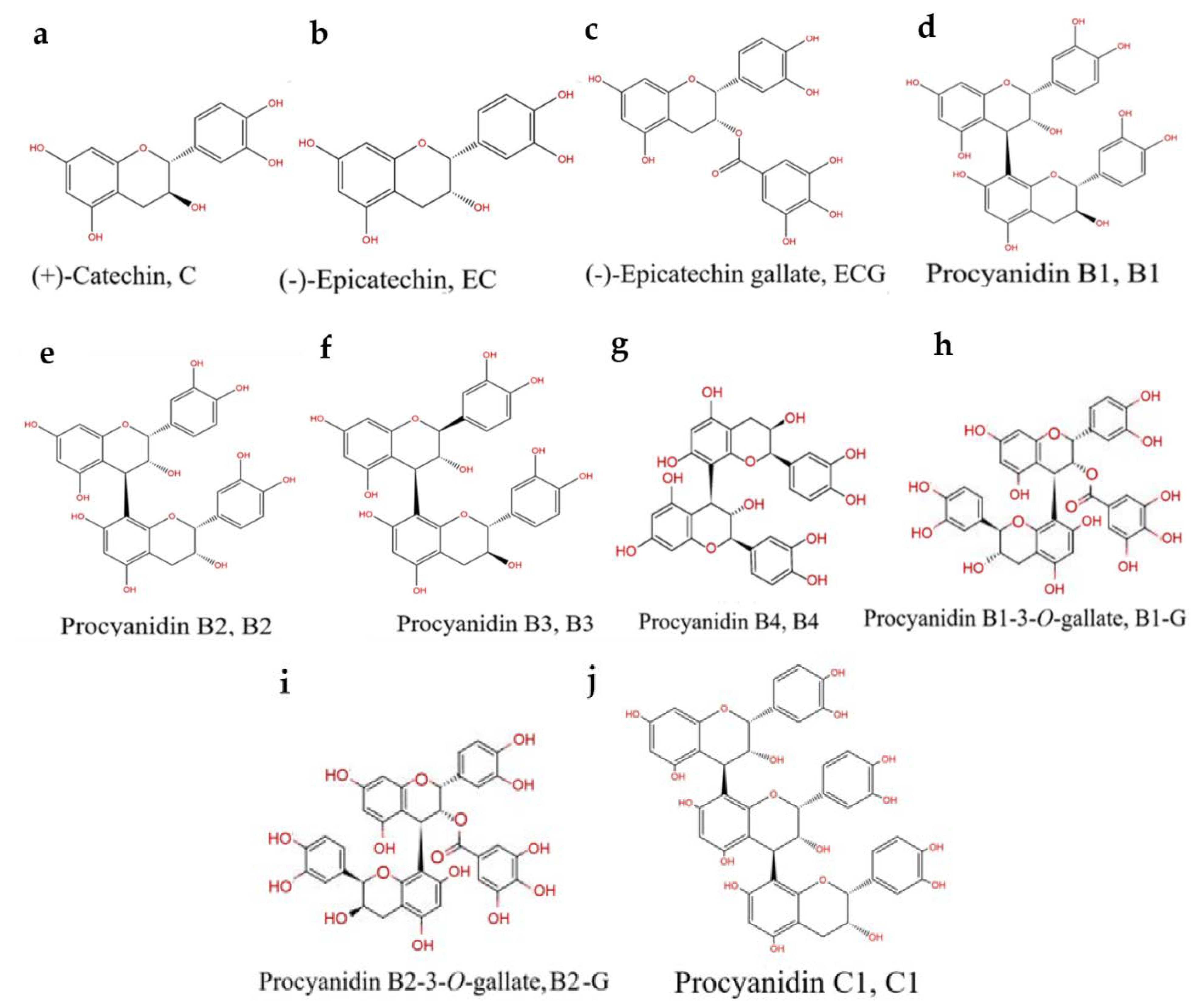
The Relationship between Procyanidin Structure and Their Protective Effect in a Parkinson’s Disease Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture
2.3. Cell Viability and Membrane Integrity Assays
2.4. Fish Maintenance and the Experimental Group
2.5. ROS Measurements
2.6. Assessment of MDA, GSH-Px, SOD, and CAT
2.7. Nrf2 siRNA Transfection
2.8. Preparation of Whole Cell, Cytoplasmic, and Nuclear Proteins
2.9. Western Blotting
2.10. Behavioral Detection of Zebrafish
2.11. Zebrafish Tyrosine hydroxylase (TH) Immunostaining
2.12. Total RNA Extraction, Reverse Transcription, and Quantitative Real-Time Polymerase Chain Reaction
2.13. Statistical Analysis
3. Results
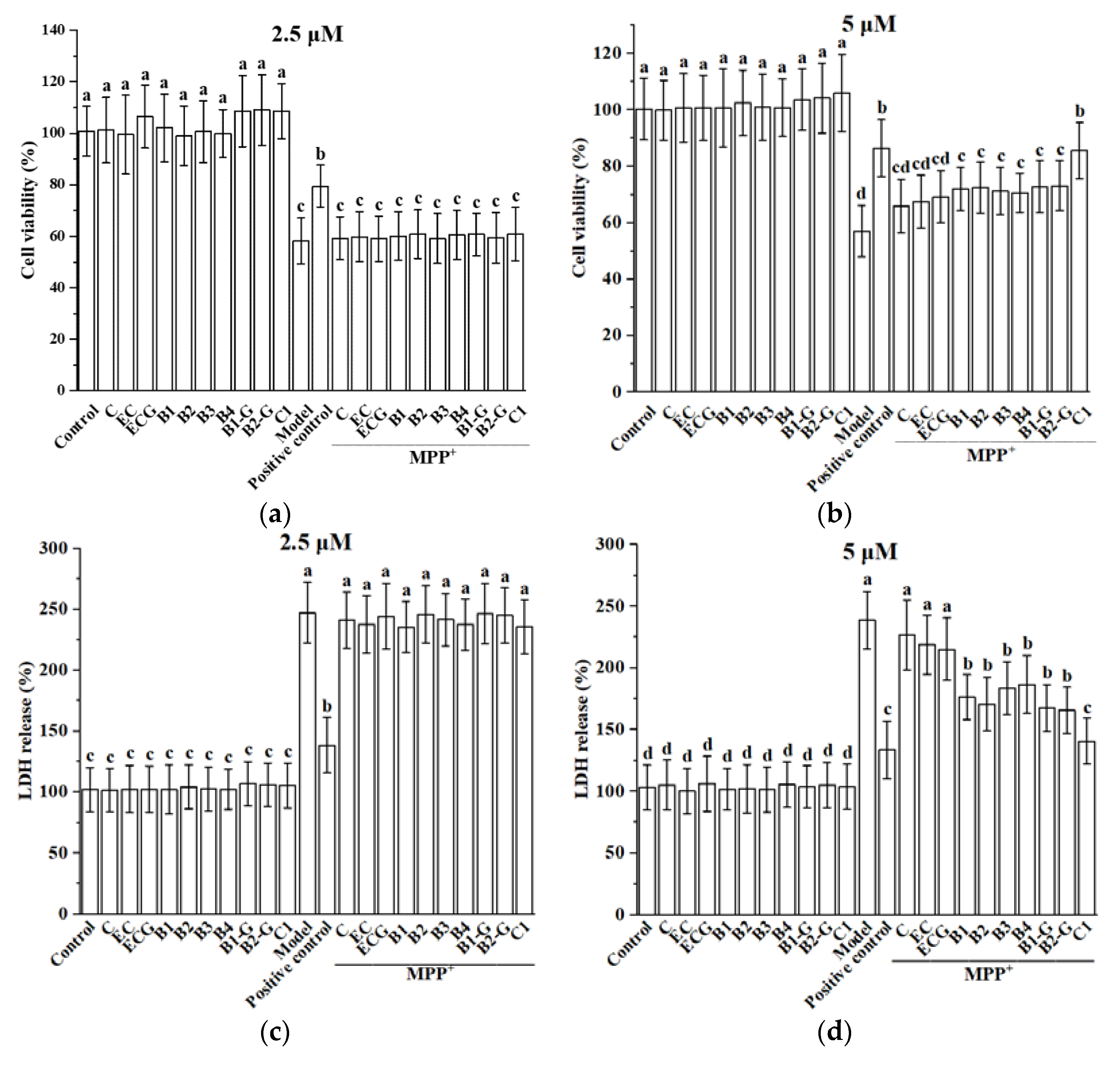
3.1. Effect of Different Structural Procyanidins on MPP+-Induced PC12 Cell Damage
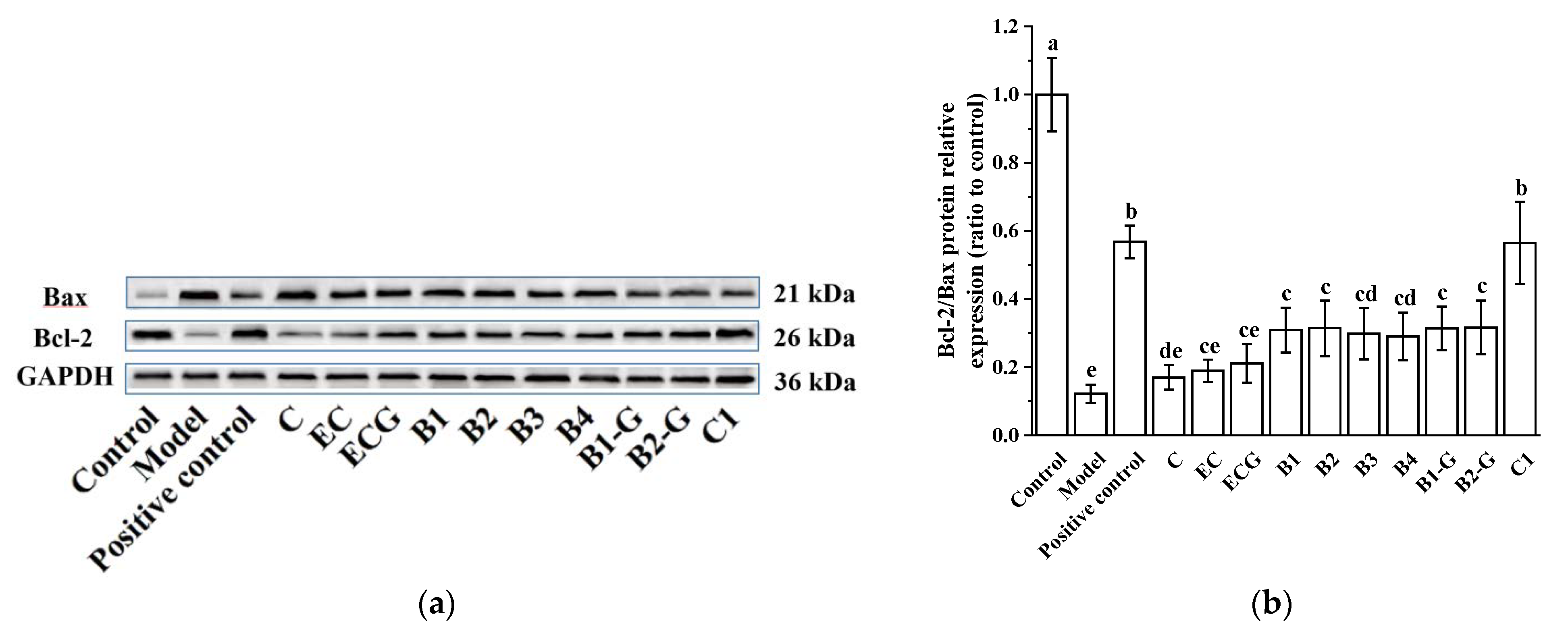
3.2. Effects of Different Structural Procyanidins on the Expression of Bax and Bcl-2 Proteins in the PC12 Cell PD Model
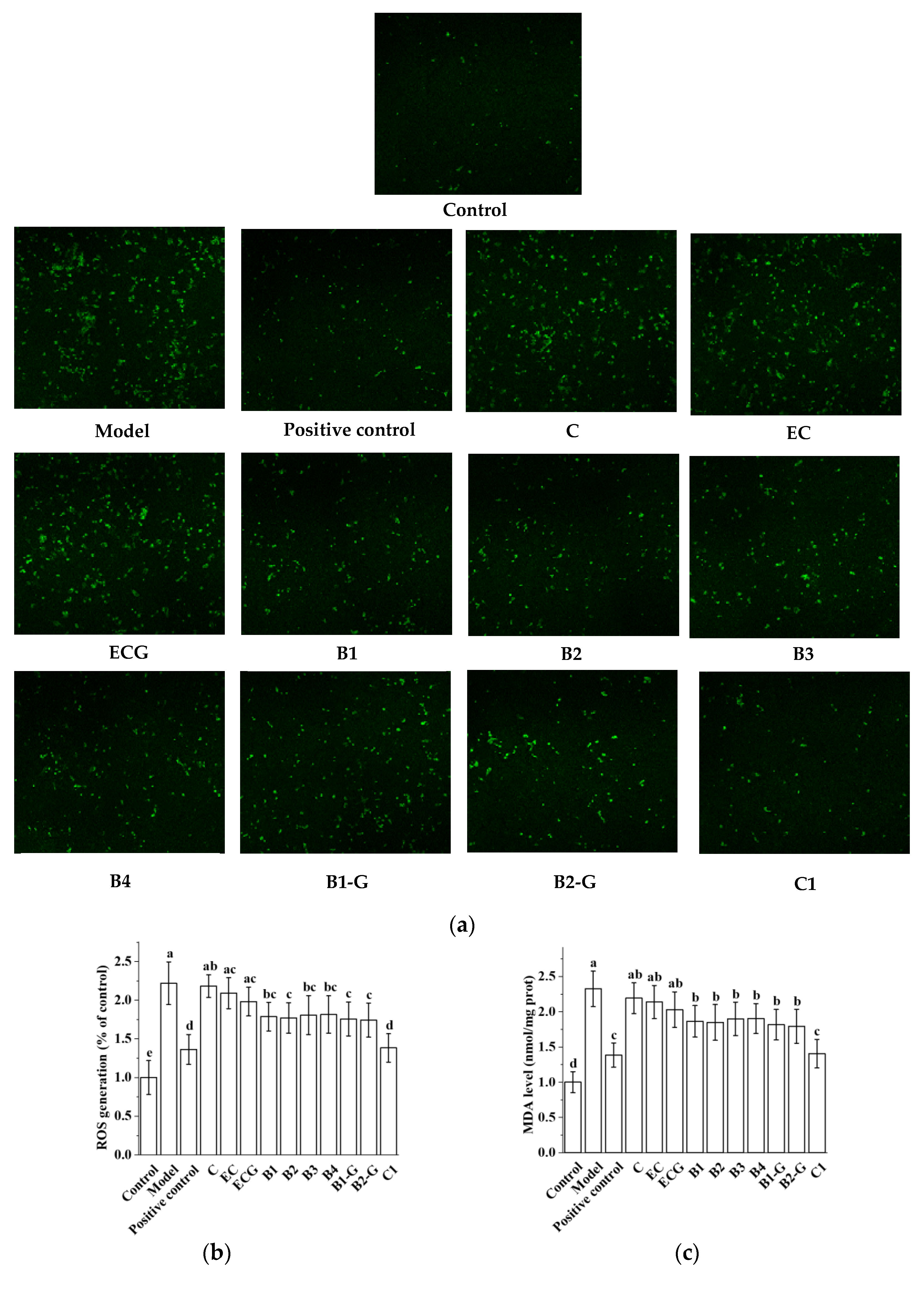
3.3. Effects of Different Structural Procyanidins on Oxidative Stress in the PC12 Cell PD Model
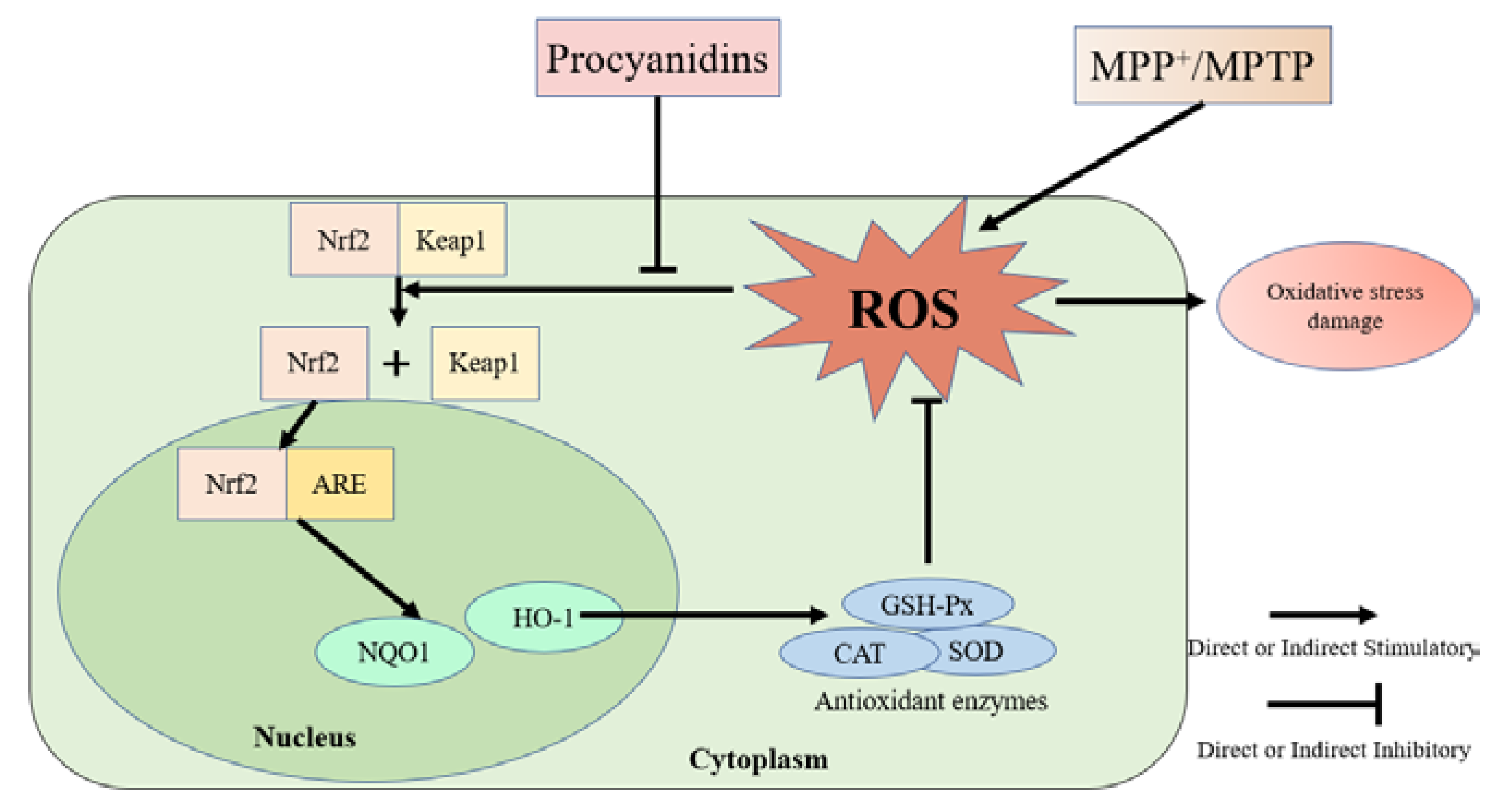
3.4. Effects of Different Structural Procyanidins on the Nrf2/ARE Pathway in the PC12 Cell PD Model
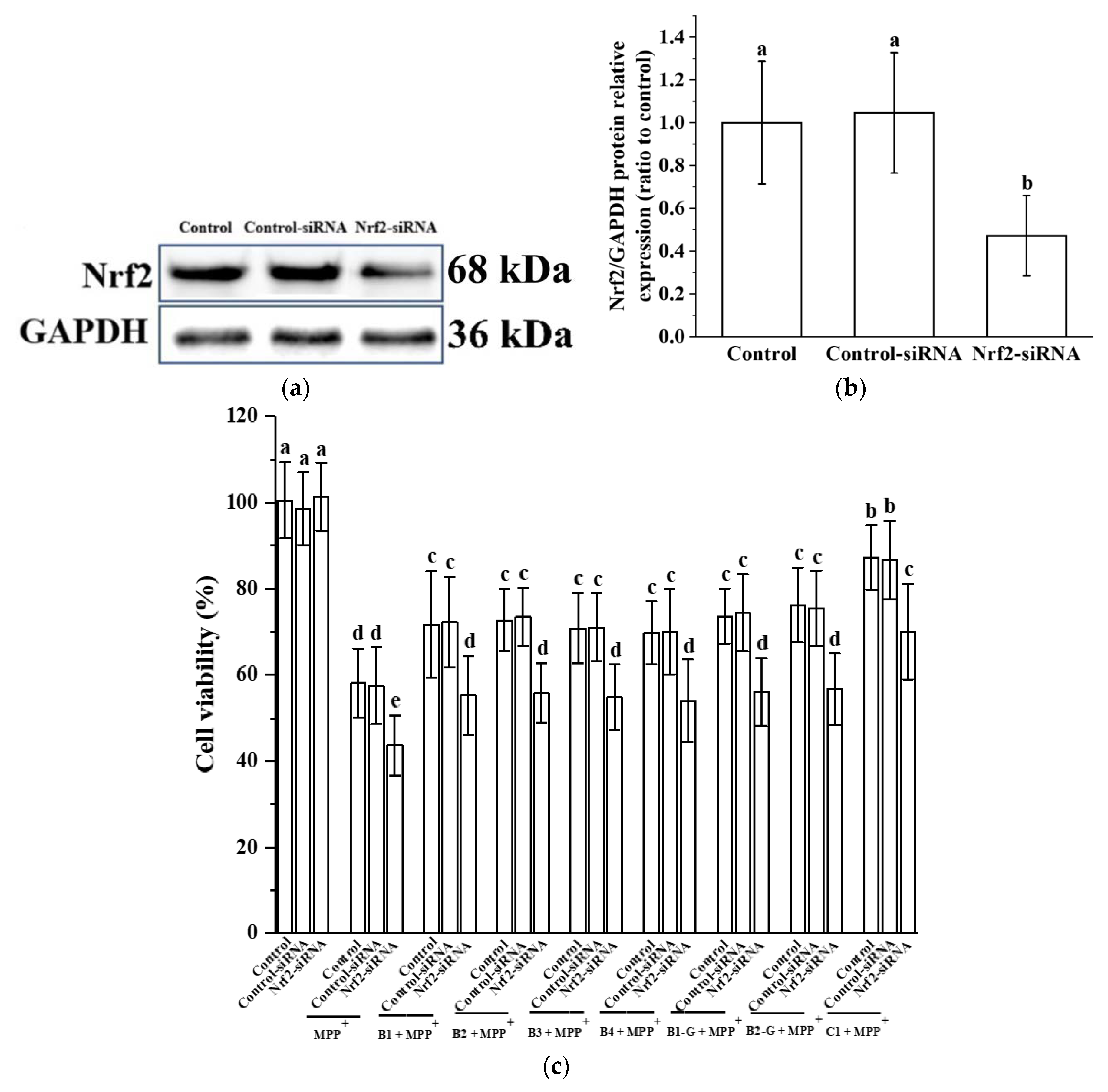
3.5. Verification of the Effect of Nrf2 on the Protective Effect of Different Structural Procyanidins in the PC12 Cell PD Model
3.6. Effects of Different Structural Procyanidins on Exercise Capacity in a Zebrafish PD Model
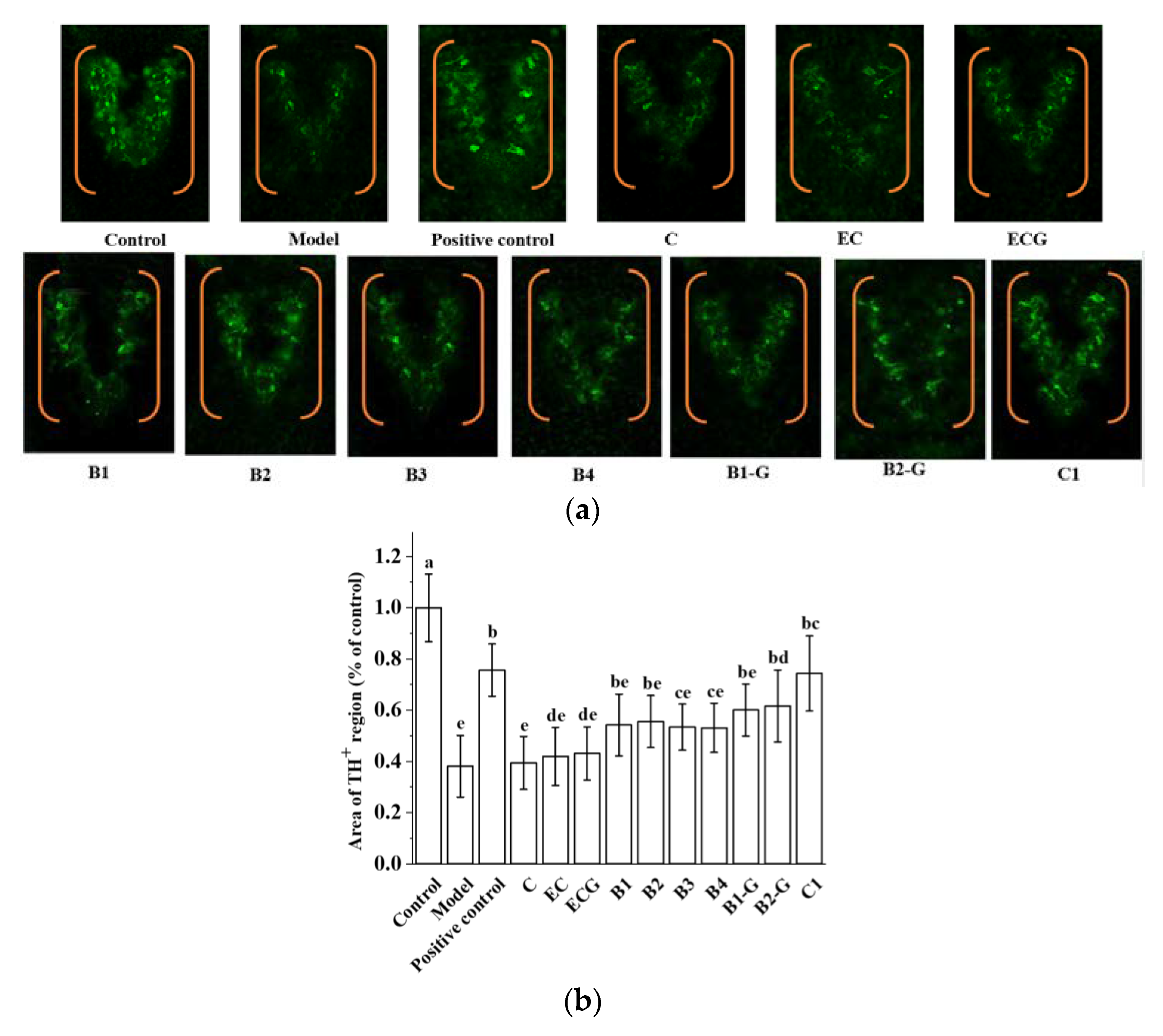
3.7. Effects of Different Structural Procyanidins on Dopaminergic Neuron Injury in the Zebrafish PD Model
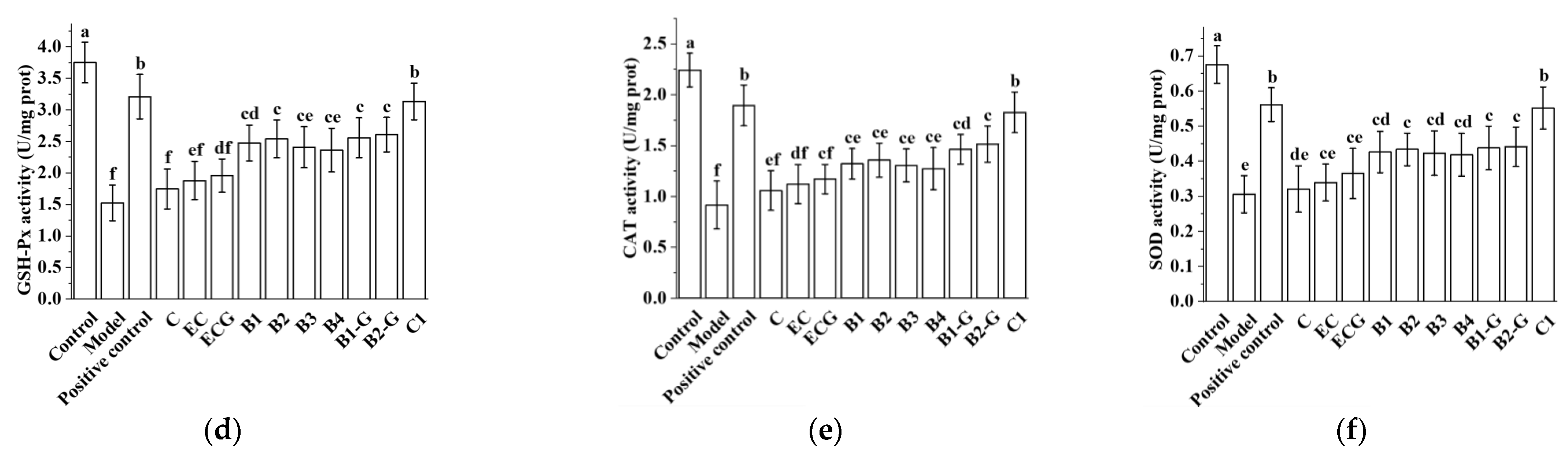
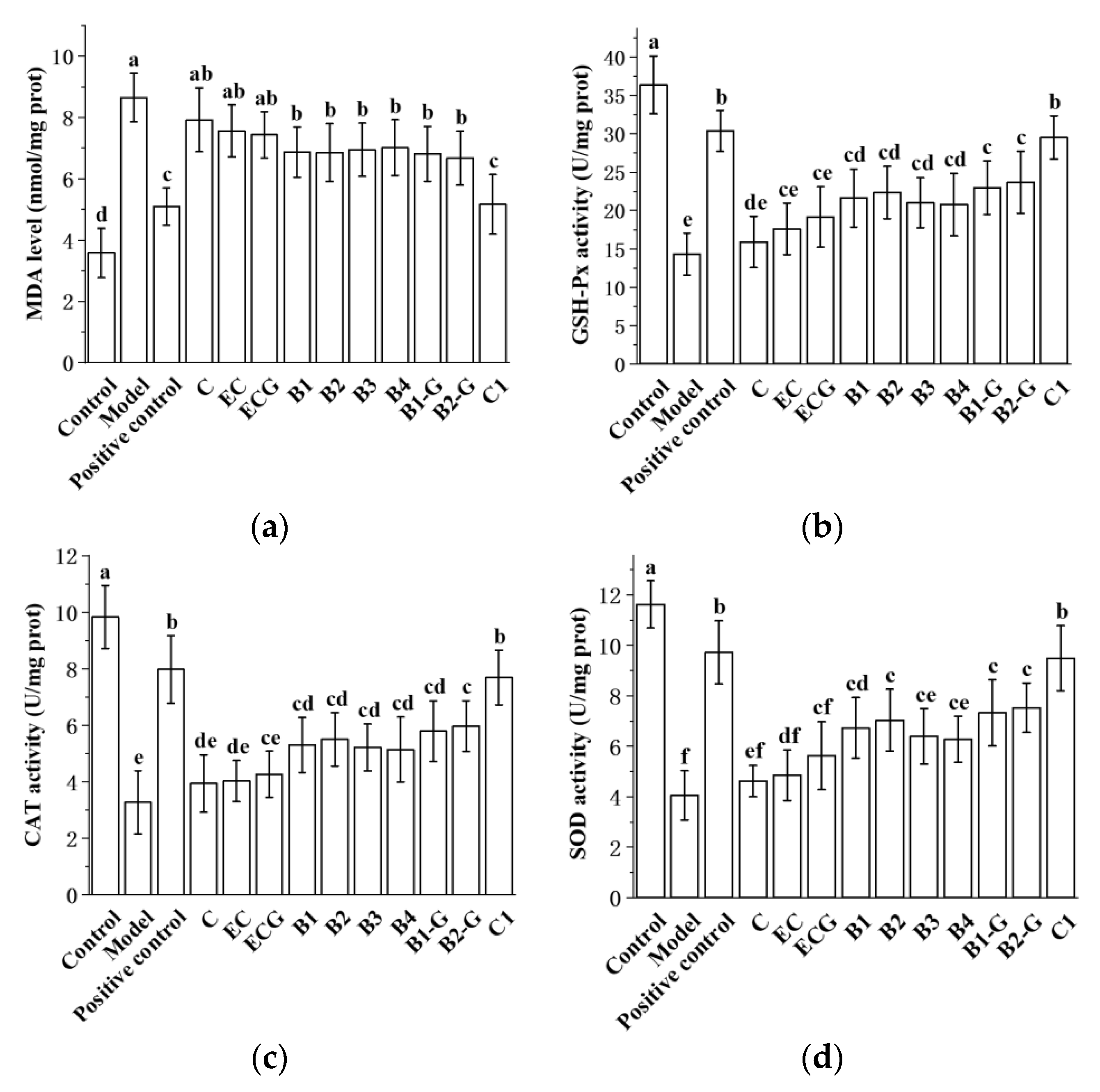
3.8. Effects of Different Structural Procyanidins on Oxidative Stress in the Zebrafish PD Model
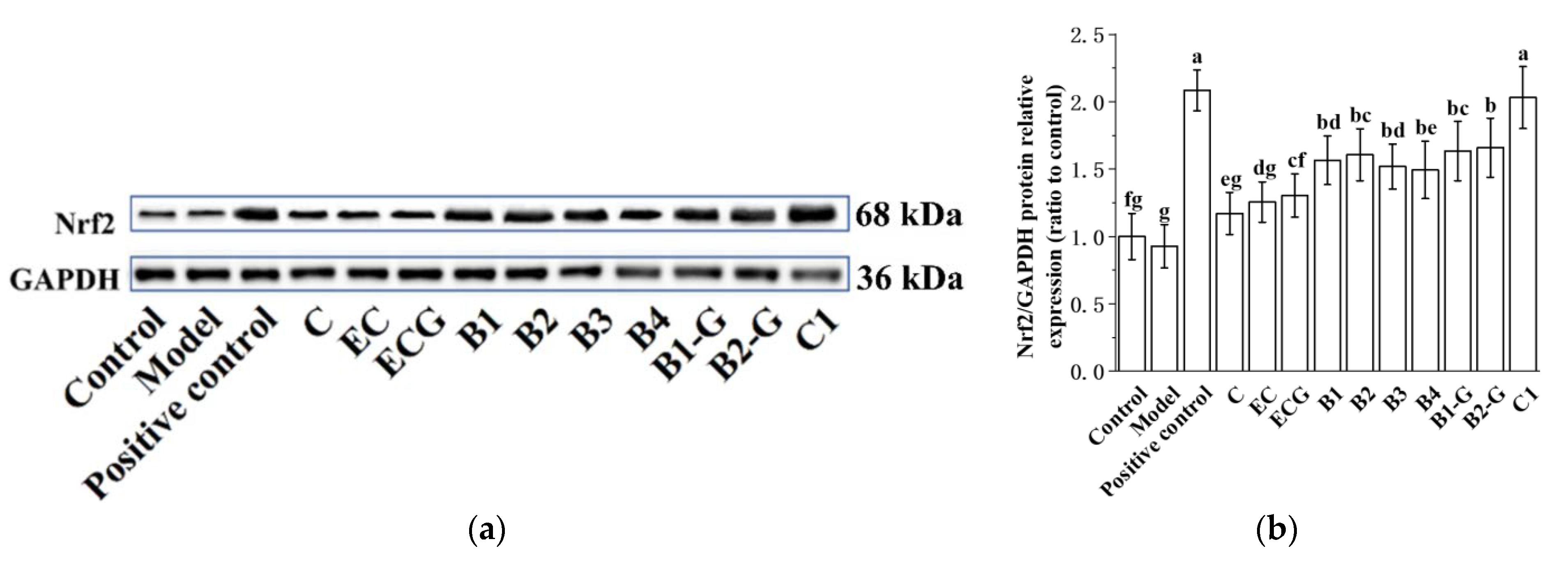
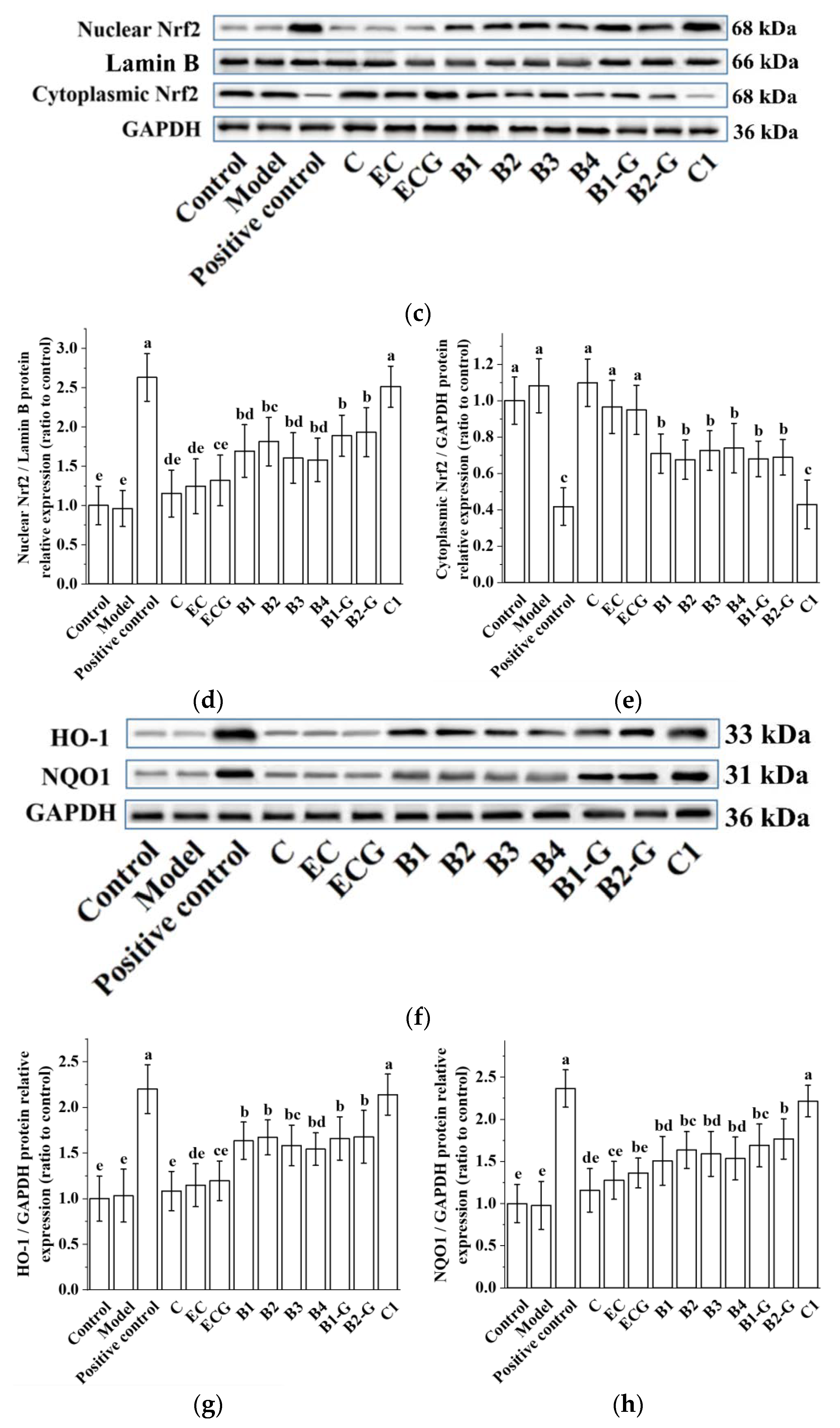
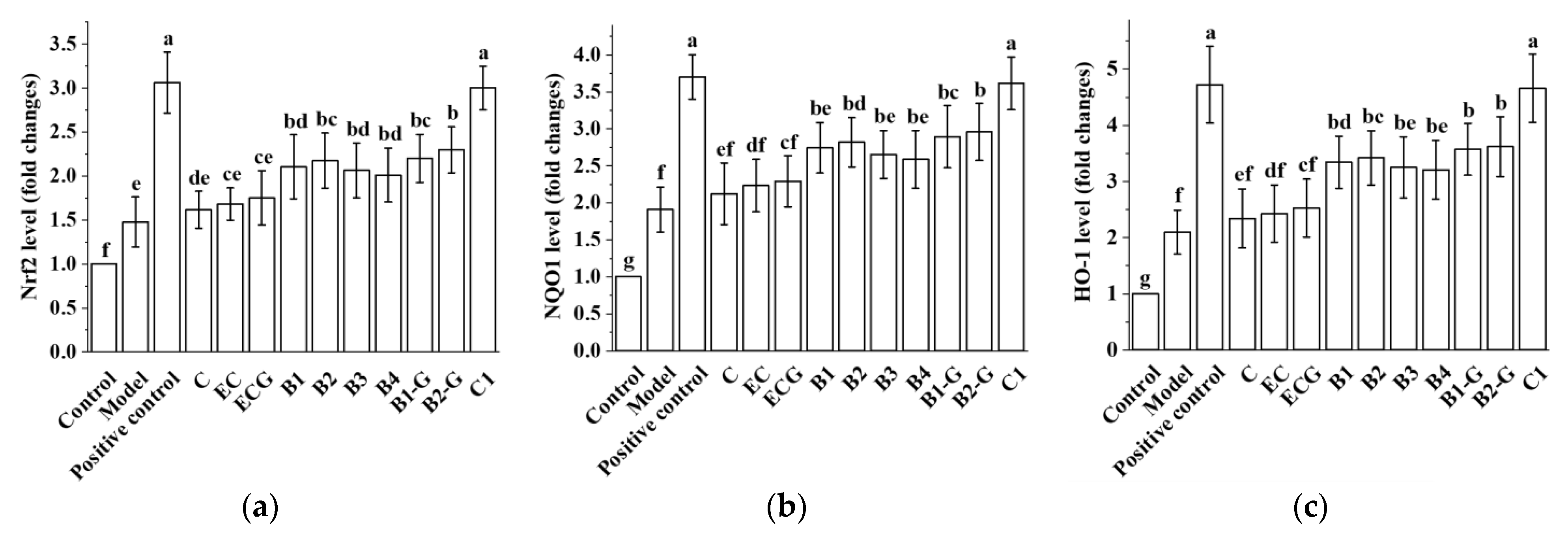
3.9. Effects of Different Structural Procyanidins on the Nrf2/ARE Pathway in the Zebrafish PD Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dawson, T.M.; Dawson, V.L. Molecular pathways of neurodegeneration in Parkinson’s disease. Science 2003, 302, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.V. Pathogenesis of Parkinson’s disease. Bailliere Clin. Neur. 1997, 6, 15–36. [Google Scholar]
- Picklo, M.J.; Olson, S.J.; Hayes, J.D.; Markesbery, W.R.; Montine, T.J. Elevation of AKR7A2 (succinic semialdehyde reductase) in neurodegenerative disease. Brain Res. 2001, 916, 229–238. [Google Scholar] [CrossRef]
- Hallett, P.J.; Engelender, S.; Isacson, O. Lipid and immune abnormalities causing age-dependent neurodegeneration and Parkinson’s disease. J. Neuroinflamm. 2019, 16, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vingerhoets, G.; Verleden, S.; Santens, P.; Miatton, M.; De Reuck, J. Predictors of cognitive impairment in advanced Parkinson’s disease. J. Neurol. Neurosur. Ps. 2003, 74, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Bosco, D.A.; Fowler, D.M.; Zhang, Q.H.; Nieva, J.; Powers, E.T.; Wentworth, P.; Lerner, R.A.; Kelly, J.W. Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate alpha-synuclein fibrilization (vol 2, pg 249, 2006). Nat. Chem. Biol. 2006, 2, 346. [Google Scholar] [CrossRef]
- Alam, Z.I.; Daniel, S.E.; Lees, A.J.; Marsden, D.C.; Jenner, P.; Halliwell, B. A generalised increase in protein carbonyls in the brain in Parkinson’s but not incidental Lewy body disease. J. Neurochem. 1997, 69, 1326–1329. [Google Scholar] [CrossRef]
- Nakabeppu, Y.; Tsuchimoto, D.; Yamaguchi, H.; Sakumi, K. Oxidative damage in nucleic acids and Parkinson’s disease. J. Neurosci. Res. 2007, 85, 919–934. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Fujikawa, K.; Zucca, F.A.; Zecca, L.; Ito, S. The structure of neuromelanin as studied by chemical degradative methods. J. Neurochem. 2003, 86, 1015–1023. [Google Scholar] [CrossRef]
- Lotharius, J.; Brundin, P. Impaired dopamine storage resulting from alpha-synuclein mutations may contribute to the pathogenesis of Parkinson’s disease. Hum. Mol. Genet. 2002, 11, 2395–2407. [Google Scholar] [CrossRef] [Green Version]
- Bagchi, D.; Garg, A.; Krohn, R.L.; Bagchi, M.; Tran, M.X.; Stohs, S.J. Oxygen free radical scavenging abilities of vitamins C and E, and A grape seed proanthocyanidin extract in vitro. Res. Commun. Mol. Pathol. Pharmacol. 1997, 95, 179–189. [Google Scholar] [PubMed]
- Chen, J.; Chen, Y.; Zheng, Y.; Zhao, J.; Yu, H.; Zhu, J.; Li, D. Protective Effects and Mechanisms of Procyanidins on Parkinson’s Disease In Vivo and In Vitro. Molecules 2021, 26, 2963. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.X.; Bai, R.F.; Zhao, Y.Q.; Li, J.; Wei, Z.M.; Wang, F.; Sun, B.S. Protective Effect of Grape Seed Procyanidins against H2O2-Induced Oxidative Stress in PC-12 Neuroblastoma Cells: Structure-Activity Relationships. J. Food Sci. 2018, 83, 2622–2628. [Google Scholar] [CrossRef]
- Gu, L.W.; Kelm, M.; Hammerstone, J.F.; Beecher, G.; Cunningham, D.; Vannozzi, S.; Prior, R.L. Fractionation of polymeric procyanidins from lowbush blueberry and quantification of procyanidins in selected foods with an optimized normal-phase HPLC-MS fluorescent detection method. J. Agric. Food Chem. 2002, 50, 4852–4860. [Google Scholar] [CrossRef] [PubMed]
- Labarbe, B.; Cheynier, V.; Brossaud, F.; Souquet, J.M.; Moutounet, M. Quantitative fractionation of grape proanthocyanidins according to their degree of polymerization. J. Agric. Food Chem. 1999, 47, 2719–2723. [Google Scholar] [CrossRef] [PubMed]
- Ricardo-Da-Silva, J.M.; Rigaud, J.; Cheynier, V.; Cheminat, A.; Moutounet, M. Procyanidin Dimers and Trimers from Grape Seeds. Phytochemistry 1991, 30, 1259–1264. [Google Scholar] [CrossRef]
- Kumar, H.; Koppula, S.; Kim, I.S.; More, S.V.; Kim, B.W.; Choi, D.K. Nuclear Factor Erythroid 2–Related Factor 2 Signaling in Parkinson Disease: A Promising Multi Therapeutic Target Against Oxidative Stress, Neuroinflammation and Cell Death. Cns Neurol. Disord. Dr. 2012, 11, 1015–1029. [Google Scholar] [CrossRef]
- Iyanagi, T.; Yamazaki, I. One-electron-transfer reactions in biochemical systems. V. Difference in the mechanism of quinone reduction by the NADH dehydrogenase and the NAD(P)H dehydrogenase (DT-diaphorase). Biochim. Biophys. Acta 1970, 216, 282–294. [Google Scholar] [CrossRef]
- Dore, S.; Snyder, S.H. Neuroprotective action of bilirubin against oxidative stress in primary hippocampal cultures. Neuroprotective Agents Fourth Int. Conf. 1999, 890, 167–172. [Google Scholar] [CrossRef]
- Stocker, R.; Yamamoto, Y.; Mcdonagh, A.F.; Glazer, A.N.; Ames, B.N. Bilirubin Is an Antioxidant of Possible Physiological Importance. Science 1987, 235, 1043–1046. [Google Scholar] [CrossRef]
- Tufekci, K.U.; Civi Bayin, E.; Genc, S.; Genc, K. The Nrf2/ARE Pathway: A Promising Target to Counteract Mitochondrial Dysfunction in Parkinson’s Disease. Parkinsons Dis. 2011, 2011, 314082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, S.; Tang, Q.; Jin, J.; Zheng, G.; Xu, J.; Huang, W.; Li, X.; Shang, P.; Liu, H. Polydatin inhibits the IL-1beta-induced inflammatory response in human osteoarthritic chondrocytes by activating the Nrf2 signaling pathway and ameliorates murine osteoarthritis. Food Funct. 2018, 9, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.D.; Verveer, P.J.; Bastiaens, P.I. Growth factor-induced MAPK network topology shapes Erk response determining PC-12 cell fate. Nat. Cell Biol. 2007, 9, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, D.A.; Jin, K. From angiogenesis to neuropathology. Nature 2005, 438, 954–959. [Google Scholar] [CrossRef]
- Shui, G.; Bao, Y.M.; Bo, J.; An, L.J. Protective effect of protocatechuic acid from Alpinia oxyphylla on hydrogen peroxide-induced oxidative PC12 cell death. Eur. J. Pharmacol. 2006, 538, 73–79. [Google Scholar] [CrossRef]
- Cheng, X.R.; Zhang, L.; Hu, J.J.; Sun, L.; Du, G.H. Neuroprotective effects of tetramethylpyrazine on hydrogen peroxide-induced apoptosis in PC12 cells. Cell Biol. Int. 2007, 31, 438–443. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, J.H.; Bao, Y.M.; An, L.J. Hydrogen peroxide-induced apoptosis in pc12 cells and the protective effect of puerarin. Cell Biol. Int. 2003, 27, 1025–1031. [Google Scholar] [CrossRef]
- Ellett, F.; Lieschke, G.J. Zebrafish as a model for vertebrate hematopoiesis. Curr. Opin. Pharmacol. 2010, 10, 563–570. [Google Scholar] [CrossRef]
- Cahill, G.M. Clock mechanisms in zebrafish. Cell Tissue Res. 2002, 309, 27–34. [Google Scholar] [CrossRef]
- Guo, S. Linking genes to brain, behavior and neurological diseases: What can we learn from zebrafish? Genes Brain Behav. 2004, 3, 63–74. [Google Scholar] [CrossRef]
- Ninkovic, J.; Bally-Cuif, L. The zebrafish as a model system for assessing the reinforcing properties of drugs of abuse. Methods 2006, 39, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Rink, E.; Wullimann, M.F. Connections of the ventral telencephalon (subpallium) in the zebrafish (Danio rerio). Brain Res. 2004, 1011, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Wullimann, M.F.; Mueller, T. Teleostean and mammalian forebrains contrasted: Evidence from genes to behavior. J. Comp. Neurol. 2004, 475, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Eisler, T.; Teravainen, H.; Nelson, R.; Krebs, H.; Weise, V.; Lake, C.R.; Ebert, M.H.; Whetzel, N.; Murphy, D.L.; Kopin, I.J.; et al. Deprenyl in Parkinson disease. Neurology 1981, 31, 19–23. [Google Scholar] [CrossRef]
- Narita, M.; Shimizu, S.; Ito, T.; Chittenden, T.; Lutz, R.J.; Matsuda, H.; Tsujimoto, Y. Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proc. Natl. Acad. Sci. USA 1998, 95, 14681–14686. [Google Scholar] [CrossRef] [Green Version]
- Tabei, Y.; Murotomi, K.; Umeno, A.; Horie, M.; Tsujino, Y.; Masutani, B.; Yoshida, Y.; Nakajima, Y. Antioxidant properties of 5-hydroxy-4-phenyl-butenolide via activation of Nrf2/ARE signaling pathway. Food Chem. Toxicol. 2017, 107, 129–137. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.P.; Wang, Y.B.; Fu, A.K.; Gong, L.; Li, W.F.; Li, Y.L. Bacillus amyloliquefaciens SC06 alleviates the oxidative stress of IPEC-1 via modulating Nrf2/Keap1 signaling pathway and decreasing ROS production. Appl. Microbiol. Biotechnol. 2017, 101, 3015–3026. [Google Scholar] [CrossRef]
- Pei, J.; Guo, S.; Zhang, C.; Zhu, Z.; Yu, L.; Xie, K.; Zhao, X. Effects of tea polyphenols on the activity of GSH-Px and NOS, and the content of MDA and NO in rats fed with high methionine diet. Wei Sheng Yan Jiu 2011, 40, 750–752. [Google Scholar]
- Gadea, J.; Selles, E.; Marco, M.A.; Coy, P.; Matas, C.; Romar, R.; Ruiz, S. Decrease in glutathione content in boar sperm after cryopreservation–Effect of the addition of reduced glutathione to the freezing and thawing extenders. Theriogenology 2004, 62, 690–701. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, R.; Li, W.M.; Niu, Y.J.; Guo, H.C.; Liu, X.H.; Hou, Y.C.; Zhao, L.J. The protective effect of grape seed procyanidin extract against cadmium-induced renal oxidative damage in mice. Environ. Toxicol. Pharmacol. 2013, 36, 759–768. [Google Scholar] [CrossRef]
- Mates, J.M.; Perez-Gomez, C.; De Castro, I.N. Antioxidant enzymes and human diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef]
- Yu, M.; Li, H.; Liu, Q.; Liu, F.; Tang, L.; Li, C.; Yuan, Y.; Zhan, Y.; Xu, W.; Li, W.; et al. Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway. Cell. Signal. 2011, 23, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Aspects Med. 2011, 32, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, S.; Chan, J.Y.; Zhang, D.D. Keap1 controls postinduction repression of the Nrf2-mediated antioxidant response by escorting nuclear export of Nrf2. Mol. Cell. Biol. 2007, 27, 6334–6349. [Google Scholar] [CrossRef] [Green Version]
- Schmidlin, C.J.; Dodson, M.B.; Madhavan, L.; Zhang, D.D. Redox regulation by NRF2 in aging and disease. Free Radic. Biol. Med. 2019, 134, 702–707. [Google Scholar] [CrossRef]
- Li, M.Q.; Xu, T.; Zhou, F.; Wang, M.M.; Song, H.X.; Xiao, X.; Lu, B.Y. Neuroprotective Effects of Four Phenylethanoid Glycosides on H2O2-Induced Apoptosis on PC12 Cells via the Nrf2/ARE Pathway. Int. J. Mol. Sci. 2018, 19, 1135. [Google Scholar] [CrossRef] [Green Version]
- Itoh, K.; Igarashi, K.; Hayashi, N.; Nishizawa, M.; Yamamoto, M. Cloning and Characterization of a Novel Erythroid Cell-Derived Cnc Family Transcription Factor Heterodimerizing with the Small Maf Family Proteins. Mol. Cell. Biol. 1995, 15, 4184–4193. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.J.; Hou, Y.N.; Yao, J.; Fang, J.G. Activation of Nrf2-driven antioxidant enzymes by cardamonin confers neuroprotection of PC12 cells against oxidative damage. Food Funct. 2017, 8, 997–1007. [Google Scholar] [CrossRef]
- Mi, Y.S.; Zhang, W.T.; Tian, H.Y.; Li, R.N.; Huang, S.X.; Li, X.Y.; Qi, G.Y.; Liu, X.B. EGCG evokes Nrf2 nuclear translocation and dampens PTP1B expression to ameliorate metabolic misalignment under insulin resistance condition. Food Funct. 2018, 9, 1510–1523. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.X.; Zheng, Y.F.; Zhao, J.W.; Yu, H.L.; Zhu, J.J. Relationship between Neuroprotective Effects and Structure of Procyanidins. Molecules 2022, 27, 2308. [Google Scholar] [CrossRef]
- Jellinger, K.A. The pathology of Parkinson’s disease. Parkinsons Dis. 2001, 86, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Solayman, M.; Islam, M.A.; Alam, F.; Khalil, M.I.; Kamal, M.A.; Gan, S.H. Natural products combating neurodegeneration: Parkinson’s disease. Curr. Drug Metab. 2017, 18, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative stress in neurodegenerative diseases: From a mitochondrial point of view. Oxid. Med. Cell. Longev. 2019, 2019, 2105607. [Google Scholar] [CrossRef] [Green Version]
- Bagchi, D.; Garg, A.; Krohn, R.L.; Bagchi, M.; Bagchi, D.J.; Balmoori, J.; Stohs, S.J. Protective effects of grape seed proanthocyanidins and selected antioxidants against TPA-induced hepatic and brain lipid peroxidation and DNA fragmentation, and peritoneal macrophage activation in mice. Gen. Pharmacol. 1998, 30, 771–776. [Google Scholar] [CrossRef]
- Barone, M.C.; Sykiotis, G.P.; Bohmann, D. Genetic activation of Nrf2 signaling is sufficient to ameliorate neurodegenerative phenotypes in a Drosophila model of Parkinson’s disease. Dis. Model. Mech. 2011, 4, 701–707. [Google Scholar] [CrossRef] [Green Version]
- Burton, N.C.; Kensler, T.W.; Guilarte, T.R. In vivo modulation of the Parkinsonian phenotype by Nrf2. Neurotoxicology 2006, 27, 1094–1100. [Google Scholar] [CrossRef]
- Rojo, A.I.; Innamorato, N.G.; Martin-Moreno, A.M.; De Ceballos, M.L.; Yamamoto, M.; Cuadrado, A. Nrf2 Regulates Microglial Dynamics and Neuroinflammation in Experimental Parkinson’s Disease. Glia 2010, 58, 588–598. [Google Scholar] [CrossRef]
- Chen, P.C.; Vargas, M.R.; Pani, A.K.; Smeyne, R.J.; Johnson, D.A.; Kan, Y.W.; Johnson, J.A. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: Critical role for the astrocyte. Proc. Natl. Acad. Sci. USA 2009, 106, 2933–2938. [Google Scholar] [CrossRef] [Green Version]
- Innamorato, N.G.; Jazwa, A.; Rojo, A.I.; Cuadrado, A. Deficiency in the Nrf2 transcription factor but not in its target gene heme oxygenase-1 sensitizes to MPTP. Mov. Disord. 2010, 25, S206–S207. [Google Scholar]
- Johnson, D.A.; Johnson, J.A. Nrf2-a therapeutic target for the treatment of neurodegenerative diseases. Free Radic. Biol. Med. 2015, 88, 253–267. [Google Scholar] [CrossRef] [Green Version]
- Jazwa, A.; Rojo, A.I.; Innamorato, N.G.; Hesse, M.; Fernandez-Ruiz, J.; Cuadrado, A. Pharmacological Targeting of the Transcription Factor Nrf2 at the Basal Ganglia Provides Disease Modifying Therapy for Experimental Parkinsonism. Antioxid. Redox Signal. 2011, 14, 2347–2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, C.M.; Ma, D.; Zhao, C.; Franklin, R.J.M.; Zhou, Z.Y.; Ai, N.; Li, C.W.; Yu, H.D.; Hou, T.J.; Sa, F.; et al. Discovery of a novel neuroprotectant, BHDPC, that protects against MPP+/MPTP-induced neuronal death in multiple experimental models. Free Radic. Biol. Med. 2015, 89, 1057–1066. [Google Scholar] [CrossRef] [PubMed]

| Genes | Forward Primer | Reverse Primer |
|---|---|---|
| β-Actin | CACTGAGGCTCCCCTGAATC | GGGTCACACCATCACCAGAG |
| Nrf2 | CTGCTGTCACTCCCAGAGTT | GCCGTAGTTTTGGGTTGGTG |
| HO-1 | AAGAGCTGGACAGAAACGCA | AGAAGTGCTCCAAGTCCTGC |
| NQO1 | AAGCCTCTGTCCTTTGCTCC | TGCTGTGGTAATGCCGTAGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Chen, Y.; Zheng, Y.; Zhao, J.; Yu, H.; Zhu, J. The Relationship between Procyanidin Structure and Their Protective Effect in a Parkinson’s Disease Model. Molecules 2022, 27, 5007. https://doi.org/10.3390/molecules27155007
Chen J, Chen Y, Zheng Y, Zhao J, Yu H, Zhu J. The Relationship between Procyanidin Structure and Their Protective Effect in a Parkinson’s Disease Model. Molecules. 2022; 27(15):5007. https://doi.org/10.3390/molecules27155007
Chicago/Turabian StyleChen, Juan, Yixuan Chen, Yangfan Zheng, Jiawen Zhao, Huilin Yu, and Jiajin Zhu. 2022. "The Relationship between Procyanidin Structure and Their Protective Effect in a Parkinson’s Disease Model" Molecules 27, no. 15: 5007. https://doi.org/10.3390/molecules27155007
APA StyleChen, J., Chen, Y., Zheng, Y., Zhao, J., Yu, H., & Zhu, J. (2022). The Relationship between Procyanidin Structure and Their Protective Effect in a Parkinson’s Disease Model. Molecules, 27(15), 5007. https://doi.org/10.3390/molecules27155007





