Detailed Chemical Prospecting of Volatile Organic Compounds Variations from Adriatic Macroalga Halopteris scoparia
Abstract
:1. Introduction
2. Results and Discussion
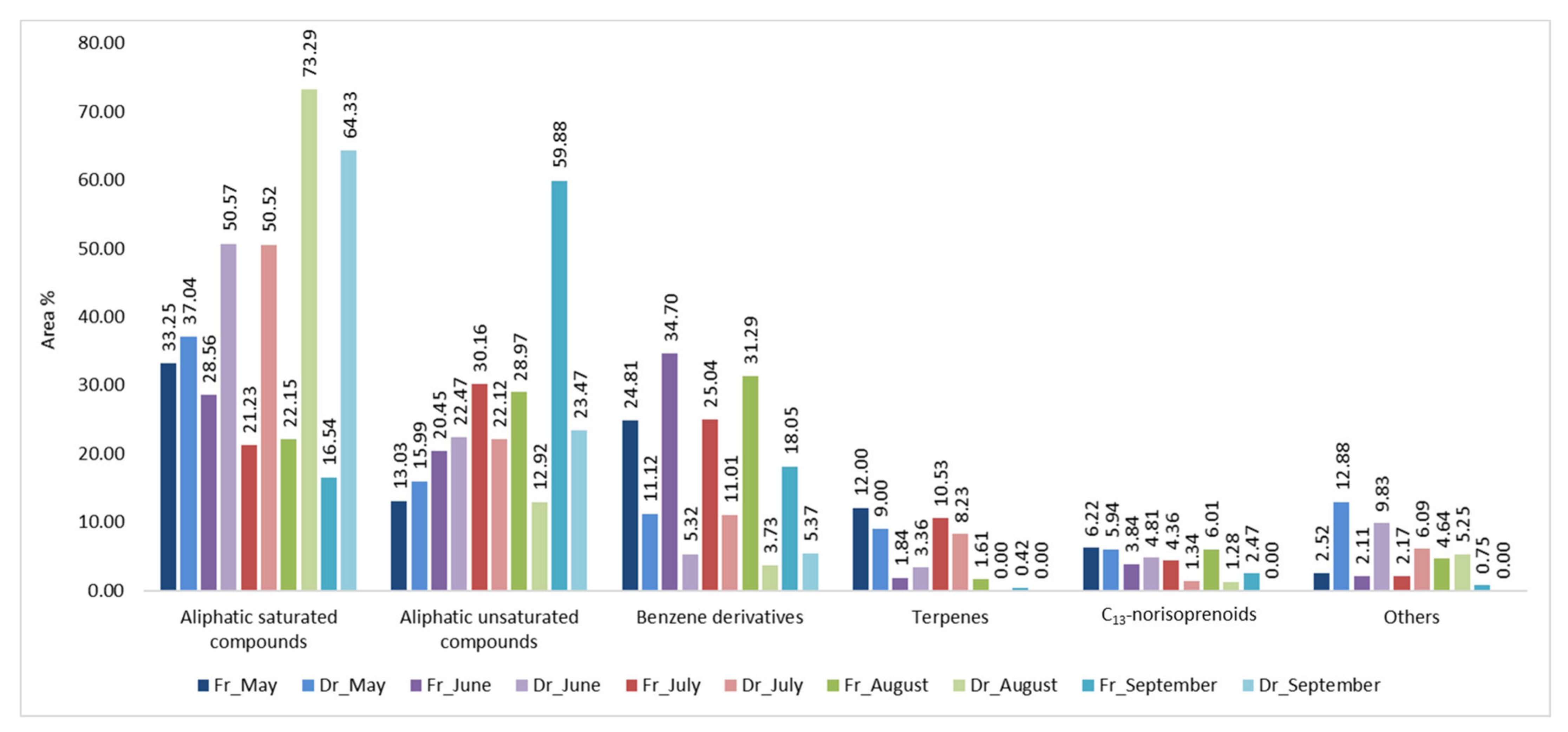
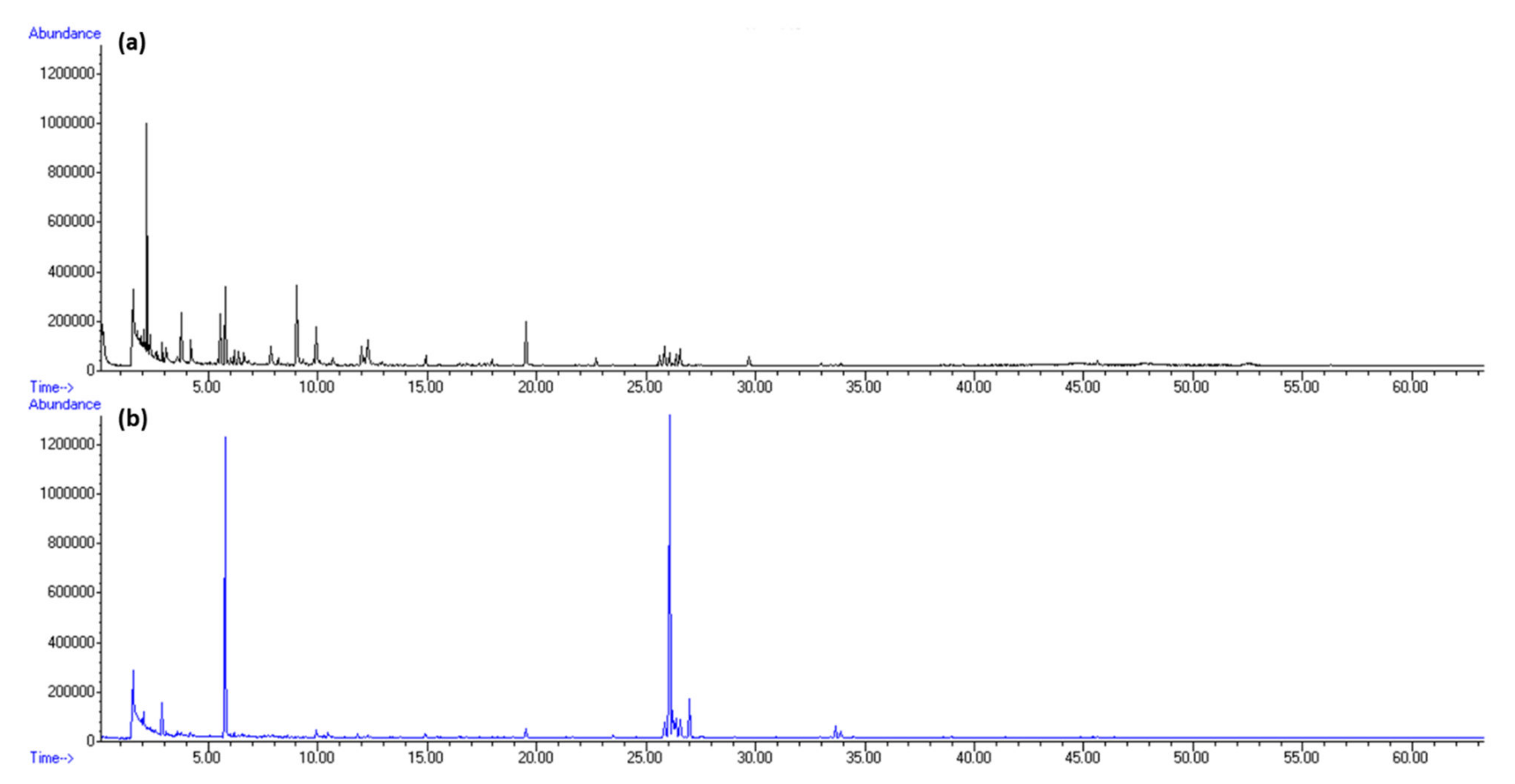
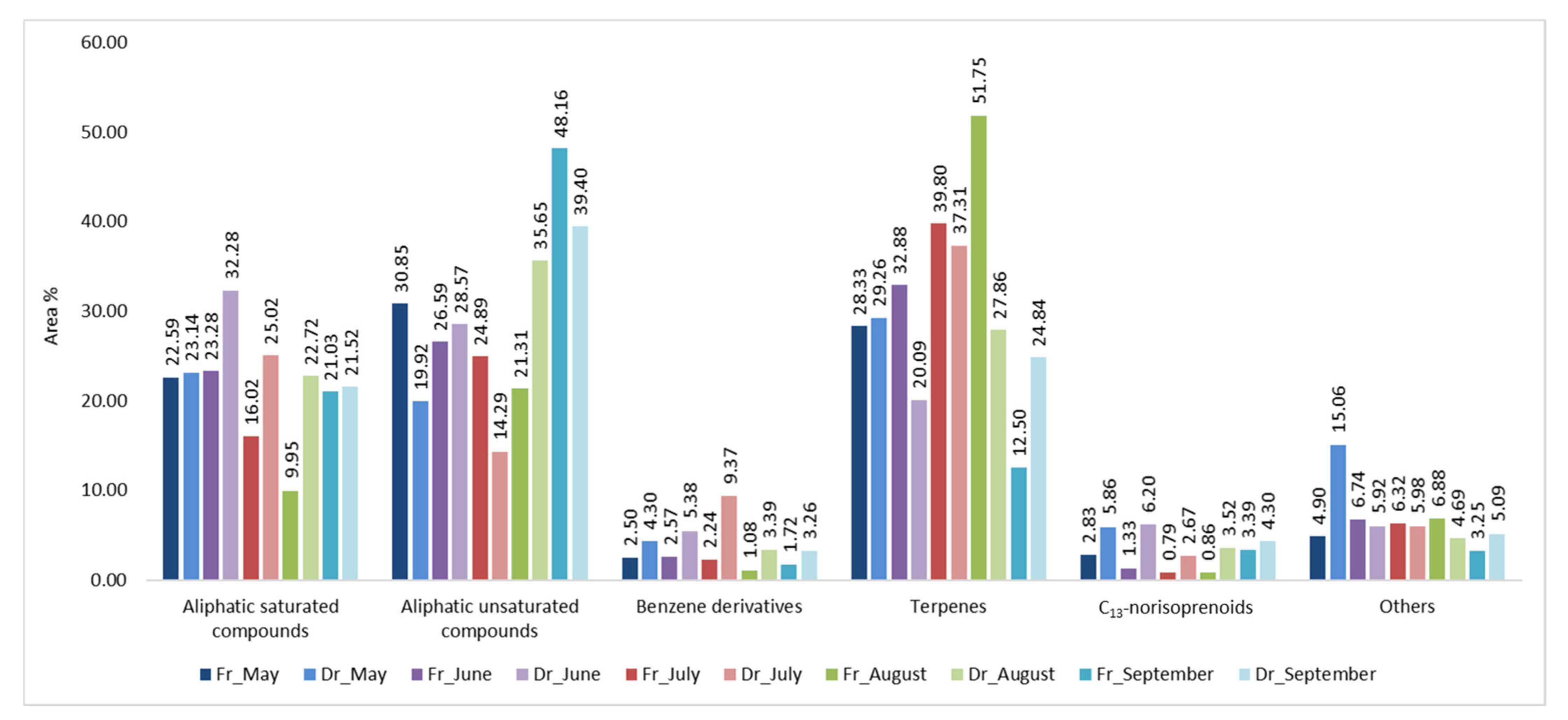
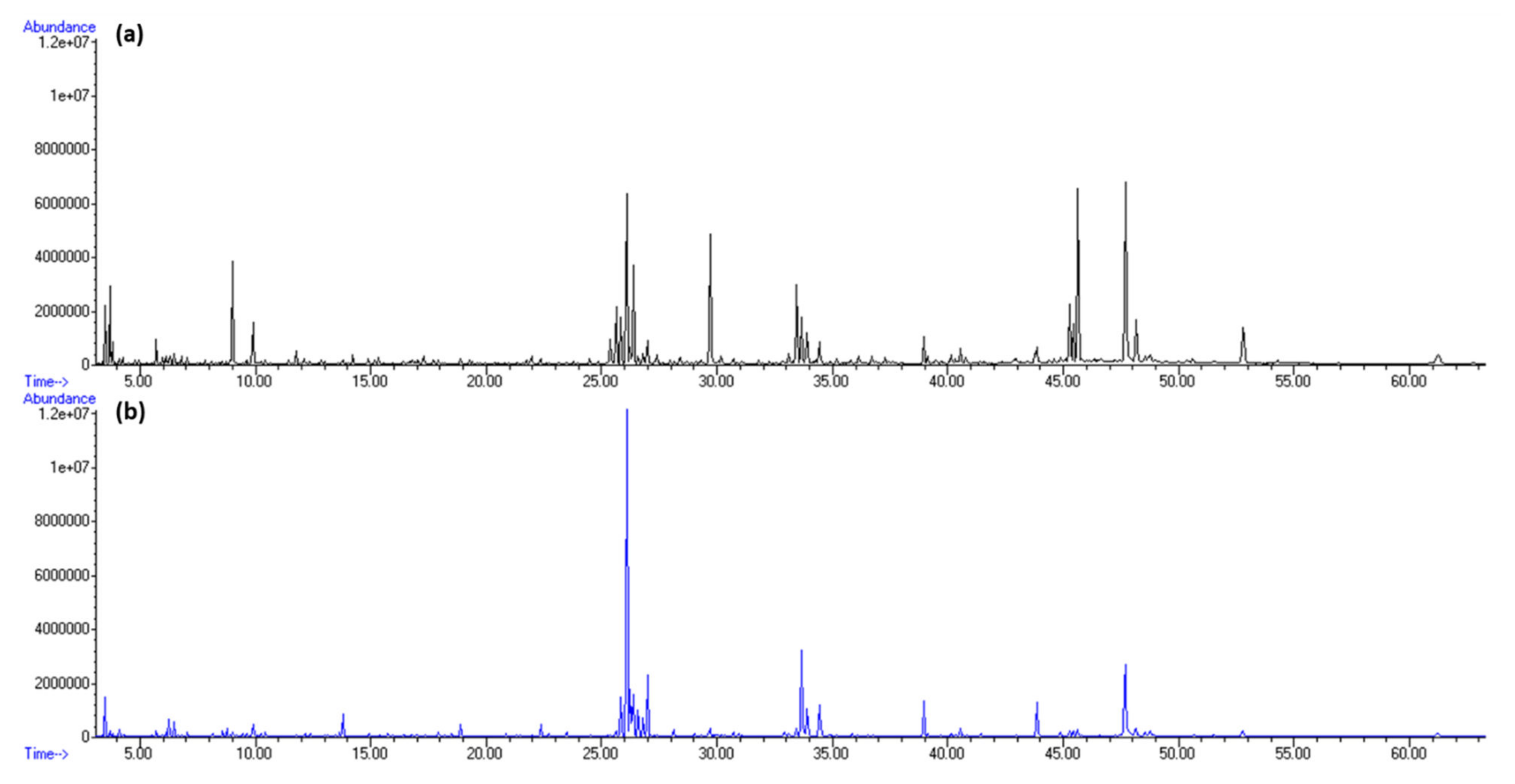
2.1. Headspace Variations of H. Scoparia
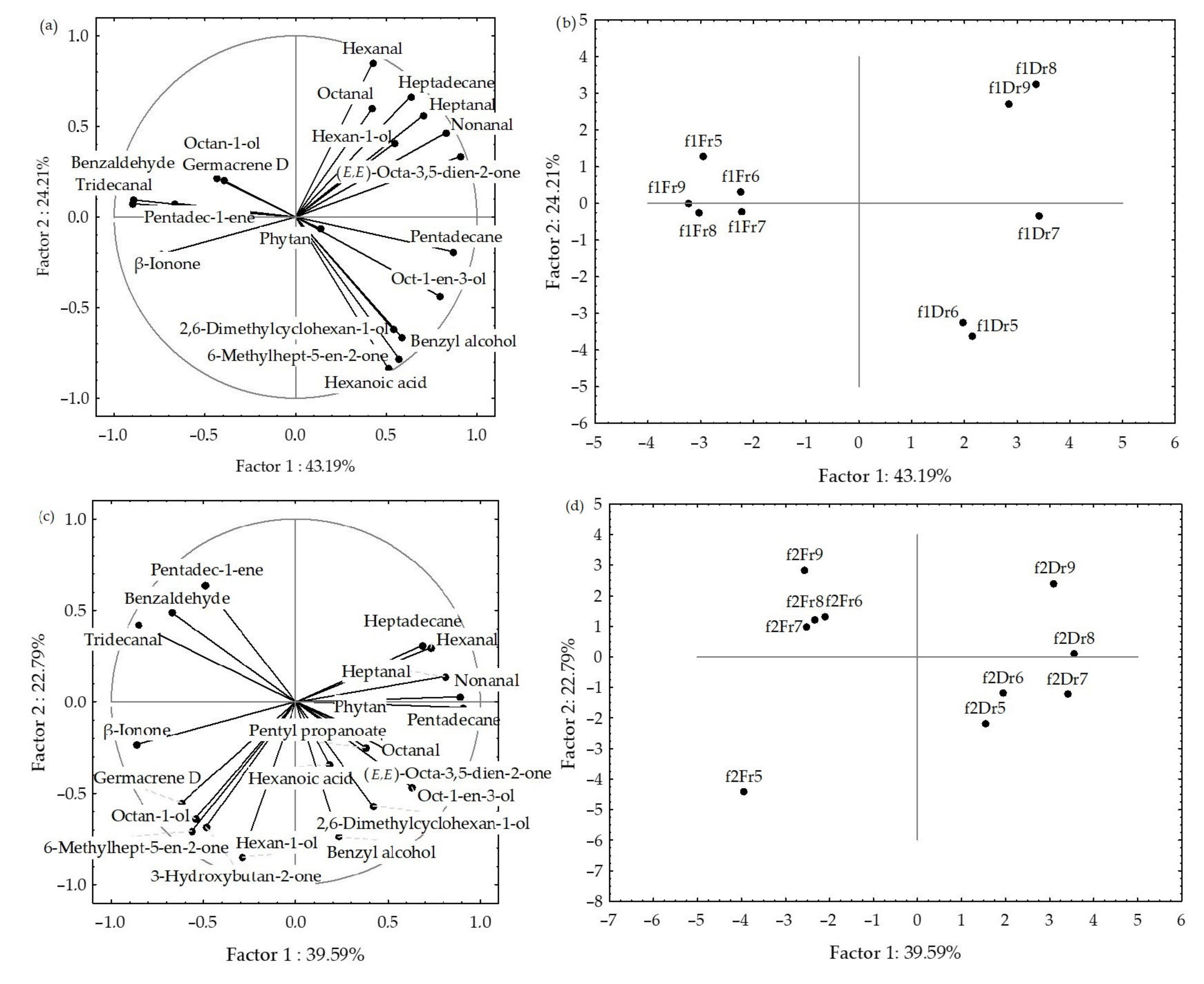
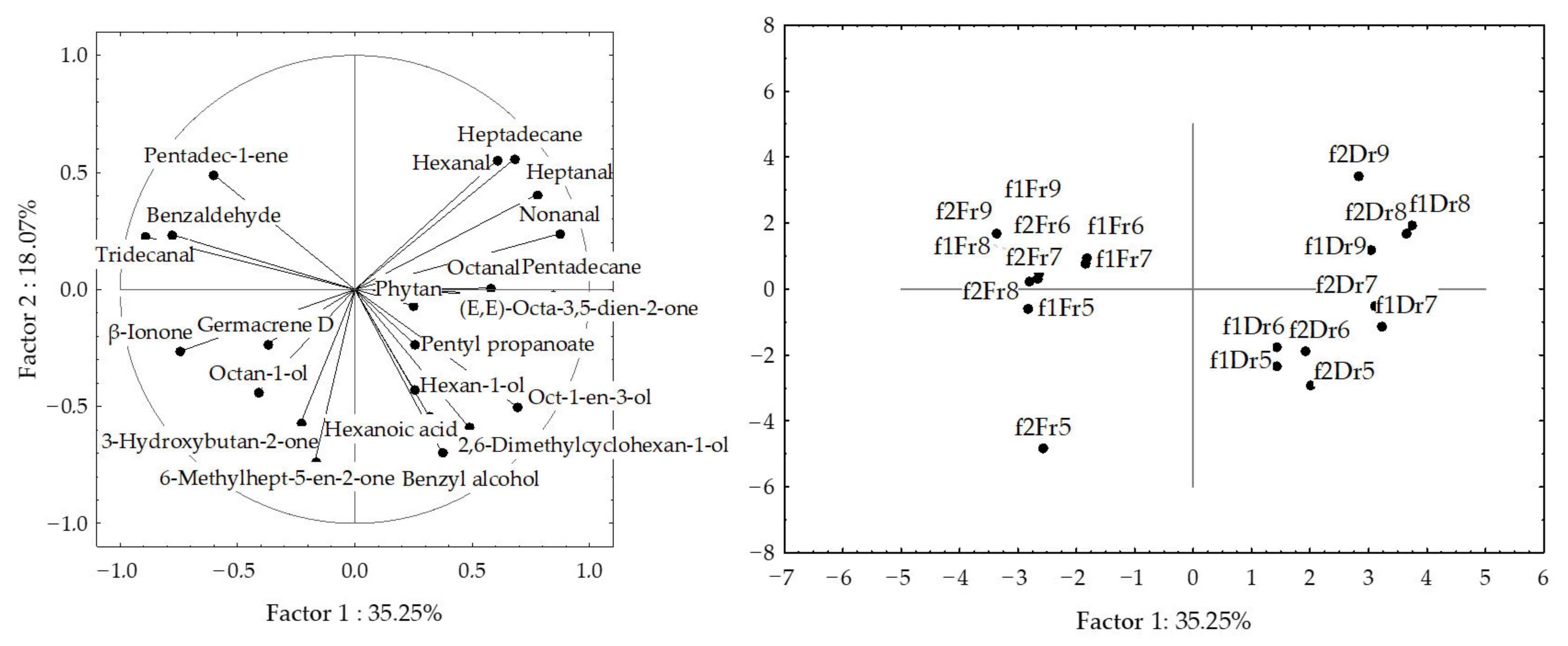
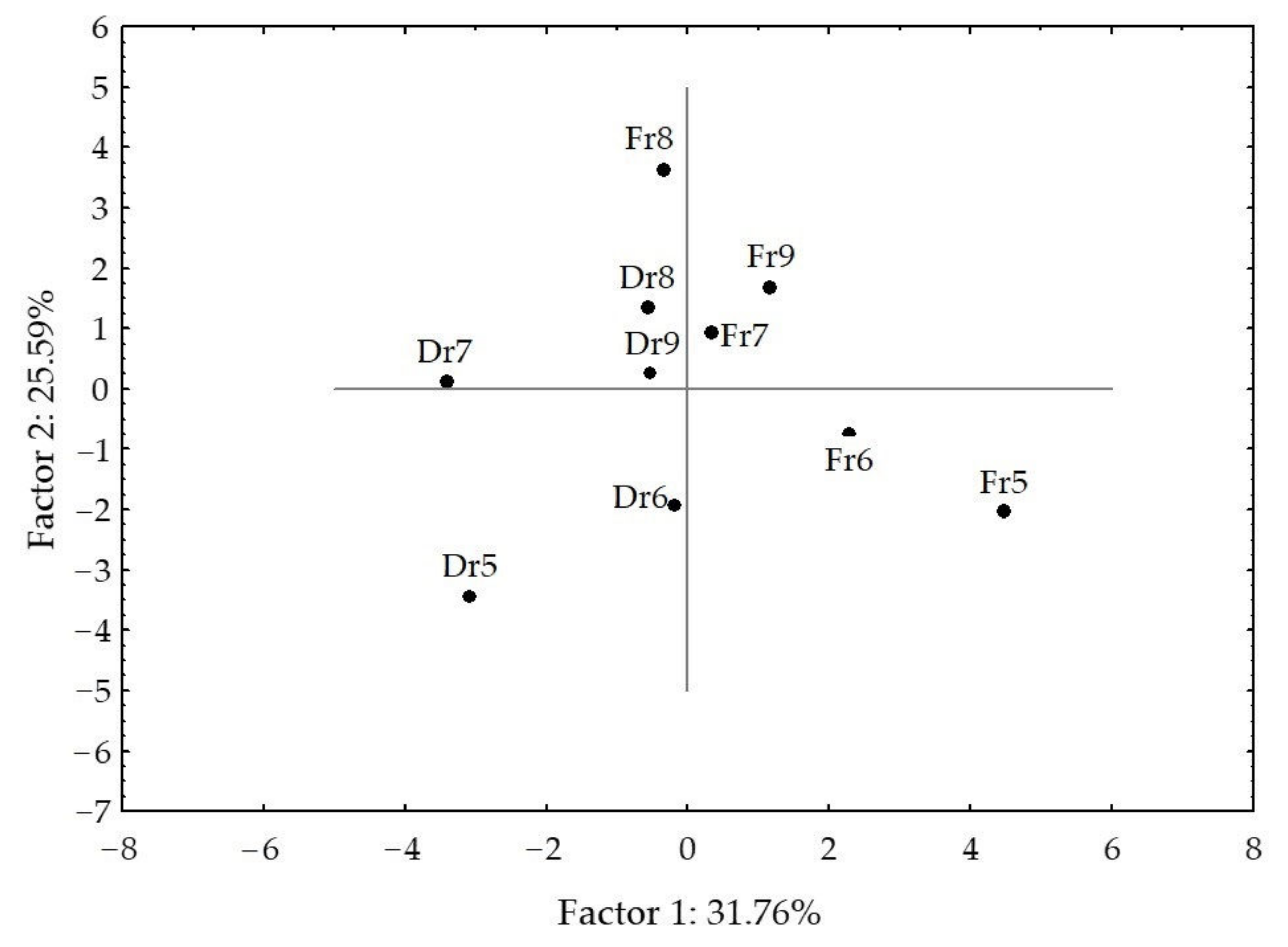
2.2. Statistical Analysis of the H. scoparia Headspace VOCs
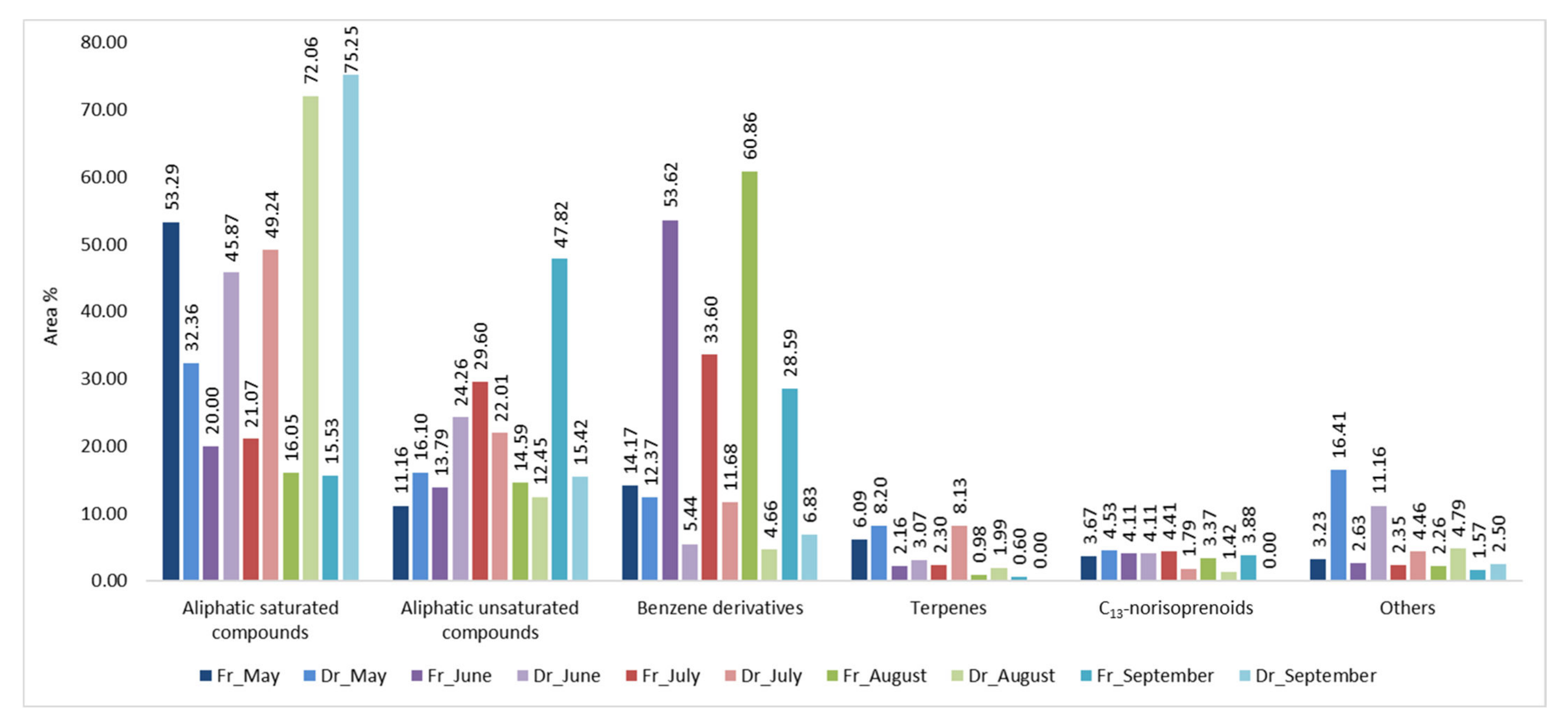
2.3. Volatile Organic Compounds Variations of H. scoparia Acquired by Hydrodistillation
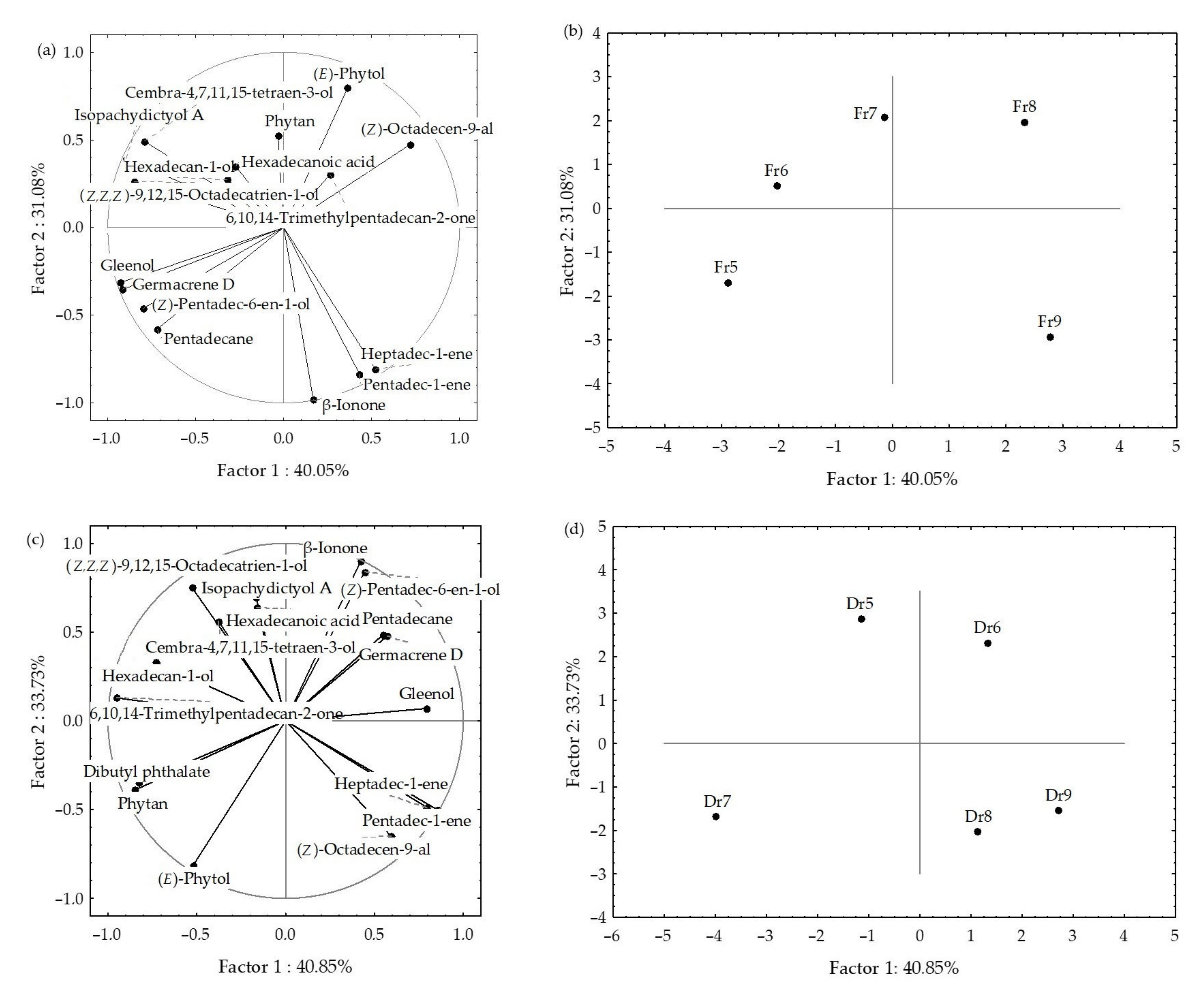
2.4. Statistical Analysis of the H. scoparia VOCs Obtained by Hydrodistillation
3. Materials and Methods
3.1. Sample Collection
3.2. Headspace Solid-Phase Microextraction (HS-SPME)
3.3. Hydrodistillation (HD)
3.4. Gas Chromatography–Mass Spectrometry Analysis (GC–MS)
3.5. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Guiry, M.D. AlgaeBase. Available online: https://www.algaebase.org (accessed on 15 June 2022).
- Orhan, I.; Sener, B.; Atıcı, T.; Brun, R.; Perozzo, R.; Tasdemir, D. Turkish Freshwater and Marine Macrophyte Extracts Show in Vitro Antiprotozoal Activity and Inhibit FabI, a Key Enzyme of Plasmodium falciparum Fatty Acid Biosynthesis. Phytomedicine 2006, 13, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Chiboub, O.; Ktari, L.; Sifaoui, I.; López-Arencibia, A.; Reyes-Batlle, M.; Mejri, M.; Valladares, B.; Abderrabba, M.; Piñero, J.E.; Lorenzo-Morales, J. In Vitro Amoebicidal and Antioxidant Activities of Some Tunisian Seaweeds. Exp. Parasitol. 2017, 183, 76–80. [Google Scholar] [CrossRef]
- Taskin, E.; Ozturk, M.; Taskin, E.; Kurt, O. Antibacterial Activities of Some Marine Algae from the Aegean Sea (Turkey). Afr. J. Biotechnol. 2007, 6, 2746–2751. [Google Scholar] [CrossRef] [Green Version]
- Heo, S.H.; Park, S.I.; Lee, J.; Jung, S.W.; Shin, M.S. Antimicrobial, Antioxidative, Elastase and Tyrosinase Inhibitory Effect of Supercritical and Hydrothermal Halopteris scoparia Extract. Turkish J. Comput. Math. Educ. 2021, 12, 407–413. [Google Scholar] [CrossRef]
- Febles, C.I.; Arias, A.; Hardisson, A.; López, A.S.; Gil-Rodríguez, M.C. Antimicrobial Activity of Extracts from Some Canary Species Of Phaeophyta And Chlorophyta. Phyther. Res. 1995, 9, 385–387. [Google Scholar] [CrossRef]
- Hellio, C.; Thomas-Guyon, H.; Culioli, G.; Piovettt, L.; Bourgougnon, N.; le Gal, Y. Marine Antifoulants from Bifurcaria bifurcata (Phaeophyceae, Cystoseiraceae) and Other Brown Macroalgae. Biofouling 2001, 17, 189–201. [Google Scholar] [CrossRef]
- Koçer, A.T.; Özçimen, D. Eco-Friendly Synthesis of Silver Nanoparticles from Macroalgae: Optimization, Characterization and Antimicrobial Activity. Biomass Convers. Biorefin. 2022, (in press). [Google Scholar] [CrossRef]
- Güner, A.; Nalbantsoy, A.; Sukatar, A.; Karabay Yavaşoğlu, N.Ü. Apoptosis-Inducing Activities of Halopteris scoparia L. Sauvageau (Brown Algae) on Cancer Cells and Its Biosafety and Antioxidant Properties. Cytotechnology 2019, 71, 687–704. [Google Scholar] [CrossRef]
- Güner, Ö.; Güner, A.; Yavaşoğlu, A.; Karabay Yavaşoğlu, N.Ü.; Kavlak, O. Ameliorative Effect of Edible Halopteris Scoparia against Cadmium-induced Reproductive Toxicity in Male Mice: A Biochemical and Histopathologic Study. Andrologia 2020, 52, e13591. [Google Scholar] [CrossRef]
- Fellah, F.; Louaileche, H.; Dehbi-Zebboudj, A.; Touati, N. Seasonal Variations in the Phenolic Compound Content and Antioxidant Activities of Three Selected Species of Seaweeds from Tiskerth Islet, Bejaia, Algeria. J. Mater. Environ. Sci. 2017, 8, 4451–4456. [Google Scholar] [CrossRef]
- Campos, A.M.; Matos, J.; Afonso, C.; Gomes, R.; Bandarra, N.M.; Cardoso, C. Azorean Macroalgae (Petalonia Binghamiae, Halopteris scoparia and Osmundea pinnatifida) Bioprospection: A Study of Fatty Acid Profiles and Bioactivity. Int. J. Food Sci. Technol. 2019, 54, 880–890. [Google Scholar] [CrossRef]
- Saidani, K.; Ziani, N.; Touati, N.; Merzouk, H.; Bedjou, F. Anticoagulant Activity of Sulfated Polysaccharides and Polyphenols Extracted from Marine Algae. Curr. Bioact. Compd. 2021, 17, 246–255. [Google Scholar] [CrossRef]
- Kartal, M.; Orhan, I.; Abu-Asaker, M.; Senol, F.; Atici, T.; Sener, B. Antioxidant and Anticholinesterase Assets and Liquid Chromatography-Mass Spectrometry Preface of Various Fresh-Water and Marine Macroalgae. Pharmacogn. Mag. 2009, 5, 291. [Google Scholar] [CrossRef]
- Nunes, N.; Valente, S.; Ferraz, S.; Barreto, M.C.; Pinheiro de Carvalho, M.A.A. Biochemical Study of Attached Macroalgae from the Madeira Archipelago and Beach-Cast Macroalgae from the Canary Islands: Multivariate Analysis to Determine Bioresource Potential. Bot. Mar. 2020, 63, 283–298. [Google Scholar] [CrossRef]
- Uslu, L.; Sayin, S.; Naz, M.; Taskin, E.; Soyler, O.; Saygili, I.; Cetin, Z.; Dinler, Z.M.; Isik, O. Proximate Analysis and Fatty Acid Profile of Some Brown Macroalgae Collected from the Northeastern Mediterranean Coast. Fresenius Environ. Bull. 2021, 30, 9433–9437. [Google Scholar]
- Pereira, H.; Barreira, L.; Figueiredo, F.; Custódio, L.; Vizetto-Duarte, C.; Polo, C.; Rešek, E.; Engelen, A.; Varela, J. Polyunsaturated Fatty Acids of Marine Macroalgae: Potential for Nutritional and Pharmaceutical Applications. Mar. Drugs 2012, 10, 1920–1935. [Google Scholar] [CrossRef] [Green Version]
- Jerković, I.; Marijanović, Z.; Roje, M.; Kuś, P.M.; Jokić, S.; Čož-Rakovac, R. Phytochemical Study of the Headspace Volatile Organic Compounds of Fresh Algae and Seagrass from the Adriatic Sea (Single Point Collection). PLoS ONE 2018, 13, e0196462. [Google Scholar] [CrossRef]
- Whitfield, F.B.; Helidoniotis, F.; Shaw, K.J.; Svoronos, D. Distribution of Bromophenols in Species of Marine Algae from Eastern Australia. J. Agric. Food Chem. 1999, 47, 2367–2373. [Google Scholar] [CrossRef]
- Radman, S.; Čagalj, M.; Šimat, V.; Jerković, I. Seasonal Variability of Volatilome from Dictyota dichotoma. Molecules 2022, 27, 3012. [Google Scholar] [CrossRef]
- Ferraces-Casais, P.; Lage-Yusty, M.A.; Rodriguez-Bernaldo De Quiros, A.; Lopez-Hernandez, J. Rapid identification of volatile compounds in fresh seaweed. Talanta 2013, 115, 798–800. [Google Scholar] [CrossRef]
- Plaza, M.; Santoyo, S.; Jaime, L.; García-Blairsy Reina, G.; Herrero, M.; Señoráns, F.J.; Ibañez, E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef]
- Radman, S.; Čižmek, L.; Babić, S.; Cikoš, A.-M.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. Bioprospecting of Less-Polar Fractions of Ericaria crinita and Ericaria amentacea: Developmental Toxicity and Antioxidant Activity. Mar. Drugs 2022, 20, 57. [Google Scholar] [CrossRef]
- Radman, S.; Cikoš, A.-M.; Flanjak, I.; Babić, S.; Čižmek, L.; Šubarić, D.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. Less Polar Compounds and Targeted Antioxidant Potential (In Vitro and In Vivo) of Codium adhaerens C. Agardh 1822. Pharmaceuticals 2021, 14, 944. [Google Scholar] [CrossRef]
- Lewis, N.G.; Yamamoto, E. Lignin: Occurrence, Biogenesis and Biodegradation. Annu. Rev. Plant Pathol. Plant. Mol. Biol. 1990, 41, 455–496. [Google Scholar] [CrossRef]
- Ragan, M.A. Fucus ‘Lignin’: A Reassessment. Phytochemistry 1984, 23, 2029–2032. [Google Scholar] [CrossRef]
- Silva, R.O.; Sousa, F.B.M.; Damasceno, S.R.B.; Carvalho, N.S.; Silva, V.G.; Oliveira, F.R.M.A.; Sousa, D.P.; Aragão, K.S.; Barbosa, A.L.R.; Freitas, R.M.; et al. Phytol, a Diterpene Alcohol, Inhibits the Inflammatory Response by Reducing Cytokine Production and Oxidative Stress. Fundam. Clin. Pharmacol. 2014, 28, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Saha, M.; Bandyopadhyay, P.K. In Vivo and in Vitro Antimicrobial Activity of Phytol, a Diterpene Molecule, Isolated and Characterized from Adhatoda vasica Nees. (Acanthaceae), to Control Severe Bacterial Disease of Ornamental Fish, Carassius auratus, Caused by Bacillus licheniformis PKBMS16. Microb. Pathog. 2020, 141, 103977. [Google Scholar] [CrossRef]
- Da Silva Almeida, J.G.; de Araujo Gomes, L.; de Andrade, T.D.; Silva, J.; de Lima, J.; Quintans-Junior, L. Phytochemical Screening and Anti-Inflammatory Activity of Cnidoscolus quercifolius (Euphorbiaceae) in Mice. Pharmacogn. Res. 2014, 6, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.-W.; Lee, H.J.; Jung, J.H.; Kim, Y.H.; Jung, D.-B.; Sohn, E.J.; Lee, J.H.; Woo, H.J.; Baek, N.-I.; Kim, Y.C.; et al. Activation of Caspase-9/3 and Inhibition of Epithelial Mesenchymal Transition Are Critically Involved in Antitumor Effect of Phytol in Hepatocellular Carcinoma Cells. Phyther. Res. 2015, 29, 1026–1031. [Google Scholar] [CrossRef]
- Siless, G.E.; García, M.; Pérez, M.; Blustein, G.; Palermo, J.A. Large-Scale Purification of Pachydictyol A from the Brown Alga Dictyota dichotoma Obtained from Algal Wash and Evaluation of Its Antifouling Activity against the Freshwater Mollusk Limnoperna Fortunei. J. Appl. Phycol. 2018, 30, 629–636. [Google Scholar] [CrossRef]
- Simas, D.L.R.; Kaiser, C.R.; Gestinari, L.M.; Duarte, H.M.; de Paula, J.C.; Soares, A.R. Diterpenes from the Brown Seaweed Dictyota caribaea (Dictyotaceae, Phaeophyceae): The Ecological and Taxonomic Significance. Biochem. Syst. Ecol. 2014, 52, 33–37. [Google Scholar] [CrossRef]
- Ayyad, S.-E.N.; Makki, M.S.; Al-kayal, N.S.; Basaif, S.A.; El-Foty, K.O.; Asiri, A.M.; Alarif, W.M.; Badria, F.A. Cytotoxic and Protective DNA Damage of Three New Diterpenoids from the Brown Alga Dictoyota dichotoma. Eur. J. Med. Chem. 2011, 46, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.-H.; You, W.-J.; Lin, C.-C.; El-Shazly, M.; Liao, Z.-J.; Su, J.-H. Anti-Inflammatory Cembranoids from the Soft Coral Lobophytum crassum. Mar. Drugs 2017, 15, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Čagalj, M.; Skroza, D.; Tabanelli, G.; Özogul, F.; Šimat, V. Maximizing the Antioxidant Capacity of Padina pavonica by Choosing the Right Drying and Extraction Methods. Processes 2021, 9, 587. [Google Scholar] [CrossRef]

| Compound | RI | Area % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| May | June | July | August | September | |||||||
| Fr | Dr | Fr | Dr | Fr | Dr | Fr | Dr | Fr | Dr | ||
| Dimethyl sulfide | <900 | - | - | - | - | - | 0.76 | - | - | - | - |
| 2-Methylpropan-1-ol | <900 | - | 0.57 | - | - | - | - | - | - | - | - |
| 3-Methylbutanal | <900 | 0.57 | - | 0.49 | - | 0.71 | - | 0.24 | - | - | - |
| Pent-1-en-3-ol | <900 | 0.97 | 1.12 | 1.09 | 2.34 | 0.60 | 1.44 | 0.48 | 0.72 | 0.44 | 1.54 |
| 2-Methylbutanol | <900 | - | 1.04 | - | - | - | - | - | - | - | - |
| Pentan-1-ol | <900 | - | 0.24 | - | 0.55 | - | 0.47 | - | 0.79 | - | - |
| (Z)-Pent-2-en-1-ol | <900 | 0.48 | - | 0.34 | 0.52 | - | 0.61 | - | - | - | - |
| Hexanal | <900 | 3.80 | 1.83 | 3.39 | 1.60 | 2.03 | 4.00 | 1.99 | 6.11 | 2.60 | 4.58 |
| 3-Methylbutanoic acid | <900 | - | 1.13 | - | 0.90 | - | - | - | - | - | - |
| 2-Methylbutanoic acid | <900 | - | 0.70 | - | 0.42 | - | - | - | - | - | - |
| (E)-Hex-2-enal | <900 | 1.11 | 1.32 | 0.88 | 0.40 | 0.39 | 0.59 | 0.42 | - | 0.38 | - |
| Hexan-1-ol | <900 | 1.26 | 0.87 | 0.44 | 0.95 | 0.94 | 3.15 | 1.29 | 1.19 | - | 5.61 |
| Tribromomethane | <900 | - | 1.80 | - | 0.77 | - | - | 0.72 | 0.93 | 0.44 | - |
| Heptan-2-one | <900 | - | 0.39 | - | 0.16 | - | 1.16 | - | 1.70 | - | - |
| Heptanal | 907 | - | 1.12 | - | 0.67 | - | 2.69 | - | 8.83 | - | 3.53 |
| 6-Methylheptan-2-one | 962 | 1.93 | - | 1.39 | 0.39 | 0.23 | 0.32 | - | - | - | - |
| Benzaldehyde | 970 | 23.85 | 5.29 | 34.24 | 3.91 | 24.63 | 6.23 | 31.29 | 3.73 | 18.05 | 5.37 |
| Heptan-1-ol | 975 | - | 0.69 | - | 0.56 | - | 0.51 | - | - | - | - |
| 3,5,5-Trimethylhex-2-ene | 980 | - | - | 0.71 | - | 0.73 | - | - | - | - | - |
| Hexanoic acid | 981 | - | 3.40 | - | 2.90 | - | 1.49 | - | - | - | - |
| Sabinene | 982 | - | - | - | - | 0.39 | - | - | - | - | - |
| Oct-1-en-3-ol | 984 | 1.44 | 3.79 | 1.00 | 6.77 | 0.78 | 5.36 | 0.55 | 2.54 | - | 2.92 |
| Octan-3-one | 991 | 1.13 | - | 1.50 | - | 0.50 | - | - | - | - | - |
| 6-Methylhept-5-en-2-one | 992 | - | 1.49 | - | 1.10 | - | 1.09 | - | - | - | - |
| Octan-2-one | 995 | - | - | 0.52 | - | 0.57 | - | 1.04 | - | - | - |
| 2-Pentylfuran | 996 | - | 1.05 | - | 0.91 | - | 2.17 | - | 1.89 | - | - |
| Octanal | 1007 | 3.10 | 0.99 | 1.40 | - | 0.66 | 2.92 | - | 3.88 | - | 1.59 |
| (E,E)-Hepta-2,4-dienal | 1017 | - | - | - | 0.39 | - | 1.41 | - | - | - | - |
| 2-Ethylhexan-1-ol | 1035 | - | - | - | 0.47 | - | 2.09 | - | 1.14 | - | - |
| (E)-3-Ethyl-2-methylhexa-1,3-diene | 1038 | - | 1.41 | - | 1.49 | - | 1.38 | - | - | - | - |
| Benzyl alcohol | 1042 | - | 5.84 | - | 1.41 | - | 4.77 | - | - | - | - |
| (E)-β-Ocimene | 1044 | - | 2.64 | - | 1.31 | 1.27 | 1.95 | - | - | - | - |
| Phenylacetaldehyde | 1052 | 0.96 | - | 0.46 | - | 0.41 | - | - | - | - | - |
| (E)-Oct-2-enal | 1064 | 0.96 | - | 0.53 | 0.62 | 0.62 | 1.50 | 0.68 | 0.98 | - | - |
| (E)-Oct-2-en-1-ol | 1074 | - | - | - | 0.81 | - | 0.76 | - | - | - | - |
| Octan-1-ol | 1076 | 13.35 | - | 2.84 | - | 1.17 | - | - | - | - | - |
| (E,E)-Octa-3,5-dien-2-one | 1077 | - | 1.35 | - | 1.76 | - | 3.63 | - | 3.41 | - | 4.59 |
| (E,Z)-Octa-3,5-dien-2-one | 1097 | - | 2.29 | - | 2.25 | - | 2.43 | - | 0.74 | - | - |
| Nonanal | 1108 | 1.08 | 2.34 | 0.69 | 0.46 | 0.56 | 5.20 | - | 5.85 | 0.24 | 4.17 |
| 2,6-Dimethylcyclohexan-1-ol | 1114 | - | 4.54 | 0.89 | 3.66 | 0.71 | 1.66 | 0.52 | 2.43 | 0.31 | - |
| 4-Ketoisophorone | 1150 | - | 2.63 | - | 1.11 | - | - | - | - | 0.27 | - |
| (Z)-Non-3-en-1-ol | 1155 | 1.75 | - | - | - | - | - | - | - | - | - |
| 6-[(1Z)-Butenyl]-cyclohepta-1,4-diene] (Dictyopterene D’) | 1158 | 2.52 | 0.27 | 1.21 | 0.27 | 1.46 | - | 2.49 | - | - | - |
| [6-Butyl-cyclohepta-1,4-diene] (Dictyopterene C’) | 1175 | - | - | - | - | - | - | 0.92 | - | - | - |
| Decan-2-one | 1196 | - | - | - | - | - | - | 0.65 | - | - | - |
| Decanal | 1209 | - | - | - | - | - | 0.92 | - | 1.13 | - | - |
| β-Cyclocitral | 1226 | 2.34 | 1.06 | 1.06 | 0.78 | 1.03 | - | 1.25 | - | 0.42 | - |
| Undecan-2-one | 1297 | - | 0.87 | - | 0.65 | - | - | - | - | - | - |
| Undecanal | 1311 | - | 0.62 | - | 0.27 | - | - | - | - | - | - |
| (E)-Undec-2-en-1-ol | 1347 | - | 0.98 | - | 1.21 | - | - | - | - | - | - |
| α-Cubebene | 1355 | - | 0.68 | - | - | - | - | - | - | - | - |
| β-Bourbonene | 1389 | - | 0.94 | - | - | - | - | - | - | - | - |
| β-Cubebene | 1393 | - | 0.93 | - | - | - | - | - | - | - | - |
| Tetradecane | 1402 | - | 0.91 | - | 0.61 | - | 0.89 | - | - | - | - |
| 6,10-Dimethylundecan-2-one | 1408 | - | 0.49 | - | 0.42 | - | - | - | - | - | - |
| α-Ionone | 1432 | - | 0.82 | - | 0.69 | 0.74 | - | 0.64 | - | 0.23 | - |
| Germacrene D | 1485 | 5.44 | - | - | - | 0.85 | - | - | - | - | - |
| β-Ionone | 1490 | 6.22 | 2.50 | 3.84 | 3.01 | 3.62 | 1.34 | 5.38 | 1.28 | 1.97 | - |
| Pentadec-1-ene | 1495 | 4.68 | 2.22 | 13.82 | 2.36 | 25.14 | 1.93 | 23.70 | 4.53 | 53.17 | 9.54 |
| (E)-Pentadec-7-ene | 1498 | 0.70 | - | 1.00 | 0.45 | 1.29 | - | 1.96 | - | 3.56 | 4.88 |
| Tridecan-2-one | 1500 | - | - | - | - | - | - | 1.29 | - | 1.94 | - |
| Pentadecane | 1500 | 3.54 | 22.06 | 9.66 | 38.46 | 9.02 | 21.22 | 7.31 | 24.99 | 4.10 | 29.33 |
| Tridecanal | 1519 | 2.78 | - | 2.64 | - | 2.02 | - | 4.11 | - | 5.92 | - |
| δ-Cadinene | 1528 | 1.10 | - | - | - | - | - | - | - | - | - |
| Dihydroactinidiolide | 1533 | - | 1.26 | - | 1.27 | - | - | - | - | - | - |
| Gleenol | 1589 | 3.11 | - | 0.78 | - | 0.55 | - | 0.36 | - | - | - |
| Hexadecane | 1603 | - | - | - | - | 0.31 | - | - | - | - | - |
| Heptadec-1-ene | 1696 | 0.94 | - | 1.09 | - | 0.61 | - | 1.19 | - | 2.34 | - |
| Heptadecane | 1700 | 0.71 | 2.00 | 3.58 | 4.35 | 2.51 | 4.97 | 4.23 | 17.67 | 1.75 | 15.52 |
| 1-Phytene (3,7,11,15-Tetramethylhexadec-1-ene) | 1791 | - | - | - | - | 2.49 | 1.82 | - | - | - | - |
| Phytane | 1813 | - | - | - | - | 3.95 | 4.46 | - | - | - | - |
| Hexahydrofarnesyl acetone (Phytone) | 1850 | - | 1.48 | - | - | - | - | - | - | - | - |
| Compound | RI | Area % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| May | June | July | August | September | |||||||
| Fr | Dr | Fr | Dr | Fr | Dr | Fr | Dr | Fr | Dr | ||
| Dimethyl sulfide | <900 | - | - | - | - | - | 0.68 | - | 0.20 | - | 2.50 |
| Butanal | <900 | - | - | 0.32 | - | 0.56 | - | 0.68 | - | 0.12 | - |
| 3-Methylbutanal | <900 | 0.51 | - | 0.77 | - | 0.61 | - | 0.70 | - | 0.16 | - |
| Pent-1-en-3-ol | <900 | 1.69 | 1.31 | 1.35 | 2.70 | 0.86 | 1.74 | 1.14 | 1.03 | 0.72 | 3.97 |
| Pentanal | <900 | - | - | - | 0.11 | - | 0.18 | - | 0.40 | - | 1.10 |
| 3-Hydroxybutan-2-one | <900 | 14.16 | - | - | - | 0.74 | - | - | - | - | - |
| 3-Methylbutanol | <900 | 1.18 | - | 0.20 | - | 0.64 | - | 0.57 | - | - | - |
| 2-Methylbutanol | <900 | - | 1.35 | - | - | - | - | - | - | - | - |
| Pyridine | <900 | - | - | - | - | - | - | 0.15 | - | 0.19 | - |
| (E)-Pent-2-enal | <900 | - | - | - | - | - | 0.44 | - | - | - | - |
| Pentan-1-ol | <900 | - | 0.58 | - | 0.74 | 0.14 | 0.62 | 0.21 | 1.12 | - | 1.25 |
| (Z)-Pent-2-en-1-ol | <900 | 0.42 | 0.40 | 0.30 | 0.80 | 0.24 | 0.79 | 0.32 | 0.61 | 0.19 | 1.82 |
| Hexanal | <900 | 1.39 | 2.46 | 2.27 | 2.09 | 1.06 | 4.98 | 1.90 | 8.22 | 1.89 | 11.47 |
| 3-Methylbutanoic acid | <900 | - | 1.94 | - | 2.43 | - | - | - | - | - | - |
| 2-Methylbutanoic acid | <900 | - | 1.58 | - | 1.58 | - | - | - | - | - | - |
| (E)-Hex-2-enal | <900 | - | 0.93 | 0.59 | 1.12 | 0.19 | 1.97 | 0.37 | 1.48 | 0.23 | - |
| Hexan-1-ol | <900 | 4.60 | 2.47 | - | 1.08 | 1.79 | 0.97 | 1.58 | 1.78 | - | - |
| Heptan-2-one | <900 | - | 0.31 | - | 0.30 | - | 0.28 | - | - | - | - |
| Tribromomethane | <900 | 0.25 | - | 0.58 | - | 0.49 | - | 0.18 | - | 0.46 | - |
| Heptanal | 907 | - | 1.94 | - | 1.31 | - | 3.94 | - | 10.04 | - | 8.14 |
| 6-Methylheptan-2-one | 962 | 5.85 | 0.52 | 0.71 | 0.43 | 1.00 | 0.58 | 1.08 | - | - | - |
| Benzaldehyde | 970 | 9.51 | 5.11 | 52.75 | 3.82 | 32.99 | 4.82 | 57.85 | 2.62 | 28.39 | 6.83 |
| Heptan-1-ol | 975 | - | 0.80 | - | 0.97 | - | 0.71 | - | 0.72 | - | - |
| Oct-1-en-3-one | 979 | 0.87 | - | 0.80 | - | 0.60 | - | 0.37 | - | 0.39 | - |
| Pentyl propanoate | 980 | - | - | - | 4.12 | - | 1.61 | - | - | - | - |
| Hexanoic acid | 982 | - | 4.80 | - | - | - | - | - | - | - | - |
| Oct-1-en-3-ol | 984 | 1.81 | 5.82 | 1.01 | 8.56 | 0.71 | 5.30 | 0.68 | 2.19 | 0.51 | 2.74 |
| Octan-3-one | 991 | 1.73 | - | 0.82 | - | 0.39 | - | 0.30 | - | - | - |
| 6-Methylhept-5-en-2-one | 992 | - | 1.30 | - | 1.18 | - | 0.80 | - | - | - | - |
| 2-Pentylfuran | 995 | - | 0.85 | 0.33 | 1.01 | 0.31 | 1.73 | 0.59 | 1.34 | 0.35 | - |
| Octanal | 1007 | 0.88 | 1.26 | 0.66 | 0.93 | - | 3.22 | - | 4.28 | - | - |
| (E,E)-Hepta-2,4-dienal | 1016 | - | - | - | 0.47 | - | 1.06 | - | - | - | - |
| 2-Ethylhexan-1-ol | 1035 | - | - | - | 0.55 | - | 2.16 | - | 2.27 | - | - |
| (E)-3-Ethyl-2-methylhexa-1,3-diene | 1038 | - | 1.62 | 0.69 | 1.60 | 1.51 | 1.39 | - | - | 0.15 | - |
| Benzyl alcohol | 1041 | 3.67 | 6.53 | 0.87 | 1.61 | 0.61 | 5.18 | 2.86 | 2.05 | - | - |
| (E)-β-Ocimene | 1044 | - | 3.55 | - | 1.42 | - | 2.57 | - | 1.99 | - | - |
| Phenylacetaldehyde | 1052 | 0.99 | 0.73 | - | - | - | 1.67 | - | - | - | - |
| γ-Caprolactone | 1062 | - | 1.17 | - | 0.48 | - | - | - | - | - | - |
| (E)-Oct-2-enal | 1064 | - | - | - | 0.65 | - | 1.74 | - | 0.90 | - | - |
| (E)-Oct-2-en-1-ol | 1074 | - | 0.50 | - | 0.87 | - | 0.79 | - | - | - | - |
| Octan-1-ol | 1076 | 13.93 | - | 3.00 | - | 0.80 | - | - | - | - | - |
| (E,E)-Octa-3,5-dien-2-one | 1077 | - | 0.95 | - | 1.35 | - | 2.75 | - | 1.84 | - | - |
| Nonan-2-one | 1096 | 0.53 | - | - | - | - | - | - | - | - | - |
| (E,Z)-Octa-3,5-dien-2-one | 1097 | - | 0.99 | - | 1.55 | - | 1.32 | - | 0.40 | - | - |
| Nonanal | 1108 | - | 2.52 | 0.53 | 0.65 | - | 4.97 | - | 4.29 | - | 5.10 |
| 2,6-Dimethylcyclohexan-1-ol | 1114 | 1.75 | 5.72 | 1.35 | 5.34 | 0.96 | 2.05 | 0.62 | 3.25 | 0.76 | - |
| 4-Ketoisophorone | 1150 | - | 2.27 | 0.54 | 1.13 | 1.01 | 0.41 | - | - | 0.61 | - |
| (Z)-Non-3-en-1-ol | 1155 | 3.76 | - | - | - | - | - | - | - | - | - |
| 6-[(1Z)-Butenyl]-cyclohepta-1,4-diene] (Dictyopterene D’) | 1158 | 1.23 | 0.35 | 0.37 | 0.33 | 0.59 | - | 0.57 | - | - | - |
| [6-Butyl-cyclohepta-1,4-diene] (Dictyopterene C’) | 1175 | - | - | - | - | - | - | 0.31 | - | - | - |
| Decan-2-one | 1196 | - | - | - | - | - | - | 0.38 | - | - | - |
| Decanal | 1209 | - | 0.33 | - | 0.21 | - | 0.90 | - | - | - | - |
| β-Cyclocitral | 1226 | 1.81 | 0.63 | 1.09 | 0.61 | 0.81 | 0.70 | 0.81 | - | 0.60 | - |
| Undecan-2-one | 1296 | 1.05 | 0.47 | - | 0.37 | - | 0.61 | - | - | - | - |
| Undecanal | 1311 | - | 0.66 | - | 0.24 | - | - | - | - | - | - |
| (E)-Undec-2-en-1-ol | 1347 | - | 0.68 | - | 0.97 | - | - | - | - | - | - |
| α-Cubebene | 1355 | - | 0.53 | - | - | - | - | - | - | - | - |
| β-Bourbonene | 1389 | - | 0.62 | - | - | - | - | - | - | - | - |
| β-Cubebene | 1393 | - | 0.48 | - | - | - | - | - | - | - | - |
| Tetradecane | 1400 | - | 0.57 | - | 0.48 | - | 0.67 | - | - | - | - |
| 6,10-Dimethylundecan-2-one | 1409 | - | 0.35 | - | 0.40 | - | - | - | - | - | - |
| Dodecanal | 1412 | 1.67 | - | - | - | - | - | - | - | - | - |
| α-Ionone | 1432 | - | 0.30 | - | 0.56 | 0.53 | - | 0.41 | - | 0.43 | - |
| Germacrene D | 1485 | 1.87 | - | 0.25 | - | 0.95 | - | - | - | - | - |
| β-Ionone | 1489 | 3.67 | 1.96 | 3.57 | 2.43 | 2.87 | 1.39 | 2.96 | 1.42 | 2.84 | - |
| Pentadec-1-ene | 1495 | 2.15 | 1.59 | 7.88 | 2.04 | 23.77 | 1.92 | 10.36 | 4.00 | 40.35 | 6.90 |
| (E)-Pentadec-7-ene | 1498 | 0.47 | - | 0.64 | 0.41 | 1.16 | - | 0.97 | - | 3.63 | - |
| Tridecan-2-one | 1500 | - | - | - | - | 0.44 | - | 0.50 | - | 1.63 | - |
| Pentadecane | 1500 | 1.95 | 14.06 | 4.91 | 27.13 | 6.41 | 17.01 | 2.41 | 20.64 | 2.68 | 24.47 |
| Tridecanal | 1514 | 3.29 | - | 4.34 | - | 4.44 | - | 4.89 | - | 8.00 | - |
| Dihydroactinidiolide | 1533 | 0.18 | 1.16 | 0.31 | 1.04 | 0.22 | - | 0.16 | - | - | - |
| Gleenol | 1589 | 2.23 | - | 0.51 | - | 0.33 | - | - | - | - | - |
| Hexadecane | 1600 | - | - | - | - | - | 0.88 | - | - | - | - |
| Heptadec-1-ene | 1696 | - | - | 0.53 | - | 0.57 | - | 0.38 | - | 1.64 | - |
| Heptadecane | 1703 | 0.56 | 1.69 | 1.48 | 3.76 | 2.06 | 4.95 | 0.84 | 18.29 | 1.05 | 23.72 |
| Phytane | 1813 | - | - | - | - | - | 4.19 | - | - | - | - |
| Hexahydrofarnesyl acetone (Phytone) | 1850 | - | 1.22 | - | - | - | 0.67 | - | - | - | - |
| Compound | RI | Area % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| May | June | July | August | September | |||||||
| Fr | Dr | Fr | Dr | Fr | Dr | Fr | Dr | Fr | Dr | ||
| 2-Ethyl-5,5-dimethylcyclopenta-1,3-diene | <900 | 0.06 | 0.14 | 0.10 | 0.23 | 0.02 | 0.09 | 0.02 | 0.22 | 0.10 | 0.19 |
| (E)-Hex-2-enal | <900 | 1.71 | 1.18 | 1.13 | 1.00 | 0.13 | 0.31 | 0.27 | 0.78 | 1.76 | 1.18 |
| Hex-3-en-1-ol | <900 | 0.06 | - | - | - | 0.14 | - | - | - | - | - |
| Hexan-1-ol | <900 | 1.72 | 0.17 | 0.81 | 0.18 | 0.18 | - | 0.22 | 0.04 | 0.24 | 0.03 |
| m-Xylene | <900 | 0.51 | - | - | - | 0.06 | - | - | - | 0.13 | - |
| 2,3-Dimethylpyridine | <900 | 0.04 | - | - | - | - | - | - | - | - | - |
| Heptan-2-one | <900 | 0.16 | 0.14 | 0.15 | 0.30 | - | 0.12 | - | 0.24 | - | 0.28 |
| Tribromomethane | <900 | - | - | - | - | 0.09 | - | 0.03 | - | 0.34 | - |
| Hept-4-enal | 902 | 0.24 | 0.14 | 0.08 | - | - | - | - | - | 0.16 | 0.10 |
| Heptanal | 904 | 0.06 | 0.71 | 0.10 | 1.11 | 0.06 | 0.25 | - | 0.34 | 0.07 | 0.21 |
| (E,Z)-Hexa-2,4-dienal (Sorbaldehyde) | 914 | 0.06 | - | - | - | - | - | 0.04 | - | - | - |
| 2-Iodopentane | 929 | 0.12 | - | 0.09 | - | - | - | - | - | 0.04 | - |
| 2,4-Dimethylpyridine | 937 | 0.16 | - | 0.09 | - | 0.03 | - | - | - | - | - |
| 6-Methylheptan-2-one | 960 | 0.05 | - | 0.11 | - | - | - | - | - | - | - |
| (E)-Hept-2-enal | 962 | - | 0.06 | - | 0.17 | - | - | - | 0.14 | 0.09 | 0.07 |
| Benzaldehyde | 969 | 0.73 | 0.69 | 0.75 | 0.95 | 0.17 | 0.45 | 0.13 | 0.41 | 0.30 | 0.47 |
| Heptan-1-ol | 973 | 0.13 | - | 0.09 | - | - | - | - | - | - | - |
| Dimethyl trisulfide | 978 | 0.22 | 0.22 | 0.36 | 0.33 | 0.39 | 0.11 | 0.05 | 0.11 | 0.13 | 0.08 |
| Oct-1-en-3-ol | 982 | 0.34 | 0.35 | 0.48 | 0.73 | 0.09 | 0.40 | 0.04 | 0.23 | 0.24 | 0.28 |
| Octan-2,3-dione | 987 | 0.18 | 0.34 | 0.53 | 0.76 | 0.15 | 0.48 | 0.25 | 0.57 | 0.90 | 0.16 |
| Octan-3-one | 989 | 0.38 | 0.24 | 0.67 | 0.44 | 0.08 | - | - | 0.16 | 0.21 | - |
| Octan-2-one | 994 | - | - | 0.16 | - | - | - | - | - | - | - |
| 2-Pentylfuran | 995 | 0.43 | 0.88 | 0.17 | 1.16 | 0.09 | 0.38 | 0.11 | 0.69 | 0.90 | 0.77 |
| Octan-3-ol | 998 | - | - | 0.23 | 0.22 | - | - | - | 0.15 | - | 0.10 |
| (E,Z)-Hepta-2,4-dienal | 1000 | - | - | - | - | 0.06 | - | 0.08 | - | 0.11 | - |
| Octanal | 1005 | 0.25 | 0.33 | 0.19 | 0.33 | - | 0.17 | - | 0.11 | 0.08 | 0.12 |
| (E,E)-Hepta-2,4-dienal | 1015 | 0.24 | 0.30 | 0.30 | 0.50 | 0.14 | 0.17 | 0.21 | 0.12 | 0.23 | 0.14 |
| p-Cymene | 1030 | 0.06 | - | - | - | - | - | - | - | - | - |
| 2-Ethylhexan-1-ol | 1033 | - | - | - | - | - | 0.07 | - | 0.23 | 0.04 | - |
| (3E)-3-Ethyl-2-methylhexa-1,3-diene | 1039 | - | - | - | - | - | - | - | - | 0.05 | - |
| Benzyl alcohol | 1041 | 0.18 | - | 0.19 | - | 0.06 | - | - | - | 0.04 | - |
| 2,2,6-Trimethylcyclohexan-1-one | 1042 | - | 0.13 | - | 0.22 | - | - | - | - | - | - |
| Phenylacetaldehyde | 1051 | 0.15 | 0.77 | 0.32 | 0.99 | 0.20 | 0.57 | 0.17 | 0.33 | 0.25 | 0.27 |
| (E)-Oct-2-enal | 1063 | 0.14 | 0.26 | 0.13 | 0.52 | 0.05 | 0.21 | - | 0.32 | 0.32 | 0.35 |
| 2-Methyldecane | 1068 | 0.11 | - | 0.08 | - | 0.06 | - | 0.12 | - | 0.50 | - |
| (E)-Oct-2-en-1-ol | 1073 | 0.20 | - | 0.15 | - | 0.08 | - | 0.05 | - | 0.13 | - |
| Acetophenone | 1073 | - | 0.21 | - | 0.25 | - | 0.16 | - | 0.17 | - | 0.20 |
| Octan-1-ol | 1075 | 3.82 | - | 1.79 | - | 0.25 | - | 0.10 | - | 0.39 | - |
| (E,E)-Octa-3,5-dien-2-one | 1075 | - | 0.31 | - | 0.58 | - | 0.20 | - | 0.20 | - | 0.25 |
| 1-Methylsulfanylpentan-3-one | 1091 | - | 0.31 | - | 0.29 | - | 0.22 | - | 0.15 | - | 0.08 |
| (E,Z)-Octa-3,5-dien-2-one | 1096 | 1.95 | 0.17 | 0.91 | 0.25 | 0.49 | - | 0.55 | - | 1.16 | 0.18 |
| Linalool | 1103 | - | - | - | 0.11 | - | - | - | - | 0.13 | 0.09 |
| Nonanal | 1107 | 0.11 | 0.44 | 0.12 | 0.61 | - | 0.28 | - | 0.24 | 0.18 | 0.21 |
| 2,6-Dimethylcyclohexan-1-ol | 1113 | 0.15 | 0.34 | 0.11 | 0.54 | 0.08 | 0.37 | 0.10 | 0.21 | 0.29 | 0.24 |
| 2-Phenylethanol | 1120 | 0.10 | - | 0.10 | - | 0.73 | - | - | - | 0.08 | - |
| (E,Z)-2,6-Dimethylocta-1,3,5,7-tetraene | 1141 | 0.21 | - | 0.08 | - | - | - | - | - | - | - |
| 4-Ketoisophorone | 1150 | 0.56 | 0.56 | 0.34 | 0.24 | 0.17 | 0.39 | 0.09 | 0.31 | 0.13 | 0.26 |
| (E,Z)-Nona-2,6-dienal | 1159 | 0.30 | 0.17 | 0.22 | 0.32 | 0.17 | 0.08 | - | 0.15 | 0.22 | 0.16 |
| 6-[(1Z)-Butenyl]-1,4-cycloheptadiene] (Dictyopterene D’) | 1161 | - | - | - | - | - | - | - | - | 0.06 | - |
| Non-2-enal | 1165 | 0.14 | 0.16 | 0.11 | 0.33 | 0.18 | 0.09 | - | 0.14 | 0.20 | 0.19 |
| [6-Butyl-1,4-cycloheptadiene] (Dictyopterene C’) | 1175 | 0.14 | - | - | - | 0.10 | - | - | - | - | - |
| 2,4-Dimethylbenzaldehyde | 1180 | 0.10 | 0.31 | 0.17 | 0.53 | - | - | 0.05 | 0.13 | 0.15 | 0.11 |
| 3,4,4-Trimethylcyclohex-2-en-1-one | 1194 | - | - | - | - | - | - | - | 0.19 | - | 0.19 |
| Dodecane | 1200 | - | 0.12 | - | 0.25 | - | - | - | - | - | - |
| Decanal | 1209 | 0.38 | - | 0.21 | 0.15 | - | 0.11 | - | 0.09 | 0.07 | 0.09 |
| β-Cyclocitral | 1225 | 0.28 | 0.36 | 0.15 | 0.53 | - | 0.11 | - | 0.21 | 0.24 | 0.18 |
| β-Cyclohomocitral | 1263 | 0.17 | 0.21 | 0.14 | 0.34 | - | - | - | 0.11 | 0.18 | 0.10 |
| (E,Z)-Deca-2,4-dienal | 1283 | 0.15 | 0.16 | - | 0.23 | 0.05 | 0.18 | 0.08 | 0.10 | 0.24 | 0.10 |
| Indole | 1296 | 0.19 | 0.36 | 0.17 | 0.27 | 0.13 | 0.25 | 0.08 | 0.26 | 0.35 | 0.22 |
| Undecanal | 1310 | - | 0.13 | - | 0.25 | 0.06 | 0.18 | - | 0.22 | 0.20 | 0.28 |
| (E,E)-Deca-2,4-dienal | 1320 | 0.38 | 0.80 | 0.48 | 1.98 | 0.15 | 0.40 | 0.25 | 0.75 | 1.03 | 0.54 |
| (E)-Undec-2-en-1-ol | 1347 | 0.11 | 0.27 | - | 0.30 | - | 0.18 | - | 0.15 | 0.07 | 0.08 |
| β-Bourbonene | 1388 | 0.19 | - | 0.09 | - | - | - | - | - | - | - |
| β-Cubebene | 1393 | 0.42 | 0.13 | 0.46 | 0.40 | 0.15 | - | - | - | 0.16 | 0.21 |
| 4-(2-Methylbutan-2-yl)phenol (p-tert-Pentylphenol) | 1400 | - | - | - | - | 0.04 | - | - | - | 0.07 | - |
| Tetradecane | 1400 | 0.27 | 0.50 | 0.26 | 0.66 | 0.07 | 0.23 | 0.09 | 1.18 | 0.91 | 0.89 |
| 6,10-Dimethylundecan-2-one | 1408 | - | 0.20 | - | 0.21 | - | - | - | 0.09 | - | 0.12 |
| Dodecanal | 1412 | - | 0.21 | - | 0.38 | 0.04 | 0.19 | - | 0.22 | 0.18 | 0.38 |
| α-Ionone | 1432 | 0.13 | 0.26 | 0.12 | 0.41 | 0.05 | 0.14 | 0.07 | 0.27 | 0.30 | 0.31 |
| (E,Z)-Dodeca-2,6-dienal | 1452 | - | - | - | - | - | - | - | 0.04 | - | 0.10 |
| (Z)-Geranylacetone | 1458 | 0.30 | 0.35 | - | 0.44 | - | - | - | 0.13 | 0.14 | 0.18 |
| Dodecan-1-ol | 1479 | 1.38 | - | 0.13 | - | - | 0.71 | - | 0.12 | 0.11 | 0.35 |
| α-Muurolene | 1481 | - | - | - | 0.37 | - | - | - | - | - | 0.10 |
| Germacrene D | 1485 | 2.67 | 0.24 | 1.71 | 1.67 | 0.56 | - | 0.06 | 0.34 | 0.39 | 0.59 |
| β-Ionone | 1489 | 2.13 | 5.04 | 0.86 | 5.56 | 0.56 | 2.14 | 0.70 | 2.94 | 2.96 | 3.72 |
| Pentadec-1-ene | 1495 | 7.70 | 2.99 | 7.56 | 8.53 | 4.41 | 1.68 | 2.88 | 17.50 | 26.46 | 18.98 |
| (E)-Pentadec-7-ene | 1499 | 0.80 | 0.58 | 0.66 | 1.33 | 0.37 | 0.35 | 0.30 | 2.00 | 3.26 | 3.10 |
| Tridecan-2-one | 1500 | 0.18 | 0.15 | - | 0.34 | 0.11 | 0.08 | 0.14 | 0.83 | 1.41 | 1.23 |
| Pentadecane | 1500 | 4.26 | 1.19 | 4.54 | 3.80 | 1.63 | 0.61 | 0.74 | 1.66 | 2.94 | 1.47 |
| Tridecanal | 1514 | 0.80 | 0.99 | 1.11 | 1.92 | 0.79 | 0.80 | 0.54 | 1.88 | 3.11 | 2.74 |
| Zonarene | 1530 | 0.42 | 0.13 | 0.36 | 0.46 | 0.16 | 0.17 | - | 0.03 | - | 0.26 |
| Dihydroactinidiolide | 1532 | - | 0.17 | - | - | - | - | - | - | - | - |
| Tetradecan-2-one | 1565 | - | 0.15 | - | - | - | - | - | - | - | - |
| Dodecanoic acid | 1573 | - | 0.19 | 0.19 | 0.27 | - | 0.18 | 0.10 | 0.17 | 0.19 | 0.21 |
| Tridecan-1-ol | 1580 | 0.28 | 0.40 | 0.04 | 0.10 | 0.18 | 0.22 | 0.08 | 0.11 | - | 0.25 |
| Gleenol | 1589 | 6.26 | 0.39 | 3.91 | 0.81 | 1.57 | 0.10 | 0.33 | 0.26 | 0.82 | 1.30 |
| Hexadec-1-ene (Cetene) | 1595 | - | - | - | - | - | - | - | - | - | 0.11 |
| Diethyl phthalate | 1597 | - | - | - | - | - | - | 0.06 | 0.05 | 0.10 | 0.24 |
| Hexadecane | 1600 | - | - | - | - | - | 0.46 | - | - | - | 0.30 |
| Tetradecanal | 1616 | 0.25 | 0.42 | 0.33 | 0.73 | 0.14 | 0.38 | 0.14 | 0.32 | 0.30 | 0.48 |
| (Z)-Hexadec-7-ene | 1622 | - | - | 0.06 | 0.14 | - | - | 0.08 | 0.35 | 0.23 | 0.33 |
| Benzophenone | 1630 | - | - | - | - | 0.11 | 0.42 | 0.10 | 0.14 | - | - |
| Cubenol | 1647 | 0.18 | - | 0.09 | - | - | - | - | - | - | - |
| (E)-Heptadec-8-ene | 1678 | - | 0.18 | 0.24 | 0.36 | - | 0.43 | 0.21 | 0.89 | 0.49 | 0.80 |
| Tetradecan-1-ol | 1681 | 0.59 | 1.70 | 0.74 | 1.87 | 0.25 | 1.54 | 0.18 | 0.85 | 0.39 | 0.94 |
| (Z)-Pentadec-6-en-1-ol | 1690 | 3.92 | 1.40 | 1.49 | 2.03 | 0.60 | 0.33 | 0.22 | 0.84 | 0.69 | 0.87 |
| Heptadec-1-ene | 1696 | 2.27 | 0.68 | 2.06 | 1.60 | 1.57 | 0.59 | 1.66 | 2.91 | 6.82 | 5.67 |
| Heptadecane | 1700 | 1.97 | 1.12 | 2.74 | 2.44 | 2.75 | 0.93 | 1.05 | 2.15 | 2.58 | 1.70 |
| 2,6,10,14-Tetramethyl-7-(3-methylpentyl)pentadecane | 1709 | 0.18 | - | - | - | 0.26 | - | - | - | - | - |
| Pentadecanal | 1718 | 1.21 | 2.03 | 3.00 | 2.95 | 2.53 | 1.61 | 2.46 | 2.12 | 2.35 | 1.68 |
| (Z)-Heptadec-3-ene | 1720 | - | - | - | - | - | - | - | - | 0.76 | 0.57 |
| Tetradecanoic acid | 1772 | - | 1.05 | - | - | - | - | - | - | - | - |
| Pentadecan-1-ol | 1782 | 0.29 | - | 0.20 | - | 0.26 | - | - | - | - | 0.20 |
| Octadec-1-ene | 1786 | - | 1.03 | - | 1.24 | - | 0.72 | - | 0.44 | - | 0.18 |
| Octadecane | 1800 | - | - | - | - | 0.14 | - | - | - | - | - |
| 2-Phenoxyethoxybenzene | 1807 | - | - | 0.08 | - | - | - | 0.07 | - | - | 0.08 |
| Phytane | 1813 | - | - | - | - | 6.11 | 2.43 | - | - | - | - |
| Hexadecanal | 1820 | - | 0.17 | 0.24 | 0.35 | 0.15 | 0.53 | 0.09 | 0.15 | - | 0.12 |
| 6,10,14-Trimethylpentadecan-2-one | 1850 | 1.19 | 6.77 | 2.27 | 5.48 | 3.84 | 7.58 | 1.69 | 5.08 | 2.68 | 5.27 |
| p-Cumylphenol | 1855 | 0.34 | 0.85 | 0.51 | 0.61 | 0.29 | 0.84 | 0.23 | 0.89 | 0.24 | 0.62 |
| (Z)-Hexadeca-1,9-diene | 1864 | 0.23 | 1.58 | - | 1.48 | 0.18 | 0.87 | 0.08 | 0.66 | - | 0.25 |
| (Z)-Hexadec-11-enal | 1868 | - | - | 0.33 | - | 0.82 | - | 0.06 | - | - | - |
| Diisobutyl phthalate | 1872 | - | 0.85 | 0.21 | 1.04 | 0.43 | 2.40 | 0.20 | 0.71 | - | 0.67 |
| Hexadecan-1-ol | 1888 | - | 2.97 | 0.73 | 4.22 | - | 4.76 | 0.20 | 1.97 | - | 1.04 |
| Nonadec-1-ene | 1896 | 0.67 | 0.93 | 2.33 | 1.38 | 3.42 | 1.10 | 1.21 | 1.17 | 0.56 | 0.80 |
| Nonadecane | 1900 | 0.29 | - | - | - | 0.52 | - | 0.15 | - | - | - |
| Heptadecan-2-one | 1905 | - | 0.13 | 0.20 | 0.32 | - | 0.28 | 0.22 | 0.24 | - | 0.31 |
| (E,E)-Farnesyl acetone | 1923 | - | 0.98 | 0.40 | 0.87 | 0.46 | 1.07 | 0.24 | 0.80 | 0.25 | 0.36 |
| Isophytol | 1953 | - | 0.41 | 0.17 | 0.27 | 0.25 | 0.37 | 0.11 | 0.23 | - | 0.10 |
| Palmitoleic acid | 1963 | - | 0.23 | - | 0.19 | - | 0.09 | - | - | - | - |
| Dibutyl phthalate | 1967 | - | 0.26 | - | 0.74 | - | 4.29 | - | 0.29 | - | 0.37 |
| Hexadecanoic acid | 1973 | 0.19 | 4.80 | 0.93 | 0.20 | 0.25 | 0.08 | 0.45 | 0.47 | 0.21 | 0.61 |
| (Z)-Octadecen-9-al | 1999 | 0.76 | 1.84 | 1.68 | 1.29 | 2.54 | 1.58 | 8.51 | 3.45 | 3.05 | 3.52 |
| Octadecanal | 2024 | 0.14 | 1.26 | 0.48 | 1.66 | 0.63 | 2.44 | 0.43 | 1.39 | - | 0.39 |
| (Z)-Falcarinol | 2032 | 0.26 | - | - | - | 0.55 | 0.54 | 0.73 | 0.70 | 0.35 | 0.85 |
| Methyl (all Z) eicosa-5,8,11,14,17-pentaenoate | 2038 | 0.15 | 0.98 | 0.48 | 0.41 | 0.22 | 0.38 | 0.20 | 0.27 | - | 0.24 |
| Methyl (all Z) eicosa-5,8,11,14-tetraenoate | 2044 | 2.85 | 2.86 | 1.86 | 1.36 | 1.51 | 0.91 | 0.83 | 0.68 | 0.46 | 0.99 |
| (Z,Z)-Octadeca-9,12-dien-1-ol | 2050 | 1.90 | 0.66 | 2.03 | 0.62 | 2.51 | 0.97 | 1.87 | 0.55 | 0.44 | 0.15 |
| (Z,Z,Z)-9,12,15-Octadecatrien-1-ol | 2056 | 8.09 | 0.86 | 4.63 | 0.66 | 6.67 | 0.56 | 2.84 | 0.45 | 0.47 | 0.10 |
| (Z)-Octadec-9-en-1-ol | 2061 | 0.18 | 0.69 | 0.25 | 0.23 | 0.54 | 1.01 | 0.35 | 0.42 | - | 0.25 |
| (Z,Z)-Octadeca-3,13-dien-1-ol | 2065 | - | 0.87 | - | 0.28 | - | 1.67 | - | 0.68 | - | - |
| Heneicos-10-ene | 2070 | - | 1.32 | - | 0.49 | - | 0.11 | - | - | - | - |
| Octadecan-1-ol | 2088 | - | - | 0.13 | - | 0.41 | - | 0.53 | - | - | - |
| γ-Palmitolactone | 2104 | - | 1.22 | 0.55 | 0.39 | 0.97 | 1.75 | 1.73 | 0.55 | - | 0.37 |
| (Z,Z)-Octadeca-9,12-dienoic acid | 2110 | - | - | 0.42 | - | 1.42 | - | 1.26 | - | 0.16 | - |
| (E)-Phytol | 2116 | 11.35 | 9.31 | 18.71 | 8.22 | 23.25 | 26.10 | 47.03 | 22.53 | 8.01 | 12.87 |
| Isopachydictyol A | 2127 | 2.73 | 9.70 | 3.07 | 2.60 | 2.80 | 2.25 | 1.57 | 1.22 | 0.96 | 3.54 |
| (Z)-Octadec-9-enoic acid (Oleic acid) | 2142 | 0.65 | 1.85 | 1.60 | 0.56 | 1.21 | 1.51 | 2.01 | 1.20 | 0.47 | 1.32 |
| Cembra-4,7,11,15-tetraen-3-ol | 2230 | 3.06 | 6.88 | 3.63 | 3.00 | 3.94 | 4.18 | 1.67 | 1.30 | 0.86 | 4.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čagalj, M.; Radman, S.; Šimat, V.; Jerković, I. Detailed Chemical Prospecting of Volatile Organic Compounds Variations from Adriatic Macroalga Halopteris scoparia. Molecules 2022, 27, 4997. https://doi.org/10.3390/molecules27154997
Čagalj M, Radman S, Šimat V, Jerković I. Detailed Chemical Prospecting of Volatile Organic Compounds Variations from Adriatic Macroalga Halopteris scoparia. Molecules. 2022; 27(15):4997. https://doi.org/10.3390/molecules27154997
Chicago/Turabian StyleČagalj, Martina, Sanja Radman, Vida Šimat, and Igor Jerković. 2022. "Detailed Chemical Prospecting of Volatile Organic Compounds Variations from Adriatic Macroalga Halopteris scoparia" Molecules 27, no. 15: 4997. https://doi.org/10.3390/molecules27154997







