Reactive Adsorption Performance and Behavior of Gaseous Cumene on MCM-41 Supported Sulfuric Acid
Abstract
:1. Introduction
2. Results
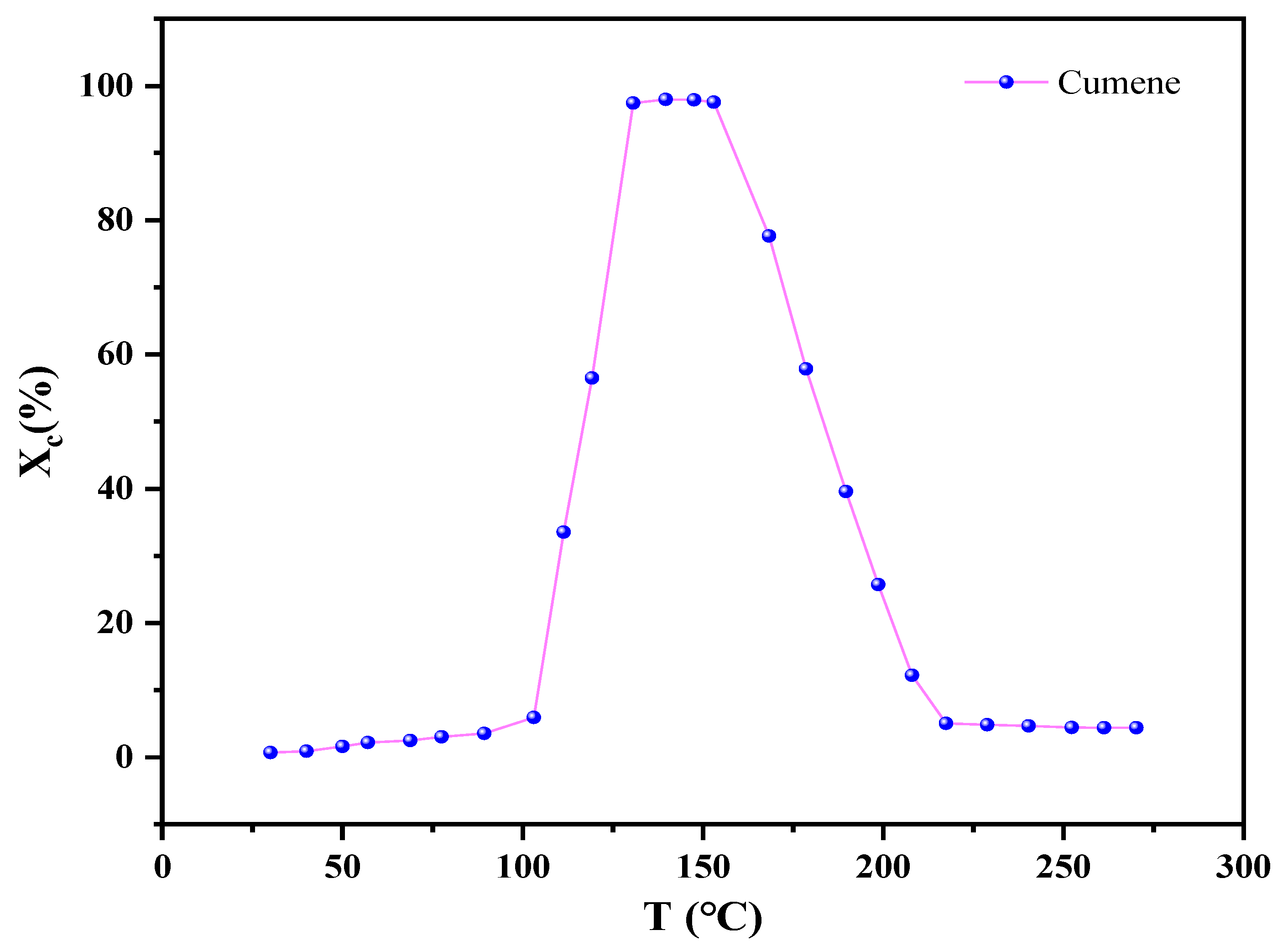
2.1. Effects of Temperature on Cumene Reaction-Type Adsorption
2.2. Comprehensive Analysis of Effects of Inlet Concentration, Bed Height, and Flow Rate
- (1)
- All breakthrough profiles presented typical S-like curves, indicating the presence of a strong interaction between the cumene molecules and the SSA/MCM-41 surface, attributable to their sulfonation reaction (see the next section).
- (2)
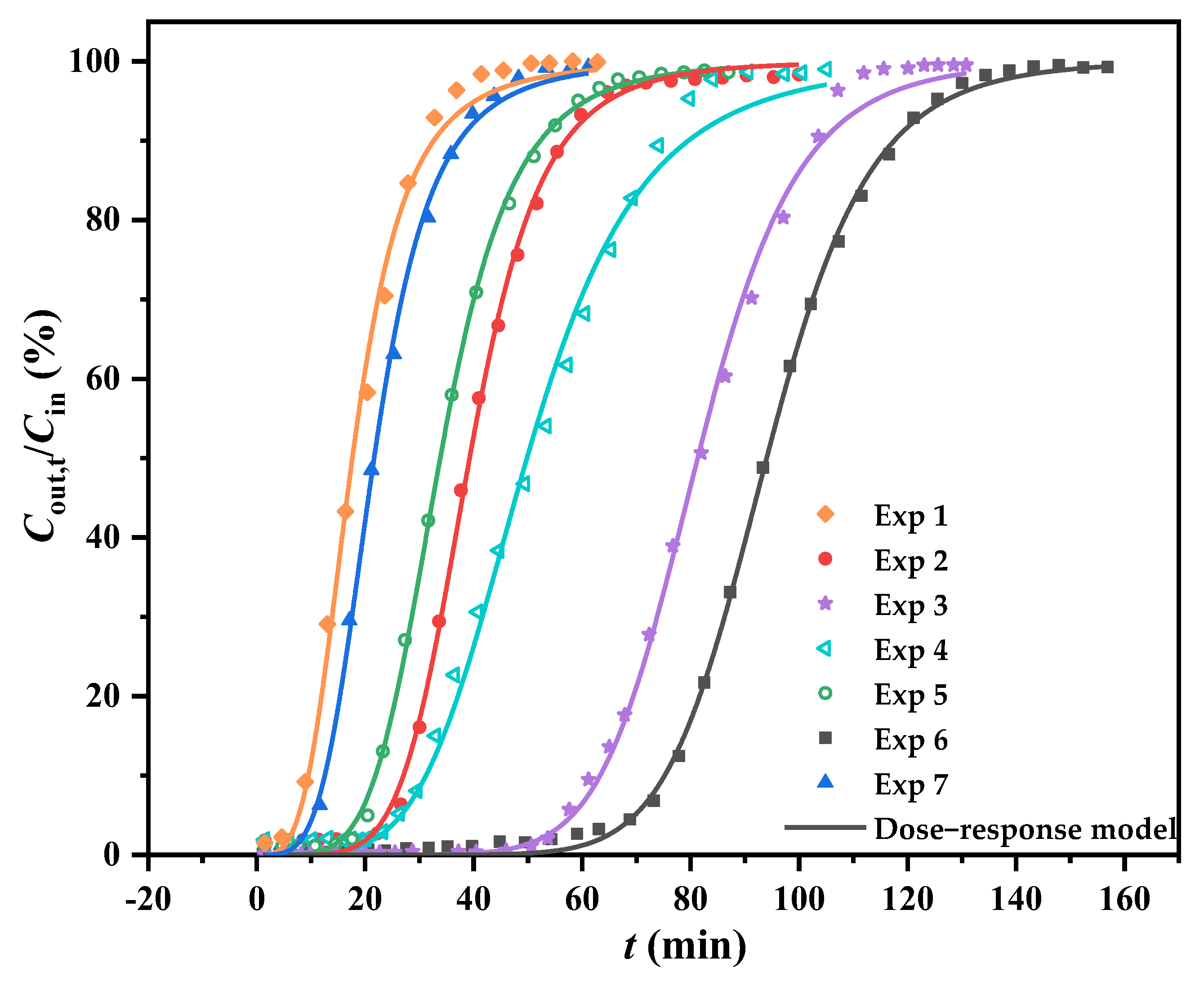
- At the stable conditions of v = 50 mL min−1 and Cin = 9.2 mg L−1, when h rose from 10.5 to 20.0 mm (Exp. nos. 1–3), the curves shifted significantly to the right-hand side, the tB,th was highly extended, and the QB,th values increased, indicating that a prolonged residence time could improve the total removal amount of cumene and enhance its specific capacity. This finding was backed up by another set of experiments (Exp. nos. 7, 2, and 6) in which the residence time was prolonged by reducing v from 75 to 25 mL min−1 under the same conditions of Cin = 9.2 mg L−1 and h = 14.3 mm. These experiments demonstrated the same response trend regarding both tB,th and QB,th metrics.
- (3)
- When controlling the residence time at the same value in the third experiment, changing Cin from 5.3 to 14.6 mg L−1 (Exp. nos. 4, 2, and 5) resulted in tB,th shortening and increased QB,th. These changes could be attributed to the relative shortage of the SSA adsorption sites and the enhancement of cumene reaction driving force caused by the Cin increase [28].
- (4)
- In the experiments mentioned above, it was found that both maximum values of tB,th (69.60 min) and QB,th (324.50 mg g−1) were obtained in Exp. No. 6, and the corresponding optimal process conditions were 9.2 mg L−1 for Cin, 14.3 mm for h, 25 mL min−1 for v, and 160 °C for T. The QB,th value identified that SSA/MCM-41 outperformed a silica-alumina material (0.82 mg g−1; 60 °C) and activated carbon (230.25 mg g−1; 20.8 °C) on the adsorption capacity of cumene [29,30], which could be related to the sulfonic acid loading on MCM-41.
2.3. Identification of the Adsorbed Products and Removal Mechanism for Cumene
3. Experimental Section
3.1. Materials
3.2. Preparation of Supported Sulfuric Acid
3.3. Characterization
3.4. Reactive Adsorption Tests
- (1)
- The removal rate of cumene (Xc) was measured while heating gradually from room temperature up to 300 °C at 5 °C min−1 under v = 50 mL min−1, Cin = 9.2 mg L−1, and h = 14.3 mm. First, the Xc values were calculated via Equation (1). Then, the values were used to plot an Xc − T curve to analyze the reactive adsorption behavior of cumene upon the variation of the adsorption bed temperature.
- (2)
- A series of transient adsorption experiments for the cumene/SSA/MCM-41 system was performed by independently changing the bed height (h), flow rate (v), or inlet concentration (Cin) with the other two variables kept constant (see Table 2). The outlet concentrations of cumene (Cout,t, mg L−1) in the gas flow at time t were determined experimentally. The extensive data collected were treated to draw the experimental breakthrough adsorption curves. These curves were then used to demonstrate the reactive behavior of SSA/MCM-41 toward cumene adsorption at various process conditions and to determine the experimental adsorption performance metrics such as breakthrough time (tB, min) and breakthrough adsorption capacity (QB, mg·g−1). Here, tB was the time corresponding to the ratio Cout,t/Cin = 0.05 on the curve, and QB was calculated from Equation (2).
- (3)
- Based on our previous work [23,29], the dose–response model (Equation (3)) was selected to fit the experimental data to more deeply understand the adsorption behavior of cumene in the SSA/MCM-41 fixed-bed column and predict the theoretical adsorption performance metrics (tB,th/min and QB,th/mg·g−1).
3.5. Desorption and Regeneration of the Spent SSA/MCM-41
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent advances in the catalytic oxidation of volatile organic compounds: A review based on pollutant sorts and sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ho, S.S.; Lu, Y.; Niu, R.; Xu, L.; Cao, J.; Lee, S. Removal of indoor volatile organic compounds via photocatalytic oxidation: A short review and prospect. Molecules 2016, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Feng, Z.; Liu, P.; He, X.; He, Z.; Chen, T.; Wang, Y.; He, H.; Mu, Y.; Liu, Y. Ozone and SOA formation potential based on photochemical loss of VOCs during the Beijing summer. Environ. Pollut. 2021, 285, 117444. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xu, Y.; Feng, Q.; Leung, D.Y.C. Low temperature catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2015, 5, 2649–2669. [Google Scholar] [CrossRef]
- Lei, R.; Sun, Y.; Zhu, S.; Jia, T.; He, Y.; Deng, J.; Liu, W. Investigation on distribution and risk assessment of volatile organic compounds in surface water, sediment, and soil in a chemical industrial park and adjacent area. Molecules 2021, 26, 5988. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, Y.; Chai, X.; Xu, L.; Zhang, L.; Ning, P.; Huang, J.; Tian, S. Interaction of inhalable volatile organic compounds and pulmonary surfactant: Potential hazards of VOCs exposure to lung. J. Hazard. Mater. 2019, 369, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhao, D.; Qian, J.; Ma, Z.; Cui, J. Utilization of hematite particles for economical removal of o−xylene in a high−temperature gas−solid reactor. Molecules 2022, 27, 1509. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhu, L.; Lu, X.; Xing, S.; Wu, Y.; Gao, Y. Catalytic ozonation of p−nitrophenol over mesoporous Mn–Co–Fe oxide. Sep. Purif. Technol. 2014, 133, 357–364. [Google Scholar] [CrossRef]
- Guo, Y.; Wen, M.; Li, G.; An, T. Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: A critical review. Appl. Catal. B 2021, 281, 119447. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, R.; Song, C.; Xing, S.; Gao, Y.; Ma, Z. Effect of reducing agent on the structure and activity of manganese oxide octahedral molecular sieve (OMS−2) in catalytic combustion of o−xylene. Catal. Today 2017, 281, 500–506. [Google Scholar] [CrossRef]
- Wu, P.; Jin, X.; Qiu, Y.; Ye, D. Recent progress of thermocatalytic and photo/thermocatalytic oxidation for VOCs purification over manganese—Based oxide catalysts. Environ. Sci. Technol. 2021, 55, 4268–4286. [Google Scholar] [CrossRef]
- Ghedini, E.; Menegazzo, F.; Manzoli, M.; Di Michele, A.; Puglia, D.; Signoretto, M. Multifunctional and environmentally friendly TiO2−SiO2 mesoporous materials for sustainable green buildings. Molecules 2019, 24, 4226. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.S. Photocatalytic oxidation technology for indoor environment air purification: The state−of−the−art. Appl. Catal. B 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Baskaran, D.; Sinharoy, A.; Pakshirajan, K.; Rajamanickam, R. Gas−phase trichloroethylene removal by Rhodococcus opacus using an airlift bioreactor and its modeling by artificial neural network. Chemosphere 2020, 247, 125806. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Ling, X. Design and dynamic behaviour investigation of a novel VOC recovery system based on a deep condensation process. Cryogenics 2020, 107, 103060. [Google Scholar] [CrossRef]
- Rodriguez Castillo, A.S.; Biard, P.F.; Guihéneuf, S.; Paquin, L.; Amrane, A.; Couvert, A. Assessment of VOC absorption in hydrophobic ionic liquids: Measurement of partition and diffusion coefficients and simulation of a packed column. Chem. Eng. J. 2019, 360, 1416–1426. [Google Scholar] [CrossRef]
- Hou, X.; Zheng, Y.; Ma, X.; Liu, Y.; Ma, Z. The effects of hydrophobicity and textural properties on hexamethyldisiloxane adsorption in reduced graphene oxide aerogels. Molecules 2021, 26, 1130. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, D.; Luo, K.H. A critical review on VOCs adsorption by different porous materials: Species, mechanisms and modification methods. J. Hazard. Mater. 2020, 389, 122102. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, X.; Qin, J.; Guo, Q.; Zhou, H.; Jin, W. Microporous polyimide VOC−rejective membrane for the separation of nitrogen/VOC mixture. J. Hazard. Mater. 2021, 402, 123817. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, Y.; Wang, J.; Gao, K.; Ma, Z. Silica supported sulfuric acid for the removal of gaseous o−xylene. J. Environ. Chem. Eng. 2019, 7, 102992. [Google Scholar] [CrossRef]
- Gao, K.; Ma, M.; Liu, Y.; Ma, Z. A comparative study of the removal of o-xylene from gas streams using mesoporous silicas and their silica supported sulfuric acids. J. Hazard. Mater. 2021, 409, 124965. [Google Scholar] [CrossRef]
- Ma, M.; Gao, K.; Ma, Z.; Ding, J. Influence of preparation method on the adsorptive performance of silica sulfuric acid for the removal of gaseous o−xylene. Sep. Purif. Technol. 2021, 265, 118484. [Google Scholar] [CrossRef]
- Ma, M.; Gao, K.; Zhao, D.; Ma, X.; Ma, Z. Effect of process conditions on reaction−type adsorption of o−xylene by MCM−41 supported sulfuric acid: Model simulations of breakthrough curves. J. Environ. Chem. Eng. 2022, 10, 106937. [Google Scholar] [CrossRef]
- Fakhfakh, N.; Dammak, N.; Benzina, M. Breakthrough modeling and experimental design for o−xylene dynamic adsorption onto clay material. Environ. Sci. Pollut. Res. Int. 2018, 25, 18263–18277. [Google Scholar] [CrossRef]
- Sun, W.; Li, J.; Li, H.; Jin, B.; Li, Z.; Zhang, T.; Zhu, X. Mechanistic insights into ball milling enhanced montmorillonite modification with tetramethylammonium for adsorption of gaseous toluene. Chemosphere 2022, 296, 133962. [Google Scholar] [CrossRef]
- Hong, T.; Wei, L.; Cui, K.; Dong, Y.; Li, R.; Zhang, T.; Zhao, Y.; Luo, L. Adsorption performance of volatile organic compounds on activated carbon fibers in a fixed bed column. J. Environ. Chem. Eng. 2021, 9, 106347. [Google Scholar] [CrossRef]
- Zhao, D.; Ma, M.; Qian, J.; Wang, Y.; Ma, Z.; Ma, X. Influence of impregnation medium on the adsorptive performance of silica sulfuric acid for the removal of gaseous o−xylene: Comparison on ethyl acetate and water. Catalysts 2022, 12, 737. [Google Scholar] [CrossRef]
- Besedová, E.; Bobok, D.; Bafrncová, S.; Steltenpohl, P. Modelling of gas phase adsorption on activated carbon I. Experiment and equilibrium model. Chem. Pap. 2004, 58, 391–396. [Google Scholar]
- Kubokawa, Y.; Miyata, H. Desorption of cumene from silica-alumina catalysts. J. Phys. Chem. 2002, 72, 356–358. [Google Scholar] [CrossRef]
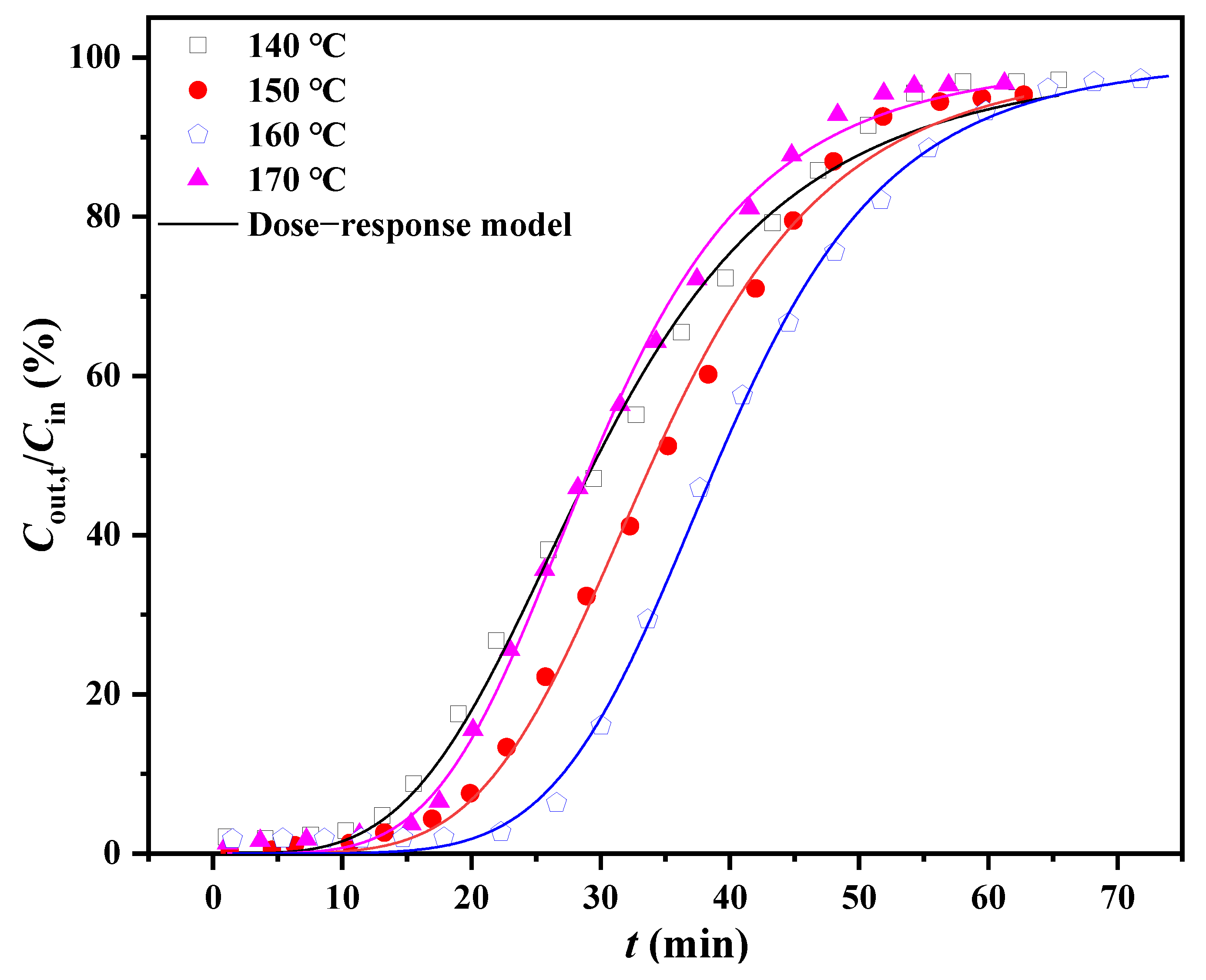

| Parameters | Temperature (°C) | |||
|---|---|---|---|---|
| 140 | 150 | 160 | 170 | |
| Experimental test: | ||||
| tB (min) | 13.11 | 16.23 | 24.68 | 14.12 |
| QB (mg g−1) | 117.30 | 146.81 | 222.41 | 127.13 |
| Dose–response model: | ||||
| q0 (mg g−1) | 0.27 | 0.31 | 0.36 | 0.27 |
| a | 3.81 | 4.89 | 5.90 | 4.57 |
| R2 | 0.995 | 0.997 | 0.999 | 0.998 |
| tB,th (min) | 13.71 | 18.71 | 24.87 | 15.50 |
| QB,th (mg g−1) | 172.42 | 218.79 | 223.13 | 198.62 |
| Exp. No. | Inlet Concentration (mg L−1) | Bed Height (mm) | Flow Rate (mL min−1) |
|---|---|---|---|
| 1 (◆) | 9.2 | 10.5 | 50 |
| 2 (●) | 9.2 | 14.3 | 50 |
| 3 (★) | 9.2 | 20.0 | 50 |
| 4 (◁) | 5.3 | 14.3 | 50 |
| 5 (○) | 14.6 | 14.3 | 50 |
| 6 (■) | 9.2 | 14.3 | 25 |
| 7 (▲) | 9.2 | 14.3 | 75 |
| Metrics | Exp. No. | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| tB,th (min) | 6.73 | 24.87 | 58.06 | 26.56 | 16.25 | 69.60 | 10.42 |
| QB,th (mg g−1) | 118.31 | 223.13 | 268.55 | 140.80 | 231.43 | 324.50 | 151.59 |
| Products | Methods & Parameters | The Data for Adsorbed Products |
|---|---|---|
| I | 1H NMR (DMF, 500 MHz), δ ppm | 7.51 (d, J = 5.0 Hz, 2 H), 7.18 (d, J = 5.0 Hz, 2 H), 3.33 (s), 2.88 (m, 1 H), 1.19 (d, J = 2.5 Hz, 6 H) |
| 13C NMR (DMF, 125 MHz), δ ppm | 23.82 (2 C), 33.18, 125.39 (2 C), 125.53 (2 C), 137.35, 148.55 | |
| FTIR (KBr), cm−1 | 3426, 2961, 2869, 1629, 1465, 1408, 1180, 1052, 1004, 831, 769, 677, 571 | |
| II | 1H NMR (DMF, 500 MHz), δ ppm | 7.85 (d, J = 5.0 Hz, 2 H), 7.33 (d, J = 2.5 Hz, 2 H), 2.94 (m, 1 H), 1.23 (d, J = 2.5 Hz, 6 H) |
| 13C NMR (DMF, 125 MHz), δ ppm | 23.63 (4 C), 34.22 (2 C), 127.36 (4 C), 127.78 (4 C), 139.32 (2 C), 154.59 (2 C) | |
| FTIR (KBr), cm−1 | 3440, 2966, 2931, 2869, 1600, 1408, 1370, 1321, 1158, 1109, 1052, 836, 788, 672, 571 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Liu, Y.; Ma, X.; Qian, J.; Ma, Z. Reactive Adsorption Performance and Behavior of Gaseous Cumene on MCM-41 Supported Sulfuric Acid. Molecules 2022, 27, 5129. https://doi.org/10.3390/molecules27165129
Zhao D, Liu Y, Ma X, Qian J, Ma Z. Reactive Adsorption Performance and Behavior of Gaseous Cumene on MCM-41 Supported Sulfuric Acid. Molecules. 2022; 27(16):5129. https://doi.org/10.3390/molecules27165129
Chicago/Turabian StyleZhao, Dandan, Yuheng Liu, Xiaolong Ma, Jinjin Qian, and Zichuan Ma. 2022. "Reactive Adsorption Performance and Behavior of Gaseous Cumene on MCM-41 Supported Sulfuric Acid" Molecules 27, no. 16: 5129. https://doi.org/10.3390/molecules27165129







