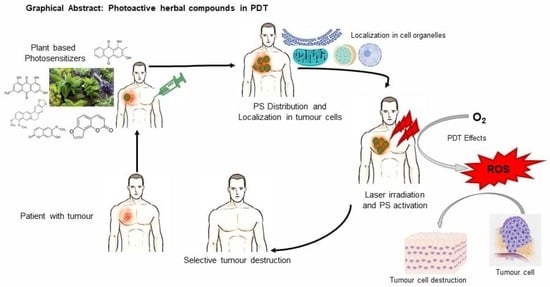
Photoactive Herbal Compounds: A Green Approach to Photodynamic Therapy
Abstract
:1. Introduction
2. Photosensitizers
3. Mechanism of Photodynamic Therapy and Photochemical Reactions
4. Natural Product Derived Photosensitizers
5. Medicinal Plants with Photosensitizing Compounds
5.1. Aloe vera (L.) Burm.f.
5.2. Berberis aristata DC.
5.3. Curcuma longa L.
5.4. Ficus religiosa L.
5.5. Ipomoea mauritiana Jacq.
5.6. Rubia cordifolia L.
6. Phytochemicals as Natural Photosensitizers
7. Conclusions and Scope for Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sulaiman, C.T.; Anju, K.; Anandan, E.M.; Balachandran, I. Synergistic Interactions of Phytochemicals in Polyherbal Formulation Enhance the Chemical Transformations of Active Constituents. J. Appl. Spectrosc. 2021, 88, 181–186. [Google Scholar] [CrossRef]
- Tripathi, Y.B. Molecular Approach to Ayurveda. Indian J. Exp. Biol. 2000, 38, 409–414. [Google Scholar] [PubMed]
- Sheth, P. Global Opportunities and Challenges for Medicinal Uses of Ayurveda, Herbal Products, Neutraceuticals and Alternatives. Health Adm. 2005, 1, 74–75. [Google Scholar]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy—Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Chota, A.; George, B.P.; Abrahamse, H. Interactions of Multidomain Pro-Apoptotic and Anti-Apoptotic Proteins in Cancer Cell Death. Oncotarget 2021, 12, 1615–1626. [Google Scholar] [CrossRef]
- Senapathy, G.J.; George, B.P.; Abrahamse, H. Exploring the Role of Phytochemicals as Potent Natural Photosensitizers in Photodynamic Therapy. Anticancer Agents Med. Chem. 2020, 20, 1831–1844. [Google Scholar] [CrossRef]
- Muniyandi, K.; George, B.; Parimelazhagan, T.; Abrahamse, H. Role of Photoactive Phytocompounds in Photodynamic Therapy of Cancer. Molecules 2020, 25, 4102. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Doustvandi, M.A.; Mohammadnejad, F.; Kamari, F.; Gjerstorff, M.F.; Baradaran, B.; Hamblin, M.R. Photodynamic Therapy for Cancer: Role of Natural Products. Photodiagn. Photodyn. Ther. 2019, 26, 395–404. [Google Scholar] [CrossRef]
- Foresto, E.; Gilardi, P.; Ibarra, L.E.; Cogno, I.S. Light-Activated Green Drugs: How We Can Use Them in Photodynamic Therapy and Mass-Produce Them with Biotechnological Tools. Phytomed. Plus 2021, 1, 100044. [Google Scholar] [CrossRef]
- Allison, R.R.; Sibata, C.H. Oncologic Photodynamic Therapy Photosensitizers: A Clinical Review. Photodiagn. Photodyn. Ther. 2010, 7, 61–75. [Google Scholar] [CrossRef]
- Chen, B.; Roskams, T.; de Witte, P.A.M. Antivascular Tumor Eradication by Hypericin-Mediated Photodynamic Therapy. Photochem. Photobiol. 2002, 76, 509–513. [Google Scholar] [CrossRef]
- Garg, A.D.; Nowis, D.; Golab, J.; Vandenabeele, P.; Krysko, D.V.; Agostinis, P. Immunogenic Cell Death, DAMPs and Anticancer Therapeutics: An Emerging Amalgamation. Biochim. Biophys. Acta 2010, 1805, 53–71. [Google Scholar] [CrossRef]
- Chota, A.; George, B.P.; Abrahamse, H. Reactive Oxygen Species Induced Cancer Cell Death—A Therapeutic Approach. In Handbook of Oxidative Stress in Cancer: Therapeutic Aspects; Chakraborti, S., Ed.; Springer: Singapore, 2021; pp. 1–17. ISBN 9789811612473. [Google Scholar]
- Abdel-Kader, M.H. Photodynamic Therapy From Theory to Application. In History of Photodynamic Therapy; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-39628-1. [Google Scholar]
- Luksiene, Z. Photodynamic Therapy: Mechanism of Action and Ways to Improve the Efficiency of Treatment. Med. Kaunas Lith. 2003, 39, 1137–1150. [Google Scholar]
- dos Santos, A.F.; de Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic Therapy in Cancer Treatment—An Update Review. J. Cancer Metastasis Treat. 2019, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Jiang, C.; Figueiró Longo, J.P.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An Updated Overview on the Development of New Photosensitizers for Anticancer Photodynamic Therapy. Acta Pharm. Sin. B 2018, 8, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Menichini, G.; Provenzano, E.; Conforti, F. Applications of Natural Compounds in the Photodynamic Therapy of Skin Cancer. Curr. Med. Chem. 2014, 21, 1371–1390. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products As Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The Re-Emergence of Natural Products for Drug Discovery in the Genomics Era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [Green Version]
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The History of Photodetection and Photodynamic Therapy. Photochem. Photobiol. 2001, 74, 656–669. [Google Scholar] [CrossRef]
- Sternberg, E.D.; Dolphin, D.; Brückner, C. Porphyrin-Based Photosensitizers for Use in Photodynamic Therapy. Tetrahedron 1998, 54, 4151–4202. [Google Scholar] [CrossRef]
- Sil, S.; Bose, T.; Roy, D.; Chakraborti, A.S. Protoporphyrin IX-Induced Structural and Functional Changes in Human Red Blood Cells, Haemoglobin and Myoglobin. J. Biosci. 2004, 29, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Manivasager, V.; Yee, K.K.L.; Heng, P.W.S.; Soo, K.C.; Olivo, M. A Study Comparing Endogenous Protoporphyrin IX Induced by 5-ALA and ALA-Methyl Ester with Exogenous PpIX and PpIX Dimethyl Ester in Photodynamic Diagnosis of Human Nasopharyngeal Carcinoma Xenografts. Int. J. Oncol. 2006, 29, 997–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.; Liang, H.; Li, Q.; Peng, C.; Li, Z.; Shi, S.; Yang, L.; Tian, Z.; Tian, Y.; Zhang, Z.; et al. Hematoporphyrin Monomethyl Ether-Mediated Photodynamic Effects on THP-1 Cell-Derived Macrophages. J. Photochem. Photobiol. B 2010, 101, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Brandis, A.S.; Salomon, Y.; Scherz, A. Chlorophyll Sensitizers in Photodynamic Therapy. In Chlorophylls and Bacteriochlorophylls: Biochemistry, Biophysics, Functions and Applications; Grimm, B., Porra, R.J., Rüdiger, W., Scheer, H., Eds.; Advances in Photosynthesis and Respiration; Springer Netherlands: Dordrecht, The Netherlands, 2006; pp. 461–483. ISBN 978-1-4020-4516-5. [Google Scholar]
- Xodo, L.E.; Rapozzi, V.; Zacchigna, M.; Drioli, S.; Zorzet, S. The Chlorophyll Catabolite Pheophorbide a as a Photosensitizer for the Photodynamic Therapy. Curr. Med. Chem. 2012, 19, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-E.; Oh, S.-H.; Kim, S.-A.; Yoon, J.-H.; Ahn, S.-G. Pheophorbide A-Mediated Photodynamic Therapy Induces Autophagy and Apoptosis via the Activation of MAPKs in Human Skin Cancer Cells. Oncol. Rep. 2014, 31, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Busch, T.; Cengel, K.A.; Finlay, J. Pheophorbide a as a Photosensitizer in Photodynamic Therapy: In Vivo Considerations. Cancer Biol. Ther. 2009, 8, 540–542. [Google Scholar] [CrossRef]
- Bellnier, D.A.; Greco, W.R.; Nava, H.; Loewen, G.M.; Oseroff, A.R.; Dougherty, T.J. Mild Skin Photosensitivity in Cancer Patients Following Injection of Photochlor (2-[1-Hexyloxyethyl]-2-Devinyl Pyropheophorbide-a; HPPH) for Photodynamic Therapy. Cancer Chemother. Pharmacol. 2006, 57, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Mody, T.D. Pharmaceutical Development and Medical Applications of Porphyrin-Type Macrocycles. J. Porphyr. Phthalocyanines 2000, 4, 362–367. [Google Scholar] [CrossRef]
- Heelis, P.F. The Photophysical and Photochemical Properties of Flavins (Isoalloxazines). Chem. Soc. Rev. 1982, 11, 15–39. [Google Scholar] [CrossRef]
- Ormond, A.B.; Freeman, H.S. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef] [Green Version]
- Mysliwa-Kurdziel, B.; Solymosi, K. Phycobilins and Phycobiliproteins Used in Food Industry and Medicine. Mini Rev. Med. Chem. 2017, 17, 1173–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.P.; Zhao, J.Q.; Jiang, L.J. Photosensitized Formation of Singlet Oxygen by Phycobiliproteins in Neutral Aqueous Solutions. Free Radic. Res. 2000, 33, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Munier, M.; Jubeau, S.; Wijaya, A.; Morançais, M.; Dumay, J.; Marchal, L.; Jaouen, P.; Fleurence, J. Physicochemical Factors Affecting the Stability of Two Pigments: R-Phycoerythrin of Grateloupia Turuturu and B-Phycoerythrin of Porphyridium Cruentum. Food Chem. 2014, 150, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Bublik, M.; Head, C.; Benharash, P.; Paiva, M.; Eshraghi, A.; Kim, T.; Saxton, R. Hypericin and Pulsed Laser Therapy of Squamous Cell Cancer in Vitro. Photomed. Laser Surg. 2006, 24, 341–347. [Google Scholar] [CrossRef]
- D’Hallewin, M.A.; De Witte, P.A.; Waelkens, E.; Merlevede, W.; Baert, L. Fluorescence Detection of Flat Bladder Carcinoma in Situ after Intravesical Instillation of Hypericin. J. Urol. 2000, 164, 349–351. [Google Scholar] [CrossRef]
- Kubin, A.; Meissner, P.; Wierrani, F.; Burner, U.; Bodenteich, A.; Pytel, A.; Schmeller, N. Fluorescence Diagnosis of Bladder Cancer with New Water Soluble Hypericin Bound to Polyvinylpyrrolidone: PVP-Hypericin. Photochem. Photobiol. 2008, 84, 1560–1563. [Google Scholar] [CrossRef]
- Bhuvaneswari, R.; Gan, Y.Y.-Y.; Yee, K.K.L.; Soo, K.C.; Olivo, M. Effect of Hypericin-Mediated Photodynamic Therapy on the Expression of Vascular Endothelial Growth Factor in Human Nasopharyngeal Carcinoma. Int. J. Mol. Med. 2007, 20, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Olivo, M.; Du, H.-Y.; Bay, B.-H. Hypericin Lights Up the Way for the Potential Treatment of Nasopharyngeal Cancer by Photodynamic Therapy. Curr. Clin. Pharmacol. 2006, 1, 217–222. [Google Scholar] [CrossRef]
- Kaihong, Z.; Lijin, J. Conversion of Hypocrellin A in Alkaline and Neutral Media. Chin. J. Org. Chem. 1989, 9, 252. [Google Scholar]
- Estey, E.P.; Brown, K.; Diwu, Z.; Liu, J.; Lown, J.W.; Miller, G.G.; Moore, R.B.; Tulip, J.; McPhee, M.S. Hypocrellins as Photosensitizers for Photodynamic Therapy: A Screening Evaluation and Pharmacokinetic Study. Cancer Chemother. Pharmacol. 1996, 37, 343–350. [Google Scholar] [CrossRef]
- Zhenjun, D.; Lown, J.W. Hypocrellins and their use in photosensitization. Photochem. Photobiol. 1990, 52, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Wakdikar, S. Global Health Care Challenge: Indian Experiences and New Prescriptions. Electron. J. Biotechnol. 2004, 7, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Bhutani, K.K.; Gohil, V.M. Natural Products Drug Discovery Research in India: Status and Appraisal. Indian J. Exp. Biol. 2010, 48, 199–207. [Google Scholar] [PubMed]
- Reynolds, T.; Dweck, A.C. Aloe Vera Leaf Gel: A Review Update. J. Ethnopharmacol. 1999, 68, 3–37. [Google Scholar] [CrossRef]
- ESHUN, K.; HE, Q. Aloe Vera: A Valuable Ingredient for the Food, Pharmaceutical and Cosmetic Industries—A Review. Crit. Rev. Food Sci. Nutr. 2004, 44, 91–96. [Google Scholar] [CrossRef]
- Medhin, L.B.; Sibhatu, D.B.; Seid, M.; Ferej, F.M.; Mohamedkassm, N.; Berhane, Y.; Kaushek, A.; Humida, M.E.; Gasmalbari, E. Comparative Antimicrobial Activities of the Gel, Leaf and Anthraquinone Fractionates of Four Aloe Species (Aloe Camperi, Aloe Elegans, Aloe Eumassawana and Aloe Scholleri). Adv. Microbiol. 2019, 9, 139–150. [Google Scholar] [CrossRef] [Green Version]
- Cogno, I.S.; Gilardi, P.; Comini, L.; Núñez-Montoya, S.C.; Cabrera, J.L.; Rivarola, V.A. Natural Photosensitizers in Photodynamic Therapy: In Vitro Activity against Monolayers and Spheroids of Human Colorectal Adenocarcinoma SW480 Cells. Photodiagn. Photodyn. Ther. 2020, 31, 101852. [Google Scholar] [CrossRef]
- Komal, S.; Ranjan, B.; Neelam, C.; Birendra, S.; Saini, N. Berberis Aristata: A Review. Int. J. Res. Ayurveda Pharm. 2011, 2, 383–388. [Google Scholar]
- Rathi, B.; Sahu, J.; Koul, S.; Kosha, R.L. Detailed Pharmacognostical Studies on Berberis Aristata DC Plant. Anc. Sci. Life 2013, 32, 234. [Google Scholar] [CrossRef]
- Luiza Andreazza, N.; Vevert-Bizet, C.; Bourg-Heckly, G.; Sureau, F.; José Salvador, M.; Bonneau, S. Berberine as a Photosensitizing Agent for Antitumoral Photodynamic Therapy: Insights into Its Association to Low Density Lipoproteins. Int. J. Pharm. 2016, 510, 240–249. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.-Q.; An, Y.-W.; Hu, A.-Z.; Li, M.-H.; Cui, G.-H. Photodynamic Therapy Enhanced the Antitumor Effects of Berberine on HeLa Cells. Open Chem. 2019, 17, 413–421. [Google Scholar] [CrossRef]
- Oliveira, P.M.; Lopes, T.Z.; Tedesco, A.C.; Rahal, P.; Calmon, M.F. Effect of Berberine Associated with Photodynamic Therapy in Cell Lines. Photodiagn. Photodyn. Ther. 2020, 32, 102045. [Google Scholar] [CrossRef] [PubMed]
- Rathaur, P.; Raja, W.; Ramteke, P.W.; Suchit, A.J. Turmeric: The Golden Spice of life. Int. J. Pharm. Sci. Res. 2012, 3, 1987–1994. [Google Scholar]
- Tung, B.T.; Nham, D.T.; Hai, N.T.; Thu, D.K. Chapter 10—Curcuma Longa, the Polyphenolic Curcumin Compound and Pharmacological Effects on Liver. In Dietary Interventions in Liver Disease; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 125–134. ISBN 978-0-12-814466-4. [Google Scholar]
- Kazantzis, K.T.; Koutsonikoli, K.; Mavroidi, B.; Zachariadis, M.; Alexiou, P.; Pelecanou, M.; Politopoulos, K.; Alexandratou, E.; Sagnou, M. Curcumin Derivatives as Photosensitizers in Photodynamic Therapy: Photophysical Properties and in Vitro Studies with Prostate Cancer Cells. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2020, 19, 193–206. [Google Scholar] [CrossRef]
- Dahl, T.A.; McGowan, W.M.; Shand, M.A.; Srinivasan, V.S. Photokilling of Bacteria by the Natural Dye Curcumin. Arch. Microbiol. 1989, 151, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Haukvik, T.; Bruzell, E.; Kristensen, S.; Tønnesen, H.H. Photokilling of Bacteria by Curcumin in Selected Polyethylene Glycol 400 (PEG 400) Preparations. Studies on Curcumin and Curcuminoids, XLI. J. Pharm. Sci. 2010, 65, 600–606. [Google Scholar]
- Singh, D.; Singh, B.; Goel, R.K. Traditional Uses, Phytochemistry and Pharmacology of Ficus Religiosa: A Review. J. Ethnopharmacol. 2011, 134, 565–583. [Google Scholar] [CrossRef]
- Pullaiah, T. Medicinal Plants in India; Regency Publication: New Delhi, India, 2006; Volume II, pp. 958–959. [Google Scholar]
- SWAMI, K.D.; BISHT, N.P.S. Constituents of Ficus Religiosa and Ficus Infectoria and Their Biological Activity. J. Indian Chem. Soc. 1996, 73, 631. [Google Scholar] [CrossRef]
- Parrish, J.; Stern, R.; Pathak, M.; Fitzpatrick, T. Science of Photomedicine. In Proceedings of the Science of Photomedicine; Plenum Press: New York, NY, USA, 1982; pp. 595–624. [Google Scholar]
- Gasparro, F. Extracorporeal Photochemotherapy: Clinical Aspects and the Molecular Basis for Efficacy. In Clinical Aspects and the Molecular Basis for Efficacy; Landes Press: Georgetown, TX, USA, 1994. [Google Scholar]
- Sulaiman, C.T.; Deepak, M.; Sunil, A.R.; Lijini, K.R.; Balachandran, I. Characterization of Coumarins from Ipomoea Mauritiana Jacq by LC-APCI-MS/MS Analysis and Evaluation of Its Anti-Amnesic Activity. Beni-Suef Univ. J. Basic Appl. Sci. 2019, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Sulaiman, C.; Geetha, S.P.; Indira, B. Identification of Phenolic Antioxidants in Ipomoea Mauritiana Jacq. Using Spectrophotometric and Mass Spectroscopic Studies. Avicenna J. Phytomed. 2014, 4, 89–96. [Google Scholar]
- Zou, Q.; Fang, Y.; Zhao, Y.; Zhao, H.; Wang, Y.; Gu, Y.; Wu, F. Synthesis and in Vitro Photocytotoxicity of Coumarin Derivatives for One- and Two-Photon Excited Photodynamic Therapy. J. Med. Chem. 2013, 56, 5288–5294. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P. Dravyaguna Vijnana; Chaukhambha Bharati Academy: Varanasi, India, 1969; Volume 928, pp. 2–3. [Google Scholar]
- Sivarajan, V.V.; Balachandran, I. Ayurvedic Drugs and Their Plant Sources; Oxford & IBH Pub. Co.: New Delhi, India, 1994. [Google Scholar]
- Rao, G.M.M.; Rao, C.V.; Pushpangadan, P.; Shirwaikar, A. Hepatoprotective Effects of Rubiadin, a Major Constituent of Rubia Cordifolia Linn. J. Ethnopharmacol. 2006, 103, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, B.S.; Prabhakara, S.; Mohan, T.; Shabeer, D.; Bhandare, B.; Nalini, M.; Sharmila, P.S.; Meghana, D.L.; Reddy, B.K.; Hanumantha Rao, H.M.; et al. Characterization of Rubia Cordifolia L. Root Extract and Its Evaluation of Cardioprotective Effect in Wistar Rat Model. Indian J. Pharmacol. 2018, 50, 12–21. [Google Scholar] [CrossRef]
- Hüll, K.; Morstein, J.; Trauner, D. In Vivo Photopharmacology. Chem. Rev. 2018, 118, 10710–10747. [Google Scholar] [CrossRef] [PubMed]
- Morten, A.G.; Martinez, L.J.; Holt, N.; Sik, R.H.; Reszka, K.; Chignell, C.F.; Tonnesen, H.H.; Roberts, J.E. Photophysical Studies on Antimalariai Drugs. Photochem. Photobiol. 1999, 69, 282–287. [Google Scholar] [CrossRef]
- Rodrigues, D.; Viotto, A.C.; Checchia, R.; Gomide, A.; Severino, D.; Itri, R.; Baptista, M.S.; Martins, W.K. Mechanism of Aloe Vera Extract Protection against UVA: Shelter of Lysosomal Membrane Avoids Photodamage. Photochem. Photobiol. Sci. 2016, 15, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Siewert, B.; Stuppner, H. The Photoactivity of Natural Products—An Overlooked Potential of Phytomedicines? Phytomedicine 2019, 60, 152985. [Google Scholar] [CrossRef] [PubMed]
- Xin, D.; Li, H.; Zhou, S.; Zhong, H.; Pu, W. Effects of Anthraquinones on Immune Responses and Inflammatory Diseases. Molecules 2022, 27, 3831. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Zheng, Z.; Chen, S.; Fang, L.; Feng, R.; Zhang, L.; Tang, Q.; Liu, X. Sensitization of Non-Small Cell Lung Cancer Cells to Gefitinib and Reversal of Epithelial–Mesenchymal Transition by Aloe-Emodin Via PI3K/Akt/TWIS1 Signal Blockage. Front. Oncol. 2022, 12, 908031. [Google Scholar] [CrossRef]
- Li, T.; Shi, L.; Liu, W.; Hu, X.; Hui, Y.; Di, M.; Xue, S.; Zheng, Y.; Yao, M.; Li, C.; et al. Aloe-Emodin Induces Mitochondrial Dysfunction and Pyroptosis by Activation of the Caspase-9/3/Gasdermin E Axis in HeLa Cells. Front. Pharmacol. 2022, 13, 854526. [Google Scholar] [CrossRef]
- Lv, B.; Zheng, K.; Sun, Y.; Wu, L.; Qiao, L.; Wu, Z.; Zhao, Y.; Zheng, Z. Network Pharmacology Experiments Show That Emodin Can Exert a Protective Effect on MCAO Rats by Regulating Hif-1α/VEGF-A Signaling. ACS Omega 2022, 7, 22577–22593. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Perlak, M.; Bromke, M.A.; Ziółkowski, P.; Woźniak, M. The Comparison of the Efficiency of Emodin and Aloe-Emodin in Photodynamic Therapy. Int. J. Mol. Sci. 2022, 23, 6276. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, J.; Kiss, A.K.; Rollinger, J.M.; Atanasov, A.G. Ethnopharmacology in Central and Eastern Europe in the Context of Global Research Developments; Frontiers Media SA: Lausanne, Switzerland, 2019; ISBN 978-2-88945-890-5. [Google Scholar]
- Chang, W.; Li, K.; Guan, F.; Yao, F.; Yu, Y.; Zhang, M.; Hatch, G.M.; Chen, L. Berberine Pretreatment Confers Cardioprotection Against Ischemia-Reperfusion Injury in a Rat Model of Type 2 Diabetes. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Liao, Q.; Li, S.; Bi, K.; Pan, B.; Xie, Z. Simultaneous Determination of Berberine, Palmatine and Jatrorrhizine by Liquid Chromatography-Tandem Mass Spectrometry in Rat Plasma and Its Application in a Pharmacokinetic Study after Oral Administration of Coptis-Evodia Herb Couple. J. Chromatogr. B Anal. Technol. Biomed. Life. Sci. 2008, 863, 195–205. [Google Scholar] [CrossRef]
- Aftab, N.; Vieira, A. Antioxidant Activities of Curcumin and Combinations of This Curcuminoid with Other Phytochemicals. Phytother. Res. PTR 2010, 24, 500–502. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2012, 15, 195–218. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, B.B.; Shishodia, S.; Takada, Y.; Banerjee, S.; Newman, R.A.; Bueso-Ramos, C.E.; Price, J.E. Curcumin Suppresses the Paclitaxel-Induced Nuclear Factor-KappaB Pathway in Breast Cancer Cells and Inhibits Lung Metastasis of Human Breast Cancer in Nude Mice. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 7490–7498. [Google Scholar] [CrossRef] [Green Version]
- Bahadori, F.; Demiray, M. A Realistic View on “The Essential Medicinal Chemistry of Curcumin”. ACS Med. Chem. Lett. 2017, 8, 893–896. [Google Scholar] [CrossRef] [Green Version]
- Chandran, B.; Goel, A. A Randomized, Pilot Study to Assess the Efficacy and Safety of Curcumin in Patients with Active Rheumatoid Arthritis. Phytother. Res. PTR 2012, 26, 1719–1725. [Google Scholar] [CrossRef]
- Sumorek-Wiadro, J.; Zając, A.; Maciejczyk, A.; Jakubowicz-Gil, J. Furanocoumarins in Anticancer Therapy—For and Against. Fitoterapia 2020, 142, 104492. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, H.; Aschner, M.; Mirzae, H.; Küpeli Akkol, E.; Capasso, R. Anticancer Potential of Furanocoumarins: Mechanistic and Therapeutic Aspects. Int. J. Mol. Sci. 2020, 21, 5622. [Google Scholar] [CrossRef] [PubMed]
- “Novel Furocoumarins as Potential HIV-1 Integrase Inhibitors” on Publons. Available online: https://publons.com/wos-op/publon/7492544/ (accessed on 20 July 2022).
- Review Scopoletin—A Coumarin Phytoalexin with Medicinal Properties. Available online: https://pdfslide.net/documents/review-scopoletin-a-coumarin-phytoalexin-with-medicinal-properties.html (accessed on 20 July 2022).
- Kashyap, P.; Ram, H.; Shukla, S.D.; Kumar, S. Scopoletin: Antiamyloidogenic, Anticholinesterase, and Neuroprotective Potential of a Natural Compound Present in Argyreia Speciosa Roots by In Vitro and In Silico Study. Neurosci. Insights 2020, 15, 2633105520937693. [Google Scholar] [CrossRef] [PubMed]
- Lodia, S.; Kansala, L. Antioxidant Activity of Rubia Cordifolia Against Lead Toxicity. Int. J. Pharm. Sci. Res. 2012, 3, 2224. [Google Scholar]
- Peng, Z.; Fang, G.; Peng, F.; Pan, Z.; Su, Z.; Tian, W.; Li, D.; Hou, H. Effects of Rubiadin Isolated from Prismatomeris Connata on Anti-Hepatitis B Virus Activity in Vitro. Phytother. Res. 2017, 31, 1962–1970. [Google Scholar] [CrossRef]
- Patil, R.; Mohan, M.; Kasture, V.; Kasture, S. Antiepileptic Activity Of Rubiadin Isolated from The Roots of Rubia Cordifolia in Mice. Int. J. Pharm. Sci. Res. 2019, 9, 1–13. [Google Scholar]
- Rumie Vittar, N.B.; Comini, L.; Fernadez, I.M.; Agostini, E.; Nuñez-Montoya, S.; Cabrera, J.L.; Rivarola, V.A. Photochemotherapy Using Natural Anthraquinones: Rubiadin and Soranjidiol Sensitize Human Cancer Cell to Die by Apoptosis. Photodiagn. Photodyn. Ther. 2014, 11, 182–192. [Google Scholar] [CrossRef] [Green Version]



| Phytochemicals | Source Plant | Absorption Wavelength (nm) | Medicinal Properties | References |
|---|---|---|---|---|
| Aloe emodin | Aloe vera | 370 to 500 | anticancer, antivirus, anti-inflammatory, antibacterial, antiparasitic, neuroprotective, and hepatoprotective | [77,78,79,80] |
| Emodin | Aloe vera | 370 to 500 | anticancer, anti-inflammatory, antioxidant, antibacterial, antivirus, anti-diabetes | [78,81,82] |
| Berberine | Berberis aristata | 250 to 350 | immunomodulatory, antioxidative, cardioprotective, hepatoprotective, and renoprotective | [83,84,85] |
| Curcumin | Curcuma longa | 350 to 450 | antioxidant, anti-inflammatory, neuroprotective, anticancer, hepatoprotective, and cardioprotective | [86,87,88,89,90] |
| Furanocoumarin | Ficus religiosa | 320 to 380 | antioxidants, antibacterial, analgesic, anticonvulsive, anticoagulant, hypotensive, antidepressants, antifungal, antiviral, anti-inflammatory, antiallergic | [91,92,93] |
| Scopoletin | Ipomoea mauritiana | 250 to 350 | antibacterial, antifungal, anti-inflammatory | [94,95] |
| Rubiadin | Rubia cordifolia | 350 to 450 | anticancer, antiosteoporotic, hepatoprotective, antiepileptic, neuroprotective, anti-inflammatory, antidiabetic, antioxidant, antibacterial, antimalarial, antifungal, and antiviral | [96,97,98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulaiman, C.; George, B.P.; Balachandran, I.; Abrahamse, H. Photoactive Herbal Compounds: A Green Approach to Photodynamic Therapy. Molecules 2022, 27, 5084. https://doi.org/10.3390/molecules27165084
Sulaiman C, George BP, Balachandran I, Abrahamse H. Photoactive Herbal Compounds: A Green Approach to Photodynamic Therapy. Molecules. 2022; 27(16):5084. https://doi.org/10.3390/molecules27165084
Chicago/Turabian StyleSulaiman, Cheruthazhakkat, Blassan P. George, Indira Balachandran, and Heidi Abrahamse. 2022. "Photoactive Herbal Compounds: A Green Approach to Photodynamic Therapy" Molecules 27, no. 16: 5084. https://doi.org/10.3390/molecules27165084
APA StyleSulaiman, C., George, B. P., Balachandran, I., & Abrahamse, H. (2022). Photoactive Herbal Compounds: A Green Approach to Photodynamic Therapy. Molecules, 27(16), 5084. https://doi.org/10.3390/molecules27165084