Development of a Lateral Flow Strip with a Positive Readout for the On-Site Detection of Aflatoxin B1
Abstract
:1. Introduction
2. Results and Discussion
2.1. Principle of the Apt-Strip
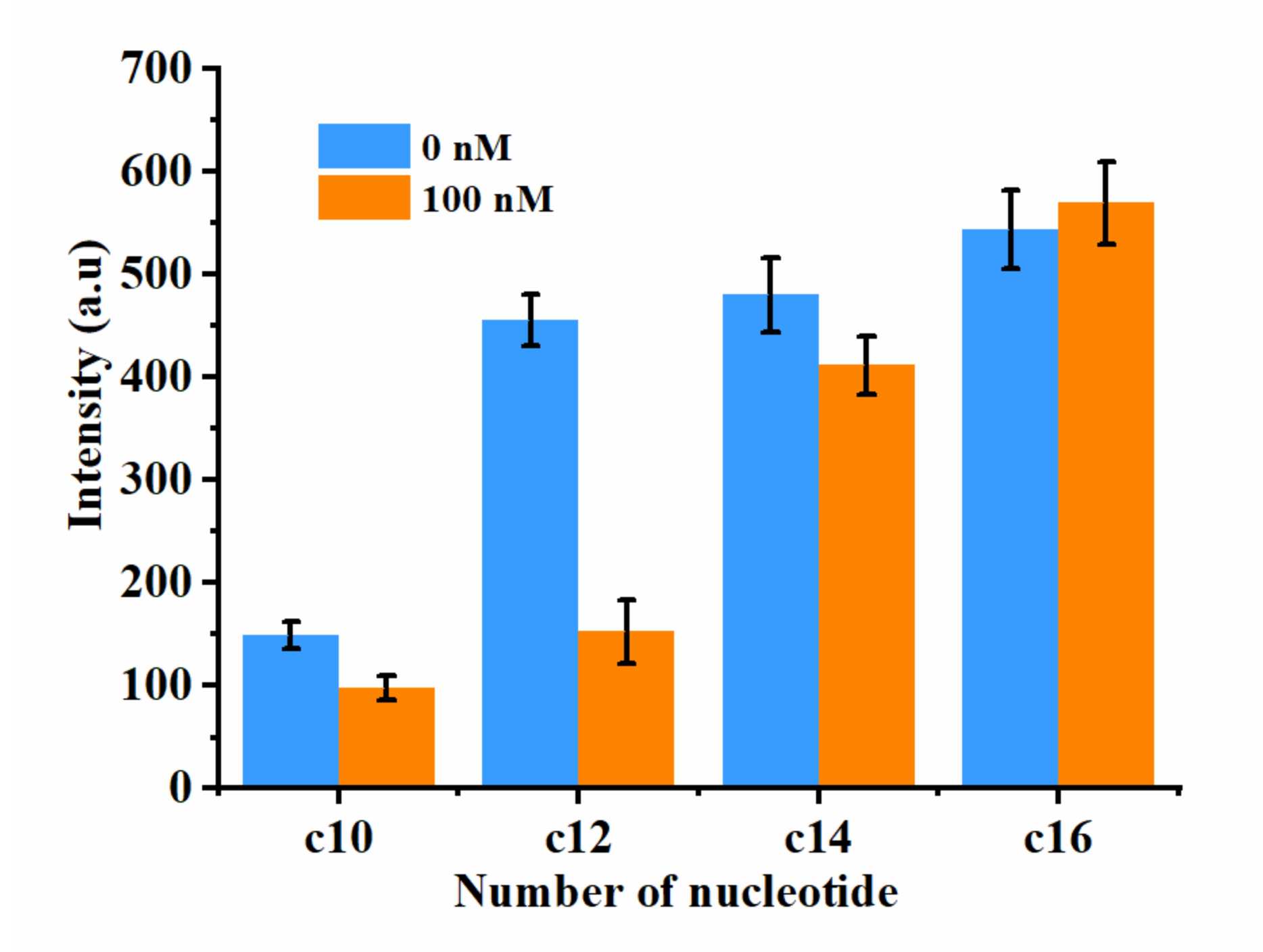
2.2. Optimization of cDNA Length
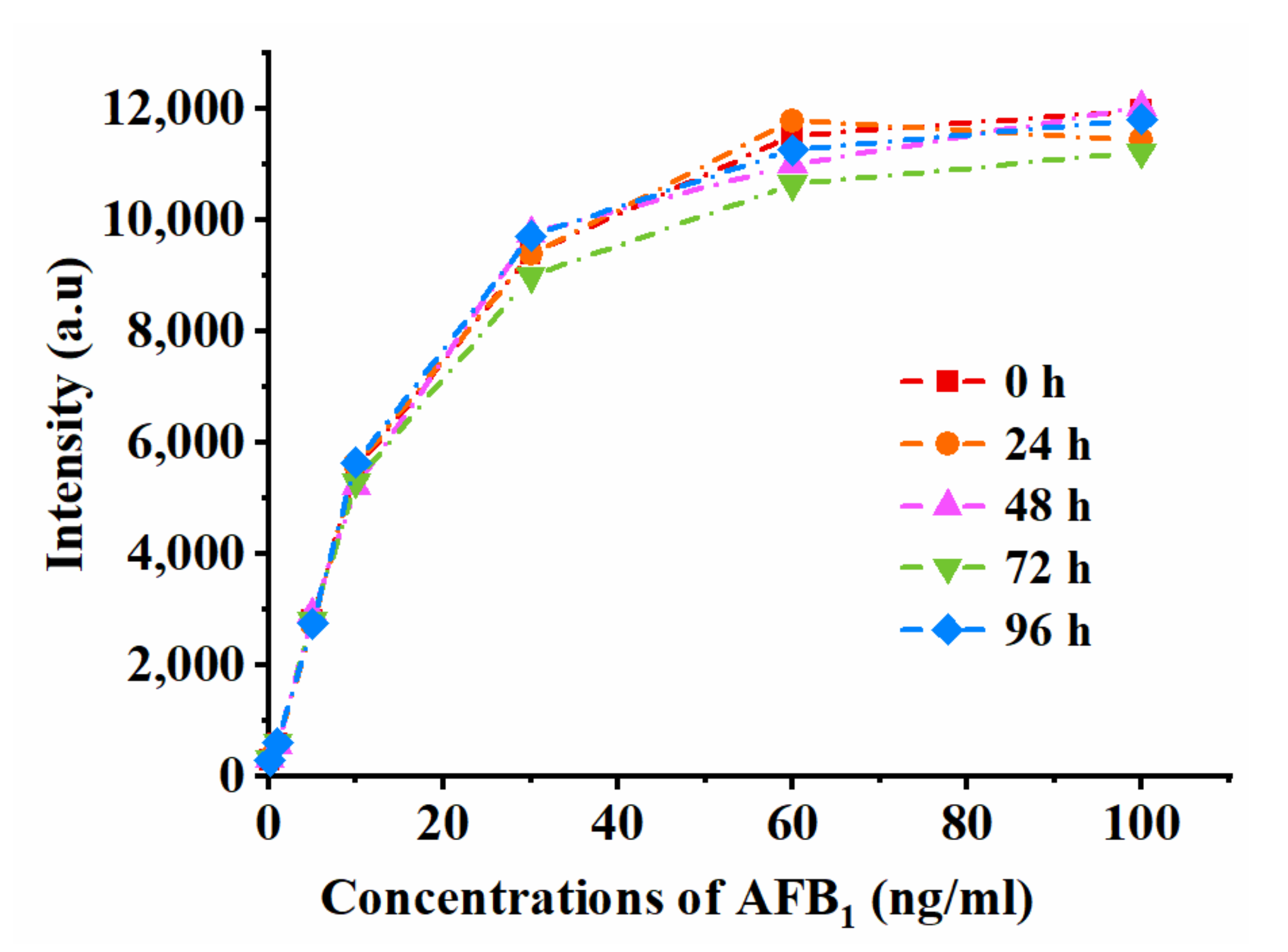
2.3. Analytical Performance of the Apt-Strip
2.4. Detection of AFB1 in Real Samples
3. Materials and Methods
3.1. Reagents and Materials
3.2. Aptamer and DNA Probes
3.3. Preparation of A35-Apt Coated Microplates
3.4. Optimization Procedure of cDNA Length
3.5. Manufacture of the Apt-Strip
3.6. Assay Procedure of AFB1 in Food Samples
3.7. HPLC-FLD Confirmation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Jiang, J.; Li, M.; Yuan, D.; Nie, C.; Sun, Y.; Zheng, P. Identification of Moldy Peanuts under Different Varieties and Moisture Content Using Hyperspectral Imaging and Data Augmentation Technologies. Foods 2022, 11, 1156. [Google Scholar] [CrossRef]
- Beitollahi, H.; Tajik, S.; Dourandish, Z.; Zhang, K.; Le, Q.V.; Jang, H.W.; Kim, S.Y.; Shokouhimehr, M. Recent Advances in the Aptamer-Based Electrochemical Biosensors for Detecting Aflatoxin B1 and Its Pertinent Metabolite Aflatoxin M1. Sensors 2020, 20, 3256. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, Q.; Wu, W. Progress on Structured Biosensors for Monitoring Aflatoxin B1 From Biofilms: A Review. Front. Microbial. 2020, 11, 408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Banerjee, K. A Review: Sample Preparation and Chromatographic Technologies for Detection of Aflatoxins in Foods. Toxins 2020, 12, 539. [Google Scholar] [CrossRef]
- Lee, S.; Kim, G.; Moon, J. Performance Improvement of the One-Dot Lateral Flow Immunoassay for Aflatoxin B1 by Using a Smartphone-Based Reading System. Sensors 2013, 13, 5109–5116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, L.; Chen, M.; Ying, Y. Development of Methods for Determination of Aflatoxins. Crit. Rev. Food Sci. Nutr. 2016, 56, 2642–2664. [Google Scholar] [CrossRef]
- Commission, E. Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union 2006, 70, 12–34. [Google Scholar]
- Wang, S.; Quan, Y.; Lee, N.; Kennedy, I.R. Rapid determination of fumonisin B1 in food samples by enzyme-linked immunosorbent assay and colloidal gold immunoassay. J. Agric. Food Chem. 2006, 54, 2491–2495. [Google Scholar] [CrossRef]
- Yu, S.; He, L.; Yu, F.; Liu, L.; Qu, C.; Qu, L.; Liu, J.; Wu, Y.; Wu, Y. A lateral flow assay for simultaneous detection of Deoxynivalenol, Fumonisin B1 and Aflatoxin B1. Toxicon 2018, 156, 23–27. [Google Scholar] [CrossRef]
- Zhao, X.; Jin, X.; Lin, Z.; Guo, Q.; Liu, B.; Yuan, Y.; Yue, T.; Zhao, X. Simultaneous Rapid Detection of Aflatoxin B1 and Ochratoxin A in Spices Using Lateral Flow Immuno-Chromatographic Assay. Foods 2021, 10, 2738. [Google Scholar] [CrossRef]
- Bu, T.; Bai, F.; Sun, X.; Tian, Y.; Zhang, M.; Zhao, S.; He, K.; Wang, X.; Jia, P.; Wang, L. An innovative prussian blue nanocubes decomposition-assisted signal amplification strategy suitable for competitive lateral flow immunoassay to sensitively detect aflatoxin B1. Food Chem. 2021, 344, 128711. [Google Scholar] [CrossRef]
- Yu, L.; Li, P.; Ding, X.; Zhang, Q. Graphene oxide and carboxylated graphene oxide: Viable two-dimensional nanolabels for lateral flow immunoassays. Talanta 2017, 165, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar]
- Ngom, B.; Guo, Y.; Wang, X.; Bi, D. Development and application of lateral flow test strip technology for detection of infectious agents and chemical contaminants: A review. Anal. Bioanal. Chem. 2010, 397, 1113–1135. [Google Scholar] [CrossRef] [PubMed]
- Bahadır, E.B.; Sezgintürk, M.K. Lateral flow assays: Principles, designs and labels. TRAC-Trend. Anal. Chem. 2016, 82, 286–306. [Google Scholar] [CrossRef]
- Focker, M.; van der Fels-Klerx, H.J.; Oude Lansink, A. Cost-Effective Sampling and Analysis for Mycotoxins in a Cereal Batch. Risk Anal. 2019, 39, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Delmulle, B.S.; De Saeger, S.M.; Sibanda, L.; Barna-Vetro, I.; Van Peteghem, C.H. Development of an immunoassay-based lateral flow dipstick for the rapid detection of aflatoxin B1 in pig feed. J. Agric. Food Chem. 2005, 53, 3364–3368. [Google Scholar] [CrossRef]
- Masinde, L.J.A.; Sheng, W.; Xu, X.; Zhang, Y.; Yuan, M.; Kennedy, I.R.; Wang, S. Colloidal gold based immunochromatographic strip for the simple and sensitive determination of aflatoxin B1 and B2 in corn and rice. Microchim. Acta 2013, 180, 921–928. [Google Scholar] [CrossRef]
- Sojinrin, T.; Liu, K.; Wang, K.; Cui, D.; JByrne, H.; Curtin, J.F.; Tian, F. Developing Gold Nanoparticles-Conjugated Aflatoxin B1 Antifungal Strips. Int. J. Mol. Sci. 2019, 20, 6260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Li, P.; Zhang, Q.; Li, R.; Zhang, W.; Ding, X.; Li, C.M. A naked-eye based strategy for semiquantitative immunochromatographic assay. Anal. Chim. Acta 2012, 740, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Xu, H.; Huang, X.; Kuang, M.; Xiong, Y.; Xu, H.; Xu, Y.; Chen, H.; Wang, A. Immunochromatographic assay for ultrasensitive detection of aflatoxin B₁ in maize by highly luminescent quantum dot beads. ACS Appl. Mater. Interfaces 2014, 6, 14215–14222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Mao, M.; Wu, F.; Li, Q.; Wei, L.; Ma, L. Amino-functionalized CdSe/ZnS quantum dot-based lateral flow immunoassay for sensitive detection of aflatoxin B1. Anal. Methods 2018, 10, 3582–3588. [Google Scholar] [CrossRef]
- Jia, B.; Liao, X.; Sun, C.; Fang, L.; Zhou, L.; Kong, W. Development of a quantum dot nanobead-based fluorescent strip immunosensor for on-site detection of aflatoxin B1 in lotus seeds. Food Chem. 2021, 356, 129614. [Google Scholar] [CrossRef]
- Shao, Y.; Duan, H.; Guo, L.; Leng, Y.; Lai, W.; Xiong, Y. Quantum dot nanobead-based multiplexed immunochromatographic assay for simultaneous detection of aflatoxin B1 and zearalenone. Anal. Chim. Acta 2018, 1025, 163–171. [Google Scholar] [CrossRef]
- Li, R.; Meng, C.; Wen, Y.; Fu, W.; He, P. Fluorometric lateral flow immunoassay for simultaneous determination of three mycotoxins (aflatoxin B1, zearalenone and deoxynivalenol) using quantum dot microbeads. Mikrochim. Acta 2019, 186, 748. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhong, X.; Xu, Y.; Zheng, Y.; Shi, X.; Li, F.; Guo, S.; Yang, J. Smartphone-based reading system integrated with phycocyanin-enhanced latex nanospheres immunoassay for on-site determination of aflatoxin B1 in foodstuffs. Food Chem. 2021, 360, 130019. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, Y.; Chen, M.; Wang, S.; Liu, K.; Lai, W. Rapid detection method for aflatoxin B1 in soybean sauce based on fluorescent microspheres probe. Food Control 2015, 50, 659–662. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, H.; Zhai, W.; Feng, X.; Fan, X.; Chen, A.; Wang, M. A Lateral Flow Strip Based on a Truncated Aptamer-Complementary Strand for Detection of Type-B Aflatoxins in Nuts and Dried Figs. Toxins 2020, 12, 136. [Google Scholar] [CrossRef] [Green Version]
- Vijitvarasan, P.; Cheunkar, S.; Oaew, S. A point-of-use lateral flow aptasensor for naked-eye detection of aflatoxin B1. Food Control 2022, 134, 108767. [Google Scholar] [CrossRef]
- Mukherjee, M.; Bhatt, P.; Manonmani, H.K. Fluorescent competitive aptasensor for detection of aflatoxin B1. J. Mol. Recognit. 2017, 30, 2650. [Google Scholar] [CrossRef] [PubMed]
- Vanrell, L.; Gonzalez-Techera, A.; Hammock, B.D.; Gonzalez-Sapienza, G. Nanopeptamers for the development of small-analyte lateral flow tests with a positive readout. Anal. Chem. 2013, 85, 1177–1182. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.; Wang, Y.; Yang, H.; Dong, Y.; Zhang, K.; Lu, Y.; Deng, R.; He, Q. Enzyme-free amplified and ultrafast detection of aflatoxin B1 using dual-terminal proximity aptamer probes. Food Chem. 2019, 283, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Y.; Zhao, Q. A signal-on electrochemical aptasensor for rapid detection of aflatoxin B1 based on competition with complementary DNA. Biosens. Bioelectron. 2019, 144, 111641. [Google Scholar] [CrossRef] [PubMed]
- Nutiu, R.; Li, Y. Structure-switching signaling aptamers. J. Am. Chem. Soc. 2003, 125, 4771. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, L.; Zhao, Q. Development of aptamer fluorescent switch assay for aflatoxin B1 by using fluorescein-labeled aptamer and black hole quencher 1-labeled complementary DNA. Anal. Bioanal. Chem. 2018, 410, 6269–6277. [Google Scholar] [CrossRef] [PubMed]
- Nutiu, R.; Li, Y. Structure-switching signaling aptamers: Transducing molecular recognition into fluorescence signaling. Chemistry 2004, 10, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Liu, N.; Zhao, Z.; Ediage, E.; Wu, S.; Sun, C.; De Saeger, S.; Wu, A. Multiplex Lateral Flow Immunoassay for Mycotoxin Determination. Anal. Chem. 2014, 86, 4995–5001. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, J.; Zhang, Z.; Zhang, Q.; Zhang, W.; Yu, L.; Jiang, J.; Chen, X.; Wang, X.; Li, P. Simultaneous Lateral Flow Immunoassay for Multi-Class Chemical Contaminants in Maize and Peanut with One-Stop Sample Preparation. Toxins 2019, 11, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Chen, Q.; Han, M.; Zhou, J.; Gong, L.; Niu, Y.; Zhang, Y.; He, L.; Zhang, L. Development and optimization of a multiplex lateral flow immunoassay for the simultaneous determination of three mycotoxins in corn, rice and peanut. Food Chem. 2016, 213, 478–484. [Google Scholar] [CrossRef]
- Cai, X.; Liang, M.; Ma, F.; Zhang, Z.; Tang, X.; Jiang, J.; Guo, C.; Ramzy Mohamed, S.; Abdel Goda, A.; Dawood, D.H.; et al. Nanozyme-strip based on MnO2 nanosheets as a catalytic label for multi-scale detection of aflatoxin B1 with an ultrabroad working range. Food Chem. 2022, 377, 131965. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, G.L.; Huang, Y.F.; Yang, S.M.; Ren, S.Y.; Gao, Z.X.; Chen, A.L. Dual-competitive lateral flow aptasensor for detection of aflatoxin B1 in food and feedstuffs. J. Hazard. Mater. 2018, 344, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.B.; Kim, M.J.; Mun, H.; Kim, M.G. An aptamer-based dipstick assay for the rapid and simple detection of aflatoxin B1. Biosens. Bioelectron. 2014, 62, 288–294. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, S.; Wang, S.; Liu, J.H.; Dong, Y.Y. Development of Lateral Flow Immunochromatographic Strips for Micropollutant Screening Using Colorants of Aptamer-Functionalized Nanogold Particles, Part II: Experimental Verification with Aflatoxin B1 and Chloramphenicol. J. AOAC Int. 2018, 101, 1408–1414. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.; Zhao, Q. Aptamer-Structure Switch Coupled with Horseradish Peroxidase Labeling on a Microplate for the Sensitive Detection of Small Molecules. Anal. Chem. 2019, 91, 2615–2619. [Google Scholar] [CrossRef] [PubMed]




| Type | T-line | Probes | Signal Readout | DL | Signal Reader | Ref. |
|---|---|---|---|---|---|---|
| Antibody-strip | AFB1-BSA | colloidal gold-mAb | negative | 5 μg/kg | Strip reader | [5] |
| Antibody-strip | AFB1-BSA | QB-mAbs | negative | 1 ng/mL | A fluorescent reader | [23] |
| Antibody-strip | AFB1-BSA | mAb@Eu- nanosphere | negative | 0.16 μg/kg | Fluorescent strip reader | [38] |
| Antibody-strip | AFB1-BSA | Ab-GNPs | negative | 0.1 μg/kg | Strip reader | [39] |
| Antibody-strip | AFB1–OVA | gold-labeled antibody | negative | 5 μg/kg | ICheck-III card reader | [10] |
| Nanozyme-strip | AFB1-BSA | MnO2NSs-mAb | negative | 15 pg/mL | Smart phone | [40] |
| Aptamer-strip | AFB1-BSA | Cy5-Aptamer | negative | 0.1 μg/kg | Fluorescent strip reader | [41] |
| Aptamer-strip | DNA single strand | Cy5-Aptamer | negative | 0.16 μg/kg | The portable multi-target reader | [28] |
| Aptamer-strip | SA | Cy5-Aptamer | negative | 0.1 ng/mL | ChemiDocTM MP System | [42] |
| Aptamer-strip | bio-DNA probe-SA | NGPs-Aptamer | negative | 0.5 μg/mL | Strip reader | [43] |
| 5 μg/mL | Naked eye | |||||
| Aptamer-strip | DNA single strand | FAM-Aptamer | Positive | <0.1 ng/mL | ChemiDocTM MP System | This work |
| Sample | AFB1 spiked (ng/g) | Detected (ng/g) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Corn | 1 | 0.5 a | 50.0 | 36.7 |
| 3 | 2.9 | 96.7 | 8.7 | |
| 10 | 9.7 | 97.0 | 6.1 | |
| Wheat | 1 | 0.5 | 50.0 | 21.7 |
| 3 | 2.5 | 83.3 | 8.4 | |
| 10 | 9.6 | 96.0 | 7.3 |
| Concentrations of AFB1 (ng/g) | Intensity (a.u) a | RSD (%) |
|---|---|---|
| 0.1 | 294.7 ± 15.4 | 5.3 |
| 1 | 582.9 ± 21.7 | 3.8 |
| 5 | 2800.7 ± 75.9 | 2.8 |
| 10 | 5460.2 ± 188.2 | 3.5 |
| 30 | 9457.6 ± 312.8 | 3.4 |
| 60 | 11,249.7 ± 444.8 | 4.0 |
| 100 | 11,700.2 ± 352.2 | 3.1 |
| Category | Sample No | Apt-Strip a (μg/kg) | HPLC-FLD (μg/kg) |
|---|---|---|---|
| Maize | 1 | ND b | ND |
| 2 | ND | ND | |
| 3 | 5.4 ± 1.2 | 6.3 ± 0.8 | |
| 4 | ND | ND | |
| 5 | 12.6 ± 1.8 | 10.8 ± 0.8 | |
| 6 | 32.4 ± 2.4 | 30.2 ± 1.0 | |
| 7 | ND | 0.1 | |
| 8 | 75.3 ± 5.3 | 80.2 ± 2.4 | |
| 9 | 18.4 ± 1.8 | 17.4 ± 0.9 | |
| 10 | 10.8 ± 0.9 | 11.6 ± 0.6 | |
| 11 | ND | ND | |
| 12 | 7.6 ± 1.6 | 8.9 ± 0.6 | |
| 13 | ND | ND | |
| Wheat | 14 | ND | ND |
| 15 | ND | ND | |
| 16 | 3.2 ± 1.5 | 3.7 ± 1.1 | |
| 17 | 23.6 ± 3.4 | 25.4 ± 0.7 | |
| 18 | ND | ND | |
| 19 | ND | ND | |
| Sorghum | 20 | ND | ND |
| 21 | ND | ND | |
| 22 | 15.3 ± 1.9 | 13.3 ± 0.6 | |
| 23 | 2.4 ± 0.7 | 1.8 ± 1.2 | |
| 24 | ND | ND | |
| 25 | ND | ND |
| Name | Sequences (5’-3’) |
|---|---|
| A35-Apt | TGCACGTGTTGTCTCTCTGTGTCTCGTGCTTTTTT-biotin-TEG |
| AFB1-Apt | TGCACGTGTTGTCTCTCTGTGTCTCGTGC |
| 10-cDNA | AACACGTGCA-6-FAM |
| 12-cDNA | ACAACACGTGCA-6-FAM |
| 14-cDNA | AGACAACACGTGCA-6-FAM |
| 16-cDNA | AGAGACAACACGTGCA-6-FAM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, K.; Hu, X.; Sun, L.; Han, C.; Yang, J. Development of a Lateral Flow Strip with a Positive Readout for the On-Site Detection of Aflatoxin B1. Molecules 2022, 27, 4949. https://doi.org/10.3390/molecules27154949
Shen K, Hu X, Sun L, Han C, Yang J. Development of a Lateral Flow Strip with a Positive Readout for the On-Site Detection of Aflatoxin B1. Molecules. 2022; 27(15):4949. https://doi.org/10.3390/molecules27154949
Chicago/Turabian StyleShen, Kemin, Xiaoqin Hu, Linlin Sun, Chun Han, and Jianzhou Yang. 2022. "Development of a Lateral Flow Strip with a Positive Readout for the On-Site Detection of Aflatoxin B1" Molecules 27, no. 15: 4949. https://doi.org/10.3390/molecules27154949
APA StyleShen, K., Hu, X., Sun, L., Han, C., & Yang, J. (2022). Development of a Lateral Flow Strip with a Positive Readout for the On-Site Detection of Aflatoxin B1. Molecules, 27(15), 4949. https://doi.org/10.3390/molecules27154949






