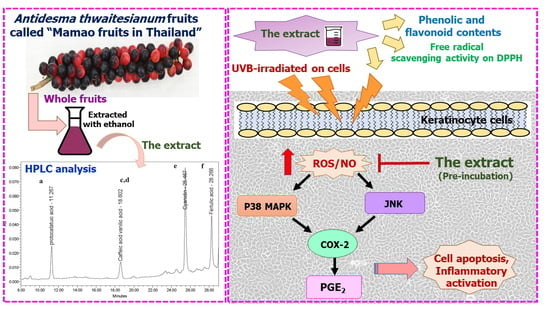
Photo-Protective and Anti-Inflammatory Effects of Antidesma thwaitesianum Müll. Arg. Fruit Extract against UVB-Induced Keratinocyte Cell Damage
Abstract
:1. Introduction
2. Results
2.1. In Vitro Antioxidant and Total Phenolic and Flavonoid Contents
2.2. Non-Toxic Concentration and Photo-Protective Effects of the Extract on UVB-Irradiated Keratinocyte Cells
2.3. The Antioxidant and Anti-Apoptotic Effects of the Extract on UVB-Irradiated Keratinocyte Cells
2.4. The Effects of the Extract on UVB-Induced Cellular p38 and JNK Phosphorylation
2.5. The Effects of the Extract on UVB-Induced COX-2 and PGE2 Production
3. Discussion and Conclusions
4. Materials and Methods
4.1. Plant Preparation and Extraction
4.2. Determination of Phenolic and Flavonoid Contents
4.3. Determination of In Vitro DPPH Scavenging Activity
4.4. High-Performance Liquid Chromatography (HPLC) Analysis of the Extract
4.5. Cell Culture and UVB Treatment
4.6. Cell Viability Determination by Resazurin Assay
4.7. Nitric Oxide (NO) Scavenging Activity by Griess Assay
4.8. Antioxidant Activity by ROS Assay
4.9. Determination of Interested Proteins by Western Blot Analysis
4.10. Determination of PGE2 Level by ELISA Assay
4.11. Determination of Gene Expression by Real-Time PCR
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guttman-Yassky, E.; Zhou, L.; Krueger, J.G. The skin as an immune organ: Tolerance versus effector responses and applications to food allergy and hypersensitivity reactions. J. Allergy Clin. Immunol. 2019, 144, 362–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neagu, M.; Constantin, C.; Caruntu, C.; Dumitru, C.; Surcel, M.; Zurac, S. Inflammation: A key process in skin tumorigenesis. Oncol. Lett. 2019, 17, 4068–4084. [Google Scholar] [CrossRef] [Green Version]
- Chouinard, N.; Valerie, K.; Rouabhia, M.; Huot, J. UVB-mediated activation of p38 mitogen-activated protein kinase enhances resistance of normal human keratinocytes to apoptosis by stabilizing cytoplasmic p53. Biochem. J. 2002, 365, 133–145. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, Y.; Wu, S.; Chen, T.; He, Y.; Sun, J.; Jiao, R.; Jiang, X.; Huang, Y.; Deng, L.; et al. Protective effect of cyanidin-3-O-glucoside against ultraviolet B radiation-induced cell damage in human HaCaT keratinocytes. Front. Pharmacol. 2016, 7, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, M.; Li, D.; Zhang, Y.; Zhou, G.; Liu, W.; Cao, Y.; Zhang, W. Protective effect of crocin on ultraviolet B-induced dermal fibroblast photoaging. Mol. Med. Rep. 2018, 18, 1439–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.H.; Wu, S.B.; Hong, C.H.; Yu, H.S.; Wei, Y.H. Molecular mechanisms of UV-induced apoptosis and its effects on skin residential cells: The implication in UV-based phototherapy. Int. J. Mol. Sci. 2013, 14, 6414–6435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andor, P.; Istvan, N.; Lajos, K. Innate immunity in the skin: How keratinocytes fight against pathogens. Curr. Immunol. Rev. 2005, 1, 29–42. [Google Scholar]
- Bito, T.; Nishigori, C. Impact of reactive oxygen species on keratinocyte signaling pathways. J. Dermatol. Sci. 2012, 68, 3–8. [Google Scholar] [CrossRef]
- Maru, G.B.; Gandhi, K.; Ramchandani, A.; Kumar, G. The role of inflammation in skin cancer. Adv. Exp. Med. Biol. 2014, 816, 437–469. [Google Scholar]
- Tang, L.; Wang, K. Chronic inflammation in skin malignancies. J. Mol. Signal. 2016, 11, 2. [Google Scholar]
- Masaki, H.; Izutsu, Y.; Yahagi, S.; Okano, Y. Reactive oxygen species in HaCaT keratinocytes after UVB iradiation are triggered by intracellular Ca2+ levels. J. Investig. Dermatol. Symp. Proc. 2009, 14, 50–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaisin, Y.; Ratanachamnong, P.; Wongsawatkul, O.; Watthammawut, A.; Malaniyom, K.; Natewong, S. Antioxidant and anti-inflammatory effects of piperine on UV-B-irradiated human HaCaT keratinocyte cells. Life Sci. 2020, 263, 118607. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, V.; Piva, T.J. A comparative study of UV-induced cell signaling pathways in human keratinocyte-derived cell lines. Arch. Dermatol. Res. 2013, 305, 817–833. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.L.; Labasi, J.M.; Zhu, Y.; Tang, X.; McClure, K.; Gabel, C.A.; Athar, M.; Bickers, D.R. Role of p38 MAPK in UVB-induced inflammatory responses in the skin of SKH-1 hairless mice. J. Investig. Dermatol. 2005, 124, 1318–1325. [Google Scholar] [CrossRef] [Green Version]
- Elmets, C.A.; Ledet, J.J.; Athar, M. Cyclooxygenases: Mediators of UV-induced skin cancer and potential targets for prevention. J. Investig. Derm. 2014, 134, 2497–2502. [Google Scholar] [CrossRef] [Green Version]
- Tudor, D.V.; Bâldea, I.; Lupu, M.; Kacso, T.; Kutasi, E.; Hopârtean, A.; Stretea, R.; Gabriela Filip, A. COX-2 as a potential biomarker and therapeutic target in melanoma. Cancer Biol. Med. 2020, 17, 20–31. [Google Scholar] [CrossRef]
- Kirkova, M.; Alexandova, A.; Kesiova, M.; Tsvetanova, E.; Georgieva, A.; Todorov, S. Potential antioxidant activity of celecoxib and amtolmetin guacyl: In vitro studies. Auton. Autacoid Pharmacol. 2007, 27, 13–18. [Google Scholar] [CrossRef]
- Quiñones, O.G.; Pierre, M.B.R. Cutaneous application of celecoxib for inflammatory and cancer diseases. Curr. Cancer Drug Targ. 2019, 19, 5–16. [Google Scholar] [CrossRef]
- Korać, R.R.; Khambholja, K.M. Potential of herbs in skin protection from ultraviolet radiation. Pharmacogn. Rev. 2011, 5, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Butkhup, L.; Samappito, S. An analysis on flavonoids contents in Mao Luang fruits of fifteen cultivars (Antidesma bunius), grown in northeast Thailand. Pak. J. Biol. Sci. 2008, 11, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.R.; Deep, A.; Abdullah, S.T. Herbal products as skincare therapeutic agents against ultraviolet radiation-induced skin disorders. J. Ayurveda Integr. Med. 2022, 13, 100500. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P. Checklist of the genus Antidesma (Euphorbiaceae) in Thailand. Thai For. Bull. 1970, 28, 139–156. [Google Scholar]
- Islam, M.A.; Ahammed, M.S.; Sukorno, F.I.; Koly, S.F.; Biswas, M.M.; Hossain, S. A review on phytochemical and pharmacological potentials of Antidesma bunius. J. Anal. Pharm. Res. 2018, 2018, 602–604. [Google Scholar]
- Puangpronpitag, D.; Yongvanit, P.; Boonsiri, P.; Suttajit, M.; Areejitranusorn, P.; Na, H.-K.; Surh, Y.-J. Molecular mechanism underlying anti-apoptotic and anti-inflammatory effects of Mamao (Antidesma thwaitesianum Müll. Arg.) polyphenolics in human breast epithelial cells. Food Chem. 2011, 127, 1450–1458. [Google Scholar] [CrossRef]
- Van Kiem, P.; Cuong, L.C.V.; Trang, D.T.; Nhiem, N.X.; Anh, H.L.T.; Tai, B.H.; Huong, L.M.; Van Minh, C.; Lee, T.H.; Kim, S.Y.; et al. New alkaloids and anti-inflammatory constituents from the leaves of Antidesma ghaesembilla. Nat. Prod. Commun. 2017, 12, 11–14. [Google Scholar] [CrossRef] [Green Version]
- Kautsar, L.; Sitorus, P.; Dalimunthe, A. Anti-inflammatory activity of ethanol and fraction of Buni Leaves (Antidesma bunius L.) on white rat in carrageenan induced paw inflammation. Asian J. Pharm. Res. Dev. 2019, 7, 1–5. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant properties of phenolic compounds: H-Atom versus electron transfer mechanism. J. Phys. Chem. A 2004, 108, 4916–4922. [Google Scholar] [CrossRef]
- Tripp, C.S.; Blomme, E.A.G.; Chinn, K.S.; Hardy, M.M.; LaCelle, P.; Pentland, A.P. Epidermal COX-2 induction following ultraviolet irradiation: Suggested mechanism for the role of COX-2 inhibition in photoprotection. J. Investig. Dermatol. 2003, 121, 853–861. [Google Scholar] [CrossRef]
- Kabashima, K.; Nagamachi, M.; Honda, T.; Nishigori, C.; Miyachi, Y.; Tokura, Y.; Narumiya, S. Prostaglandin E2 is required for ultraviolet B-induced skin inflammation via EP2 and EP4 receptors. Lab. Investig. 2007, 87, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Rundhaug, J.E.; Mikulec, C.; Pavone, A.; Fischer, S.M. A role for cyclooxygenase-2 in ultraviolet light-induced skin carcinogenesis. Mol. Carcinog. 2007, 46, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Greenhough, A.; Smartt, H.J.M.; Moore, A.E.; Roberts, H.R.; Williams, A.C.; Paraskeva, C.; Kaidi, A. The COX-2/PGE 2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009, 30, 377–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombo, I.; Sangiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Corsetto, P.A.; Scaccabarozzi, D.; et al. HaCaT cells as a reliable in vitro differentiation model to dissect the inflammatory/repair response of human keratinocytes. Mediat. Inflamm. 2017, 2017, 7435621. [Google Scholar] [CrossRef]
- Lee, C.; Park, G.H.; Ahn, E.M.; Park, C.-I.; Jang, J.-H. Sargassum fulvellum protects HaCaT cells and BALB/c mice from UVB-induced proinflammatory responses. Evid. Based Complement. Altern. Med. 2013, 2013, 747846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balupillai, A.; Prasad, R.N.; Ramasamy, K.; Muthusamy, G.; Shanmugham, M.; Govindasamy, K.; Gunaseelan, S. Caffeic acid inhibits UVB-induced inflammation and photocarcinogenesis through activation of peroxisome proliferator-activated receptor-γ in mouse skin. Photochem. Photobiol. 2015, 91, 1458–1468. [Google Scholar] [CrossRef]
- Peres, D.D.; Sarruf, F.D.; de Oliveira, C.A.; Velasco, M.V.R.; Baby, A.R. Ferulic acid photoprotective properties in association with UV filters: Multifunctional sunscreen with improved SPF and UVA-PF. J. Photochem. Photobiol. B 2018, 185, 46–49. [Google Scholar] [CrossRef]
- Li, K.; Zhang, M.; Chen, H.; Peng, J.; Jiang, F.; Shi, X.; Bai, Y.; Jian, M.; Jia, Y. Anthocyanins from black peanut skin protect against UV-B induced keratinocyte cell and skin oxidative damage through activating Nrf 2 signaling. Food Funct. 2019, 10, 6815–6828. [Google Scholar] [CrossRef]
- Daré, R.G.; Oliveira, M.M.; Truiti, M.C.T.; Nakamura, C.V.; Ximenes, V.F.; Lautenschlager, S.O.S. Abilities of protocatechuic acid and its alkyl esters, ethyl and heptyl protocatechuates, to counteract UVB-induced oxidative injuries and photoaging in fibroblasts L929 cell line. J. Photochem. Photobiol. B Biol. 2020, 203, 111771. [Google Scholar] [CrossRef]
- Taqvi, S.; Ahmed Bhat, E.; Sajjad, N.; Sabir, J.S.M.; Qureshi, A.; Rather, I.A.; Rehman, S. Protective effect of vanillic acid in hydrogen peroxide-induced oxidative stress in D.Mel-2 cell line. Saudi J. Biol. Sci. 2021, 28, 1795–1800. [Google Scholar] [CrossRef]
- Goradel, N.H.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cyclooxygenase-2 in cancer: A review. J. Cell Physiol. 2019, 234, 5683–5699. [Google Scholar] [CrossRef]
- Puangpronpitag, D.; Areejitranusorn, P.; Boonsiri, P.; Suttajit, M.; Yongvanit, P. Antioxidant activities of polyphenolic compounds isolated from Antidesma thwaitesianum Müll. Arg. seeds and marcs. J. Food Sci. 2008, 73, C648–C653. [Google Scholar] [CrossRef] [PubMed]
- Orengo, I.F.; Gerguis, J.; Phillips, R.; Guevara, A.; Lewis, A.T.; Black, H.S. Celecoxib, a cyclooxygenase 2 inhibitor as a potential chemopreventive to UV-induced skin cancer: A study in the hairless mouse model. Arch. Dermatol. 2002, 138, 751–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wulff, B.C.; Thomas-Ahner, J.M.; Schick, J.S.; Oberyszyn, T.M. Celecoxib reduces the effects of acute and chronic UVB exposure in mice treated with therapeutically relevant immunosuppressive drugs. Int. J. Cancer Res. 2010, 126, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaisin, Y.; Ratanachamnong, P.; Prachayasittikul, S.; Watanapokasin, R.Y.; Kuanpradit, C. Protective effects of ethyl acetate extract of Eclipta prostrata against 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells. Sci. As. 2016, 42, 259. [Google Scholar] [CrossRef] [Green Version]
- Namchaiw, P.; Jaisin, Y.; Niwaspragrit, C.; Malaniyom, K.; Auvuchanon, A.; Ratanachamnong, P. The leaf extract of Coccinia grandis (L.) Voigt accelerated in vitro wound healing by reducing oxidative stress injury. Oxid. Med. Cell. Longev. 2021, 2021, 3963510. [Google Scholar] [CrossRef]
- Jaisin, Y.; Ratanachamnong, P.; Kuanpradit, C.; Khumpum, W.; Suksamrarn, S. Protective effects of γ-mangostin on 6-OHDA-induced toxicity in SH-SY5Y cells. Neurosci. Lett. 2018, 665, 229–235. [Google Scholar] [CrossRef]
- Hutabarat, R.P.; Xiao, Y.D.; Wu, H.; Wang, J.; Li, D.J.; Huang, W.Y. Identification of anthocyanins and optimization of their extraction from rabbiteye blueberry fruits in Nanjing. J. Food Qual. 2019, 2019, 6806790. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Lima, J.L.F.C. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef]








| Compounds | A. thwaitesianum Fruit Extract (μg/mL) |
|---|---|
| Ferulic acid | 798.13 |
| Caffeic acid plus Vanillic acid | 510.79 |
| Protocatechuic | 487.76 |
| Cyanidin | 52.70 |
| A. thwaitesianum Fruit Extract | Phenolic Content (mg Gallic Acid Equivalent/g Crude Extract) | Flavonoid Content (mg Quercetin Equivalent/g Crude Extract) | IC50 of DPPH (mg/mL) |
|---|---|---|---|
| EtOH extract | 29.115 ± 0.528 | 1.237 ± 0.104 | 10.94 |
| Trolox | - | - | 0.17 |
| Genes | Forward Primer | Reverse Primer |
|---|---|---|
| COX-2 | TGAGCATCTACGGTTTGCTG | TGCTTGTCTGGAACAACTGC |
| GAPDH | TGAGCATCTACGGTTTGCTG | TGCTTGTCTGGAACAACTGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natewong, S.; Niwaspragrit, C.; Ratanachamnong, P.; Samatiwat, P.; Namchaiw, P.; Jaisin, Y. Photo-Protective and Anti-Inflammatory Effects of Antidesma thwaitesianum Müll. Arg. Fruit Extract against UVB-Induced Keratinocyte Cell Damage. Molecules 2022, 27, 5034. https://doi.org/10.3390/molecules27155034
Natewong S, Niwaspragrit C, Ratanachamnong P, Samatiwat P, Namchaiw P, Jaisin Y. Photo-Protective and Anti-Inflammatory Effects of Antidesma thwaitesianum Müll. Arg. Fruit Extract against UVB-Induced Keratinocyte Cell Damage. Molecules. 2022; 27(15):5034. https://doi.org/10.3390/molecules27155034
Chicago/Turabian StyleNatewong, Sutthibhon, Cholticha Niwaspragrit, Piyanee Ratanachamnong, Papavee Samatiwat, Poommaree Namchaiw, and Yamaratee Jaisin. 2022. "Photo-Protective and Anti-Inflammatory Effects of Antidesma thwaitesianum Müll. Arg. Fruit Extract against UVB-Induced Keratinocyte Cell Damage" Molecules 27, no. 15: 5034. https://doi.org/10.3390/molecules27155034
APA StyleNatewong, S., Niwaspragrit, C., Ratanachamnong, P., Samatiwat, P., Namchaiw, P., & Jaisin, Y. (2022). Photo-Protective and Anti-Inflammatory Effects of Antidesma thwaitesianum Müll. Arg. Fruit Extract against UVB-Induced Keratinocyte Cell Damage. Molecules, 27(15), 5034. https://doi.org/10.3390/molecules27155034