Optical Properties of New Third-Order Nonlinear Materials Modified by Click Chemistry
Abstract
:1. Introduction
2. Experimental
2.1. 4,4′-(1,4-Phenylenebis(ethyne-2,1-diyl))bis(N,N-dihexadecylaniline) (B)
2.2. 2-(4-(Dihexadecylamino)phenyl)-3-(4-((4(dihexadecylamino)phenyl)ethynyl)phenyl)buta-1,3-diene-1,1,4,4-tetracarbonitrile (B-1)
2.3. 3′-(1,4-Phenylene)bis(2-(4-(dihexadecylamino)phenyl)buta-1,3-diene-1,1,4,4-tetracarbonitrile) (B-11)
2.4. 2-(4-(3,3-Dicyano-2-(4-(dihexadecylamino)phenyl)-1-(4-((4-(dihexadecylamino)phenyl)ethynyl)phenyl)allylidene)cyclohexa-2,5-dien-1-ylidene)malononitrile (B-2)
2.5. 2-(4-(3,3-Dicyano-1-(4-(1,1-dicyano-3-(4-(dicyanomethylene)cyclohexa-2,5-dien-1-ylidene)-3-(4-(dihexadecylamino)phenyl)prop-1-en-2-yl)phenyl)-2-(4-(dihexadecylamino)phenyl)allylidene)cyclohexa-2,5-dien-1-ylidene)malononitrile (B-22)
2.6. 2-(4-(3,3-Dicyano-2-(4-(dihexadecylamino)phenyl)-1-(4-((4-(dihexadecylamino)phenyl)ethynyl)phenyl)allylidene)-2,3,5,6-tetrafluorocyclohexa-2,5-dien-1-ylidene)malononitrile (B-3)
2.7. 2-(4-(3,3-Dicyano-1-(4-(1,1-dicyano-3-(4-(dicyanomethylene)-2,3,5,6-tetrafluorocyclohexa-2,5-dien-1-ylidene)-3-(4-(dihexadecylamino)phenyl)prop-1-en-2-yl)phenyl)-2-(4-(dihexadecylamino)phenyl)allylidene)-2,3,5,6-tetrafluorocyclohexa-2,5-dien-1-ylidene)malononitrile (B-33)
2.8. 2-(4-(3,3-Dicyano-1-(4-(dicyanomethylene)cyclohexa-2,5-dien-1-ylidene)-2-(4-(dihexadecylamino)phenyl)allyl)phenyl)-3-(4-(dihexadecylamino)phenyl)buta-1,3-diene-1,1,4,4-tetracarbonitrile (B-12)
2.9. 2-(4-(3,3-Dicyano-1-(4-(dicyanomethylene)-2,3,5,6-tetrafluorocyclohexa-2,5-dien-1-ylidene)-2-(4-(dihexadecylamino)phenyl)allyl)phenyl)-3-(4-(dihexadecylamino)phenyl)buta-1,3-diene-1,1,4,4-tetracarbonitrile (B-13)
2.10. (E)-2-(4-(3,3-Dicyano-1-(4-(3,3-dicyano-1-(4-(dicyanomethylene)-2,3,5,6-tetrafluorocyclohexa-2,5-dien-1-ylidene)-2-(4-(dihexadecylamino)phenyl)allyl)phenyl)-2-(4-(dihexadecylamino)phenyl)allylidene)cyclohexa-2,5-dien-1-ylidene)malononitrile (B-23)
3. Result and Discussion
3.1. Preparation Method
3.2. UV-Vis Spectroscopy
3.3. Electrochemistry
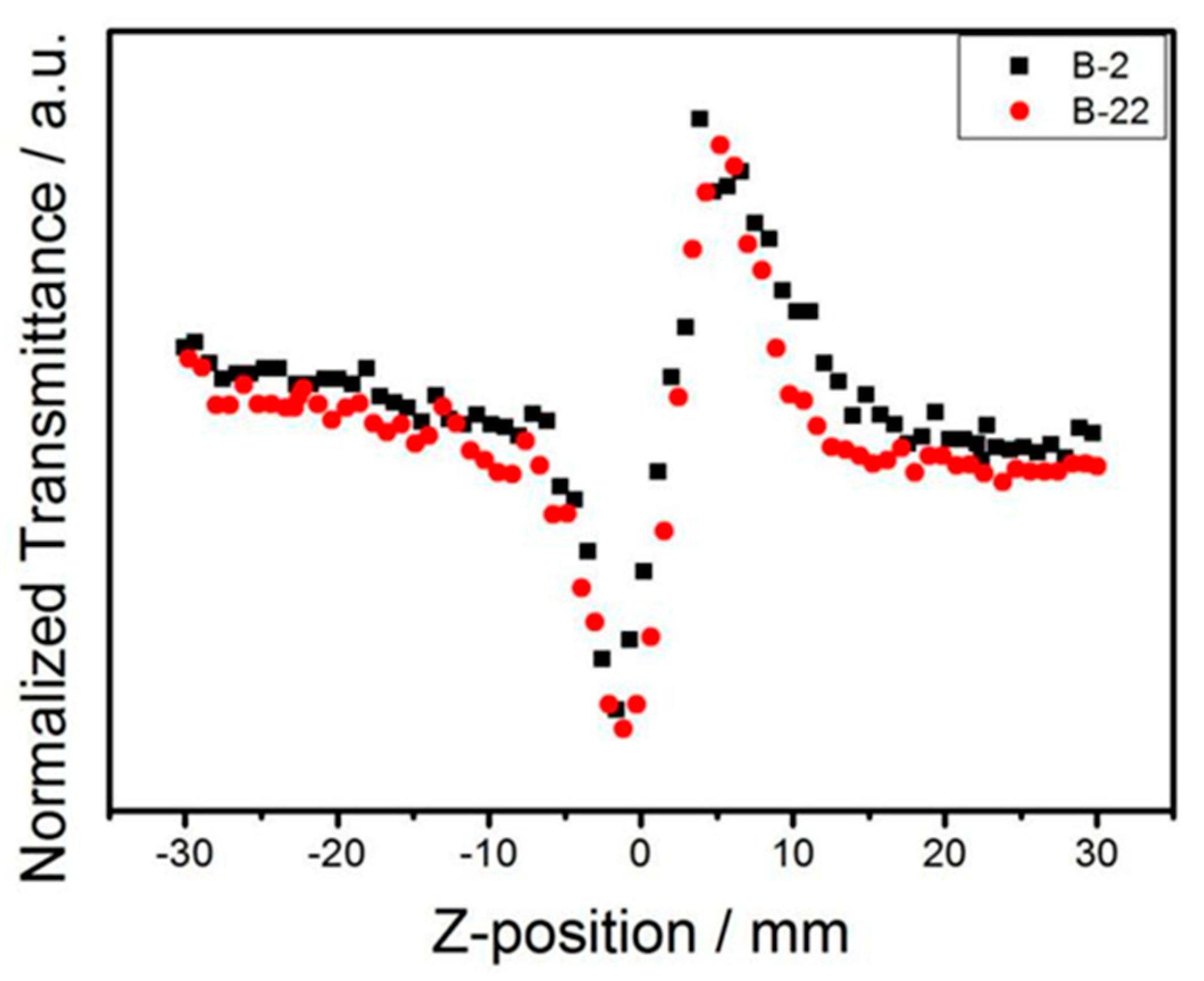
3.4. Non-Linear Optics (NLOs)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, D.; Gou, Q.S.; Guo, H.; Yang, Z.; Cao, H.; He, W.L.; Wang, H.H. Facile synthesis of functional poly(vinylene sulfide)s con-taining donor–acceptor chromophores by a double click reaction. RSC Adv. 2016, 6, 59327–59332. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Babu, M.; Kalluraya, B.; Chandrasekharan, K. Third-order nonlinear optical response of newly synthesized accept-er/donor substituted propylidene aryloxy acet hydrazide. Optik 2012, 123, 21–25. [Google Scholar] [CrossRef]
- Zhou, G.J.; Zhang, S.; Wu, P.J.; Ye, C. Optical limiting properties of soluble poly(thienyleneethynylene)s. Chem. Phys. Lett. 2002, 363, 610–614. [Google Scholar] [CrossRef]
- Li, R.; Pang, C.; Li, Z.Q.; Chen, F. Plasmonic Nanoparticles in Dielectrics Synthesized by Ion Beams: Optical Properties and Photonic Applications. Adv. Opt. Mater. 2020, 8, 1902087. [Google Scholar] [CrossRef]
- Li, P.H.; Zhou, Z.H.; Zhao, Y.S.; Yan, Y.L. Recent advances in luminescent metal–organic frameworks and their photonic applications. Chem. Commun. 2021, 57, 13678–13691. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Guo, Q.; Gao, H.; Yang, Z.; Xing, Y.; Cao, H.; He, W.; Wang, H.; Gu, J.; Hu, H. The application of double click to synthesize a third-order nonlinear polymer containing donor-acceptor chromophores. Polym. Chem. 2016, 7, 3714–3721. [Google Scholar] [CrossRef]
- Huang, J.X.; Yang, Y.H.; He, J.Z.; He, Z.; Wu, H. The important role of tetraphenylethene on designing bichromophores for organic nonlinear optical materials. Mater. Lett. 2021, 1, 129521. [Google Scholar] [CrossRef]
- Jagadesan, A.; Sivakumar, N.; Arjunan, S.; Parthipan, G. Growth, structural, optical, thermal and dielectric behaviour of a novel organic nonlinear optical (NLO) material: Benzimidazolium trichloroacetate monohydrate. Opt. Mater. 2020, 109, 110285. [Google Scholar] [CrossRef]
- Keinan, S.; Therien, M.J.; Beratan, D.N.; Yang, W.T. Molecular Design of Porphyrin-Based Nonlinear Optical Materials. J. Phys. Chem. A 2008, 112, 12203–12207. [Google Scholar] [CrossRef]
- Ganivada, M.N.; Kumar, P.; Shunmugam, R. A unique polymeric gel by thiol–alkyne click chemistry. RSC Adv. 2015, 5, 50001–50004. [Google Scholar] [CrossRef]
- Karna, S.P.; Yeates, A.T. Nonlinear Optical Materials: Theory and Modeling. J. ACS Symp. Ser. 1996, 628, 1–22. [Google Scholar]
- Zhou, G.J.; Wong, W.Y.; Lin, Z.Y.; Ye, C. White Metallopolyynes for Optical Limiting/Transparency Trade-off Optimization. Angew. Chem. Int. Ed. 2006, 45, 6189–6193. [Google Scholar] [CrossRef]
- Ray, P.C.; Sainudeen, Z. Very Large Infrared Two-Photon Absorption Cross Section of Asymmetric Zinc Porphyrin Aggregates: Role of Intermolecular Interaction and Donor-Acceptor Strengths. J. Phys. Chem. A 2006, 110, 12342–12347. [Google Scholar] [CrossRef]
- Liu, X.; Wang, D.; Gao, H.; Yang, Z.; Xing, Y.; Cao, H.; He, W.L.; Wang, H.H.; Gu, J.M.; Hu, H.Y. Nonlinear optical properties of symmetrical and asymmetrical porphyrin derivatives with click chemistry modification. Dyes Pigment. 2016, 134, 155–163. [Google Scholar] [CrossRef]
- Aurelio, M.A.; Kostas, L.; Stelios, C.; Maurizio, P. Efficient Modulation of the Third Order Nonlinear Optical Properties of Fullerene Derivatives. J. Am. Chem. Soc. 2008, 130, 1534–1535. [Google Scholar]
- Zhang, W.S.; Wang, D.; Cao, H.; Yang, H. Energy level tunable pre-click functionalization of [60]fullerene for nonlinear optics. Tetrahedron 2014, 70, 573–577. [Google Scholar] [CrossRef]
- Finn, M.G.; Fokin, V.V. Click chemistry: Function follows form. Chem. Soc. Rev. 2010, 39, 1231–1232. [Google Scholar] [CrossRef]
- Michinobu, T.; May, J.C.; Lim, J.H.; Boudon, C.; Gisselbrecht, J.P.; Seiler, P.; Gross, M.; Biaggio, I.; Diederich, F. A new class of organic donor-acceptor molecules with large third-order optical nonlinearities. Chem. Commun. 2005, 6, 737–739. [Google Scholar] [CrossRef]
- Miao, Z.C.; Han, H.H.; Wang, D.; Gao, H.; Gu, J.M.; Hu, H.Y. Nonlinear optical and energy-level modulation of organic alkynes by click chemistry. Tetrahedron 2016, 72, 4039–4046. [Google Scholar] [CrossRef]
- Liu, X.; Wang, D.; Gao, H.; Yang, Z.; Xing, Y.; Cao, H.; He, W.; Wang, H.; Gu, J.; Hu, H. Click chemistry functionalization improving the wideband optical-limiting performance of fullerene derivatives. Phys. Chem. Chem. Phys. 2016, 18, 7341–7348. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X.K.; Zhao, Y.Z.; Gao, H.; Xing, Y.; Yang, H. Ladder-type poly(benzopentalene) derivatives with tunable energy levels by “click” reaction. Polym. Chem. 2012, 3, 914–919. [Google Scholar] [CrossRef]
- Wu, J.Y.; Li, Z.A.; Luo, J.D.; Jen, A.K.Y. High-performance organic second- and third-order nonlinear optical materials for ultrafast information processing. J. Mater. Chem. C 2020, 8, 15009–150026. [Google Scholar] [CrossRef]
- Mi, Y.S.; Liang, P.X.; Jin, Z.K.; Wang, D.; Yang, Z. Self-Assembly Micro-Nanostructures of Discotic Organic Molecules. Chem. Phys. Chem. 2013, 331, 567–571. [Google Scholar] [CrossRef]
- Michinobu, T.; Li, Y.R.; Hyakutake, T. Polymeric ion sensors with multiple detection modes achieved by a new type of click chemistry reaction. Phys. Chem. Chem. Phys. 2013, 15, 2623–2631. [Google Scholar] [CrossRef] [PubMed]
- Ouazzani, E.I.; Iliopoulos, K.; Pranaitis, M.; Krupka, O.; Smokal, V.; Kolendo, A.; Sahraoui, B. Second- and third-order nonlinearities of novel push-pull azobenzene polymers. J. Phys. Chem. B 2011, 115, 1944–1949. [Google Scholar] [CrossRef] [Green Version]
- Gazvoda, M.; Virant, M.; Pevec, A.; Urankar, D.; Bolje, A.; Kočevar, M.; Košmrlj, J. A mesoionic bis(Py-tzNHC) palladium(II) complex catalyses “green” Sonogashira reaction through an unprecedented mechanism. Chem. Commun. 2016, 52, 1571–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Y.; Liu, J. Sequential Sonogashira and Glaser coupling reactions: Facile access to 1,4-disubstituted 1,3-butadiynes from arylbromide. Tetrahedron Lett. 2016, 57, 2143–2146. [Google Scholar] [CrossRef]
- Probst, N.P.; Deprez, B.; Willand, N. Palladium-free Sonogashira-type cross-coupling reaction of bromoisoxazolines or N-alkoxyimidoyl bromides and alkynes. Tetrahedron Lett. 2016, 57, 1066–1070. [Google Scholar] [CrossRef]
- Crouch, R.D. Selective deprotection of silyl ethers. Tetrahedron 2013, 69, 2383. [Google Scholar] [CrossRef]
- Shah, S.T.; Singh, S.; Guiry, P.J. Microwave-Assisted Synthesis of Substituted Tetrahydropyrans Catalyzed by ZrCl4 and Its Application in the Asymmetric Synthesis of exo- and endo-brevicomin. J. Org. Chem. 2009, 74, 5758–5761. [Google Scholar]
- Varotto, A.; Nam, C.Y.; Radivojevic, I.; Tomé, J.; Cavaleiro, J.A.S.; Black, C.T.; Drain, C.M. Phthalocyanine Blends Improve Bulk Heterojunction Solar Cells. J. Am. Chem. Soc. 2010, 132, 2552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Lee, J.; Chae, S.; Shim, J.Y.; Lee, D.Y.; Kim, I.; Kim, H.J.; Kim, S.H.; Suh, H. Conjugated polymers containing pyrimidine with electron withdrawing substituents for organic photovoltaics with high open-circuit voltage. Polymer 2016, 83, 53. [Google Scholar] [CrossRef]
- Powell, B.J.; Baruah, T.; Bernstein, N.; Brake, K.; McKenzie, R.H.; Meredith, P.; Pederson, M.R. A first-principles density-functional calculation of the electronic and vibrational structure of the key melanin monomers. J. Chem. Phys. 2004, 120, 8608–8615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshioka, K.; Minami, Y.; Shudo, K.; Dao, T.D.; Nagao, T.; Kitajima, M.; Takeda, J.; Katayama, I. Terahertz-Field-Induced Nonlinear Electron Delocalization in Au Nanostructures. J. Am. Chem. Soc. 2015, 15, 1036. [Google Scholar] [CrossRef] [Green Version]
- Hatano, T.; Stopa, M.; Tarucha, S. Single-Electron Delocalization in Hybrid Vertical-Lateral Double Quantum Dots. Science 2005, 309, 268–271. [Google Scholar] [CrossRef]
- Michinobu, T.; Seo, C.; Noguchid, K.; Moric, T. Effects of click postfunctionalization on thermal stability and field effect transistor performances of aromatic polyamines. Polym. Chem. 2012, 3, 1427. [Google Scholar] [CrossRef]
- Michinobu, T. Adapting semiconducting polymer doping techniques to create new types of click postfunctionalization. Cheminform 2011, 40, 2306. [Google Scholar] [CrossRef]
- Wu, S.T.; Chen, L.Y.; Yin, B.L.; Li, Y.W. “Click” post-functionalization of a metal-organic framework for engineering active single-site heterogeneous Ru (III) catalysts. Chem. Commun. 2015, 51, 9884. [Google Scholar] [CrossRef]
- Yildirim, M.; Kaya, I. Soluble semi-conductive chelate polymers containing Cr(III) in the backbone: Synthesis, characterization, optical, electrochemical, and electrical properties. Polymer 2009, 50, 5653–5660. [Google Scholar] [CrossRef]
- Li, Y.R.; Tsuboi, K.; Michinobu, T. Double click synthesis and second-order nonlinearities of polystyrenes bearing donor-acceptor chromophores. Macromolecules 2010, 43, 5277–5286. [Google Scholar] [CrossRef]
- Sheik-Bahae, M.; Said, A.A.; Wei, T.H.; Hagan, D.J.; Van Stryland, E.W. Sensitive measurement of optical nonlinearities using a single beam. IEEE J. Quantum Electron. 1990, 26, 760–769. [Google Scholar] [CrossRef] [Green Version]
- Zaanen, J. Band gaps and electronic structure of transition-metal compounds. Phys. Rev. Lett. 1985, 55, 418. [Google Scholar] [CrossRef]
- Ghanavatkar, C.W.; Mishra, V.R.; Sekar, N. Review of NLO phoric azo dyes—Developments in hyperpolarizabilities in last two dec ades. Dyes Pigment. 2021, 191, 109367. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, H.; Zhang, H.; Li, D.; Su, J.; Zhang, S.; Tian, Y. A series of stilbazolium salts with A-pi-A model and their third-order nonlinear optical response in the near-IR region. Spectrochim. Acta A 2017, 175, 92–99. [Google Scholar] [CrossRef]
- May, J.C.; Lim, J.H.; Biaggio, I.; Moonen, N.N.P.; Michinobu, T.; Diederich, F. Highly efficient third-order optical nonlinearities in donor-substituted cyanoethynylethene molecules. Opt. Lett. 2005, 30, 3057–3059. [Google Scholar] [CrossRef]

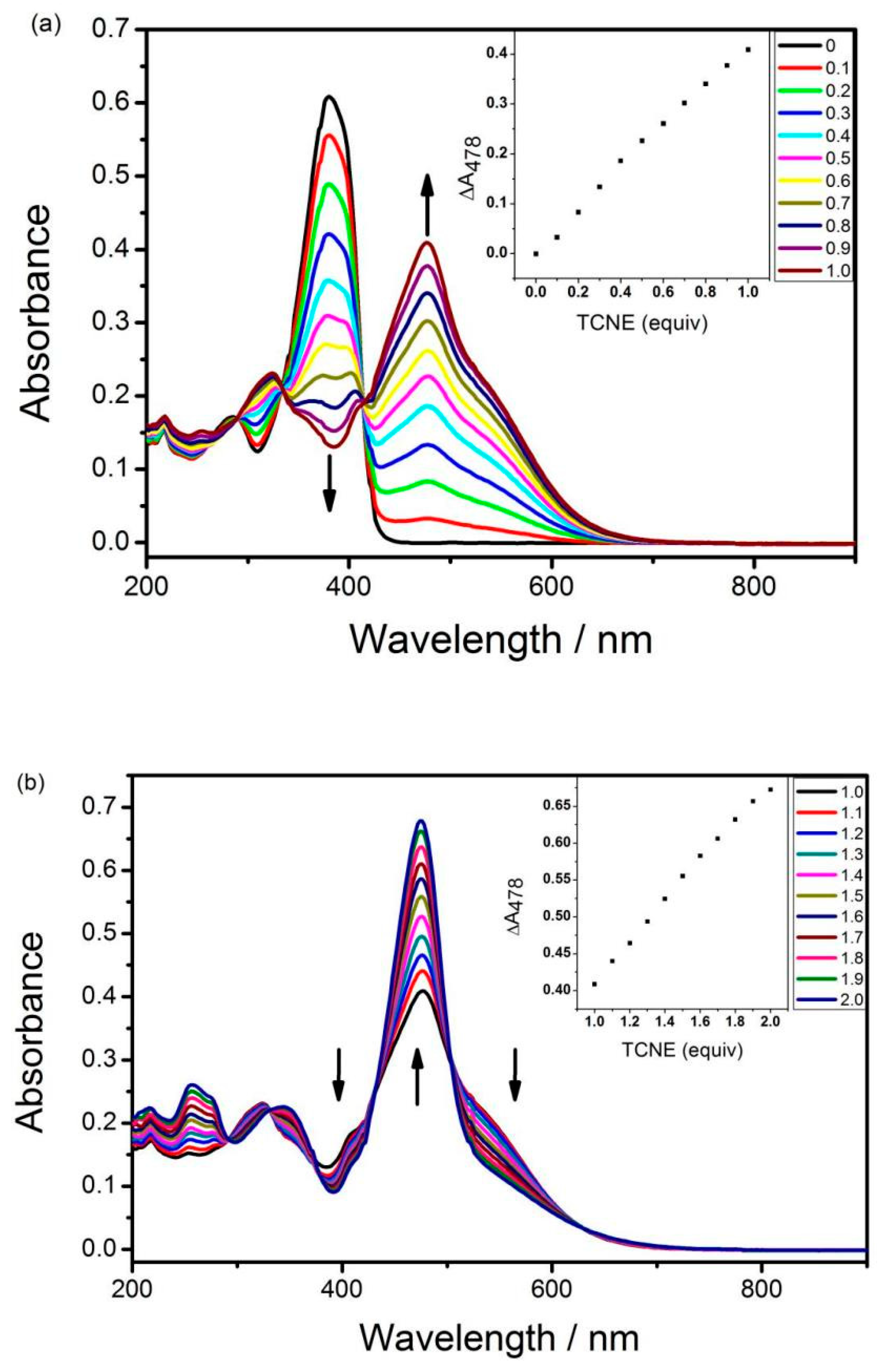
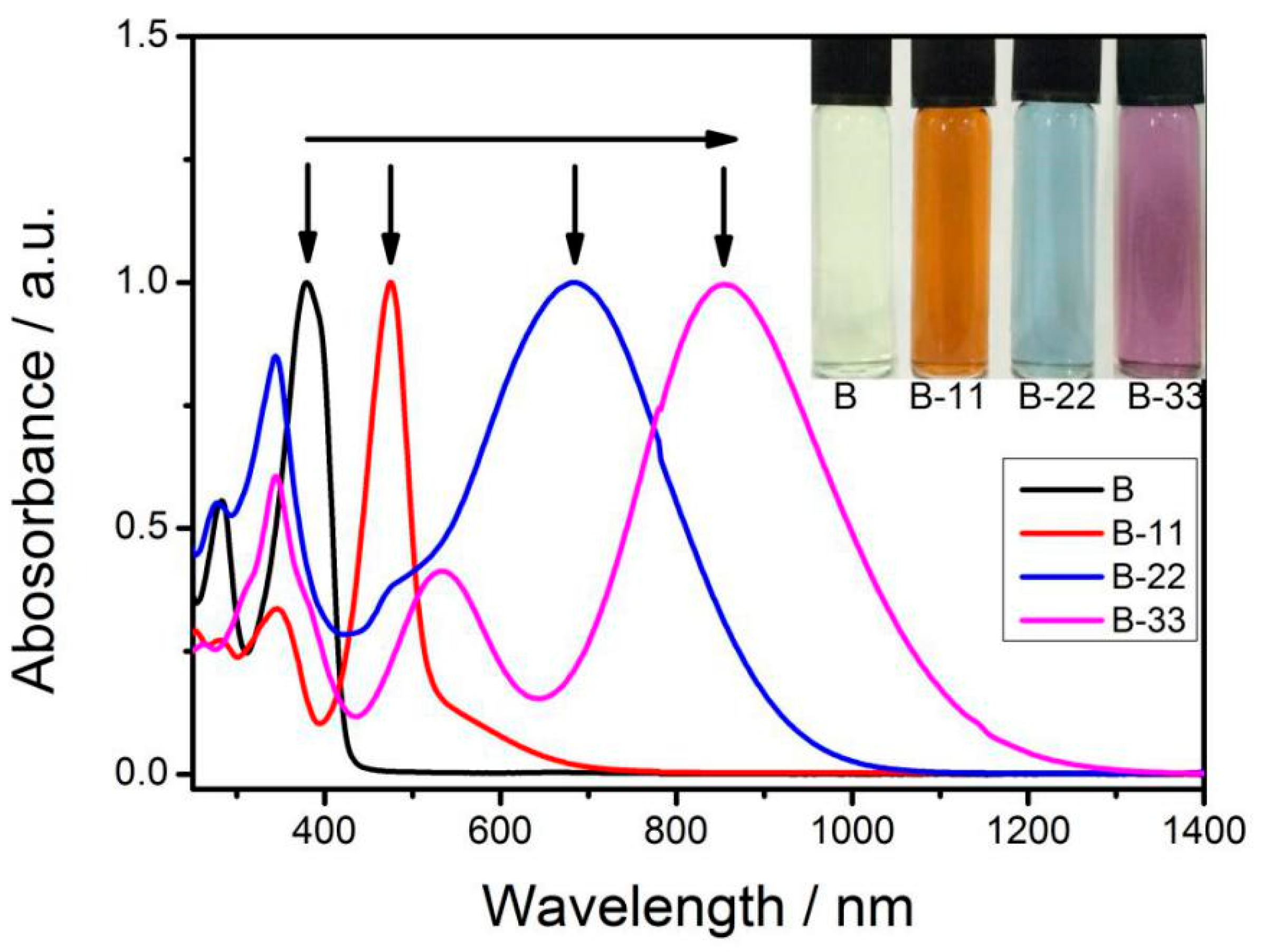
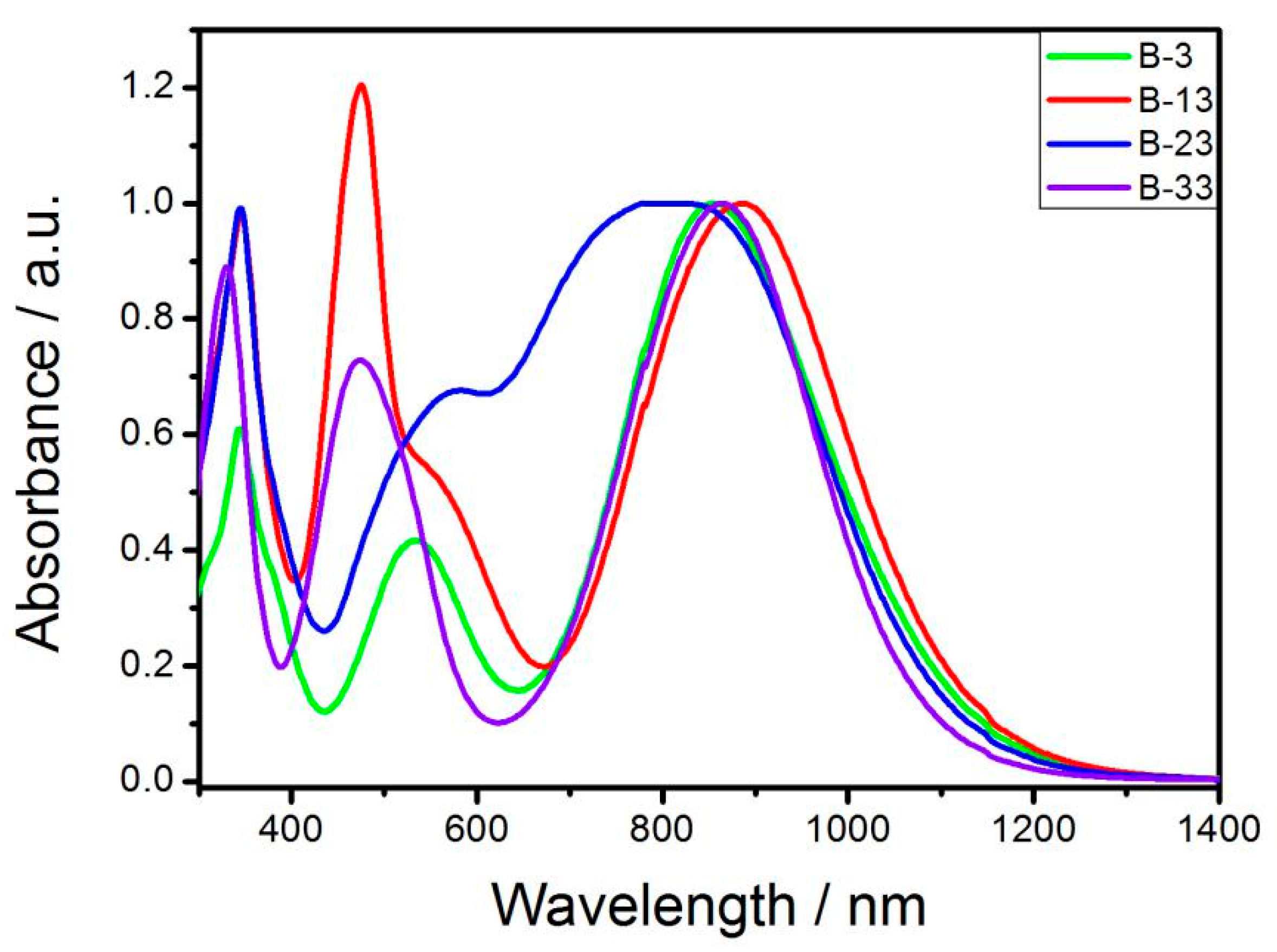

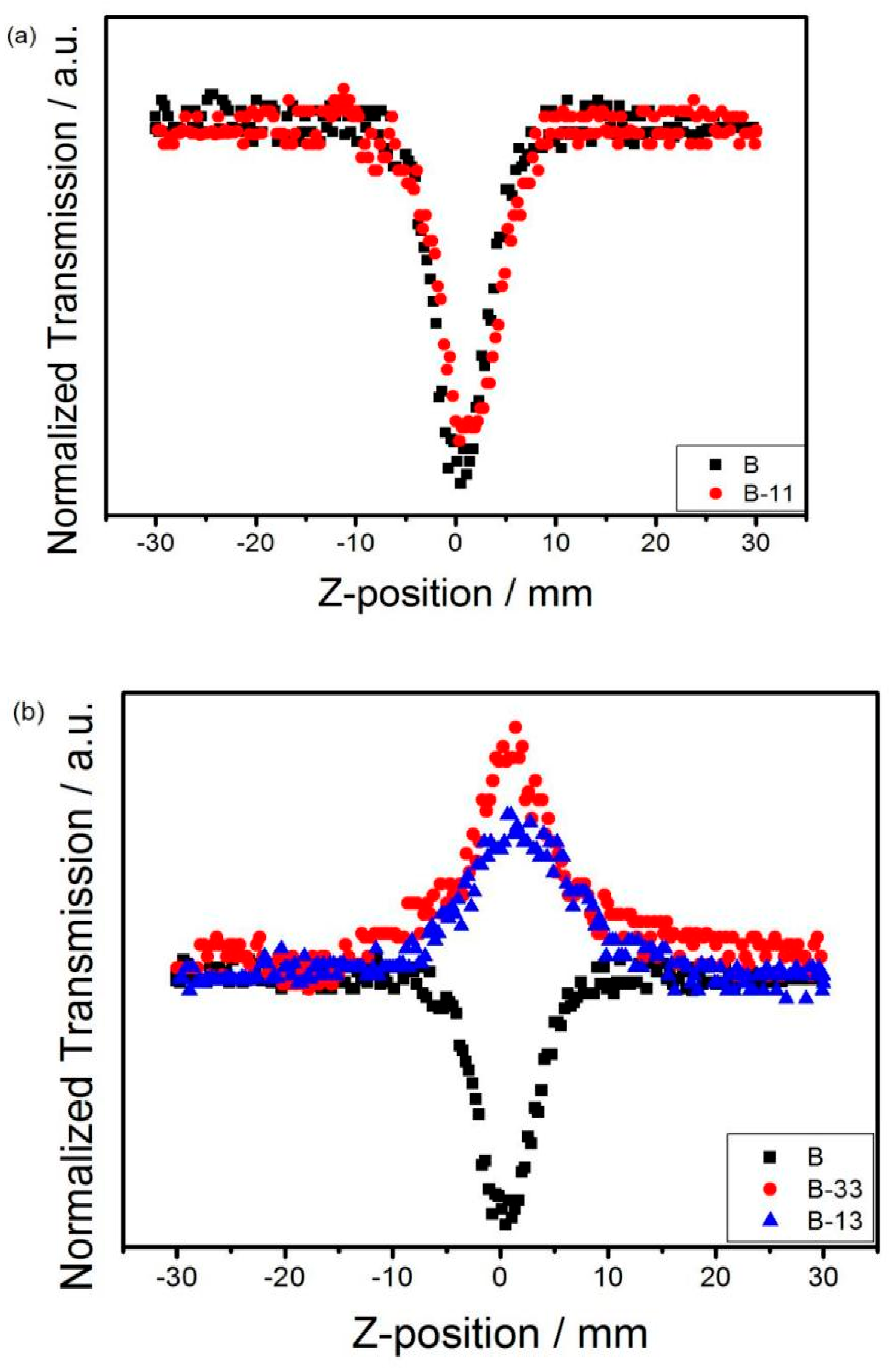
| Materials | λmax[a] (nm) | λend[b] (nm) | Eg [c] (eV) | Eonox [d] (V) | Eonred [d] (V) | Eg [e] (eV) |
|---|---|---|---|---|---|---|
| B | 382 | 490 | 2.53 | 1.10 | −1.10 | 2.20 |
| B-1 | 478 | 734 | 1.69 | 1.00 | −0.63 | 1.63 |
| B-11 | 478 | 781 | 1.59 | 1.05 | −0.49 | 1.54 |
| B-2 | 698 | 1026 | 1.21 | 0.62 | −0.44 | 1.06 |
| B-22 | 691 | 1092 | 1.14 | 0.77 | −0.42 | 1.19 |
| B-3 | 867 | 1334 | 0.93 | 0.63 | −0.24 | 0.87 |
| B-33 | 867 | 1416 | 0.88 | 0.67 | −0.22 | 0.89 |
| B-12 | 475 | 1150 | 1.08 | 0.62 | −0.49 | 1.11 |
| B-13 | 475 | 1394 | 0.89 | 0.75 | −0.18 | 0.93 |
| B-23 | 823 | 1386 | 0.89 | 0.66 | −0.24 | 0.90 |
| Sample | β | n2 | Imχ(3) | Reχ(3) | χ(3) |
|---|---|---|---|---|---|
| ×10−12 m/W | ×10−19 m/W | ×10−13 esu | ×10−13 esu | ×10−13 esu | |
| B | 1.1 | 2 | 0.24 | 1.02 | 1.05 |
| B-1 | −4.5 | - | −0.98 | - | - |
| B-11 | 1 | −3.6 | 0.22 | −1.84 | 1.85 |
| B-2 | - | 2.5 | - | 1.28 | - |
| B-22 | - | 2.5 | - | 1.28 | - |
| B-3 | - | 1.8 | - | 0.92 | - |
| B-33 | −1.2 | - | −0.26 | - | - |
| B-12 | - | - | — | - | - |
| B-13 | −0.9 | 2.3 | −0.20 | 1.18 | 2.28 |
| B-23 | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Li, Z.; Li, Q.; Zhao, Y.; Yao, R.; Ma, C.; Zhang, Y.; Wang, D. Optical Properties of New Third-Order Nonlinear Materials Modified by Click Chemistry. Molecules 2022, 27, 5006. https://doi.org/10.3390/molecules27155006
Zhao Y, Li Z, Li Q, Zhao Y, Yao R, Ma C, Zhang Y, Wang D. Optical Properties of New Third-Order Nonlinear Materials Modified by Click Chemistry. Molecules. 2022; 27(15):5006. https://doi.org/10.3390/molecules27155006
Chicago/Turabian StyleZhao, Yuzhen, Zhenhua Li, Qing Li, Yang Zhao, Ruijuan Yao, Cheng Ma, Yongming Zhang, and Dong Wang. 2022. "Optical Properties of New Third-Order Nonlinear Materials Modified by Click Chemistry" Molecules 27, no. 15: 5006. https://doi.org/10.3390/molecules27155006
APA StyleZhao, Y., Li, Z., Li, Q., Zhao, Y., Yao, R., Ma, C., Zhang, Y., & Wang, D. (2022). Optical Properties of New Third-Order Nonlinear Materials Modified by Click Chemistry. Molecules, 27(15), 5006. https://doi.org/10.3390/molecules27155006





