Abstract
Aquaculture environment plays important roles in regulating the growth, morphology, nutrition, and flavor of aquatic products. The present study investigated growth, morphology, nutrition, and flavor formation in largemouth bass (Micropterus salmoides) cultured in the ponds with (EM group) and without (M group) the submerged macrophytes (Elodea nuttallii). Fish in the EM group showed a significantly greater body length, higher growth rate, and lower hepatosomatic index than those in the M group (p < 0.05). Moreover, compared with fish in the M group, those in the EM group showed improved muscle quality with significantly elevated levels of crude protein, total free and hydrolysable amino acids, and polyunsaturated fatty acids (p < 0.05). Specifically, certain amino acids related to flavor (Glu, Asp, Ala, and Arg) and valuable fatty acids (C18:2, C18:3n3, C20:3n3, and C22:6) were more abundant in the EM group (p < 0.05). In addition, the levels of 19 volatile (p < 0.05) were significantly higher in the EM group than in the M group. Therefore, E. nuttallii significantly improved growth, morphological traits, nutritional components, and characteristic flavor in largemouth bass, indicating the superior nutritional value and palatability of fish cultured with submerged macrophytes.
1. Introduction
China is one of the largest producers and consumers of aquatic products in the world, accounting for over 60% of the global aquaculture output [1]. Among aquatic products, the annual production of largemouth bass (Micropterus salmoides)—an economically important freshwater aquaculture species [2]—reached about 0.62 million tons [3] with an output value of over $1.76 billion in 2020. This species is native to lakes and rivers in North America [4]. Owing to its rapid growth, delicious flavor, and lack of intermuscular bones, largemouth bass has become widely popular in China since its introduction in 1983 [5]. At present, traditional pond systems remain the only acceptable mode of largemouth bass culture in China [6]. During pond culture, artificial compound feed is supplied, which leads to eutrophication, thereby promoting the outbreak of bloom-forming cyanophytes and diseases and ultimately deteriorating the quality of aquaculture products [7,8]. Therefore, these challenges must be addressed by developing and promoting new ecological aquaculture models.
Ecological aquaculture not only protects the environment but also fulfills the market demand. In recent times, with increased affluence, the focus of consumption has shifted from quantity to quality [9]. Fish meat quality traits, including shape, taste, and flavor, are important factors related to consumer preferences [10], and these attributes are affected by both external and internal drivers, including the culture environment, nutrition, and genetics [11,12,13]. In particular, the nutritional value and sensory traits of fish muscle are affected by the culture environment [14,15]. In bighead carp (Hypophthalmichthys nobilis), due to the presence of abundant volatiles with distinct aromas, fish cultured in cold water reservoirs and common culture ponds developed a greater umami intensity than those captured from a natural lake [16].
Therefore, development of effective approaches to produce high-quality aquatic products is a key target in the aquaculture industry [15,17]. In this context, bio-floating beds and submerged plant technology have been used in aquaculture as ecological remediation methods to purify water in situ, thereby improving the meat quality of aquaculture species [15,18]. For instance, floating beds planted with Ipomoea aquatica were installed in ponds to assimilate excess nutrients; improve water quality; and promote crab growth, yield, and quality [19]. In grass carp, growth performance and muscle quality improved in the presence of bio-floating beds in culture ponds [15]. In terms of the nutritional quality of aquatic products, the polyculture mode with an in situ ecological floating bed system was proven superior to the conventional monoculture mode [20]. An artificial composite ecosystem with aquatic plants and fish is a reliable eco-agricultural model. To date, however, ecological aquaculture of largemouth bass with aquatic plants has received little attention, and the effects of water quality factors on flavor have seldom been reported.
Volatile compounds significantly affect food flavor, further influencing the overall evaluation of food. Various techniques, including gas chromatography coupled to ion mobility spectrometry (GC-IMS), have been developed to investigate volatile organic compound emissions. In this technique, GC is applied for pre-separation, followed by IMS, and this method does not require time for sample pretreatment [21,22]. Therefore, GC-IMS is a rapid, nondestructive, sensitive, and reliable detection method, and it has gained popularity for exploring trace toxic chemicals and drug residues [23], particularly in the flavor analysis of food and agricultural products [21,24].
However, no study has investigated differences in the morphology and nutritional quality of largemouth bass cultured in the presence or absence of submerged plants (e.g., Elodea sp.). Therefore, in the present study, we compared the water quality as well as largemouth bass morphology and nutritional value between two aquaculture modes and constructed a fingerprint of flavor substances using GC-IMS. Further exploration of the key water quality parameters affecting flavor and nutritional value is warranted. Overall, our findings offer novel insights into the effects of submerged plants on the nutritional value and flavor formation of cultured largemouth bass, providing a reference for healthy, sustainable, and ecological culture of this species.
2. Materials and Methods
2.1. Experimental Design and Sampling
Largemouth bass juveniles of the same age were purchased from Zhanglin Fishery Co., Ltd. (Anhui, China). Six ponds, each covering an area of 0.17 ha, were selected for two treatments with three replicates at the Yang Zhong experimental base: Freshwater Fisheries Research Center (FFRC) and Chinese Academy of Fishery Sciences (CAFS). Three ponds lacked aquatic vegetation (M group), whereas in the remaining three ponds, submerged macrophytes (Elodea nuttallii) covered 20% of the superficial area of the bottom (EM group). The experimental period was 90 days (28 June 2021–28 September 2021). At the start of the trial, fish with an initial body weight of 14.50 ± 0.23 g were randomly distributed into six ponds at a stocking density of 43.48 g·m−3. The fish were supplied a commercial floating feed containing ≥47% crude protein and ≥5% crude lipid (Xinxin Tianen Aquafeed, Zhejiang, China). Feeding (stopped when >80% largemouth bass were no longer feeding) was performed twice a day at 8–9 a.m. and 5–6 p.m., with 5% feeding ratio.
Samples were collected every month to record growth performance. Body weight, body length, and liver and visceral weights of 30 fish in each group were measured after 24 h of starvation, and the fish anaesthetized with 100 mg·L−1 MS-222. At the end of the experimental period, dorsal muscles of 12 fish in each group were sampled, and stored at −80 °C for subsequent analysis. Water samples were collected from the experimental ponds to determine water quality parameters. All animal experiments conformed to the ARRIVE guidelines and were performed following the U.K. Animals (Scientific Procedures) Act, 1986, and the associated guidelines of the EU Directive 2010/63/EU for animal experimentation.
2.2. Water Quality Determination
Dissolved oxygen (DO) and pH were measured in situ using a portable multimeter (HQ30D; HACH, Ames, IA, USA) and the YSI Professional Plus system (YSI Inc., Yellow Springs, OH, USA). To test quality, triplicate water samples from each of the treatment sets were transferred to 500 mL polyethylene bottles and the physicochemical parameters of total nitrogen (TN), total phosphorus (TP), and chemical oxygen demand (CODMn) were analyzed as described previously [25]. All samples were filtered using Whatman filter papers with a pore size of 0.45 μm before laboratory analyses.
2.3. Biological, Color and Muscle Nutrients Measurements
In the present study, we calculated the weight gain rate (WGR), specific growth rate (SGR), and hepatosomatic index (HSI) as parameters reflecting growth performance, as follows.
where W0 is the initial body weight and Wt is the final body weight.
WGR (%) = (Wt − W0) × 100/W0
SGR (% day−1) = (lnWt − lnW0) × 100/days
HSI (%) = (hepatosomatic weight/body weight) × 100
Dorsal and abdominal skin color of largemouth bass cultured under different conditions was measured using a colorimeter (NR10QC Shenzhen Sanen Time Technology Co., Ltd., Shenzhen, China), calibrated with a standard white tile. L*, a*, and b* values were recorded, and the color results were expressed as L* (lightness), a* (−a*: greenness, +a*: redness), and b* (−b*: blueness, +b*: yellowness) [26].
The approximate composition of muscles was investigated following the national standard methods, with three parallel measurements per group. Moisture content was determined according to the AOAC Official Method 930.15 (drying at 105 °C to a constant weight). Ash content was measured according to the AOAC Official Method 942.05 (burning at 550 °C in a muffle furnace) [27]. Crude protein content was determined according to the AOAC Official Method 968.08 (Kjeldahl nitrogen determination method) [28], and crude fat was determined according to the AOAC Official Method 996.06 (Soxhlet extraction method).
For fatty acid analysis, fatty acid methyl esters (FAMEs) were prepared by transesterification with boron trifluoride and methanol, then dissolved in hexane, and the upper organic phase was collected for analysis with an Agilent 7820 A Gas Chromatograph (Agilent Technologies, Inc., Santa Clara, CA, USA) [29].
To determine amino acid content, muscle samples were freeze-dried and ground to powder. Next, 0.1 g samples were accurately weighed and used for amino acid determination. Briefly, the samples were treated with 6 M HCl for acid hydrolysis at 120 °C for 22 h, and then then neutralized with NaOH, and the supernatant was collected for analysis. Free amino acid were adjusted to an appropriate volume with 5% trichloroacetic acid, mixed well, then allowed to stand for 2 h and filtered. Finally, the supernatant was collected for analysis. Amino acid analyses were performed using high-performance liquid chromatography (HPLC) (Ag 1260 HPLC, American Agilent Company), according to the method described by Harimana [5].
2.4. Comparison of Fish Muscle Volatile Substances
Volatile compounds were identified using GC-IMS [30]. Muscle samples from each group were weighed and chopped evenly. Each sample was analyzed in triplicate to ensure the reliability of results. Briefly, 3 g samples in 20 mL headspace bottles were randomly selected. The analytical conditions were as follow: headspace incubation = 15 min, temperature = 60 °C, speed = 500 rpm, injection volume = 500 µL, and syringe temperature = 110 °C. GC conditions were as follows: chromatographic column = MXT-5 (15.00 m × 0.53 mm, 1.00 µm i.d.), column temperature = 60 °C, run time = 20 min, and carrier gas = N2 (purity ≥ 99.999%). The initial flow rate of the carrier gas was 2 mL·min−1 for 2 min, which was increased to 100 mL·min−1, and the total run time was 20 min. IMS conditions were as follows: temperature = 45 °C and drift gas flow rate = 150 mL·min−1. The retention index (RI) of each compound was calculated. The analytical software supporting the measurement instruments were vocal, three plug-ins (Reporter, Gallery Plot, and Dynamic PCA), and GC-IMS Library Search, which can analyze samples from different perspectives. Spiked and non-spiked samples were measured five times in parallel to calculate the recovery rate and relative standard deviation (RSD).
2.5. Statistical Methods and Data Processing
Data collated using Microsoft Excel were expressed as mean ± standard deviation (SD). In SPSS v26.0. (IBM Corporation, Armonk, NY, USA), t-test was performed to determine significant differences between the groups. A p < 0.05 indicated significant (*), p < 0.01 indicated highly significant (**), and p < 0.001 indicated extremely highly significant (***) difference.
3. Results
3.1. Growth Performance and Morphological Characteristics
The monthly growth performance of fish is summarized in Table 1. On day 30, there were no significant differences in BL, BT, BW, WGR, or SGR (p > 0.05), whereas fish in the EM group showed a significantly lower HSI than those in the M group (p < 0.05). On day 60, compared with fish in the M group, those in the EM group showed a significantly higher BW and SGR (p < 0.05) and a lower BT and HSI (p < 0.05). One month later (at 90 days), the BL, BW, WGR, and SGR of fish in the EM group significantly increased, while HSI continued to decrease significantly (p < 0.05). Moreover, fish in the EM group were slender and presented a green body (Figure 1), with darker dorsal skin, as evidenced by significantly lower L* values (Table 2, p < 0.01). Regardless of the origin (dorsal or abdominal skin), significant differences were observed in a* and b*.

Table 1.
Growth performance and morphological indices of largemouth bass.

Figure 1.
Comparative photographs of Micropterus salmoides in the EM (A) and M (B) groups.

Table 2.
Chroma values of largemouth bass skin.
3.2. Water Quality and Dominant Phytoplankton
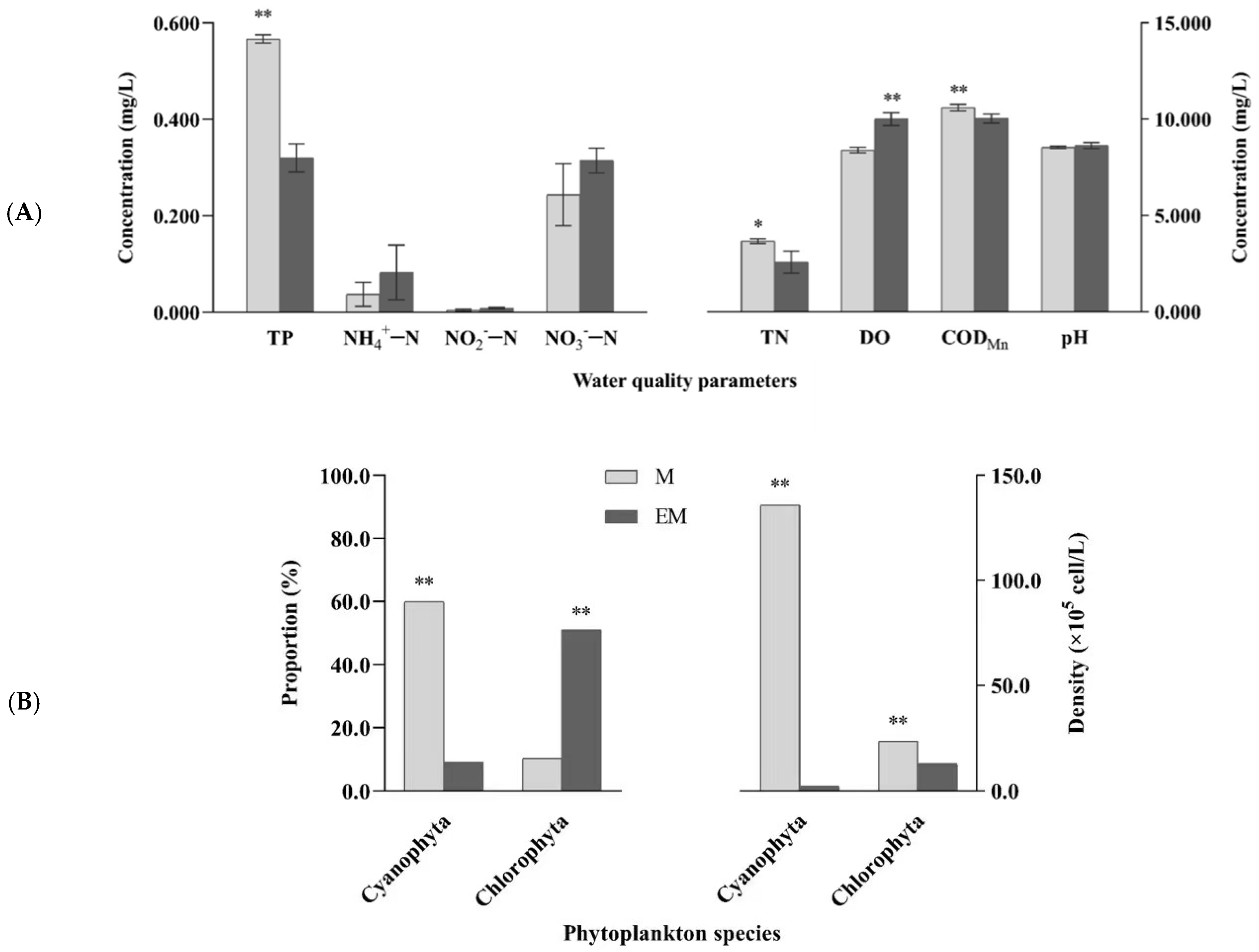
Water chemical indices and dominant phytoplankton, including Cyanophyta and Chlorophyta, are shown in Figure 2. At the end of the 90-day experimental period, significant differences in four water quality indices (TN, TP, DO, and CODMn) and dominant phytoplankton reflected the variations in ecological factors for aquaculture water between groups. Compared with values in the M group, TN, TP, and CODMn in the EM group were significantly decreased, while DO was significantly increased (p < 0.05). Furthermore, in the M group, cyanobacteria accounted for 60% of all phytoplankton, with a density of 1.36 × 107 cells·L−1, which was significantly higher than that in the EM group (2.28 × 105 cells·L−1; p < 0.01). More specifically, nearly 60-fold difference was noted between the two groups.
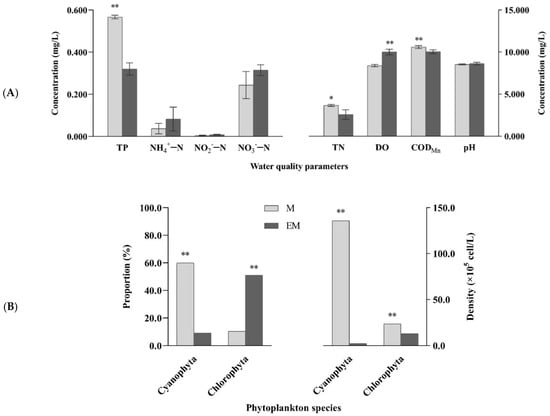
Figure 2.
(A) Water quality parameters and (B) dominant phytoplankton (Cyanophyta and Chlorophyta) in the M and EM group. Note: p < 0.05 indicated significant (*), p < 0.01 indicated highly significant (**).
3.3. Nutritional Components
The proximate compositions of samples varied (Table 3). All samples were rich sources of proteins. The crude protein content of samples in the M and EM groups was respectively 21.13% and 23.07% (p < 0.05). Moisture content was significantly higher in the M group (p < 0.01). Ash and crude fat content did not significantly differ between the two groups (p > 0.05).

Table 3.
Proximate chemical composition of fish meat (%, n = 3).
Seventeen free and hydrolysable amino acids were detected in different samples (Table 4). Levels of free amino acids, including Gly, Thr, Tyr, Phe, and Ile, in the muscles of largemouth bass were significantly higher in the EM group than in the M group. Moreover, levels of essential, no−essential, and total free amino acids significantly differed between the two groups (EM > M). Among hydrolysable amino acids, Glu content was the highest in different samples, and Glu content in the EM group was significantly higher than that in the M group. In addition, Asp, Ala, Arg, Ser, His, Thr, Val, Phe, IIe, and Leu levels were higher in the EM group than in the M group.

Table 4.
Amino acid profile (n = 3).
Furthermore, 22 fatty acids were detected (Table 5), including eight saturated fatty acids (ΣSFAs), five monounsaturated fatty acids (ΣMUFAs), and nine polyunsaturated fatty acids (ΣPUFAs). ΣPUFA levels were higher but ΣSFA and ΣMUFA levels were lower in the EM group than in the M group. In addition, the levels of C18:2, C18:3n3, C20:3n3, and C22:6 (docosahexaenoic acid, DHA), which are important indicators for evaluating the nutritional value of fatty acids, were significantly higher in the EM group. Based on these results, largemouth bass cultured with submerged macrophytes shows a relatively higher nutritional value.

Table 5.
Fatty acid profile (%, n = 3).
3.4. Volatile Compounds
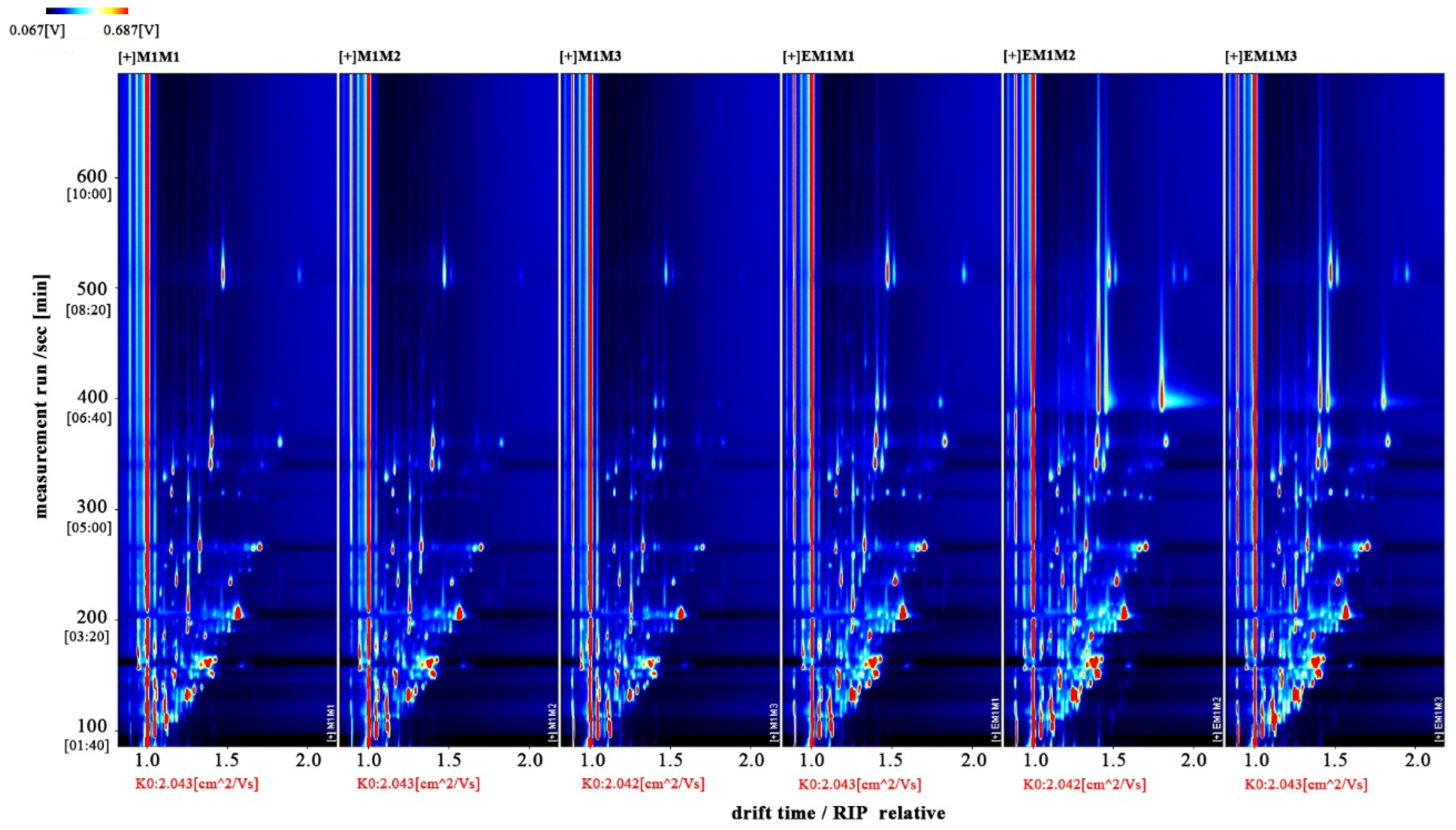
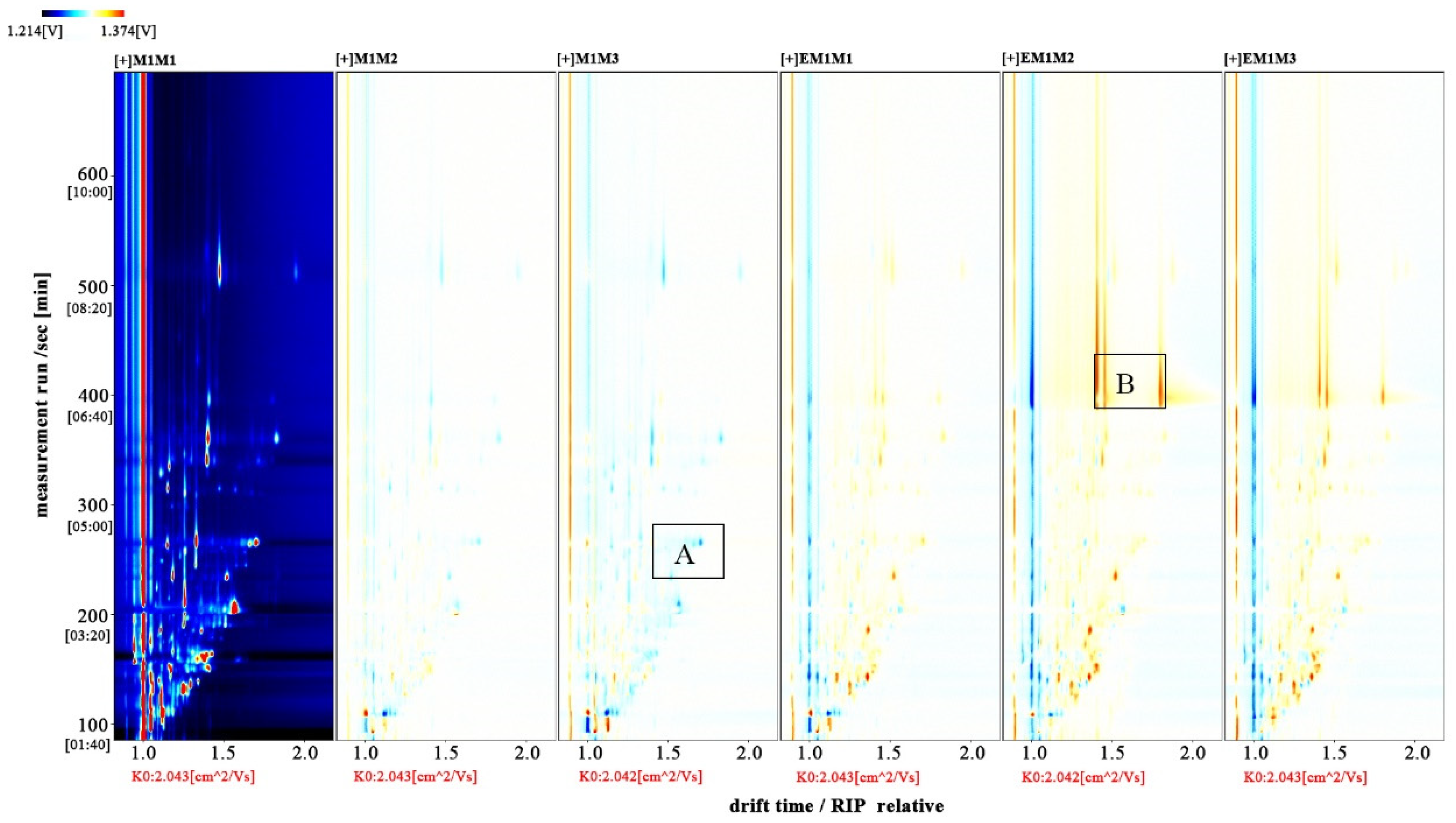
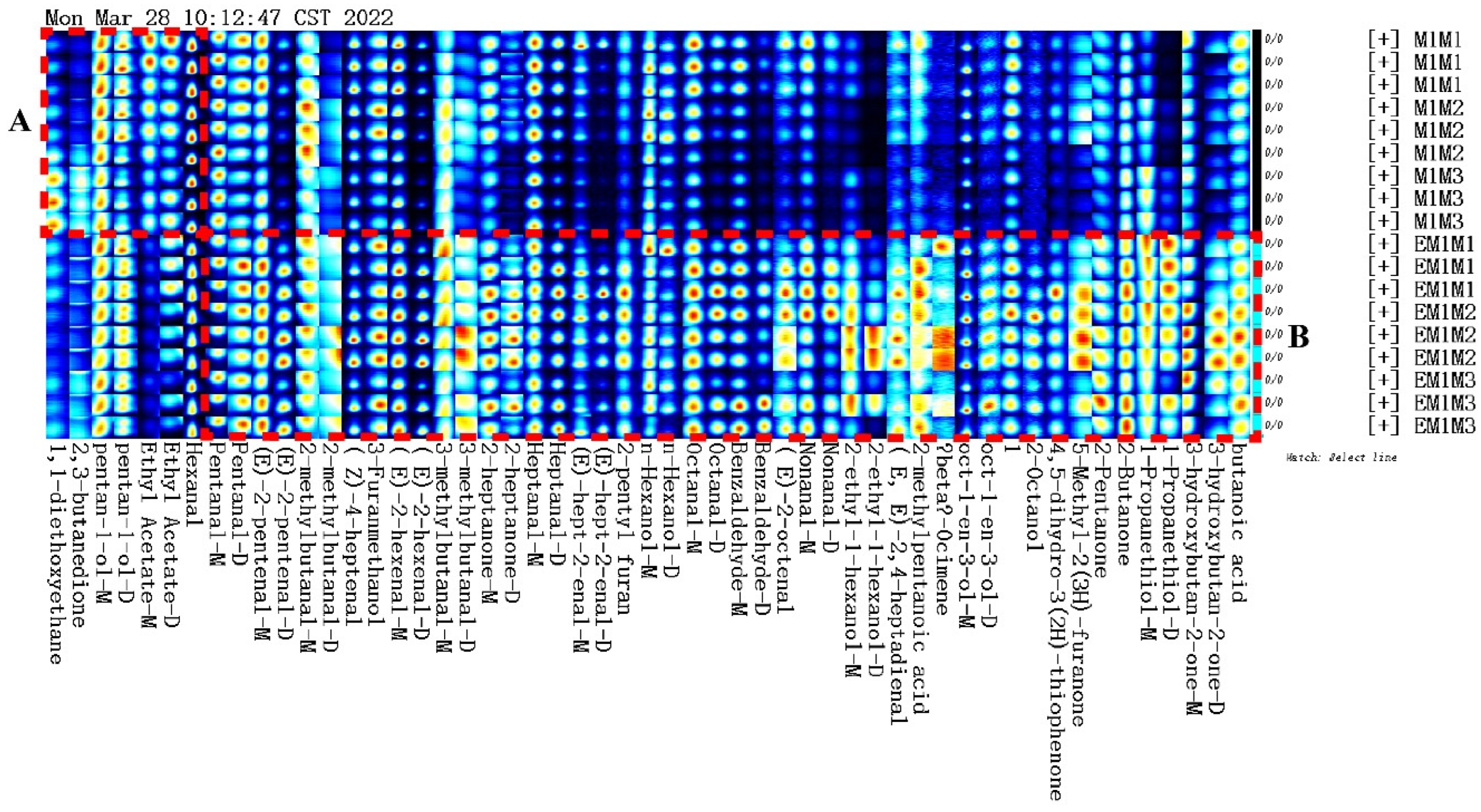
The entire spectrum representing total volatile substances was presented as two-dimensional topographical visualization. Figure 3 shows significant differences in the gas-phase ion migration spectra of muscle samples. The concentration of volatile substances was significantly lower in the M group than in the EM group. We used a different comparison system to visualize the differences between samples. Taking M1 as the reference, the remaining spectral values were deducted from the signal peaks in M1 to obtain the differences in spectra (Figure 4). Substances with levels lower than those in M1 are shown in blue (region A), whereas those with levels higher than those in M1 are shown in red (region B). If the levels of volatile substances are comparable, the background after deduction is white. The deeper the color, the greater the difference. Differences between Figure 3 and Figure 4 clearly demonstrate that the concentration of volatile organic compounds was consistently higher in the EM group. The galleryplot plug-in of the LAV software was used to automatically generate fingerprints of all peaks for determining characteristic differences in volatile substances. As shown in Figure 5, substances related to flavor presented characteristic and common peak areas in the two groups. Regions A and B in Figure 5 represent the characteristic peak areas of the M and EM group, respectively. Therefore, the flavor of samples significantly differed between the M and EM groups.
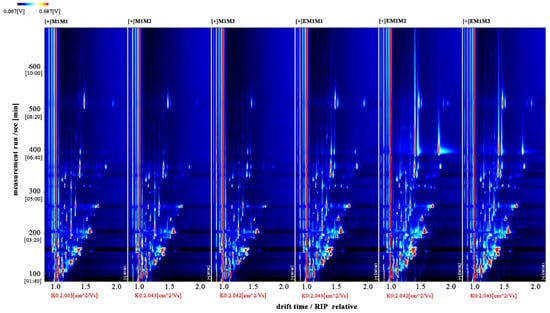
Figure 3.
Gas-phase ion mobility spectra of Micropterus salmoides muscles. Note: M1M1, M1M2, and M1M3: three samples from the M group. EM1M1, EM1M2, and EM1M3: three samples from the EM group. The background is blue, and the red vertical line on the abscissa (1.0) is the normalized reaction peak. The y-axis represents the retention time of gas chromatography, and the x-axis represents the ion relative drift time. Points on both sides of the reaction peak represent volatile organic compounds. Colors indicate the concentration of substance, with white and red representing a low and high concentration, respectively. The deeper the color, the higher the concentration. For the interpretation of references to colors in this figure legend, please refer the web version of this article.
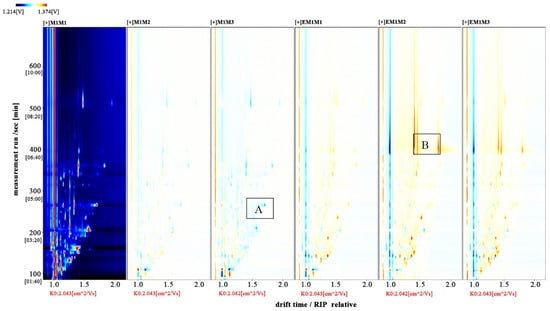
Figure 4.
Difference diagram of gas-phase ion mobility spectra of Micropterus salmoides muscles. Note: M1M1, M1M2, and M1M3: three samples from the M group. EM1M1, EM1M2, and EM1M3: three samples from the EM group.
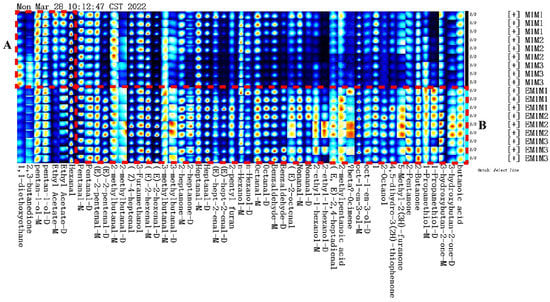
Figure 5.
Gallery plot of selected volatile organic compounds in the gas-phase ion migration spectrum. Note: Regions A and B represent the characteristic peak areas of the M and EM group, respectively.
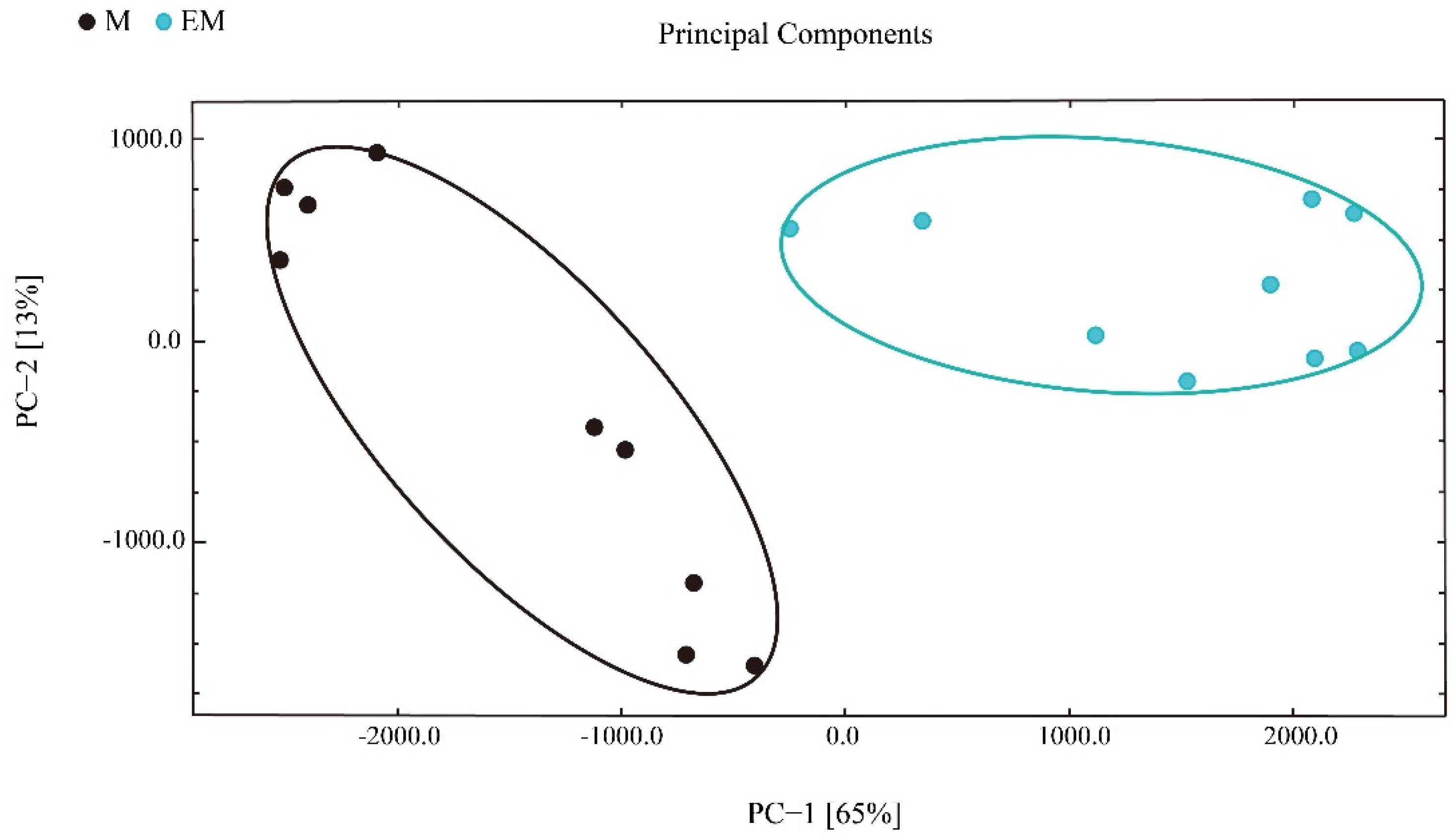
Next, principal component analysis (PCA) was applied to understand the correlations in largemouth bass muscle samples. PC1 explained 36% sample variance, whereas PC2 explained 28% sample variance (Figure 6). Based on these data, the samples were divided into two groups, and the between-group difference was greater than the within-group difference. Therefore, GC-IMS is suitable to distinguish largemouth bass from different culture models.
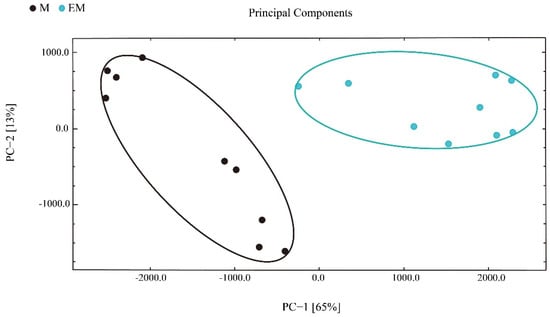
Figure 6.
Principal component analysis of flavor compounds in largemouth bass muscle samples.
In the present study, 55 volatile compounds were identified, of which 54 were qualitative substances, primarily comprising aldehydes, alcohols, ketones, acids, esters, and miscellaneous compounds (Table 6). Twenty-four aldehydes accounted for 43.64% of all volatile compounds and were the most abundant volatile compounds. Thus, different culture environments affect flavor composition. Compared with those in the M group, the levels of aldehydes, namely nonanal-M, nonanal-D, octanal-D, benzaldehyde-M, heptanal-D, 2-methylbutanal-D, and 2-methylbutanal-D, were significantly higher in the EM group. Furthermore, the most abundant alcohols were 2-ethyl-1-hexanol-M, 2-ethyl-1-hexanol-D, 1-propanethiol-D, 1-propanethiol-M, 3-furanmethanol, pentan-1-ol-D, oct-1-en-3-ol-D, and 2-octanol, and their levels were higher in the EM group. Similarly, the levels of ketones, such as 2-butanone, 2-pentanone, 3-hydroxybutan-2-one-D, 3-hydroxybutan-2-one-M, (E)-3-penten-2-one-M, and (E)-3-penten-2-one-D, were significantly higher in the EM group than in the M group. Finally, 2-butanone accounted for approximately 50% of all ketones and was the most abundant ketone in bass muscle in the present study. Thus, 2-butanone appears to be a characteristic volatile compound of largemouth bass.

Table 6.
Qualitative results of the gas-phase ion mobility spectra of Micropterus salmoides muscles (n = 3).
3.5. Correlation Analysis
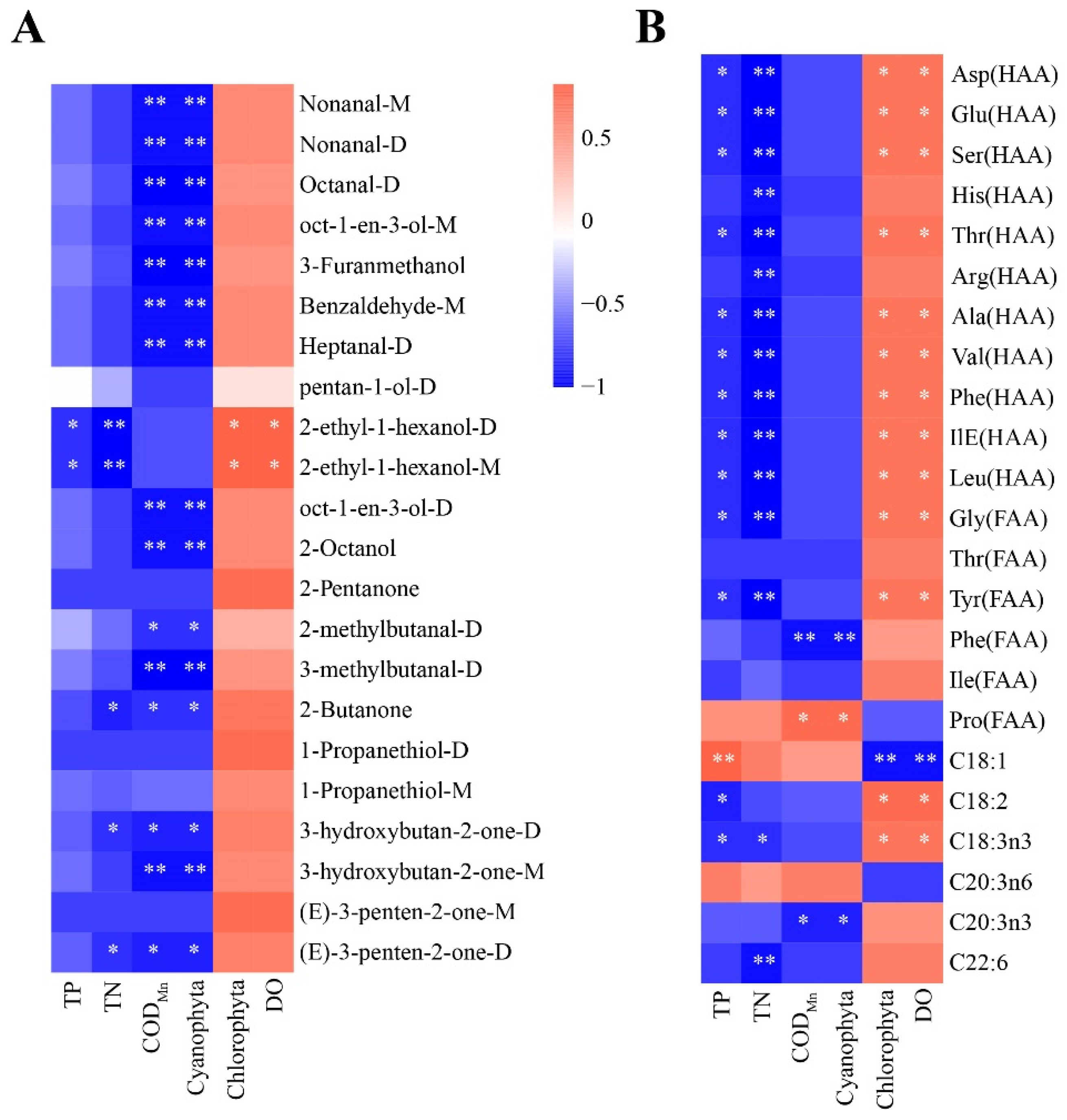
To better understand the key factors affecting the flavor and nutritional quality of largemouth bass, we performed Spearman’s correlation analysis. As shown in Figure 7A, the abundance of all volatile substances analyzed was positively correlated with that of DO and chlorophyta, but negatively correlated with that of TN, TP, and CODMn. In addition, 2-ethyl-1-hexanol-D and 2-ethyl-1-hexanol-M were significantly and positively correlated with DO and Chlorophyta but negatively correlated with TN and TP (p < 0.05). Cyanobacteria and CODMn are important biotic and abiotic factors affecting aquatic animals, respectively, and they were significantly but negatively correlated with 68% of the analyzed volatile substances (nonanal-M, nonanal-D, octanal-D, oct-1-en-3-ol-M, 3-furanmethanol, benzaldehyde-M, heptanal-D, oct-1-en-3-ol-D, 2-octanol, 2-methylbutanal-D, 3-methylbutanal-D, 2-Butanone, 3-hydroxybutan-2-one-D,3-hydroxybutan-2-one-M, and (E)-3-penten-2-one-D). Regarding nutritional components (Figure 7B), free and hydrolysable most amino acids were significantly and positively correlated with DO and Chlorophyta and significantly but negatively correlated with TN and TP (p < 0.05). ΣPUFAs (C18:2 and C18:3n3) were significantly and positively correlated with DO and Chlorophyta (p < 0.05). C20:3n3 levels were significantly but negatively correlated with cyanobacteria and CODMn (p < 0.05), and C22:6 levels were significantly but negatively correlated with TN (p < 0.05). Based on these results, cyanobacteria, Chlorophyta, CODMn, DO, TN, and TP may be the key biotic and abiotic factors affecting the flavor and nutritional value of largemouth bass.
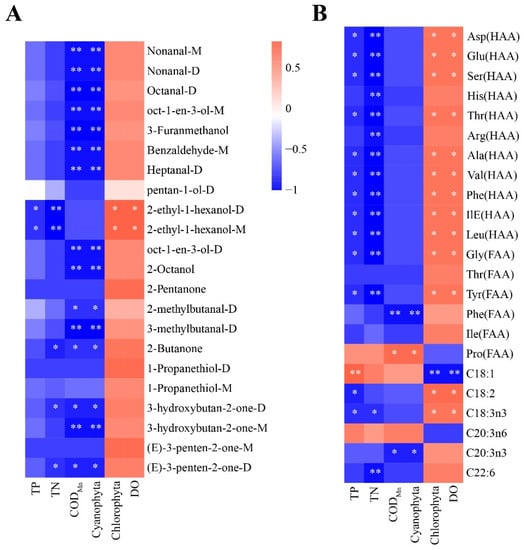
Figure 7.
Correlation of significantly different environmental factors with (A) volatile compounds and (B) nutritional components. Different colors represent the strength of correlation; the red line represents a significant positive correlation, and the blue line represents a significant negative correlation. Note: p < 0.05 indicated significant (*), p < 0.01 indicated highly significant (**).
4. Discussion
4.1. Effects of Submerged Macrophytes on the Growth and Morphology of Largemouth Bass
Water quality is a critical factor in aquaculture, as poor-quality water can significantly impede growth and production [31]. In the present study, the higher content of TN, TP, and CODMn in the M group, which lacked submerged macrophytes, led to cyanobacterial outbreak (1.36 × 107 cells·L−1). Conversely, this phenomenon was not observed in the EM group, which comprised submerged macrophytes. TN and TP are the two most important indices of the eutrophication of water bodies [32], while CODMn is an indicator of organic pollution [33]. Higher values of these indices promote the proliferation of phytoplankton and outbreak of cyanobacterial blooms [34]. Cyanobacteria can produce abundant toxic secondary metabolites, such as dermatoxins, hepatotoxins, and cytotoxins [35], which affect the feeding, growth, and immunity of exposed cultured species [36]. In our experiment, fish in the EM group showed a significantly higher growth rate, corroborating previously reported experimental findings. For instance, Yao showed that the inclusion of live submerged macrophytes in tanks improved the growth of Macrobrachium nipponense [37]. Meanwhile, in the present experiment, largemouth bass cultured in the presence of submerged macrophytes were slender, with a green body. Our observations are consistent with reported findings in largemouth bass cultured in an aquaculture system using land-based containers with recycled water [6]. Moreover, fish in the M group showed a significantly higher HSI, suggesting that largemouth bass cultured in the conventional model produced excess body energy, which led to lipid and glycogen accumulation in the liver. Our results are consistent with previous reports from pond and ecological cultures [21]. Overall, aquaculture with submerged macrophytes significantly affected the growth performance and morphology of largemouth bass in the present study, indicating the potential of this model as a reference for farmers.
4.2. Effects of sSubmerged Macrophytes on the Nutrient Composition of Largemouth Bass Muscles
Amino acids present a high nutritive value and are important regulators of key metabolic pathways essential for maintenance, growth, feed intake, nutrient utilization, immunity, behavior, and reproduction [38,39]. In the present study, most free and hydrolysable amino acids were more abundant in the EM group; among these, Glu, Asp, Ala, and Arg are well-known as delicious amino acids and contribute significantly to the characteristic flavor of aquatic products [40]. In addition, amino acid content in fish muscles is closely related to their living environment [5,40]. In the present study, levels of 65% amino acids analyzed were significantly and positively correlated with DO and Chlorophyta but significant and negatively correlated with TN and TP (p < 0.05). Furthermore, a significant correlation was noted between amino acids and water environmental factors. Chlorella is highly effective in counteracting fish enteropathy, maintaining a healthy intestine to balance gene expression [41].
Aquatic products are considered to be nutritionally high-quality foods, because they are rich in amino acids and are an excellent source of unsaturated fatty acids, which are beneficial against cardiovascular disease and promote physiological processes [42,43]. In the present study, the content of C18:2, C18:3n3, C20:3n3, and C22:6 (DHA), which can improve human health and nutritional status [44,45], was significantly higher in the EM group. In particular, DHA is beneficial for optimal brain and neuronal development [46] and is an important indicator for evaluating the nutritional value of fatty acids. Moreover, PUFAs (C18:2 and C18:3n3) were significantly and positively correlated with DO and Chlorophyta (p < 0.05). C20:3n3 level was significantly but negatively correlated with cyanobacteria and CODMn (p < 0.05), while C22:6(DHA) level was significantly but negatively correlated with TN (p < 0.05). In a previous study on channel catfish, long-term exercise was shown to increase bacterial diversity and richness as well as alter the intestinal microbial composition and unsaturated fatty acid and amino acid biosynthesis [47]. Interestingly, water quality (ammonia) affected swimming activity and feeding behavior [48].
4.3. Effects of Submerged Macrophytes on Volatile Compounds in Largemouth Bass Muscles
Each food product has a distinct odor imbued by hundreds of volatile organic compounds, and odor change is one of the most sensitive indicators of food quality. Thus, accurately describing the composition of volatile substances can help assess the quality of agri-food products [23,47]. Various flavor components of largemouth bass meat have been documented [5,6]. In the present study, 24 aldehydes accounted for 43.64% of all components and were the most abundant volatile compounds in largemouth bass muscles. These results confirm that different culture environments indeed affect flavor composition. Aldehydes are mainly generated through lipid oxidation and considered to make the greatest contribution to the flavor of meat products because of their higher content and lower odor detection threshold [49,50].
Compared with values in the M group, the levels of aldehydes, such as nonanal-M, nonanal-D, octanal-D, benzaldehyde-M, heptanal-D, 2-methylbutanal-D, and 2-methylbutanal-D, were significantly higher in the EM group. Such differences in aldehydes and other flavor components have been detected in many aquatic products [51]. Benzaldehyde generates pleasant almondy, fruity, and nutty notes [52] and is an important source of the special aroma of crayfish [53]. Meanwhile, the content of hexanal, which was the most abundant aldehyde, was not significantly different between the two groups. Hexanal has previously been identified as an aroma-active compound providing a green note [54].
Typically, alcohols produce a relatively soft odor, similar to the aroma of fruits [55]. Oct-1-en-3-ol is mainly responsible for the green, plant-like aroma and mushroom-like odor and is formed by the oxidation of arachidonic acid by 12-lipoxygenase [56]. Furthermore, ketones are produced through lipid oxidation and generate creamy and fruity notes [57]. 2-Butanone was abundant in the muscles of largemouth bass in a recirculatory aquaculture system [6]. In the present study, 2-butanone accounted for approximately 50% of all ketones, being the most abundant one. Thus, 2-butanone appears to be a characteristic volatile compound in largemouth bass.
The higher contents of odor compounds in the muscles of fish from the EM group suggest a more pleasant aroma, which may be another reason for their more delicious meat. The differences in volatile substances between the two culture modes can be explained based on two aspects: (1) improvement of water quality by submerged macrophytes through absorption of excess nitrogen and phosphorus, preventing the outbreak of cyanobacteria and (2) abundance of unsaturated fatty acids in fish muscles. Previous studies have shown that cyanobacterial density in ponds and unsaturated fatty acids in fish muscle are linked to the composition of volatile substances [58,59]. In the present study, cyanobacteria and CODMn were important biotic and abiotic factors affecting fish, respectively, and these factors were significantly but negatively correlated with 68% of the volatile substances analyzed. Thus, our data suggest that submerged plants play an important role in improving the nutritional composition and characteristic flavor profile of Micropterus salmoides.
5. Conclusions
In summary, largemouth bass cultured in an ecological pond with submerged macrophytes (Elodea nuttallii) showed optimal growth, with a slender body shape and significantly higher contents of crude protein, total free and hydrolysable amino acids, and ΣPUFA, compared with fish cultured in conventional ponds. Seven aldehydes, nine alcohols, and six ketones were identified as characteristic volatile components in the muscles of largemouth bass cultured in an ecological pond with submerged macrophytes. Variations in the profiles of volatile components between the two groups are closely linked to the diverse water environments caused by the different aquaculture models. Furthermore, cyanobacteria, Chlorophyta, CODMn, DO, TN, and TP may be the key factors affecting the flavor and nutritional value of largemouth bass. In general, aquaculture with live submerged macrophytes can not only bioremediate the water in situ without producing aquaculture wastewater but also improve the nutritional quality and flavor of aquatic products. Therefore, this is an environmentally friendly and high-value-added ecological aquaculture model worthy of extensive application and popularization.
Author Contributions
Conceptualization, Z.N. and G.X.; methodology, Z.Z. and X.T.; software, Z.Z. and H.Z.; formal analysis, Z.N. and Y.Z.; investigation, Y.S. and J.G.; writing—original draft preparation, Z.Z. and Z.N.; supervision, G.X. and P.X.; project administration, Z.N. and G.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key Research and Development Program of China, grant number 2019YFD0900301 and the Modern Agriculture Industry System Construction of Special Funds (CARS-46).
Institutional Review Board Statement
All animal experiments complied with the ARRIVE guidelines and were carried out following U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, W.; Shen, H. A statistical analysis of China’s fisheries in the 12th five-year period. Aquac. Fish. 2016, 1, 41–49. [Google Scholar] [CrossRef]
- Bai, J.J.; Li, S.J.; Dong, G.C.; Xie, J. Culture Status and Technology of Micropterus salmoides in China. Sci. Fish Farming 2009, 6, 20–21. [Google Scholar]
- Yearbook, C.F.S. Fishery Bureau of Ministry of Agriculture of the People’s Republic of China; China Agriculture Press: Beijing, China, 2020; p. 24. [Google Scholar]
- Coyle, S.D.; Tidwell, J.H.; Webster, C.D. Response of largemouth bass Micropterus slmoides to dietary supplementation of lysine, methionine, and highly unsaturated fatty acids. J. World Aquac. Soc. 2000, 31, 89–95. [Google Scholar] [CrossRef]
- Harimana, Y.; Tang, X.; Xu, P.; Xu, G.; Karangwa, E.; Zhang, K.; Sun, Y.; Li, Y.; Ma, S.; Uriho, A.; et al. Effect of long-term moderate exercise on muscle cellularity and texture, antioxidant activities, tissue composition, freshness indicators and flavor characteristics in largemouth bass (Micropterus salmoides). Aquaculture 2019, 510, 100–108. [Google Scholar] [CrossRef]
- Jia, S.P.; Wang, L.; Zhang, J.M.; Zhang, L.; Ma, F.R.; Huang, M.L.; Liu, S.-S.; Gong, J.-H.; Zhang, M.; Yu, M.; et al. Comparative study on the morphological characteristics and nutritional quality of largemouth bass (Micropterus salmoides) cultured in an aquaculture system using land-based container with recycling water and a traditional pond system. Aquaculture 2022, 549, 737721. [Google Scholar] [CrossRef]
- Munni, M.A.; Fardus, Z.; Mia, M.Y.; Afrin, R. Assessment of pond water quality for fish culture: A case study of Santosh region in Tangail, Bangladesh. J. Environ. Sci. Nat. Resour. 2013, 6, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Costa, S.M.; Appel, E.; Macedo, C.F.; Huszar, V.L. Low water quality in tropical fishponds in southeastern Brazil. An. Acad. Bras. Ciências 2014, 86, 1181–1195. [Google Scholar] [CrossRef]
- Liu, Y.T.; Zhang, W.; Chen, Q.S.; Zhang, Q.; Yin, P.; Guo, Y.Z. On the Quality and Safety of Agricultural Products in Tianjin. J. Agric. 2021, 11, 97–100. [Google Scholar] [CrossRef]
- Jorge, F.; Paulo, V.; Câmara, J.S. From aquaculture production to consumption: Freshness, safety, traceability and authentication, the four pillars of quality. Aquaculture 2019, 518, 734857. [Google Scholar] [CrossRef]
- Valente, L.M.P.; Cornet, J.; Donnay-Moreno, C.; Gouygou, J.P.; Bergé, J.P.; Bacelar, M.; Escórcio, C.; Rocha, E.; Malhão, F.; Cardinal, M. Quality differences of gilthead sea bream from distinct production systems in Southern Europe: Intensive, integrated, semi-intensive or extensive systems. Food Control. 2011, 22, 708–717. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Tong, F.; Tang, T.; Ao, Z.; Wei, Z.; Yang, F.; Shu, Y.; Liu, S.; Mai, K. Comparative evaluation of nutritional value and flavor quality of muscle in triploid and diploid common carp: Application of genetic improvement in fish quality. Aquaculture 2021, 541, 736780. [Google Scholar] [CrossRef]
- Sobczak, M.; Panicz, R.; Eljasik, P.; Sadowski, J.; Tórz, A.; Żochowska-Kujawska, J.; Barbosa, V.L.; Domingues, V.; Marques, A.; Dias, J. Quality improvement of common carp (Cyprinus carpio L.) meat fortified with n-3 PUFA. Food Chem. Toxicol. 2020, 139, 111261. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.H.; Sun, D.W.; Han, Z.; Zeng, X.A. Texture and structure measurements and analyses for evaluation of fish and fillet freshness quality: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 52–61. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Tang, R.; He, X.; Li, L.; Takagi, Y.; Li, D. Improvement of muscle quality of grass carp (Ctenopharyngodon idellus) with a bio-floating bed in culture ponds. Front. Physiol. 2019, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhou, J.; Qiu, H.; Lai, X.; Li, J.; Wu, D.; Sheng, J.; Hong, Y. Comparison of nutritional quality and volatile flavor compounds among bighead carp from three aquaculture systems. Saudi J. Biol. Sci. 2021, 28, 4291–4299. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.H.; Xia, J.G.; Zhang, X.; He, X.G.; Li, L.; Tang, R.; Chi, W.; Li, D. Diet affects muscle quality and growth traits of grass carp (Ctenopharyngodon idellus): A comparison between grass and artificial feed. Front. Physiol. 2018, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Z.; Zhang, Y.; Liu, G.Y.; Huang, C. Effects of Two Kinds of Submerged Plant on the Quality of Waters Cultured with Pseudobagrus fulvidraco Fry. J. Lake Sci. 2003, 15, 184–188. [Google Scholar]
- Zhan, J.; Yang, X. Standard Aquaculture Techniques for Chinese Mitten Crab; Chemical Industry Press: Beijing, China, 2015. [Google Scholar]
- Zhai, Z.; Feng, J.X.; Gao, S.S.; Chen, J.F.; Huang, J.R.; Li, Z.F. Effects of ecological floating bed on content of heavy metals and nutritional quality in aquatic products. J. Fish. China 2017, 41, 88–98. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography–ion mobility spectrometry (GC–IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Xu, X.; Sun, C.; Liu, B.; Zhou, Q.; Xu, P.; Liu, M.; Wang, A.; Tian, H.; Luo, W.; Jiang, Q. Flesh flavor of red swamp crayfish (Procambarus clarkii Girard, 1852) processing by GS-IMS and electronic tongue is changed by dietary animal and plant protein. Food Chem. 2022, 373, 131453. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Zhang, J.; Wang, J.; Wang, X.; Du, D. Recent development of HS-GC-IMS technology in rapid and non-destructive detection of quality and contamination in agri-food products. TrAC Trends Anal. Chem. 2021, 144, 116435. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, B.; Fu, Y.; Shi, Y.G.; Chen, F.L.; Guan, H.N.; Liu, L.-L.; Zhang, C.-Y.; Zhu, P.-Y.; Liu, Y.; et al. HS-GC-IMS with PCA to analyze volatile flavor compounds across different production stages of fermented soybean whey tofu. Food Chem. 2021, 346, 128880. [Google Scholar] [CrossRef] [PubMed]
- State EPA of China. Monitoring and Determination Methods for Water and Wastewater, 4th ed.; China Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- Ninwichian, P.; Phuwan, N.; Limlek, P. Effects of tank color on the growth, survival rate, stress response, and skin color of juvenile hybrid catfish (Clarias macrocephalus × Clarias gariepinus). Aquaculture 2022, 554, 738129. [Google Scholar] [CrossRef]
- Cunniff, P. Official methods of analysis of AOAC International, Method 923.03. Trends Food Sci. Technol. 1995, 6, 382. [Google Scholar]
- Gao, J.; Tai, X.; Shao, N.; Sun, Y.; Nie, Z.; Wang, Y.; Li, Q.; Xu, P.; Xu, G. Effects of effective microorganisms on the growth performance, nutritional composition and flavour quality of the pond-cultured Eriocheir sinensis. Aquaculture 2021, 52, 871–880. [Google Scholar] [CrossRef]
- Sun, C.; Zou, X.; Yao, Y.; Jin, J.; Xia, Y.; Huang, J.; Jin, Q.; Wang, X. Evaluation of fatty acid composition in commercial infant formulas on the Chinese market: A comparative study based on fat source and stage. Int. Dairy J. 2016, 63, 42–51. [Google Scholar] [CrossRef]
- Liu, D.; Bai, L.U.; Feng, X.I.; Chen, Y.P.; Zhang, D.; Yao, W.; Zhang, H.; Chen, G.; Liu, Y. Characterization of Jinhua ham aroma profiles in specific to aging time by gas chromatography-ion mobility spectrometry (GC-IMS). Meat Sci. 2020, 168, 108178. [Google Scholar] [CrossRef] [PubMed]
- Viadero, R.C. Factors affecting fish growth and production. Water Encycl. 2005, 3, 129–133. [Google Scholar] [CrossRef]
- Shang, W.; Jin, S.; He, Y.; Zhang, Y.; Li, J. Spatial–Temporal Variations of Total Nitrogen and Phosphorus in Poyang, Dongting and Taihu Lakes from Landsat-8 Data. Water 2021, 13, 1704. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, Y.L.; Liu, X.H.; Zhu, G.W.; Qin, B.Q.; Shi, Z.Q.; Feng, L. Temporal and spatial variations of chemical oxygen demand in lake taihu, China, from 2005 to 2009. Hydrobiologia 2011, 665, 129–141. [Google Scholar] [CrossRef]
- Zhao, C.S.; Shao, N.F.; Yang, S.T.; Ren, H.; Ge, Y.R.; Zhang, Z.S.; Feng, P.; Liu, W. Quantitative assessment of the effects of human activities on phytoplankton communities in lakes and reservoirs. Sci. Total Environ. 2019, 665, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Boyer, G.L.; Zimba, P.V. A review of cyanobacterial odorous and bioactive metabolites: Impacts and management alternatives in aquaculture. Aquaculture 2008, 280, 5–20. [Google Scholar] [CrossRef]
- Drobac, D.; Tokodi, N.; Lujić, J.; Marinović, Z.; Subakov-Simić, G.; Dulić, T.; Važić, T.; Nybom, S.; Meriluoto, J.; Codd, G.A.; et al. Cyanobacteria and cyanotoxins in fishponds and their effects on fish tissue. Harmful Algae 2016, 55, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.L.; Zhao, Y.; Wang, H.; Chen, H.J.; Ji, X.S. Growth promotion and dietary contribution assessment of three submerged macrophytes to Macrobrachium nipponense. Aquaculture 2019, 504, 70–80. [Google Scholar] [CrossRef]
- Li, X.; Zheng, S.; Wu, G. Nutrition and functions of amino acids in fish. Amino Acids Nutr. Health 2021, 1285, 133–168. [Google Scholar] [CrossRef]
- Sarma, D.; Akhtar, M.S.; Das, P.; Das, P.; Shahi, N.; Ciji, A.; Mahanta, P.C.; Yengkokpam, S.; Debnath, D. Nutritional quality in terms of amino acid and fatty acid of five coldwater fish species: Implications to human health. Natl. Acad. Sci. Lett. 2013, 36, 385–391. [Google Scholar] [CrossRef]
- Fuentes, A.; Fernández-Segovia, I.; Serra, J.A.; Barat, J.M. Comparison of wild and cultured sea bass (Dicentrarchus labrax) quality. Food Chem. 2010, 119, 1514–1518. [Google Scholar] [CrossRef]
- Grammes, F.; Reveco, F.E.; Romarheim, O.H.; Landsverk, T.; Mydland, L.T.; Øverland, M. Candida utilis and Chlorella vulgaris counteract intestinal inflammation in Atlantic salmon (Salmo salar L.). PLoS ONE 2013, 8, e83213. [Google Scholar] [CrossRef] [Green Version]
- Pasupuleti, R.R.; Tsai, P.C.; Ponnusamy, V.K.; Chen, N.C. Green sample pre-treatment technique coupled with UHPLC-MS/MS for the rapid biomonitoring of dietary poly-unsaturated (omega) fatty acids to predict health risks. Chemosphere 2022, 291, 132685. [Google Scholar] [CrossRef]
- Strobel, C.; Jahreis, G.; Kuhnt, K. Survey of n-3 and n-6 polyunsaturated fatty acids in fish and fish products. Lipids Health Dis. 2012, 11, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candiloro, F.; Borioli, V.; Borsellino, G.; Picozza, M.; Pellini, R.; Cereda, E.; Gargano, F.; Caraccia, M.; Nardi, M.T.; Bellu, L.; et al. Influence of different lipid emulsions on specific immune cell functions in head and neck cancer patients receiving supplemental parenteral nutrition: An exploratory analysis. Nutrition 2021, 86, 111178. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, J. Intake or blood levels of n-3 polyunsaturated fatty acids and risk of colorectal cancer: A systematic review and meta-analysis of prospective studies. Cancer Epidemiol. Prev. Biomark. 2020, 29, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harsløf, L.; Ciappolino, V.; Agostoni, C. DHA effects in brain development and function. Nutrients 2016, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yu, Y.; Dong, L.; Gan, J.; Mao, T.; Liu, T.; Li, X.; He, L. Effects of moderate exercise on hepatic amino acid and fatty acid composition, liver transcriptome, and intestinal microbiota in channel catfish (Ictalurus punctatus). Comp. Biochem. Physiol. Part D 2021, 40, 100921. [Google Scholar] [CrossRef]
- Soler, P.; Faria, M.; Barata, C.; García-Galea, E.; Lorente, B.; Vinyoles, D. Improving water quality does not guarantee fish health: Effects of ammonia pollution on the behaviour of wild-caught pre-exposed fish. PLoS ONE 2021, 16, e0243404. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Qian, Y.L.; Alcazar Magana, A.; Xiong, S.; Qian, M.C. Comparative characterization of aroma compounds in silver carp (Hypophthalmichthys molitrix), pacific whiting (Merluccius productus), and alaska pollock (Theragra chalcogramma) surimi by aroma extract dilution analysis, odor activity value, and aroma recombination studies. J. Agric. Food Chem. 2020, 68, 10403–10413. [Google Scholar] [CrossRef]
- Varlet, V.; Prost, C.; Serot, T. Volatile aldehydes in smoked fish: Analysis methods, occurence and mechanisms of formation. Food Chem. 2007, 105, 1536–1556. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, Y.; Cao, W.; Zhou, L.; Zhang, C.; Qin, X.; Zheng, H.; Lin, H.; Gao, J. Novel insight into the role of processing stages in nutritional components changes and characteristic flavors formation of noble scallop Chlamys nobilis adductors. Food Chem. 2022, 378, 132049. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, J.; Zhang, W.; Wæhrens, S.S.; Tøstesen, M.; Hansen, E.T.; Bredie, W.L.; Lametsch, R. Exopeptidase treatment combined with Maillard reaction modification of protein hydrolysates derived from porcine muscle and plasma: Structure–taste relationship. Food Chem. 2020, 306, 125613. [Google Scholar] [CrossRef]
- Zhou, M.Z.; Xiong, G.Q.; Qiao, Y.; Wang, C.; Wang, L.; Wu, W.J.; Li, X.; Shi, L.; Ding, A.Z.; Li, C. Analysis on flavor components of different edible parts of Procambarus clarkii. Meat Res. 2020, 34, 52–58. [Google Scholar] [CrossRef]
- Salum, P.; Guclu, G.; Selli, S. Comparative evaluation of key aromaactive compounds in raw and cooked red mullet (Mullus barbatus) by aroma extract dilution analysis. J. Agric. Food Chem. 2017, 65, 8402–8408. [Google Scholar] [CrossRef]
- Jiang, J.J.; Xie, P.H.; Ren, F.; Lai, Z.J.; Ou, A.F. Flavor analysis of Xinhui citrus Pu’er tea based on gas-phase ion migration spectrum and headspace solid phase microextraction. Sci. Technol. Food Ind. 2020, 41, 214–220. [Google Scholar] [CrossRef]
- Hsieh, R.J.; Kinsella, J.E. Lipoxygenase generation of specific volatile flavor carbonyl compounds in fish tissues. J. Agric. Food Chem. 1989, 37, 279–286. [Google Scholar] [CrossRef]
- Calkins, C.R.; Hodgen, J.M. A fresh look at meat flavor. Meat Sci. 2007, 77, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Tucker, C.S. Off-flavor problems in aquaculture. Rev. Fish. Sci. 2000, 8, 45–88. [Google Scholar] [CrossRef]
- Grigorakis, K. Compositional and organoleptic quality of farmed and wild gilthead sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) and factors affecting it: A review. Aquaculture 2007, 272, 55–75. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).