J.; Zhang, T.; Xu, H.; Wong, M.; Xu, B.; Zheng, C. The occurrence, potential toxicity, and toxicity mechanism of bisphenol S, a substitute of bisphenol A: A critical review of recent progress. Ecotoxicol. Environ. Saf. 2019, 173, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Fei, Y.; Wang, M.; Xue, Y.; Chen, H.; Liu, Y. Study on the Joint Toxicity of BPZ, BPS, BPC and BPF to Zebrafish. Molecules 2021, 26, 4180. [Google Scholar] [CrossRef]
- Gao, C.; He, H.; Qiu, W.; Zheng, Y.; Chen, Y.; Hu, S.; Zhao, X. Oxidative Stress, Endocrine Disturbance, and Immune Interference in Humans Showed Relationships to Serum Bisphenol Concentrations in a Dense Industrial Area. Environ. Sci. Technol. 2021, 55, 1953–1963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Quan, Q.; Zhang, M.; Zhang, N.; Zhang, W.; Zhan, M.; Xu, W.; Lu, L.; Fan, J.; Wang, Q. Occurrence of bisphenol A and its alternatives in paired urine and indoor dust from Chinese university students: Implications for human exposure. Chemosphere 2020, 247, 125987. [Google Scholar] [CrossRef]
- Liao, C.; Liu, F.; Kannan, K. Bisphenol S, a New Bisphenol Analogue, in Paper Products and Currency Bills and Its Association with Bisphenol A Residues. Environ. Sci. Technol. 2012, 46, 6515–6522. [Google Scholar] [CrossRef]
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Yates, B.S.; Breed, C.S.; Williams, E.S.; Brooks, B.W. Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation. Dose-Response Publ. Int. Hormesis Soc. 2015, 13, 15593258–15598308. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Fernández, J.; Lopez-Martinez, J.; Barceló, D. Quantification and elimination of substituted synthetic phenols and volatile organic compounds in the wastewater treatment plant during the production of industrial scale polypropylene. Chemosphere 2021, 263, 128027. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, J. Quantification of arsine and phosphine in industrial atmospheric emissions in Spain and Colombia. Implementation of modified zeolites to reduce the environmental impact of emissions. Atmospheric Pollut. Res. 2021, 12, 167–176. [Google Scholar] [CrossRef]
- Hernández-Fernández, J. Quantification of oxygenates, sulphides, thiols and permanent gases in propylene. A multiple linear regression model to predict the loss of efficiency in polypropylene production on an industrial scale. J. Chromatogr. A 2020, 1628, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Joaquin, H.-F.; Juan, L. Quantification of poisons for Ziegler Natta catalysts and effects on the production of polypropylene by gas chromatographic with simultaneous detection: Pulsed discharge helium ionization, mass spectrometry and flame ionization. J. Chromatogr. A 2020, 1614, 460736. [Google Scholar] [CrossRef]
- Joaquin, H.-F.; Juan, L.-M. Autocatalytic influence of different levels of arsine on the thermal stability and pyrolysis of polypropylene. J. Anal. Appl. Pyrolysis 2022, 161, 105385. [Google Scholar] [CrossRef]
- Hernández-Fernandez, J.; Rodríguez, E. Determination of phenolic antioxidants additives in industrial wastewater from polypropylene production using solid phase extraction with high-performance liquid chromatography. J. Chromatogr. A 2019, 1607, 460442. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; López-Martínez, J. Experimental study of the auto-catalytic effect of triethylaluminum and TiCl4 residuals at the onset of non-additive polypropylene degradation and their impact on thermo-oxidative degradation and pyrolysis. J. Anal. Appl. Pyrolysis 2021, 155, 105052. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Lopez-Martinez, J.; Barceló, D. Development and validation of a methodology for quantifying parts-per-billion levels of arsine and phosphine in nitrogen, hydrogen and liquefied petroleum gas using a variable pressure sampler coupled to gas chromatography-mass spectrometry. J. Chromatogr. A 2021, 1637, 461833. [Google Scholar] [CrossRef]
- Lee, S.; Liao, C.; Song, G.-J.; Ra, K.; Kannan, K.; Moon, H.-B. Emission of bisphenol analogues including bisphenol A and bisphenol F from wastewater treatment plants in Korea. Chemosphere 2015, 119, 1000–1006. [Google Scholar] [CrossRef]
- Wang, W.; Abualnaja, K.O.; Asimakopoulos, A.G.; Covaci, A.; Gevao, B.; Johnson-Restrepo, B.; Kumosani, T.A.; Malarvannan, G.; BinhMinh, T.; Moon, H.-B.; et al. A comparative assessment of human exposure to tetrabromobisphenol A and eight bisphenols including bisphenol A via indoor dust ingestion in twelve countries. Environ. Int. 2015, 83, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Barroso, P.J.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Evaluation of the airborne pollution by emerging contaminants using bitter orange (Citrus aurantium) tree leaves as biosamplers. Sci. Total Environ. 2019, 677, 484–492. [Google Scholar] [CrossRef]
- Geens, T.; Roosens, L.; Neels, H.; Covaci, A. Assessment of human exposure to Bisphenol-A, Triclosan and Tetrabromobisphenol-A through indoor dust intake in Belgium. Chemosphere 2009, 76, 755–760. [Google Scholar] [CrossRef]


| Country | Sampling Point | Parameters | BPA | D-BPA-1 | D-BPA-2 | D-BPA-3 | D-BPA-4 | ∑BPs |
|---|---|---|---|---|---|---|---|---|
| Colombia | EC-MFI5 | Production MFI 5 (n = 16) | ||||||
| Mean | 239.71 | 83.29 | 21.81 | 27.29 | 16.97 | 389.07 | ||
| Median | 214 | 64 | 16.7 | 25.9 | 13.2 | 393.7 | ||
| Range | 45–546 | 15–341 | 0.57–81.6 | 15.6–43.5 | 4.26–51.6 | 92.45–976.75 | ||
| DR | 100 | 100 | 100 | 100 | 100 | 100 | ||
| EC-MFI50 | Production MFI 50 (n = 16) | |||||||
| Mean | 264.88 | 104.06 | 49.14 | 52.68 | 16.97 | 487.72 | ||
| Median | 246 | 88 | 46.6 | 49.4 | 13.2 | 510.9 | ||
| Range | 73–576 | 26–421 | 1.85–134.5 | 29.3–75.3 | 4.26–51.6 | 160.16–1129.15 | ||
| DR | 100 | 100 | 100 | 100 | 100 | 100 | ||
| EC-MFI80 | Production MFI 80 (n = 16) | |||||||
| Mean | 324.76 | 136.35 | 78.72 | 86.51 | 44.49 | 670.83 | ||
| Median | 345 | 99 | 76.9 | 91.6 | 34.6 | 698.6 | ||
| Range | 95–624 | 42–542 | 6.5–172.5 | 46.7–121.1 | 19.4–99.7 | 337.6–1383.4 | ||
| DR | 100 | 100 | 100 | 100 | 100 | 100 | ||
| Brazil | EB-MFI5 | Production MFI 5 (n = 16) | ||||||
| Mean | 301.01 | 105.53 | 35.09 | 45.66 | 35.99 | 523.28 | ||
| Median | 316 | 87 | 34.6 | 42.1 | 33.9 | 530 | ||
| Range | 79–615 | 34–369 | 2.43–99.3 | 31.2–74.3 | 9.75–81.3 | 186.75–1123.2 | ||
| DR | 100 | 100 | 100 | 100 | 100 | 100 | ||
| EB-MFI50 | Production MFI 50 (n = 16) | |||||||
| Mean | 377.12 | 151.71 | 51.13 | 70.41 | 57.75 | 708.11 | ||
| Median | 419 | 141 | 46.5 | 66.5 | 55.6 | 699.8 | ||
| Range | 99–721 | 49–455 | 4.65–112.1 | 47.6–99.2 | 25.4–98.4 | 308.05–1339.7 | ||
| DR | 100 | 100 | 100 | 100 | 100 | 100 | ||
| EB-MFI80 | Production MFI 80 (n = 16) | |||||||
| Mean | 523.88 | 207.00 | 75.95 | 89.90 | 83.86 | 980.59 | ||
| Median | 536 | 196 | 75.3 | 88.5 | 77.2 | 1002.6 | ||
| Range | 184–947 | 75–523 | 10.8–201.5 | 66.2–124.3 | 46.5–142.5 | 430.5–1565.1 | ||
| DR | 100 | 100 | 100 | 100 | 100 | 100 | ||
| Total (n = 40) | ||||||||
| Mean | 338.56 | 131.32 | 51.97 | 62.07 | 42.67 | 626.60 | ||
| Median | 294 | 99 | 44.35 | 64.8 | 35.5 | 542.95 | ||
| Range | 45–947 | 15–542 | 0.6–201 | 16–124 | 4–142 | 92–1565 | ||
| DR | 100 | 100 | 100 | 100 | 100 | 100 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, J.H.; Guerra, Y.; Cano, H. Detection of Bisphenol A and Four Analogues in Atmospheric Emissions in Petrochemical Complexes Producing Polypropylene in South America. Molecules 2022, 27, 4832. https://doi.org/10.3390/molecules27154832
Fernández JH, Guerra Y, Cano H. Detection of Bisphenol A and Four Analogues in Atmospheric Emissions in Petrochemical Complexes Producing Polypropylene in South America. Molecules. 2022; 27(15):4832. https://doi.org/10.3390/molecules27154832
Chicago/Turabian StyleFernández, Joaquín Hernández, Yoleima Guerra, and Heidi Cano. 2022. "Detection of Bisphenol A and Four Analogues in Atmospheric Emissions in Petrochemical Complexes Producing Polypropylene in South America" Molecules 27, no. 15: 4832. https://doi.org/10.3390/molecules27154832
APA StyleFernández, J. H., Guerra, Y., & Cano, H. (2022). Detection of Bisphenol A and Four Analogues in Atmospheric Emissions in Petrochemical Complexes Producing Polypropylene in South America. Molecules, 27(15), 4832. https://doi.org/10.3390/molecules27154832







