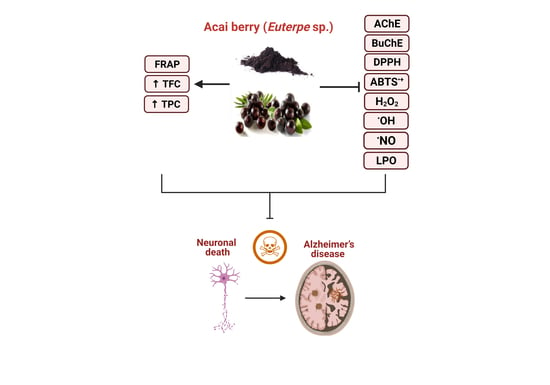
A Preliminary Assessment of the Nutraceutical Potential of Acai Berry (Euterpe sp.) as a Potential Natural Treatment for Alzheimer’s Disease
Abstract
:1. Introduction
2. Results
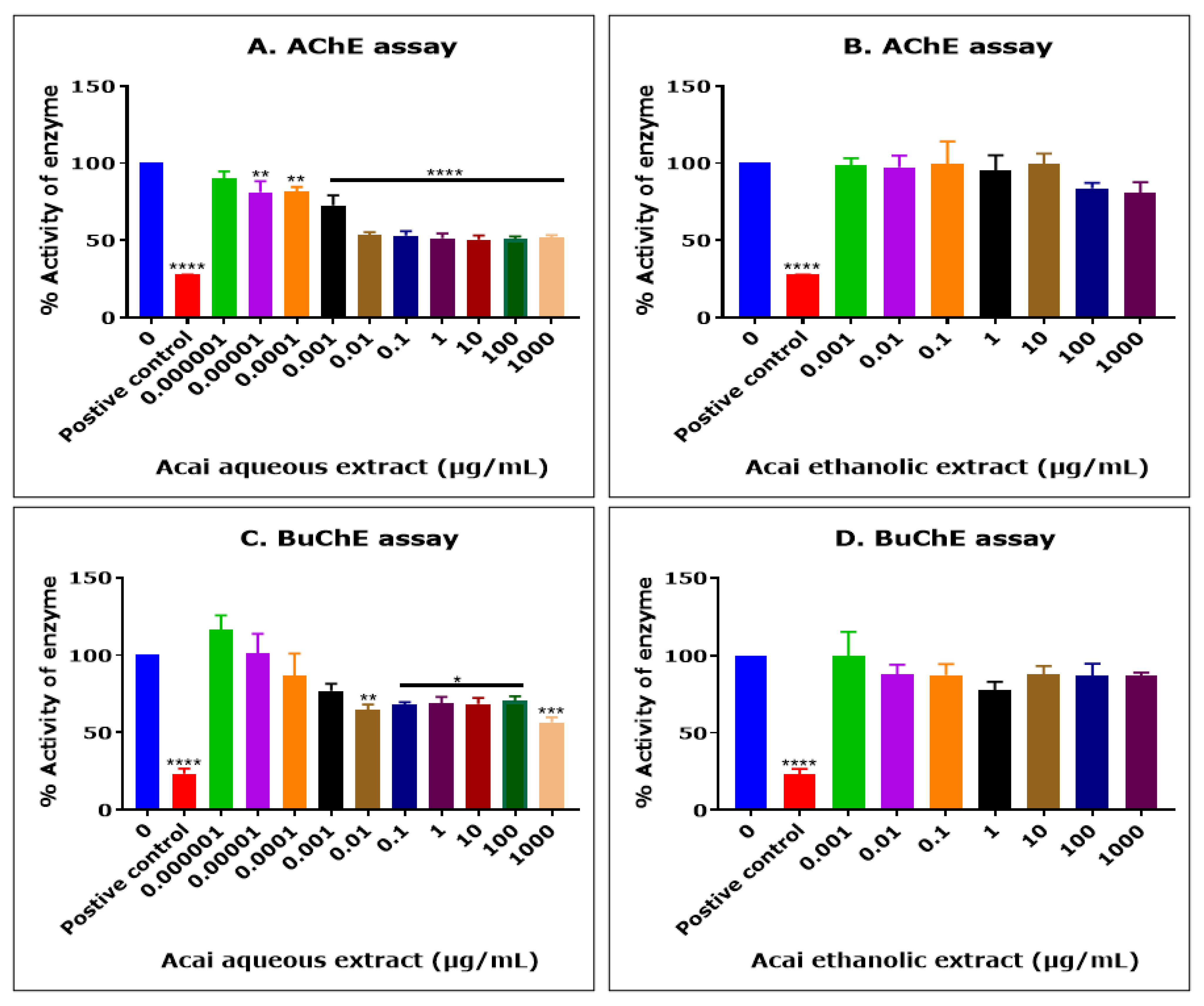
2.1. Acai Aqueous Extract Is a Cholinesterase Inhibitor
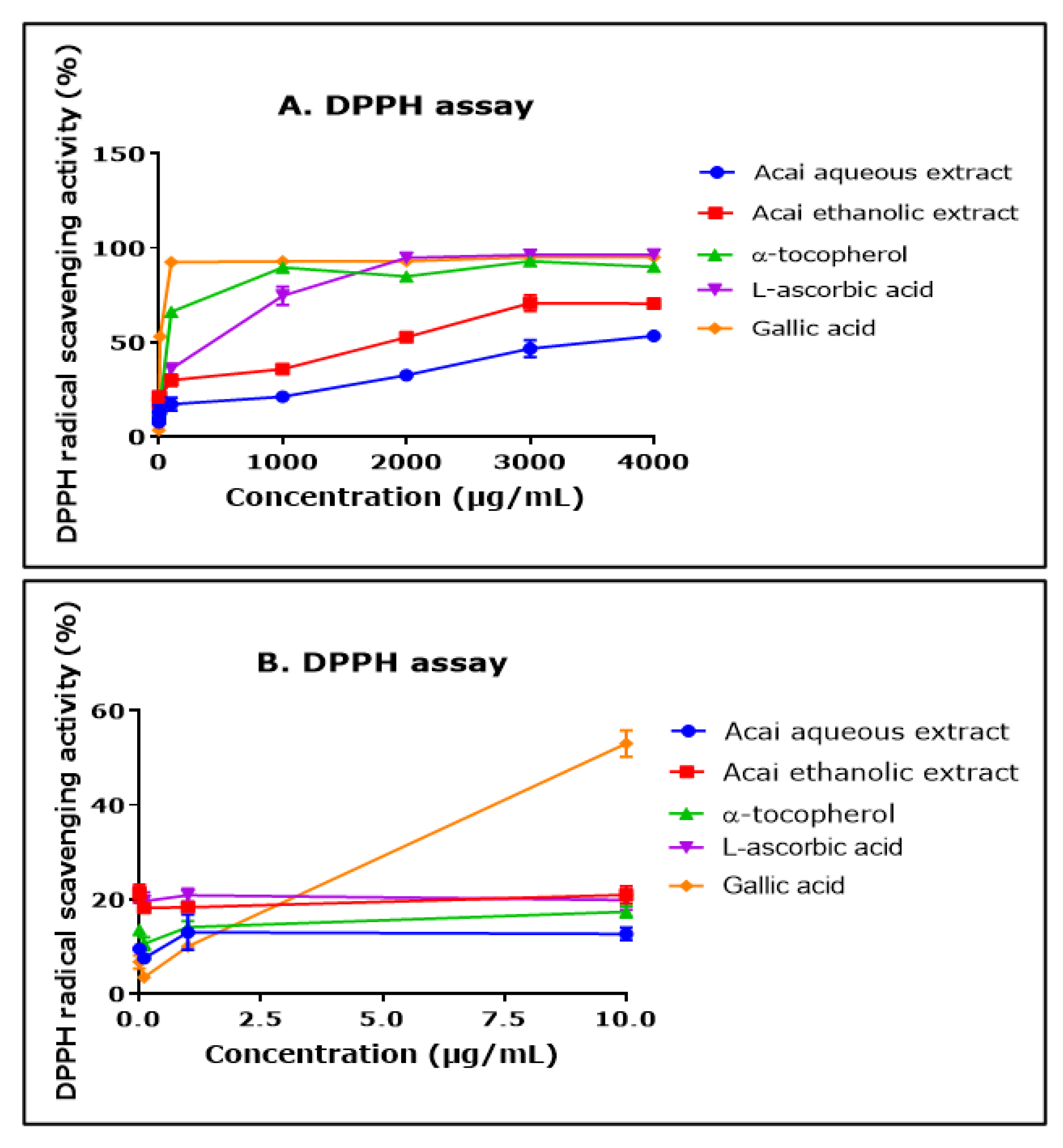
2.2. Acai Aqueous and Ethanolic Extracts Exhibit 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Free Radical Scavenging Activity
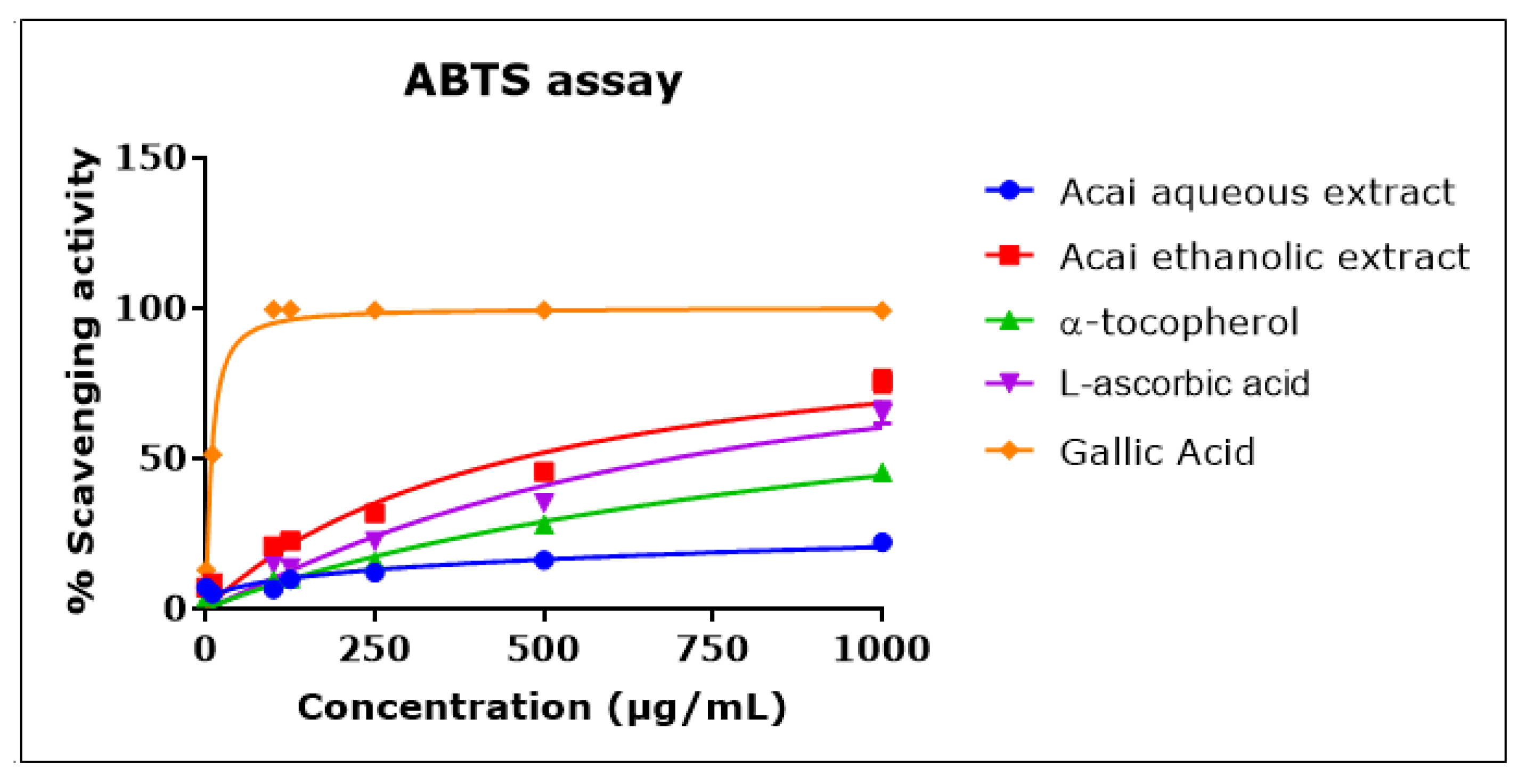
2.3. Acai Aqueous and Ethanolic Extracts Exhibit 2,2′-Azino-bis-3-ethylbenzthiazoline-6-sulphonic acid Radical Cation (ABTS•+) Scavenging Activity
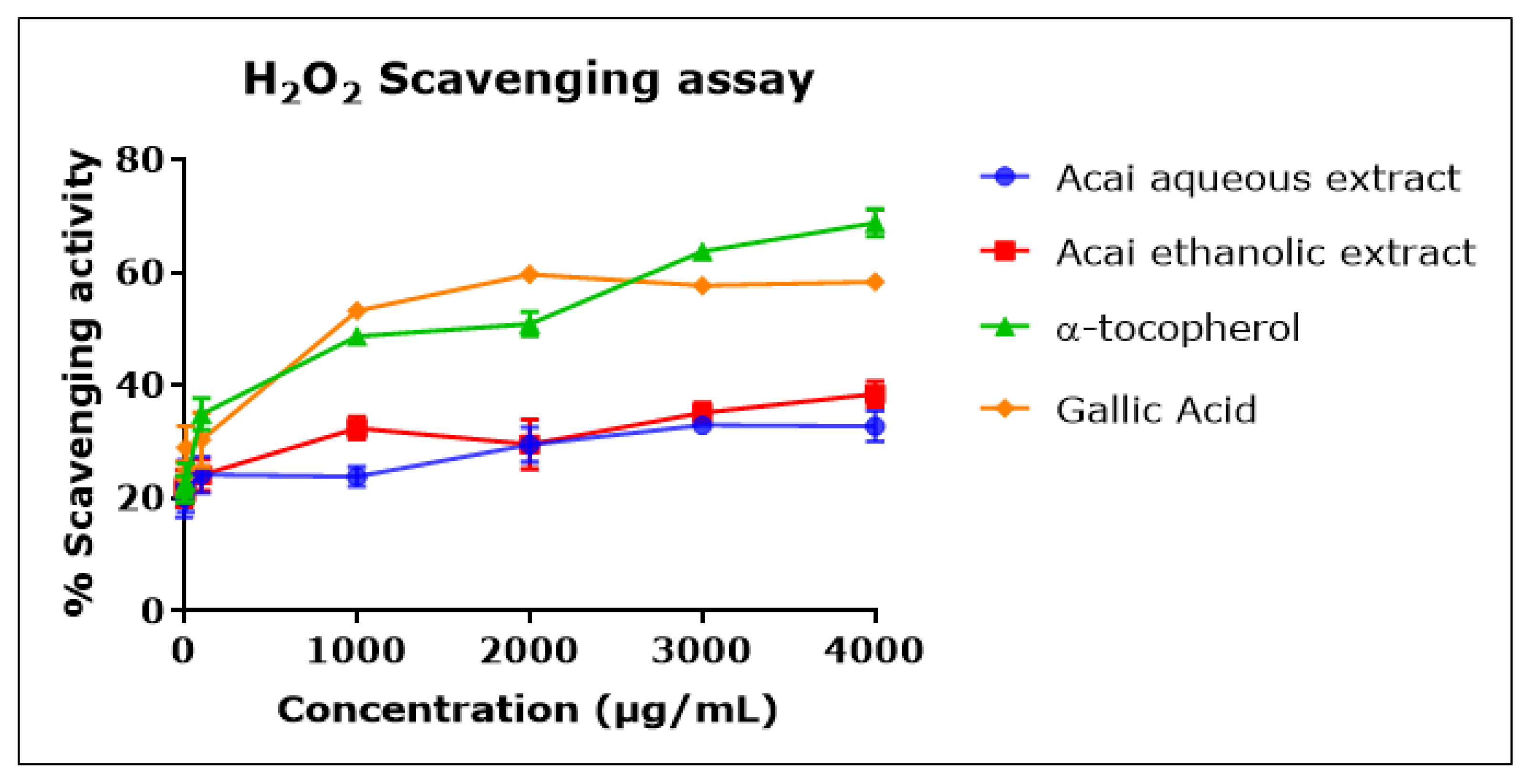
2.4. Acai Aqueous and Ethanolic Extracts Exhibit Hydrogen Peroxide (H2O2) Scavenging Activity
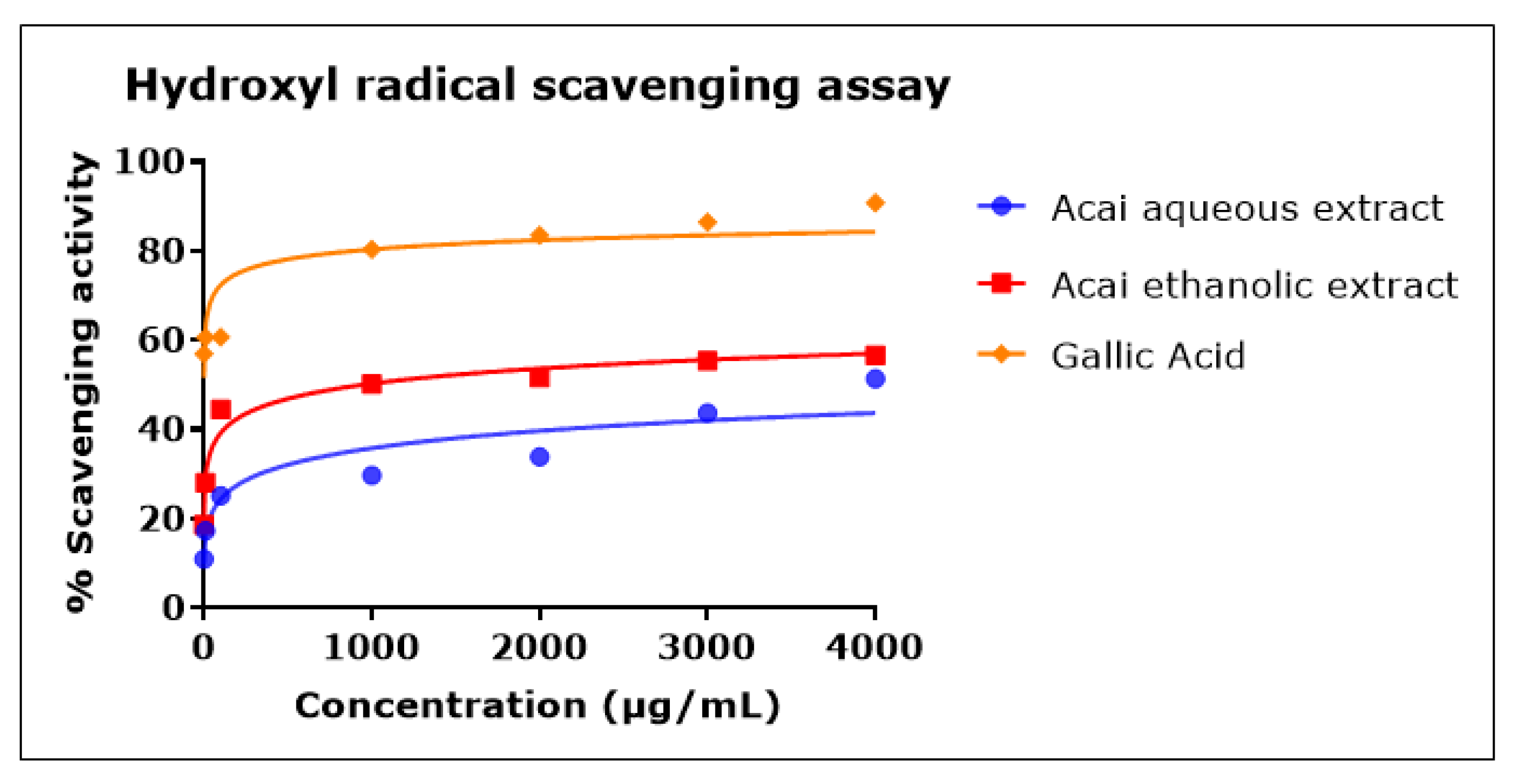
2.5. Acai Aqueous and Ethanolic Extracts Exhibit Hydroxyl Radical (•OH) Scavenging Activity
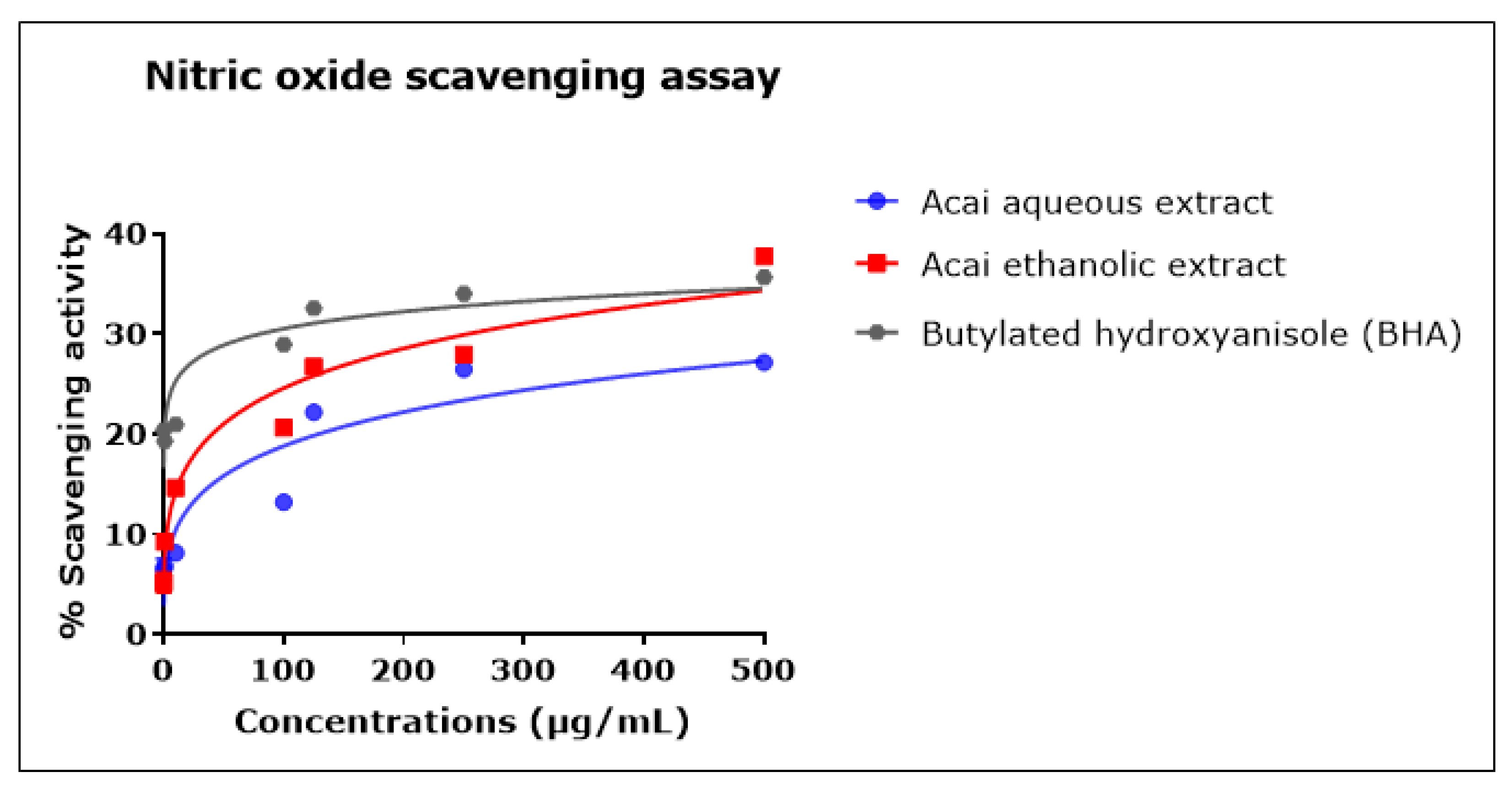
2.6. Acai Aqueous and Ethanolic Extracts Exhibit Nitric Oxide (•NO) Scavenging Activity
2.7. Acai Aqueous and Ethanolic Extracts Exhibit Lipid Peroxidation Inhibitory Activity
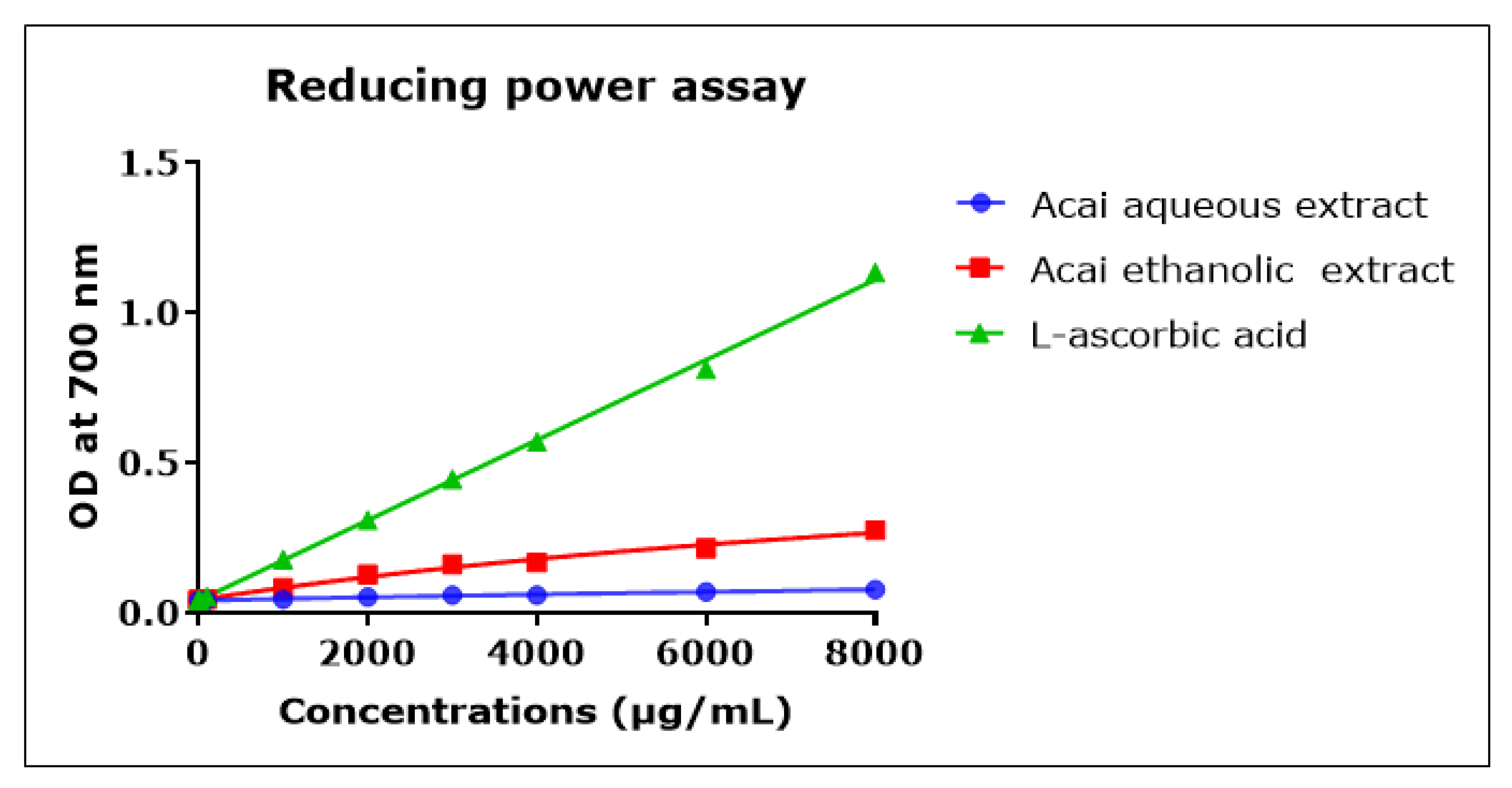
2.8. Acai Aqueous and Ethanolic Extracts Exhibit Reducing Power Activity
2.9. Total Phenolic and Total Flavonoid Content of Acai Berry Extracts
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Ethanolic and Aqueous Extracts of Acai Berry (Euterpe oleracea)
4.3. Cholinesterase Activity Assessments
4.4. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Free Radical Scavenging Activity
4.5. Radical 2,2′-Azino-bis-3-ethylbenzthiazoline-6-sulphonic acid Cation (ABTS•+) Scavenging Activity
4.6. Hydrogen Peroxide (H2O2) Scavenging Activity
4.7. Hydroxyl Radical (•OH) Scavenging Activity
4.8. Nitric Oxide Radical (•NO) Scavenging Activity
4.9. Lipid Peroxidation (LPO) Inhibitory Activity
4.10. Ferric-Reducing Antioxidant Power (FRAP) Assay
4.11. Total Phenolic Content Determination
4.12. Total Flavonoid Content Determination
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Alzheimer’s-Association. 2019 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- World-Alzheimer-Report. World Alzheimer’s Report 2018: The State of the Art of Dementia Research: New Frontiers. Available online: https://www.alz.co.uk/research/WorldAlzheimerReport2018.pdf (accessed on 26 June 2022).
- El-Hayek, Y.; Wiley, R.; Khoury, C.; Daya, R.; Ballard, C.; Evans, A.; Karran, M.; Molinuevo, J.; Norton, M.; Atri, A. Tip of the iceberg: Assessing the global socioeconomic costs of Alzheimer’s disease and related dementias and strategic implications for stakeholders. J. Alzheimers Dis. 2019, 70, 323–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzhemier’s-Association-Report. 2021 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2021, 17, 327–406. [Google Scholar] [CrossRef] [PubMed]
- Alzhemier’s-Association-Report. 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020, 16, 391–460. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Lopez, J.; Yachnis, A.; Prokop, S. Neuropathology of Alzheimer’s disease. Neurotherapeutics 2021, 19, 173–185. [Google Scholar] [CrossRef]
- Grand, J.; Caspar, S.; Macdonald, S. Clinical features and multidisciplinary approaches to dementia care. J. Multidiscip. Healthc. 2011, 4, 125–147. [Google Scholar] [CrossRef] [Green Version]
- Reitz, C.; Mayeux, R. Alzheimer disease: Epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem. Pharmacol. 2014, 88, 640–651. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural. Regen. Res. 2012, 7, 376. [Google Scholar] [CrossRef] [PubMed]
- Denzer, I.; Muench, G.; Friedland, K. Modulation of mitochondrial dysfunction in neurodegenerative diseases via activation of nuclear factor erythroid-2-related factor 2 by food-derived compounds. Pharmacol. Res. 2016, 103, 80–94. [Google Scholar] [CrossRef]
- Kovacs, G. Molecular pathological classification of neurodegenerative diseases: Turning towards precision medicine. Int. J. Mol. Sci. 2016, 17, 189. [Google Scholar] [CrossRef]
- Lewerenz, J.; Maher, P. Chronic glutamate toxicity in neurodegenerative diseases—what is the evidence? Front. Neurosci. 2015, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jiang, L. Neuroinflammation in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2015, 11, 243–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mufson, E.; Counts, S.; Perez, S.; Ginsberg, S. Cholinergic system during the progression of Alzheimer’s disease: Therapeutic implications. Expert Rev. Neurother. 2008, 8, 1703–1718. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Zhang, D.; Zeng, Y.; Huang, T.; Xu, H.; Zhao, Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Misrani, A.; Tabassum, S.; Yang, L. Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease. Front. Aging Neurosci. 2021, 13, 617588. [Google Scholar] [CrossRef]
- ALNasser, M.; Mellor, I.; Carter, W. Is L-Glutamate toxic to neurons and thereby contributes to neuronal loss and neurodegeneration? A systematic review. Brain Sci. 2022, 12, 577. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.; Croteau, D.; Bohr, V. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Loy, C.; Schofield, P.; Turner, A.; Kwok, J. Genetics of dementia. Lancet 2014, 383, 828–840. [Google Scholar] [CrossRef]
- Green, R.; Cupples, A.; Go, R.; Benke, K.; Edeki, T.; Griffith, P.; Williams, M.; Hipps, Y.; Graff-Radford, N.; Bachman, D. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA 2002, 287, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Jakaria, M.; Park, S.; Haque, M.; Karthivashan, G.; Kim, I.; Ganesan, P.; Choi, D. Neurotoxic agent-induced injury in neurodegenerative disease model: Focus on involvement of glutamate receptors. Front. Mol. Neurosci. 2018, 11, 307. [Google Scholar] [CrossRef]
- Modgil, S.; Lahiri, D.; Sharma, V.; Anand, A. Role of early life exposure and environment on neurodegeneration: Implications on brain disorders. Transl. Neurodegener. 2014, 3, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, R. Risk factors for Alzheimer’s disease. Folia Neuropathol 2019, 57, 87–105. [Google Scholar] [CrossRef] [Green Version]
- Massoud, F.; Léger, G. Pharmacological treatment of Alzheimer disease. Can. J. Psychiatry 2011, 56, 579–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winslow, B.; Onysko, M.; Stob, C.; Hazlewood, K. Treatment of Alzheimer disease. Am Fam Physician 2011, 83, 1403–1412. [Google Scholar] [PubMed]
- Yiannopoulou, K.; Papageorgiou, S. Current and future treatments for Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2013, 6, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Féger, J.; Hirsch, E. In search of innovative therapeutics for neuropsychiatric disorders: The case of neurodegenerative diseases. Ann. Pharm. Fr. 2015, 73, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-D.; Zhang, Y.-H.; Zhang, W.; Zhao, P. Meta-analysis of randomized controlled trials on the efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease. Front. Neurosci. 2019, 13, 472. [Google Scholar] [CrossRef] [PubMed]
- Atri, A. Current and future treatments in Alzheimer’s disease. Semin. Neurol. 2019, 39, 227–240. [Google Scholar] [CrossRef]
- Yiannopoulou, K.; Papageorgiou, S. Current and future treatments in Alzheimer disease: An update. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520907397. [Google Scholar] [CrossRef] [Green Version]
- Sutherland, G.; Chami, B.; Youssef, P.; Witting, P. Oxidative stress in Alzheimer’s disease: Primary villain or physiological by-product? Redox. Rep. 2013, 18, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Barbagallo, M.; Marotta, F.; Dominguez, L. Oxidative stress in patients with Alzheimer’s disease: Effect of extracts of fermented papaya powder. Mediators Inflamm. 2015, 2015, 624801. [Google Scholar] [CrossRef] [Green Version]
- Su, B.; Wang, X.; Nunomura, A.; Moreira, P.; Lee, H.; Perry, G.; Smith, M.; Zhu, X. Oxidative stress signaling in Alzheimer’s disease. Curr. Alzheimer. Res. 2008, 5, 525–532. [Google Scholar] [CrossRef]
- Cai, Z.; Zhao, B.; Ratka, A. Oxidative stress and β-Amyloid protein in Alzheimer’s disease. Neuromolecular. Med. 2011, 13, 223–250. [Google Scholar] [CrossRef]
- Youssef, P.; Chami, B.; Lim, J.; Middleton, T.; Sutherland, G.; Witting, P. Evidence supporting oxidative stress in a moderately affected area of the brain in Alzheimer’s disease. Sci. Rep. 2018, 8, 11553. [Google Scholar] [CrossRef] [Green Version]
- Lovell, M.; Markesbery, W. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer’s disease. Nucleic. Acids Res. 2007, 35, 7497–7504. [Google Scholar] [CrossRef] [Green Version]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar]
- AlAli, M.; Alqubaisy, M.; Aljaafari, M.; AlAli, A.; Baqais, L.; Molouki, A.; Abushelaibi, A.; Lai, K.; Lim, S. Nutraceuticals: Transformation of conventional foods into health promoters/disease preventers and safety considerations. Molecules 2021, 26, 2540. [Google Scholar] [CrossRef] [PubMed]
- Bigford, G.; Del Rossi, G. Supplemental substances derived from foods as adjunctive therapeutic agents for treatment of neurodegenerative diseases and disorders. Adv. Nutr. 2014, 5, 394–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macready, A.; Kennedy, O.; Ellis, J.; Williams, C.; Spencer, J.; Butler, L. Flavonoids and cognitive function: A review of human randomized controlled trial studies and recommendations for future studies. Genes Nutr. 2009, 4, 227. [Google Scholar] [CrossRef] [Green Version]
- Van de Rest, O.; Berendsen, A.; Haveman-Nies, A.; de Groot, L. Dietary patterns, cognitive decline, and dementia: A systematic review. Adv. Nutr. 2015, 6, 154–168. [Google Scholar] [CrossRef]
- Krikorian, R.; Nash, T.; Shidler, M.; Shukitt-Hale, B.; Joseph, J. Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Br. J. Nutr. 2010, 103, 730–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kean, R.; Lamport, D.; Dodd, G.; Freeman, J.; Williams, C.; Ellis, J.; Butler, L.; Spencer, J. Chronic consumption of flavanone-rich orange juice is associated with cognitive benefits: An 8-wk, randomized, double-blind, placebo-controlled trial in healthy older adults. Am. J. Clin. Nutr. 2015, 101, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, A.; Salo, I.; Plaza, M.; Björck, I. Effects of a mixed berry beverage on cognitive functions and cardiometabolic risk markers; A randomized cross-over study in healthy older adults. PLoS ONE 2017, 12, e0188173. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.J.; Hoggard, N.; Aceves-Martins, M. The effect of grape interventions on cognitive and mental performance in healthy participants and those with mild cognitive impairment: A systematic review of randomized controlled trials. Nutr. Rev. 2021, 80, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, J.; Zafrilla, M.; Marhuenda, J. Cognitive function and consumption of fruit and vegetable polyphenols in a young population: Is there a relationship? Foods 2019, 8, 507. [Google Scholar] [CrossRef] [Green Version]
- Grodzicki, W.; Dziendzikowska, K. The role of selected bioactive compounds in the prevention of Alzheimer’s disease. Antioxidants 2020, 9, 229. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Huang, J.; Song, D.; Deng, R.; Wei, J.; Zhang, Z. Increased consumption of fruit and vegetables is related to a reduced risk of cognitive impairment and dementia: Meta-analysis. Front. Aging Neurosci. 2017, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, K.; Pereira, L.; Lamarão, C.; Lima, E.; da Veiga-Junior, V. Amazon acai: Chemistry and biological activities: A review. Food Chem. 2015, 179, 137–151. [Google Scholar] [CrossRef]
- De Souza, F.; de Araújo, F.; de Paulo Farias, D.; Zanotto, A.; Neri-Numa, I.; Pastore, G. Brazilian fruits of Arecaceae family: An overview of some representatives with promising food, therapeutic and industrial applications. Food Res. Int. 2020, 138, 109690. [Google Scholar] [CrossRef] [PubMed]
- Benatrehina, A.; Pan, L.; Naman, B.; Li, J.; Kinghorn, D. Usage, biological activity, and safety of selected botanical dietary supplements consumed in the United States. J. Tradit. Complement Med. 2018, 8, 267–277. [Google Scholar] [CrossRef]
- Jensen, G.; Wu, X.; Patterson, K.; Barnes, J.; Carter, S.; Scherwitz, L.; Beaman, R.; Endres, J.; Schauss, A. In Vitro and in vivo antioxidant and anti-inflammatory capacities of an antioxidant-rich fruit and berry juice blend. results of a pilot and randomized, double-blinded, placebo-controlled, crossover study. J. Agric. Food Chem. 2008, 56, 8326–8333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulose, S.; Fisher, D.; Larson, J.; Bielinski, D.; Rimando, A.; Carey, A.; Schauss, A.; Shukitt-Hale, B. Anthocyanin-rich açai (Euterpe oleracea Mart.) fruit pulp fractions attenuate inflammatory stress signaling in mouse brain BV-2 microglial cells. J. Agric. Food Chem. 2012, 60, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Spada, P.; Dani, C.; Bortolini, G.; Funchal, C.; Henriques, J.; Salvador, M. Frozen fruit pulp of Euterpe oleraceae Mart. (acai) prevents hydrogen peroxide-induced damage in the cerebral cortex, cerebellum, and hippocampus of rats. J. Med. Food 2009, 12, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Fe3+–Fe2+ transformation method: An important antioxidant assay. In Advanced Protocols in Oxidative Stress III; Springer: Berlin/Heidelberg, Germany, 2015; pp. 233–246. [Google Scholar]
- Ferreira-Vieira, T.; Guimaraes, I.; Silva, F.; Ribeiro, F. Alzheimer’s disease: Targeting the cholinergic system. Curr Neuropharmacol 2016, 14, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Cummings, J.; Tong, G.; Ballard, C. Treatment combinations for Alzheimer’s disease: Current and future pharmacotherapy options. J. Alzheimers. Dis. 2019, 67, 779–794. [Google Scholar] [CrossRef] [Green Version]
- Amat-Ur-Rasool, H.; Ahmed, M.; Hasnain, S.; Carter, W. Anti-cholinesterase combination drug therapy as a potential treatment for Alzheimer’s disease. Brain Sci. 2021, 11, 184. [Google Scholar] [CrossRef]
- Collins, A.; Saleh, T.; Kalisch, B. Naturally occurring antioxidant therapy in Alzheimer’s disease. Antioxidants 2022, 11, 213. [Google Scholar] [CrossRef]
- Wang, E.; Wu, M.; Lu, J. Ferulic acid in animal models of Alzheimer’s disease: A systematic review of preclinical studies. Cells 2021, 10, 2653. [Google Scholar] [CrossRef]
- Dvir, H.; Silman, I.; Harel, M.; Rosenberry, T.; Sussman, J. Acetylcholinesterase: From 3D structure to function. Chem. Biol. Interact. 2010, 187, 10–22. [Google Scholar] [CrossRef] [Green Version]
- Rosenberry, T. Strategies to resolve the catalytic mechanism of acetylcholinesterase. J. Mol. Neurosci. 2010, 40, 32–39. [Google Scholar] [CrossRef]
- Bajda, M.; Więckowska, A.; Hebda, M.; Guzior, N.; Sotriffer, C.; Malawska, B. Structure-based search for new inhibitors of cholinesterases. Int. J. Mol. Sci. 2013, 14, 5608–5632. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.; Kiametis, A.; Treptow, W. Donepezil inhibits acetylcholinesterase via multiple binding modes at room temperature. J. Chem. Inf. Model 2020, 60, 3463–3471. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.; Lightfoot, D. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Chung, H.-J. Physiological activity of acai berry (Euterpe oleracea Mart.) extracted with different solvents. J. Korean Soc. Food Cult. 2012, 27, 75–81. [Google Scholar] [CrossRef] [Green Version]
- López de Dicastillo, C.; Piña, C.; Garrido, L.; Arancibia, C.; Galotto, M. Enhancing thermal stability and bioaccesibility of Açaí fruit polyphenols through electrohydrodynamic encapsulation into zein electrosprayed particles. Antioxidants 2019, 8, 464. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Huang, G. The biological activities of butyrylcholinesterase inhibitors. Biomed. Pharmacother. 2022, 146, 112556. [Google Scholar] [CrossRef]
- Li, Q.; Yang, H.; Chen, Y.; Sun, H. Recent progress in the identification of selective butyrylcholinesterase inhibitors for Alzheimer’s disease. Eur. J. Med. Chem. 2017, 132, 294–309. [Google Scholar] [CrossRef]
- Amat-ur-Rasool, H.; Ahmed, M.; Hasnain, S.; Ahmed, A.; Carter, W. In silico design of dual-binding site anti-cholinesterase phytochemical heterodimers as treatment options for Alzheimer’s disease. Curr. Issues Mol. Biol. 2022, 44, 152–175. [Google Scholar] [CrossRef]
- Mushtaq, G.; Greig, N.; Khan, J.; Kamal, M. Status of acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol. Disord. Drug Targets 2014, 13, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Han, Y. Pharmacological profile of huperzine a, a novel acetylcholinesterase inhibitor from Chinese herb. CNS Drug Rev. 2006, 5, 281–300. [Google Scholar] [CrossRef] [Green Version]
- Ogura, H.; Kosasa, T.; Kuriya, Y.; Yamanishi, Y. Comparison of inhibitory activities of donepezil and other cholinesterase inhibitors on acetylcholinesterase and butyrylcholinesterase in vitro. Methods Find Exp. Clin. Pharmacol. 2000, 22, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.; Gomes, T.; Pinto, B.; Camara, A.; Paes, A. Naturally occurring acetylcholinesterase inhibitors and their potential use for Alzheimer’s disease therapy. Front. Pharmacol. 2018, 9, 1192. [Google Scholar] [CrossRef] [Green Version]
- Schauss, A.; Wu, X.; Prior, R.; Ou, B.; Huang, D.; Owens, J.; Agarwal, A.; Jensen, G.; Hart, A.; Shanbrom, E. Antioxidant capacity and other bioactivities of the freeze-dried Amazonian palm berry, Euterpe oleraceae Mart. (acai). J. Agric. Food Chem. 2006, 54, 8604–8610. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Andreazza, A.; da Silva, T.; Boligon, A.; do Nascimento, V.; Scola, G.; Duong, A.; Cadona, F.; Ribeiro, E.; da Cruz, I. Neuroprotective effects of acai (Euterpe oleracea Mart.) against rotenone in vitro exposure. Oxid. Med. Cell Longev. 2016, 2016, 8940850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matheus, M.; Fernandes, S.; Silveira, C.; Rodrigues, V.; Menezes, F.; Fernandes, P. Inhibitory effects of Euterpe oleracea Mart. on nitric oxide production and iNOS expression. J. Ethnopharmacol. 2006, 107, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Mertens-Talcott, S.; Rios, J.; Jilma-Stohlawetz, P.; Pacheco-Palencia, L.; Meibohm, B.; Talcott, S.; Derendorf, H. Pharmacokinetics of anthocyanins and antioxidant effects after the consumption of anthocyanin-rich açai juice and pulp (Euterpe oleracea Mart.) in human healthy volunteers. J. Agric. Food Chem. 2008, 56, 7796–7802. [Google Scholar] [CrossRef] [PubMed]
- Poulose, S.; Bielinski, D.; Carey, A.; Schauss, A.; Shukitt-Hale, B. Modulation of oxidative stress, inflammation, autophagy and expression of Nrf2 in hippocampus and frontal cortex of rats fed with açaí-enriched diets. Nutr. Neurosci. 2017, 20, 305–315. [Google Scholar] [CrossRef]
- Kang, J.; Xie, C.; Li, Z.; Nagarajan, S.; Schauss, A.; Wu, T.; Wu, X. Flavonoids from acai (Euterpe oleracea Mart.) pulp and their antioxidant and anti-inflammatory activities. Food Chem. 2011, 128, 152–157. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.; Mertens-Talcott, S.; Talcott, S. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from açai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2008, 56, 4631–4636. [Google Scholar] [CrossRef]
- Nwidu, L.; Elmorsy, E.; Aprioku, J.; Siminialayi, I.; Carter, W. In Vitro anti-Cholinesterase and antioxidant activity of extracts of Moringa oleifera plants from Rivers State, Niger Delta, Nigeria. Medicines 2018, 5, 71. [Google Scholar] [CrossRef] [Green Version]
- Wong, D.; Musgrave, I.; Harvey, B.; Smid, S. Açaí (Euterpe oleraceae Mart.) berry extract exerts neuroprotective effects against β-amyloid exposure in vitro. Neurosci. Lett. 2013, 556, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.; Courtney, K.; Andres, V.; Feather-Stone, R. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Carter, W.; Tarhoni, M.; Rathbone, A.; Ray, D. Differential protein adduction by seven organophosphorus pesticides in both brain and thymus. Hum. Exp. Toxicol. 2007, 26, 347–353. [Google Scholar] [CrossRef]
- Dorling, J.; Clayton, D.; Jones, J.; Carter, W.; Thackray, A.; King, J.; Pucci, A.; Batterham, R.; Stensel, D. A randomized crossover trial assessing the effects of acute exercise on appetite, circulating ghrelin concentrations, and butyrylcholinesterase activity in normal-weight males with variants of the obesity-linked FTO rs9939609 polymorphism. Am. J. Clin. Nutr. 2019, 110, 1055–1066. [Google Scholar] [CrossRef]
- Nwidu, L.; Elmorsy, E.; Thornton, J.; Wijamunige, B.; Wijesekara, A.; Tarbox, R.; Warren, A.; Carter, W. Anti-acetylcholinesterase activity and antioxidant properties of extracts and fractions of Carpolobia lutea. Pharm. Biol. 2017, 55, 1875–1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acharya, K. Simplified methods for microtiter based analysis of in vitro antioxidant activity. Asian J. Pharm. 2017, 11, S327–S335. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.; Bristi, N.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, V.; Park, Y.; Agrawal, P. Studies on phytochemical analysis, antioxidant and lipid peroxidation inhibitory effects of a medicinal plant, Coleus forskohlii. Front. Life Sci. 2015, 8, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B.; Gutteridge, J. Formation of a thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts. FEBS Lett. 1981, 128, 347–352. [Google Scholar] [CrossRef] [Green Version]
- Unuofin, J.; Otunola, G.; Afolayan, A. Polyphenolic content, antioxidant and antimicrobial activities of Vernonia mespilifolia Less. Used in folk medicine in the Eastern Cape Province, South Africa. J. Evid. Based Integr. Med. 2018, 23, 2515690X18773990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimoh, M.; Afolayan, A.; Lewu, F. Antioxidant and phytochemical activities of Amaranthus caudatus L. harvested from different soils at various growth stages. Sci. Rep. 2019, 9, 12965. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.; Esteras, N.; Abramov, A. Mitochondria and lipid peroxidation in the mechanism of neurodegeneration: Finding ways for prevention. Med. Res. Rev. 2021, 41, 770–784. [Google Scholar] [CrossRef]
- Akomolafe, S.; Oboh, G.; Akindahunsi, A.; Akinyemi, A.; Tade, O. Inhibitory effect of aqueous extract of stem bark of Cissus populnea on ferrous sulphate- and sodium nitroprusside-induced oxidative stress in rat’s testes in vitro. ISRN Pharmacol. 2013, 2013, 130989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, L.; Theodoridou, K.; Sheldrake, G.; Walsh, P. A critical review of analytical methods used for the chemical characterisation and quantification of phlorotannin compounds in brown seaweeds. Phytochem. Anal. 2019, 30, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Da Silveira, T.; de Souza, T.; Carvalho, A.; Ribeiro, A.; Kuhnle, G.; Godoy, H. White açaí juice (Euterpe oleracea): Phenolic composition by LC-ESI-MS/MS, antioxidant capacity and inhibition effect on the formation of colorectal cancer related compounds. J. Funct. Foods 2017, 36, 215–223. [Google Scholar] [CrossRef]
- ALNasser, M.; Mellor, I. Neuroprotective activities of acai berries (Euterpe sp.): A review. J. Herbmed. Pharmacol. 2022, 11, 166–181. [Google Scholar] [CrossRef]
- Alqurashi, R.; Commane, D.; Rowland, I. Açai fruit as a source of bioactive phytochemicals. J. Life Sci. 2016, 10, 391–404. [Google Scholar] [CrossRef] [Green Version]
- Schauss, A.; Wu, X.; Prior, R.; Ou, B.; Patel, D.; Huang, D.; Kababick, J. Phytochemical and nutrient composition of the freeze-dried Amazonian palm berry, Euterpe oleraceae Mart. (acai). J. Agric. Food Chem. 2006, 54, 8598–8603. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, S.; Zargaham, M.; Khan, A.; Khan, S.; Hussain, A.; Uddin, J.; Khan, A.; Al-Harrasi, A. Potential therapeutic natural products against Alzheimer’s disease with reference of acetylcholinesterase. Biomed. Pharmacother. 2021, 139, 111609. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Marya; Amin, S.; Kamal, M.A.; Patel, S. Flavonoids as acetylcholinesterase inhibitors: Current therapeutic standing and future prospects. Biomed. Pharmacother. 2018, 101, 860–870. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Szymanowska, D.; Maciejewska, P.; Kmiecik, D.; Gramza-Michałowska, A.; Kulczyński, B.; Cielecka-Piontek, J. In Vitro screening for acetylcholinesterase and butyrylcholinesterase inhibition and antimicrobial activity of chia seeds (Salvia hispanica). Electron. J. Biotechnol. 2019, 37, 1–10. [Google Scholar] [CrossRef]
- Birsan, R.; Wilde, P.; Waldron, K.; Rai, D. Anticholinesterase activities of different solvent extracts of brewer’s spent grain. Foods 2021, 10, 930. [Google Scholar] [CrossRef]
- Budryn, G.; Majak, I.; Grzelczyk, J.; Szwajgier, D.; Rodríguez-Martínez, A.; Pérez-Sánchez, H. Hydroxybenzoic acids as acetylcholinesterase inhibitors: Calorimetric and docking simulation studies. Nutrients 2022, 14, 2476. [Google Scholar] [CrossRef]
- Orhan, I.; Kartal, M.; Tosun, F.; Sener, B. Screening of various phenolic acids and flavonoid derivatives for their anticholinesterase potential. J. Biosci. 2007, 62, 829–832. [Google Scholar] [CrossRef]
- Woo, Y.-J.; Lee, B.-H.; Yeun, G.-H.; Kim, H.-J.; Won, M.-H.; Kim, S.-H.; Lee, B.-H.; Park, J.-H. Selective butyrylcholinesterase inhibitors using polyphenol-polyphenol hybrid molecules. Bull Korean Chem. Soc. 2011, 32, 2593–2598. [Google Scholar] [CrossRef] [Green Version]
- Salau, V.; Erukainure, O.; Ibeji, C.; Olasehinde, T.; Koorbanally, N.; Islam, M. Vanillin and vanillic acid modulate antioxidant defense system via amelioration of metabolic complications linked to Fe2+-induced brain tissues damage. Metab. Brain Dis. 2020, 35, 727–738. [Google Scholar] [CrossRef]
- Jabir, N.; Khan, F.; Tabrez, S. Cholinesterase targeting by polyphenols: A therapeutic approach for the treatment of Alzheimer’s disease. CNS Neurosci. Ther. 2018, 24, 753–762. [Google Scholar] [CrossRef]
- Oh, J.; Jang, H.; Kang, M.; Song, S.; Kim, D.; Kim, J.; Noh, J.; Park, J.; Park, D.; Yee, S.; et al. Acetylcholinesterase and monoamine oxidase-B inhibitory activities by ellagic acid derivatives isolated from Castanopsis cuspidata var. sieboldii. Sci. Rep. 2021, 11, 13953. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Kwon, H.; Cho, E.; Jeon, J.; Kang, R.; Youn, K.; Jun, M.; Lee, Y.; Ryu, J.; Kim, D. The effects of pinoresinol on cholinergic dysfunction-induced memory impairments and synaptic plasticity in mice. Food Chem. Toxicol. 2019, 125, 376–382. [Google Scholar] [CrossRef] [PubMed]
- El-Hassan, A.; El-Sayed, M.; Hamed, A.; Rhee, I.; Ahmed, A.; Zeller, K.; Verpoorte, R. Bioactive constituents of Leptadenia arborea. Fitoterapia 2003, 74, 184–187. [Google Scholar] [CrossRef]
- Tang, H.-Y.; Bai, M.-M.; Tian, J.-M.; Pescitelli, G.; Ivšić, T.; Huang, X.-H.; Lee, H.; Son, Y.N.; Kim, J.H.; Kim, Y. Chemical components from the seeds of Catalpa bungei and their inhibitions of soluble epoxide hydrolase, cholinesterase and nuclear factor kappa B activities. RSC Adv. 2016, 6, 40706–40716. [Google Scholar] [CrossRef]
- Geiss, J.; Sagae, S.; Paz, E.; de Freitas, M.; Souto, N.; Furian, A.; Oliveira, M.; Guerra, G. Oral administration of lutein attenuates ethanol-induced memory deficit in rats by restoration of acetylcholinesterase activity. Physiol. Behav. 2019, 204, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Xu, J.; Zhao, H.; Jiang, W.; Guo, X.; Zhao, M.; Sun-Waterhouse, D.; Zhao, Q.; Su, G. Antioxidant and anti-acetylcholinesterase activities of anchovy (Coilia mystus) protein hydrolysates and their memory-improving effects on scopolamine-induced amnesia mice. Int. J. Food Sci. Technol. 2017, 52, 504–510. [Google Scholar] [CrossRef]
- Cásedas, G.; Les, F.; González-Burgos, E.; Gómez-Serranillos, M.; Smith, C.; López, V. Cyanidin-3-O-glucoside inhibits different enzymes involved in central nervous system pathologies and type-2 diabetes. S. Afr. J. Bot. 2019, 120, 241–246. [Google Scholar] [CrossRef]
- Uriarte-Pueyo, I.; Calvo, M. Flavonoids as acetylcholinesterase inhibitors. Curr. Med. Chem. 2011, 18, 5289–5302. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, S.-H.; Lee, H.W.; Sun, Y.; Jang, W.-H.; Yang, S.-Y.; Jang, H.-D.; Kim, Y. (−)-Epicatechin derivate from Orostachys japonicus as potential inhibitor of the human butyrylcholinesterase. Int. J. Biol. Macromol. 2016, 91, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Okello, E.; Mather, J. Comparative kinetics of acetyl- and butyryl-cholinesterase inhibition by green tea catechins|relevance to the symptomatic treatment of Alzheimer’s disease. Nutrients 2020, 12, 1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucukboyacı, N.; Orhan, I.; Şener, B.; Nawaz, S.; Choudhary, M. Assessment of Enzyme Inhibitory and Antioxidant Activities of Lignans from Taxus baccata L. J. Biosci. 2010, 65, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D.; Borowiec, K. Phenolic acids from malt are efficient acetylcholinesterase and butyrylcholinesterase inhibitors. J. Inst. Brew. 2012, 118, 40–48. [Google Scholar] [CrossRef]
- Chunhakant, S.; Chaicharoenpong, C. Antityrosinase, Antioxidant, and Cytotoxic Activities of Phytochemical Constituents from Manilkara zapota L. Bark. Molecules 2019, 24, 2798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaoglan, E.; Koca, M. Tyrosinase and cholinesterase inhibitory activities and molecular docking studies on apigenin and vitexin. J. Pharm. Istanbul. Univ. 2020, 50, 268–272. [Google Scholar] [CrossRef]
- Choi, J.; Nurul Islam, M.; Yousof Ali, M.; Kim, E.; Kim, Y.; Jung, H. Effects of C-glycosylation on anti-diabetic, anti-Alzheimer’s disease and anti-inflammatory potential of apigenin. Food Chem. Toxicol. 2014, 64, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Rigano, D.; Menichini, F.; Loizzo, M.; Senatore, F. Protection against neurodegenerative diseases of Iris pseudopumila extracts and their constituents. Fitoterapia 2009, 80, 62–67. [Google Scholar] [CrossRef]
- Khaw, K.-Y.; Murugaiyah, V.; Khairuddean, M.; Tan, W.-N. Garcinexanthone G, a Selective Butyrylcholinesterase Inhibitor from the Stem Bark of Garcinia atroviridis. Nat. Prod. Sci. 2018, 24, 88–92. [Google Scholar] [CrossRef]
- Fang, Z.; Jeong, S.; Jung, H.; Choi, J.; Min, B.; Woo, M. Anticholinesterase and antioxidant constituents from Gloiopeltis furcata. Chem. Pharm. Bull. 2010, 58, 1236–1239. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Su, X.; Lu, J.; Wu, M.; Yang, S.; Mai, Y.; Deng, W.; Xue, Y. In Vitro and in silico analysis of phytochemicals from fallopia dentatoalata as dual functional cholinesterase inhibitors for the treatment of Alzheimer’s disease. Front. Pharmacol. 2022, 13, 905708. [Google Scholar] [CrossRef]
- Ayaz, M.; Junaid, M.; Ullah, F.; Subhan, F.; Sadiq, A.; Ali, G.; Ovais, M.; Shahid, M.; Ahmad, A.; Wadood, A.; et al. Anti-Alzheimer’s Studies on β-Sitosterol Isolated from Polygonum hydropiper L. Front. Pharmacol. 2017, 8, 697. [Google Scholar] [CrossRef] [Green Version]
- Sultana, N.; Khalid, A. Phytochemical and enzyme inhibitory studies on indigenous medicinal plant Rhazya stricta. Nat. Prod. Res. 2010, 24, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Kissling, J.; Ioset, J.R.; Marston, A.; Hostettmann, K. Bio-guided isolation of cholinesterase inhibitors from the bulbs of Crinum x powellii. Phytother. Res. 2005, 19, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Copper, aluminum, iron and calcium inhibit human acetylcholinesterase in vitro. Environ. Toxicol. Pharmacol. 2014, 37, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Mercado, A.T.; Vazquez-Armenta, F.J.; Tapia-Rodriguez, M.R.; Islas-Osuna, M.A.; Mata-Haro, V.; Gonzalez-Aguilar, G.A.; Lopez-Zavala, A.A.; Ayala-Zavala, J.F. Comparison of Single and Combined Use of Catechin, Protocatechuic, and Vanillic Acids as Antioxidant and Antibacterial Agents against Uropathogenic Escherichia Coli at Planktonic and Biofilm Levels. Molecules 2018, 23, 2813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common Trends and Differences in Antioxidant Activity Analysis of Phenolic Substances Using Single Electron Transfer Based Assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.-T.; Wu, J.-H.; Kuo, Y.-H.; Chang, S.-T. Antioxidant activities of natural phenolic compounds from Acacia confusa bark. Bioresour. Technol. 2007, 98, 1120–1123. [Google Scholar] [CrossRef]
- Kawabata, J.; Okamoto, Y.; Kodama, A.; Makimoto, T.; Kasai, T. Oxidative Dimers Produced from Protocatechuic and Gallic Esters in the DPPH Radical Scavenging Reaction. J. Agric. Food Chem. 2002, 50, 5468–5471. [Google Scholar] [CrossRef]
- Alcalde, B.; Granados, M.; Saurina, J. Exploring the Antioxidant Features of Polyphenols by Spectroscopic and Electrochemical Methods. Antioxidants 2019, 8, 523. [Google Scholar] [CrossRef] [Green Version]
- McCann, M.J.; Dalziel, J.E.; Bibiloni, R.; Barnett, M.P. An integrated approach to assessing the bio-activity of nutrients in vitro: The anti-oxidant effects of catechin and chlorogenic acid as an example. Integr. Food Nutr. Metab. 2015, 2, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.-G.; Hu, Q.-P.; Liu, Y. Antioxidant and DNA-Protective Activities of Chlorogenic Acid Isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef]
- Spagnol, C.M.; Assis, R.P.; Brunetti, I.L.; Isaac, V.L.B.; Salgado, H.R.N.; Corrêa, M.A. In Vitro methods to determine the antioxidant activity of caffeic acid. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2019, 219, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.; Allen, P.; Brunton, N.; O’Grady, M.; Kerry, J. Phenolic composition and in vitro antioxidant capacity of four commercial phytochemical products: Olive leaf extract (Olea europaea L.), lutein, sesamol and ellagic acid. Food Chem. 2010, 126, 948–955. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Masuda, M.; Kishi, E.; Ozaki, M.; Kondo, K.; Kanai, A.; Shiomi, K.; Furuta, T.; Mizu, M.; Nagai, Y. Components for Inhibiting Lipid Oxidation Related to Discoloration of Carotenoid Contained in Sugarcane Extract. Food Sci. Technol. Res. 2019, 25, 715–725. [Google Scholar] [CrossRef]
- Veselova, M.V.; Fedoreev, S.A.; Vasilevskaya, N.A.; Denisenko, V.A.; Gerasimenko, A.V. Antioxidant activity of polyphenols from the far-east plant Taxus cuspidata. Pharm. Chem. J. 2007, 41, 88–93. [Google Scholar] [CrossRef]
- Chambers, C.S.; Biedermann, D.; Valentová, K.; Petrásková, L.; Viktorová, J.; Kuzma, M.; Křen, V. Preparation of Retinoyl-Flavonolignan Hybrids and Their Antioxidant Properties. Antioxidants 2019, 8, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambigaipalan, P.; Oh, W.Y.; Shahidi, F. Epigallocatechin (EGC) esters as potential sources of antioxidants. Food Chem. 2019, 309, 125609. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, X.; Liu, G.; Li, J.; Zhang, J.; Cao, Y.; Miao, J. Antioxidant Activity and Mechanism of Resveratrol and Polydatin Isolated from Mulberry (Morus alba L.). Molecules 2021, 26, 7574. [Google Scholar] [CrossRef]
- Wei, C.-C.; Yen, P.-L.; Chang, S.-T.; Cheng, P.-L.; Lo, Y.-C.; Liao, V.H.-C. Antioxidative Activities of Both Oleic Acid and Camellia tenuifolia Seed Oil Are Regulated by the Transcription Factor DAF-16/FOXO in Caenorhabditis elegans. PLoS ONE 2016, 11, e0157195. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, Y.; Niki, E. Antioxidant Effects of Phytosterol and Its Components. J. Nutr. Sci. Vitaminol. 2003, 49, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Gulaboski, R.; Bogeski, I.; Mirčeski, V.; Saul, S.; Pasieka, B.; Haeri, H.H.; Stefova, M.; Stanoeva, J.P.; Mitrev, S.; Hoth, M.; et al. Hydroxylated derivatives of dimethoxy-1,4-benzoquinone as redox switchable earth-alkaline metal ligands and radical scavengers. Sci. Rep. 2013, 3, srep01865. [Google Scholar] [CrossRef] [Green Version]
- Rohmah, M.; Rahmadi, A.; Raharjo, S. Bioaccessibility and antioxidant activity of β-carotene loaded nanostructured lipid carrier (NLC) from binary mixtures of palm stearin and palm olein. Heliyon 2022, 8, e08913. [Google Scholar] [CrossRef]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Murillo, A.G.; Hu, S.; Fernandez, M.L. Zeaxanthin: Metabolism, Properties, and Antioxidant Protection of Eyes, Heart, Liver, and Skin. Antioxidants 2019, 8, 390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Y.; Song, J.; Zhang, M.; Wang, H.; Zhang, Y.; Suo, H. Comparison of In Vitro and In Vivo Antioxidant Activities of Six Flavonoids with Similar Structures. Antioxidants 2020, 9, 732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, W.; Liu, J.; Liu, H.; Lv, Z.; Zhang, C.; Chen, D.; Jiao, Z. Identification of Six Flavonoids as Novel Cellular Antioxidants and Their Structure-Activity Relationship. Oxidative Med. Cell. Longev. 2020, 2020, 4150897. [Google Scholar] [CrossRef]
- Sordon, S.; Popłoński, J.; Milczarek, M.; Stachowicz, M.; Tronina, T.; Kucharska, A.Z.; Wietrzyk, J.; Huszcza, E. Structure–Antioxidant–Antiproliferative Activity Relationships of Natural C7 and C7–C8 Hydroxylated Flavones and Flavanones. Antioxidants 2019, 8, 210. [Google Scholar] [CrossRef] [Green Version]
- Mishra, B.; Priyadarsini, K.; Kumar, M.; Unnikrishnan, M.; Mohan, H. Effect of O -glycosilation on the antioxidant activity and free radical reactions of a plant flavonoid, chrysoeriol. Bioorganic Med. Chem. 2003, 11, 2677–2685. [Google Scholar] [CrossRef]
- He, J.-W.; Yang, L.; Mu, Z.-Q.; Zhu, Y.-Y.; Zhong, G.-Y.; Liu, Z.-Y.; Zhou, Q.-G.; Cheng, F. Anti-inflammatory and antioxidant activities of flavonoids from the flowers of Hosta plantaginea. RSC Adv. 2018, 8, 18175–18179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Fang, X.; Ge, L.; Cao, F.; Zhao, L.; Wang, Z.; Xiao, W. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS ONE 2018, 13, e0197563. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Cheng, J.; Jin, C.; Zhang, Y. The reduction effect of dietary flavone C- and O-glycosides on the formation of acrylamide and its correlation and prediction with the antioxidant activity of Maillard reaction products. RSC Adv. 2014, 4, 24147–24155. [Google Scholar] [CrossRef]
- Jung, Y.H.; Chae, C.W.; Choi, G.E.; Shin, H.C.; Lim, J.R.; Chang, H.S.; Park, J.; Cho, J.H.; Park, M.R.; Lee, H.J.; et al. Cyanidin 3-O-arabinoside suppresses DHT-induced dermal papilla cell senescence by modulating p38-dependent ER-mitochondria contacts. J. Biomed. Sci. 2022, 29, 17. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Kwak, H.; Hwang, K.T. Antioxidant and anti-inflammatory activities of cyanidin-3-glucoside and cyanidin-3-rutinoside in hydrogen peroxide and lipopolysaccharide treated RAW264.7 cells (830.23). FASEB J. 2014, 28, 830.23. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-D.; Wu, D.-G.; Cai, X.-H.; Kennelly, E.J. New Antioxidant Phenolic Glycosides fromWalsura yunnanensis. Chem. Biodivers. 2006, 3, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Alam, M.B.; Quan, K.T.; Kwon, K.-R.; Ju, M.-K.; Choi, H.-J.; Lee, J.S.; Yoon, J.-I.; Majumder, R.; Rather, I.A.; et al. Antioxidant efficacy and the upregulation of Nrf2-mediated HO-1 expression by (+)-lariciresinol, a lignan isolated from Rubia philippinensis, through the activation of p38. Sci. Rep. 2017, 7, srep46035. [Google Scholar] [CrossRef] [Green Version]
- Saito, S.; Okamoto, Y.; Kawabata, J. Effects of Alcoholic Solvents on Antiradical Abilities of Protocatechuic Acid and Its Alkyl Esters. Biosci. Biotechnol. Biochem. 2004, 68, 1221–1227. [Google Scholar] [CrossRef]
- Li, L.; Seeram, N.P. Further Investigation into Maple Syrup Yields 3 New Lignans, a New Phenylpropanoid, and 26 Other Phytochemicals. J. Agric. Food Chem. 2011, 59, 7708–7716. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, K.; Tani, S.; Baba, C.; Hashimoto, T. Phenylpropanoid, Sapnol A, Lignan and Neolignan Sophorosides, Saposides A and B, Isolated from Canadian Sugar Maple Sap. Molecules 2013, 18, 9641–9649. [Google Scholar] [CrossRef] [Green Version]
- Xu, N.; Chen, G.; Liu, H. Antioxidative Categorization of Twenty Amino Acids Based on Experimental Evaluation. Molecules 2017, 22, 2066. [Google Scholar] [CrossRef] [Green Version]
- XueJiao, Z.; DengYong, L.; DavidWang, H.J.S.K.F.S. Antioxidant activity in vitro of hydroxyproline peptides. Shipin Kexue/Food Sci. 2021, 42, 55–60. [Google Scholar]

| Sample | AChE | BuChE | DPPH | ABTS | H2O2 | •OH | •NO | LPO |
|---|---|---|---|---|---|---|---|---|
| Acai aqueous extract | 0.014 | 6.378 | 11.550 | 30.541 | 7.803 | 11.604 | 12.932 | 4.862 |
| Acai ethanolic extract | NS | NS | 0.791 | 0.462 | 1.479 | 0.946 | 4.544 | 438.8 |
| α-tocopherol | - | - | 0.050 | 1.270 | 0.676 | - | - | - |
| L-ascorbic acid | - | - | 0.115 | 0.690 | - | - | - | - |
| Gallic acid | - | - | 0.008 | 0.008 | 0.737 | 0.001 | - | - |
| Butylated hydroxyanisole | - | - | - | - | - | 135.437 | 0.004 |
| Acai Berry Extracts | Total Phenolic Content (mg GAE/g) | Total Flavonoid Content (mg QUER E/g) |
|---|---|---|
| Acai aqueous extracts | 19.42 ± 0.40 | 1.26 ± 0.11 |
| Acai ethanolic extracts | 101.39 ± 4.61 | 11.78 ± 1.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
ALNasser, M.N.; Mellor, I.R.; Carter, W.G. A Preliminary Assessment of the Nutraceutical Potential of Acai Berry (Euterpe sp.) as a Potential Natural Treatment for Alzheimer’s Disease. Molecules 2022, 27, 4891. https://doi.org/10.3390/molecules27154891
ALNasser MN, Mellor IR, Carter WG. A Preliminary Assessment of the Nutraceutical Potential of Acai Berry (Euterpe sp.) as a Potential Natural Treatment for Alzheimer’s Disease. Molecules. 2022; 27(15):4891. https://doi.org/10.3390/molecules27154891
Chicago/Turabian StyleALNasser, Maryam N., Ian R. Mellor, and Wayne G. Carter. 2022. "A Preliminary Assessment of the Nutraceutical Potential of Acai Berry (Euterpe sp.) as a Potential Natural Treatment for Alzheimer’s Disease" Molecules 27, no. 15: 4891. https://doi.org/10.3390/molecules27154891
APA StyleALNasser, M. N., Mellor, I. R., & Carter, W. G. (2022). A Preliminary Assessment of the Nutraceutical Potential of Acai Berry (Euterpe sp.) as a Potential Natural Treatment for Alzheimer’s Disease. Molecules, 27(15), 4891. https://doi.org/10.3390/molecules27154891