Effect of Biofunctional Green Synthesized MgO-Nanoparticles on Oxidative-Stress-Induced Tissue Damage and Thrombosis
Abstract
:1. Introduction
2. Results
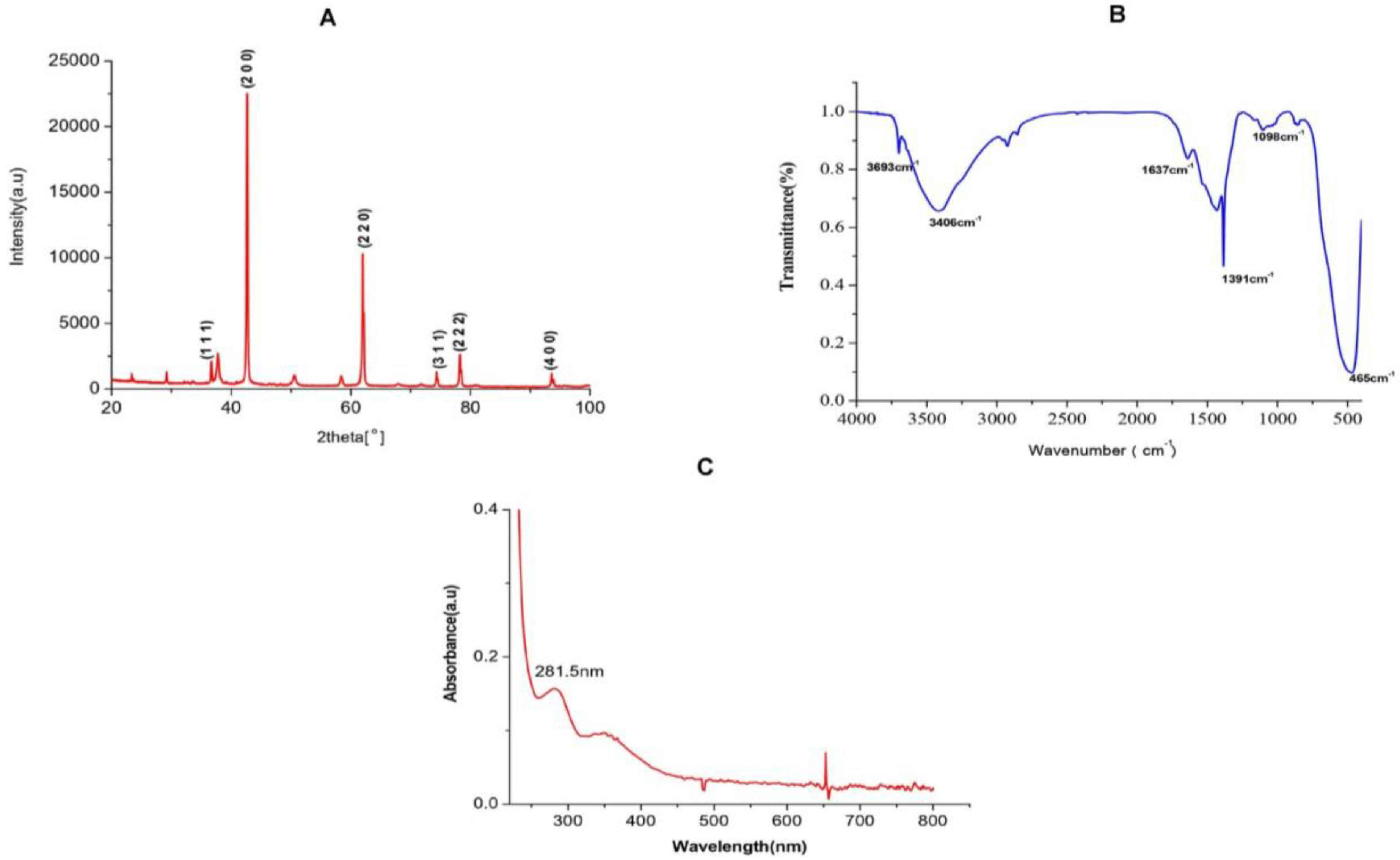
2.1. Characterization Studies of MgO NPs
2.1.1. XRD Analysis of TAFEMgO NPs
2.1.2. FTIR Analysis of TAFEMgO NPs
2.1.3. UV-Vis Spectral Analysis of TAFEMgO NPs
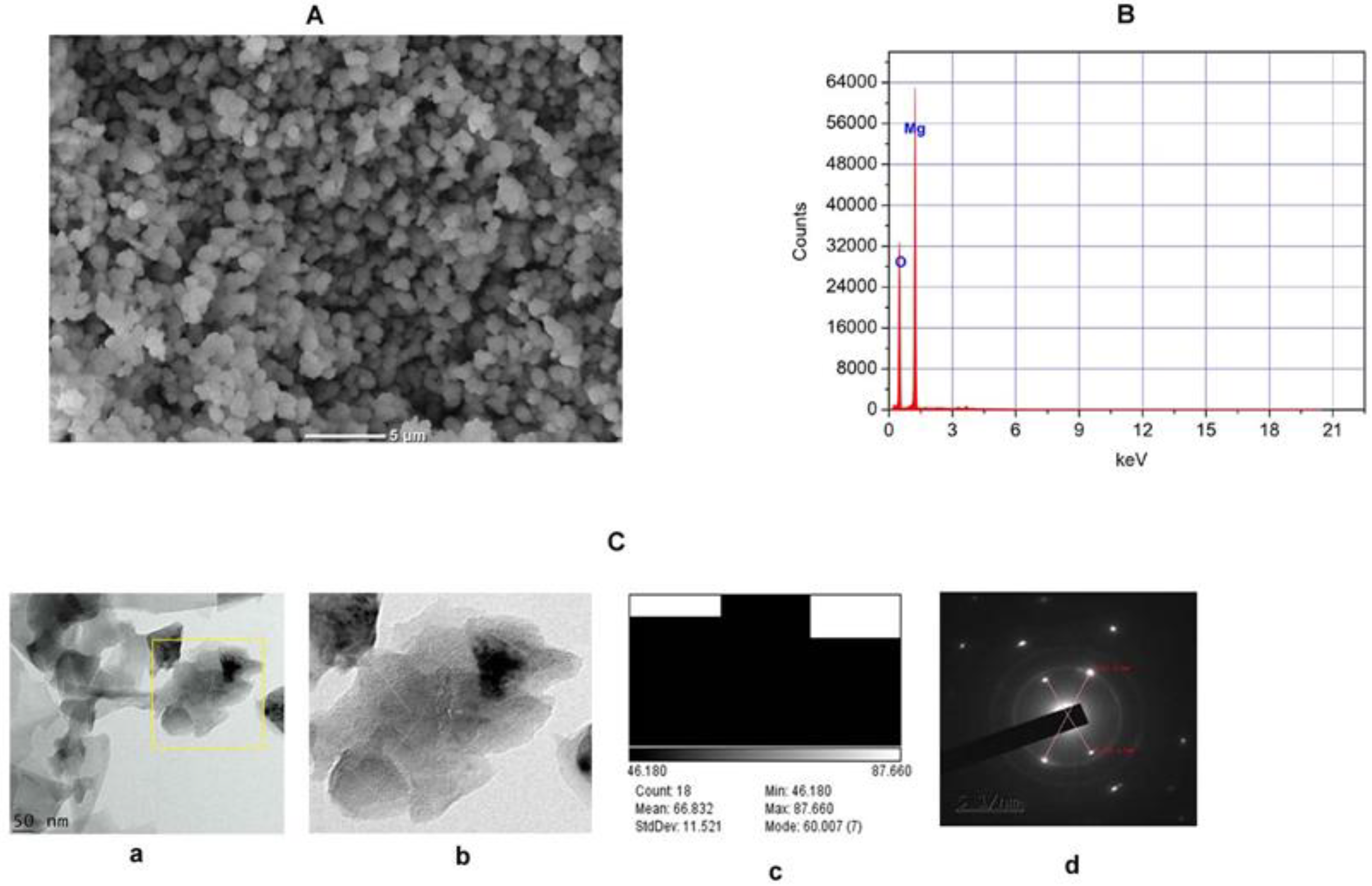
2.1.4. SEM, EDX-ray Diffraction, TEM, and Selective Electron Diffraction (SED) Study of TAFEMgO NPs
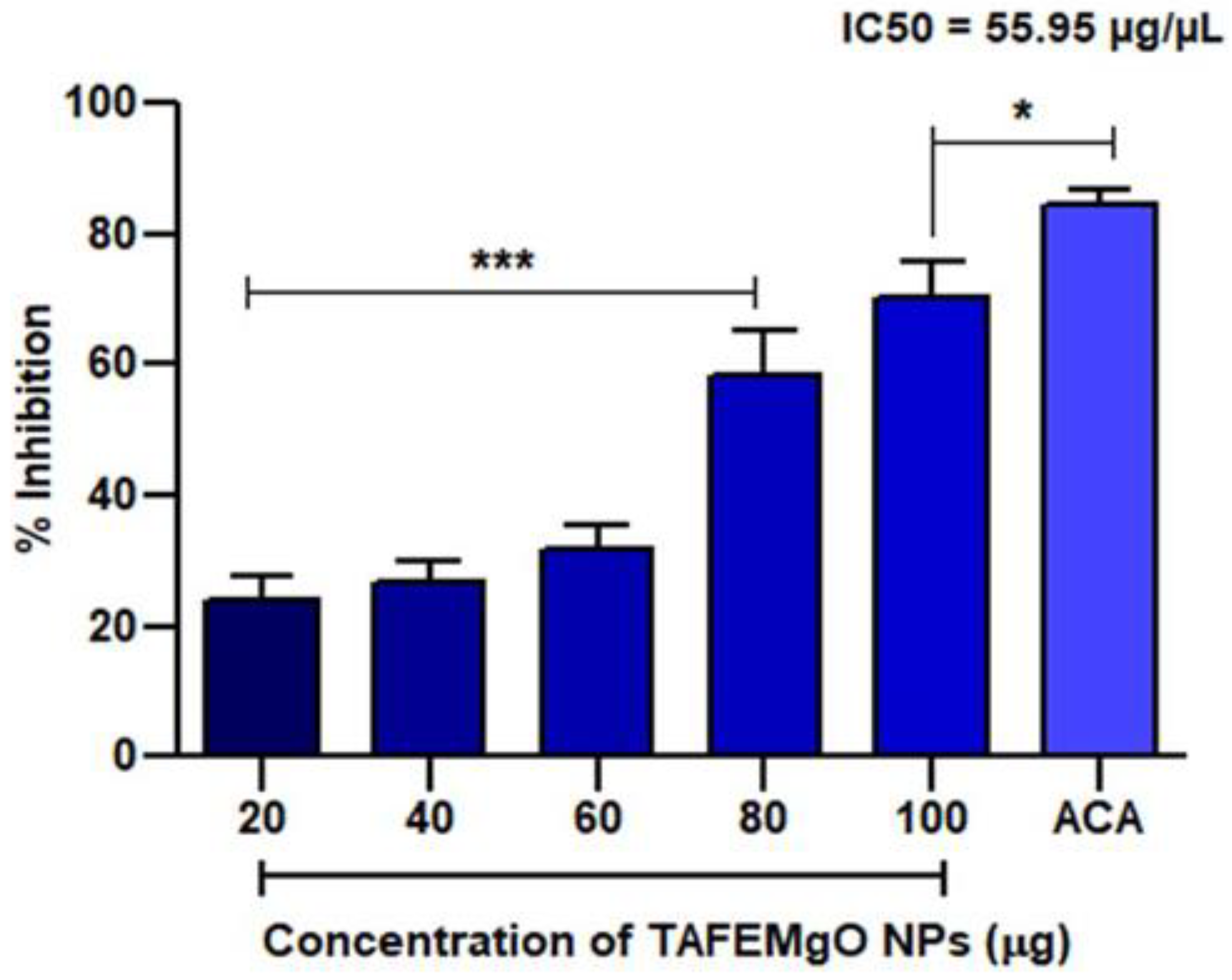
2.2. TAFEMgO NPs Scavenge Free Radicals In Vitro
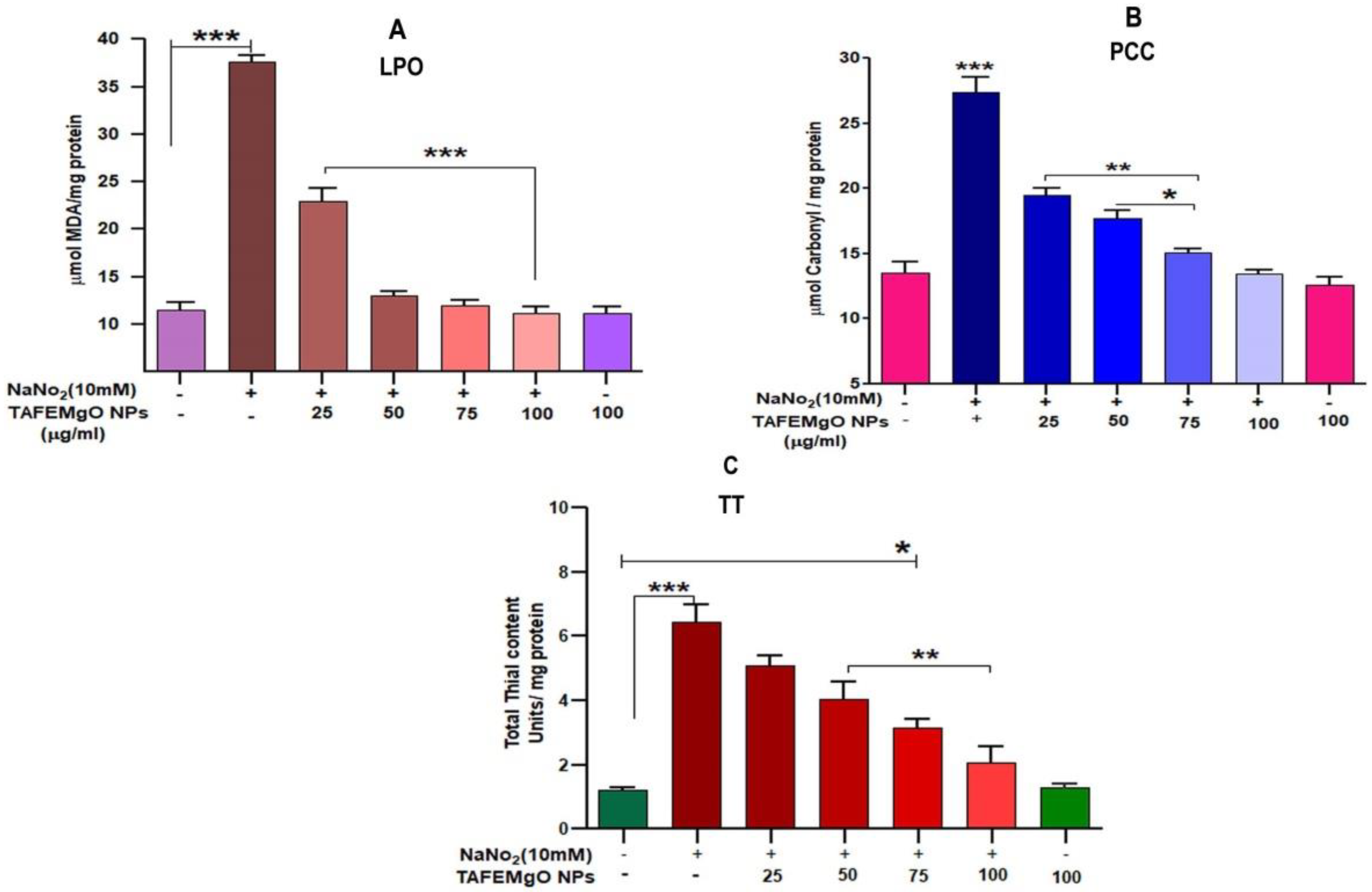
2.3. TAFEMgO NPs Ameliorate NaNO2-Induced Stress Markers in the RBC Model (In Vitro)
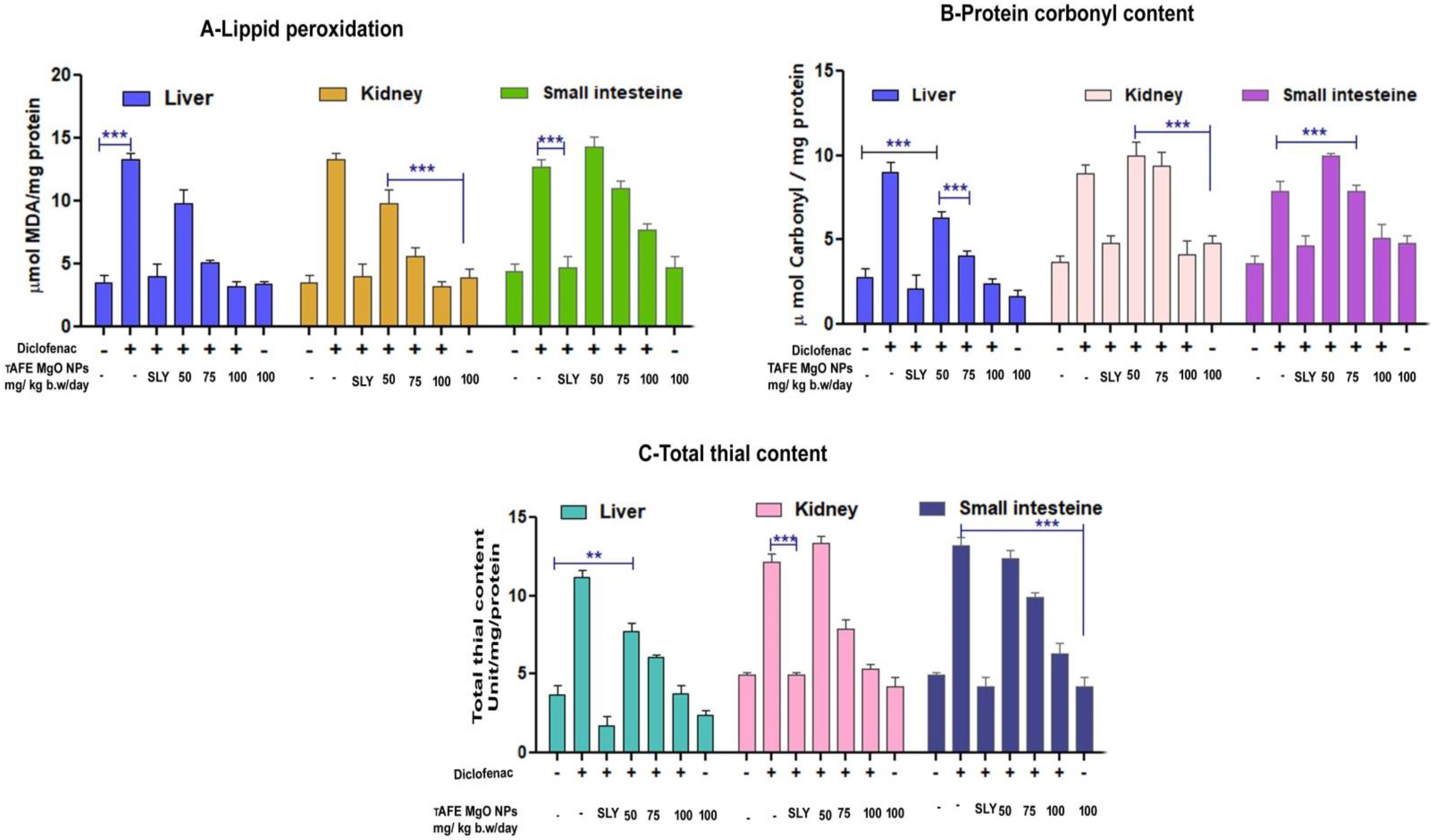
2.4. TAFEMgO NPs Ameliorate the Diclofenac-Induced Oxidative Stress Markers (In Vivo)
2.5. TAFEMgO NPs Restore the Biochemical Parameters In Vivo
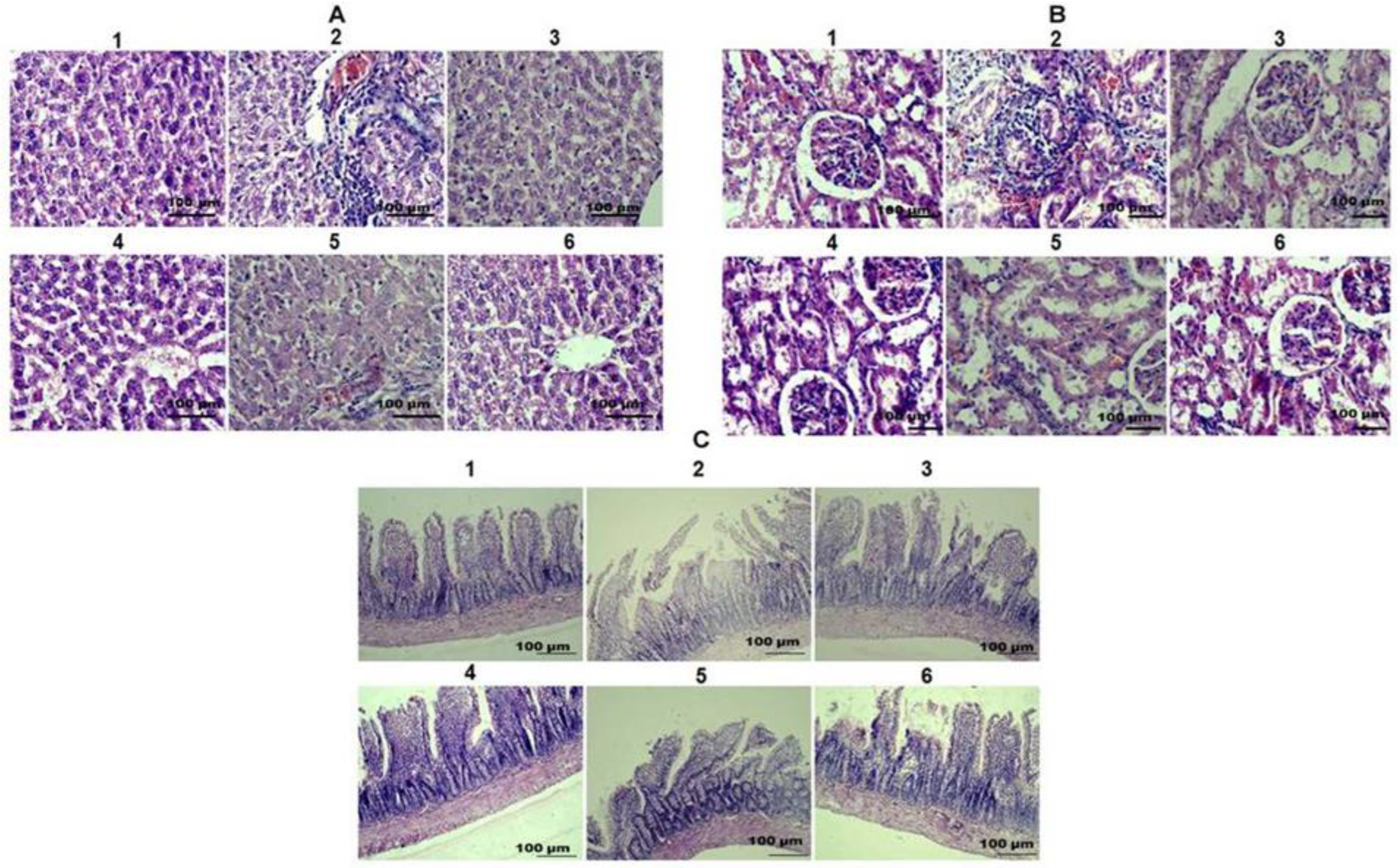
2.6. TAFEMgO NPs Restore the Liver, Kidney, and Small Intestine Morphology
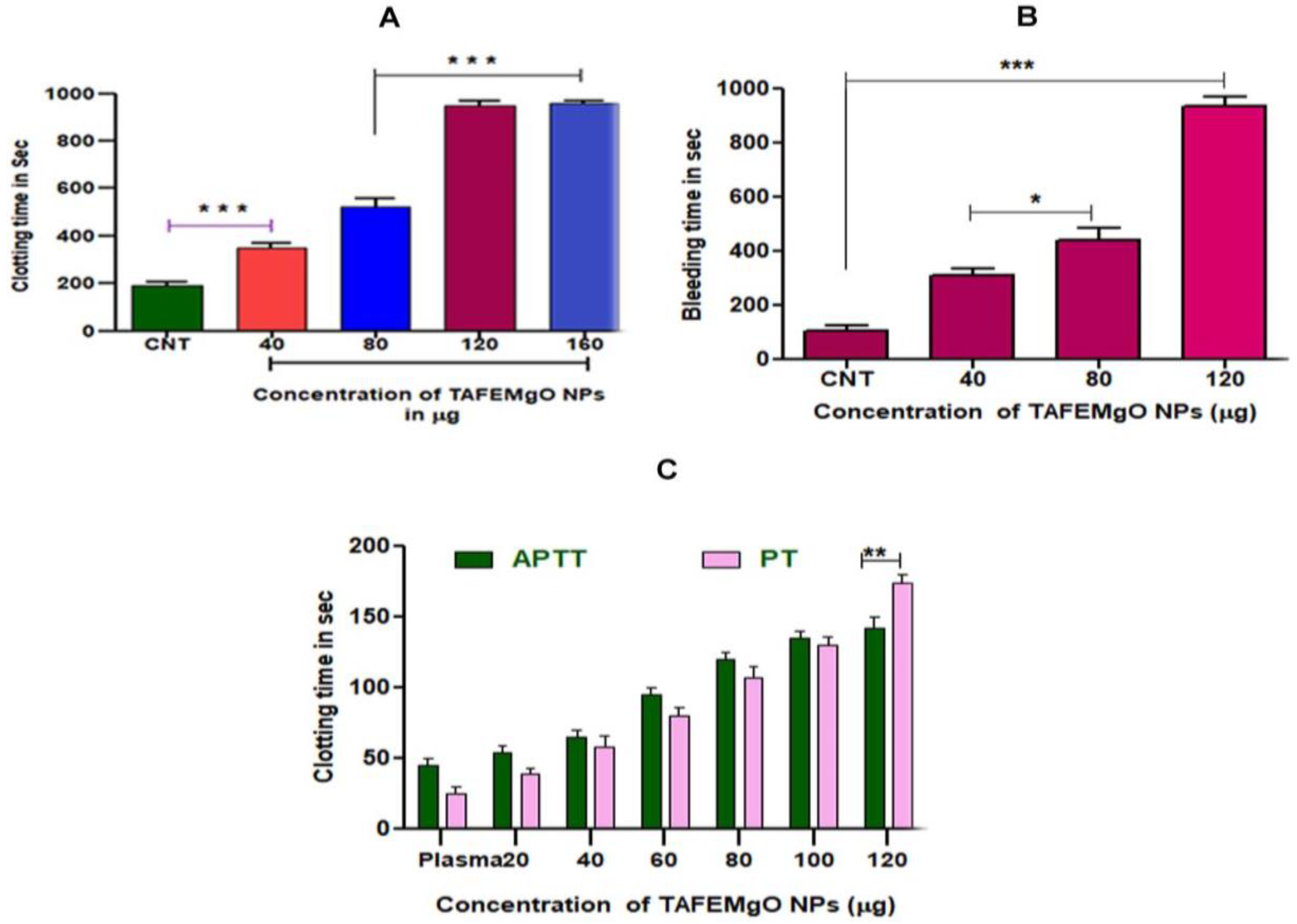
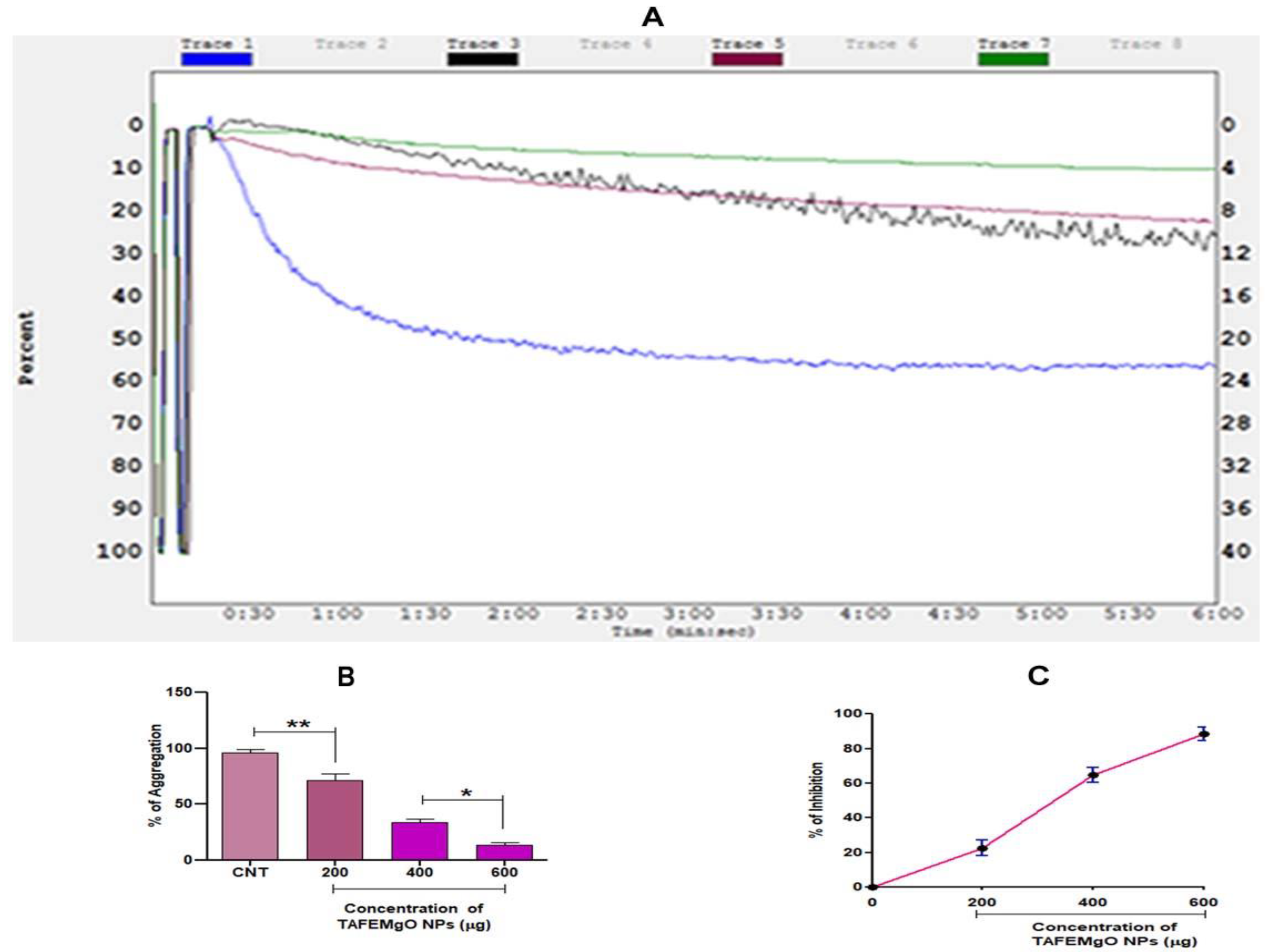
2.7. TAFEMgO NPs Indicated Anticoagulant and Antiplatelet Properties
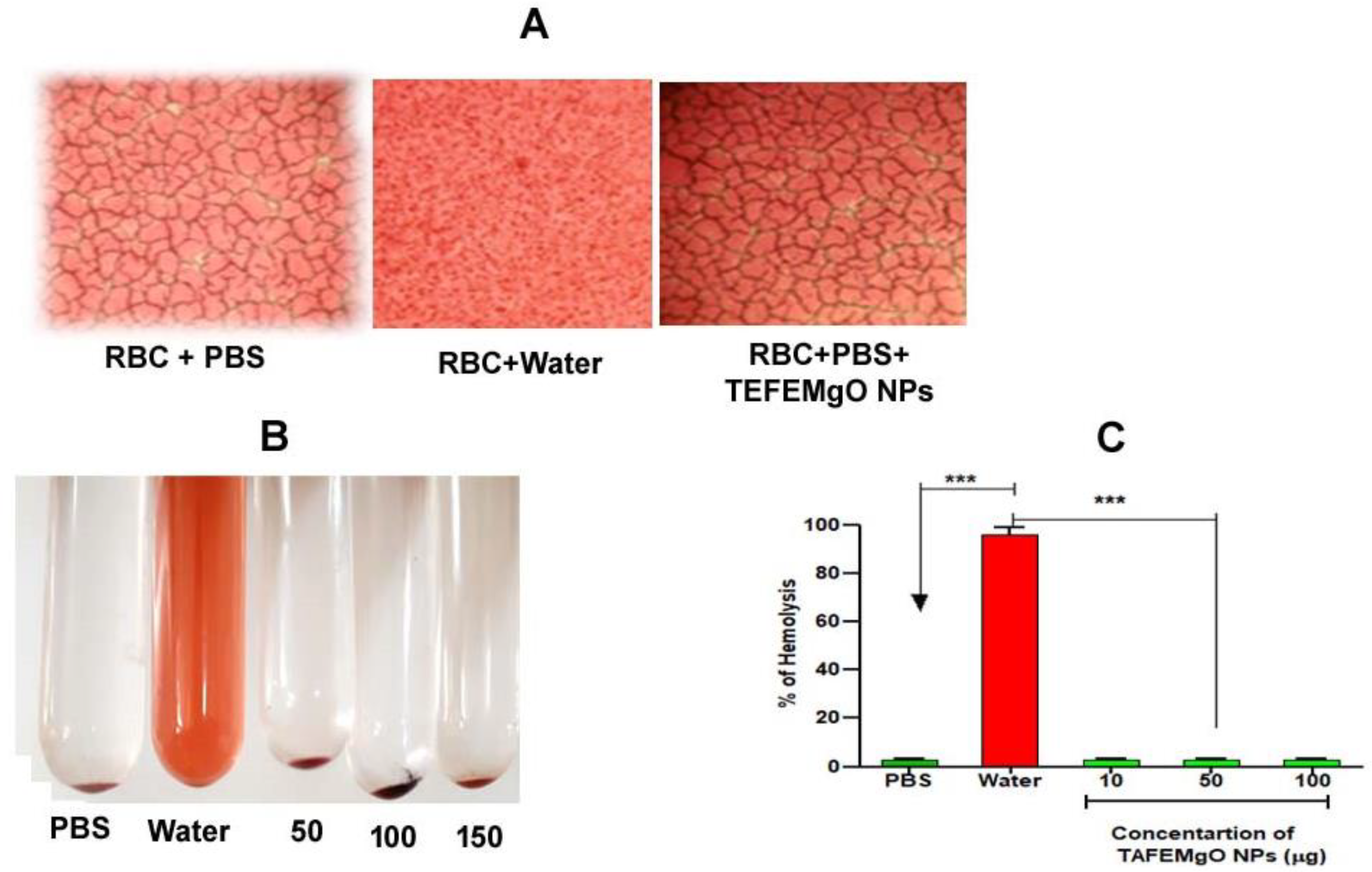
2.8. TAFEMgO NPs Are Nontoxic Biogenic Molecules
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparations of Terenna Asiatica Fruit Extract (TAFE)
4.3. Green Biosynthesis of TAFEMgO NPs
4.4. Characterization of TAFEMgO NPs
4.5. Estimation of Antioxidant Property by DPPH Method
4.6. Human Blood Collection and 2% Hematocrit Preparation
4.7. Oxidative Stress-Induced by NaNO2
4.8. Determination of Lipid Peroxidation (LPO)
4.9. Estimation of Protein Carbonyl Content (PCC)
4.10. Estimation of Total Thiol (TT)
4.11. Determination of Activities of CAT and SOD
4.12. Animal Grouping and Sample Dose Administration
| Group I | Control (normal saline). |
| Group II | Diclofenac alone. |
| Group III | Silymarin (25 mg/kg body weight/day) was injected intraperitonially, and after 45 min diclofenac (50 mg/kg body weight/day) was administered. |
| Group IV | TAFEMgO NPs (50 mg/kg body weight/day) was injected intraperitonially, and after 45 min diclofenac (50 mg/kg body weight/day) was administered. |
| Group V | TAFEMgO NPs (75 mg/kg body weight/day) was injected intraperitonially, and after 45 min diclofenac (50 mg/kg body weight/day) was administered. |
| Group VI | TAFEMgO NPs (100 mg/kg body weight/day) was injected intraperitonially, and after 45 min diclofenac (50 mg/kg body weight/day) was administered. |
| Group VII | TAFEMgO NPs (100 mg/kg body weight/day) alone administered. |
4.13. Histopathological Examination
4.14. Determination of In Vivo Antioxidant Activities
4.15. Plasma Recalcification Time
4.16. Prediction of Prothrombin Time and Thromboplastin Time
4.17. Bleeding Time
4.18. Determination of Platelet Aggregation
4.19. Determination of Direct Hemolytic Property
4.20. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Leung, Y.H.; Ng, A.M.C.; Xu, X.; Shen, Z.; Gethings, L.A.; Wong, M.T.; Chan, C.M.N.; Guo, M.Y.; Ng, Y.H.; Djurišić, A.B.; et al. Mechanisms of Antibacterial Activity of MgO: Non-ROS Mediated Toxicity of MgO Nanoparticles Towards Escherichia Coli. Small 2014, 10, 1171–1183. [Google Scholar] [CrossRef]
- Kengaiah, J.; Nandish, S.K.M.; Ramachandraiah, C.; Chandramma; Shivaiah, A.; Vishalakshi, G.J.; Paul, M.; Santhosh, M.S.; Shankar, R.L.; Sannaningaiah, D. Protective Effect of Tamarind Seed Coat Ethanol Extract on Eryptosis Induced by Oxidative Stress. Biochem. Mosc. 2020, 85, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Elfeky, A.S.; Salem, S.S.; Elzaref, A.S.; Owda, M.E.; Eladawy, H.A.; Saeed, A.M.; Awad, M.A.-Y.-Y.; Abou-Zeid, R.E.; Fouda, A. Multifunctional Cellulose Nanocrystal/Metal Oxide Hybrid, Photo-Degradation, Antibacterial and Larvicidal Activities. Carbohydr. Polym. 2020, 230, 115711. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Li, J.; Li, S.; Han, B.; Wu, P.; Deng, N.; Guo, X.; Lv, Z.; Zhang, Z. Inorganic Mercury Induces Liver Oxidative Stress Injury in Quails by Inhibiting Akt/Nrf2 Signal Pathway. Inorg. Chem. Commun. 2022, 142, 109603. [Google Scholar] [CrossRef]
- Radwan, N.R.E.; El-Shall, M.S.; Hassan, H.M.A.-Y.-Y. Synthesis and Characterization of Nanoparticle Co3O4, CuO and NiO Catalysts Prepared by Physical and Chemical Methods to Minimize Air Pollution. Appl. Catal. A Gen. 2007, 331, 8–18. [Google Scholar] [CrossRef]
- Kumari, R.; Nath, M. Synthesis and Characterization of Novel Trimethyltin(IV) and Tributylltin(IV) Complexes of Anticoagulant, WARFARIN: Potential DNA Binding and Plasmid Cleaving Agents. Inorg. Chem. Commun. 2018, 95, 40–46. [Google Scholar] [CrossRef]
- Iravani, S. Green Synthesis of Metal Nanoparticles Using Plants. Green Chem. 2011, 13, 2638. [Google Scholar] [CrossRef]
- Nejati, M.; Rostami, M.; Mirzaei, H.; Rahimi-Nasrabadi, M.; Vosoughifar, M.; Nasab, A.S.; Ganjali, M.R. Green Methods for the Preparation of MgO Nanomaterials and Their Drug Delivery, Anti-Cancer and Anti-Bacterial Potentials: A Review. Inorg. Chem. Commun. 2022, 136, 109107. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu; Chaudhry, S.A.; Ikram, S. A Review on Biogenic Synthesis of ZnO Nanoparticles Using Plant Extracts and Microbes: A Prospect towards Green Chemistry. J. Photochem. Photobiol. B Biol. 2017, 166, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, B.; Singh, P.; Kumar, A.; Kumar, S.; Budhwar, V. Eco-Friendly Greener Synthesis of Nanoparticles. Adv. Pharm. Bull. 2020, 10, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Biosynthesis and Characterization of MgO Nanoparticles from Plant Extracts via Induced Molecular Nucleation. New J. Chem. 2017, 41, 2800–2814. [Google Scholar] [CrossRef]
- Amjad, R.; Mubeen, B.; Ali, S.S.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Alzarea, S.I.; Rasool, R.; Ullah, I.; Nadeem, M.S.; et al. Green Synthesis and Characterization of Copper Nanoparticles Using Fortunella Margarita Leaves. Polymers 2021, 13, 4364. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Deborah, S.; Velmurugan, G. Evaluation of In vitro anticancer activity of Tarenna asiatica (L.) fruits ethanolic extract against human breast cancer. Int. J. Herbal Med. 2017, 5, 110–113. [Google Scholar]
- Anjanadevi, N.; Abirami, P.; Sharmila, S. Antibacterial activity of leaf extract of Tarenna asiatica (L.). Int. J. Curr. Microbiol. Appl. Sci. 2014, 10, 48–51. [Google Scholar]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Lenaz, G. The Mitochondrial Production of Reactive Oxygen Species: Mechanisms and Implications in Human Pathology. IUBMB Life (Int. Union Biochem. Mol. Biol. Life) 2001, 52, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Nanomaterial-Based Amplified Transduction of Biomolecular Interactions. Small 2005, 1, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.-F. Non-Conjugated Polymers with Intrinsic Luminescence for Drug Delivery. J. Drug Deliv. Sci. Technol. 2020, 59, 101916. [Google Scholar] [CrossRef]
- Obireddy, S.R.; Lai, W.-F. Multi-Component Hydrogel Beads Incorporated with Reduced Graphene Oxide for PH-Responsive and Controlled Co-Delivery of Multiple Agents. Pharmaceutics 2021, 13, 313. [Google Scholar] [CrossRef]
- Singh, H. Nanotechnology Applications in Functional Foods; Opportunities and Challenges. Prev. Nutr. Food Sci. 2016, 21, 1–8. [Google Scholar] [CrossRef]
- Li, T.; Yin, W.; Gao, S.; Sun, Y.; Xu, P.; Wu, S.; Kong, H.; Yang, G.; Wei, G. The Combination of Two-Dimensional Nanomaterials with Metal Oxide Nanoparticles for Gas Sensors: A Review. Nanomaterials 2022, 12, 982. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ Synthesis of Metals and Their Oxide Nanoparticles: Applications for Environmental Remediation. J Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Hamidian, K.; Sarani, M.; Barani, M.; Khakbaz, F. Cytotoxic Performance of Green Synthesized Ag and Mg Dual Doped ZnO NPs Using Salvadora Persica Extract against MDA-MB-231 and MCF-10 Cells. Arab. J. Chem. 2022, 15, 103792. [Google Scholar] [CrossRef]
- Husseiny, M.I.; El-Aziz, M.A.-Y.-Y.; Badr, Y.; Mahmoud, M.A.-Y.-Y. Biosynthesis of Gold Nanoparticles Using Pseudomonas Aeruginosa. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Ezhilarasi, A.A.; Vijaya, J.J.; Kaviyarasu, K.; Maaza, M.; Ayeshamariam, A.; Kennedy, L.J. Green Synthesis of NiO Nanoparticles Using Moringa Oleifera Extract and Their Biomedical Applications: Cytotoxicity Effect of Nanoparticles against HT-29 Cancer Cells. J. Photochem. Photobiol. B Biol. 2016, 164, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.J.P.; Finub, J.S.; Narayanan, A. Synthesis of Silver Nanoparticles Using Piper Longum Leaf Extracts and Its Cytotoxic Activity against Hep-2 Cell Line. Colloids Surf. B Biointerfaces 2012, 91, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Velidandi, A.; Pabbathi, N.P.P.; Dahariya, S.; Baadhe, R.R. Catalytic and Eco-Toxicity Investigations of Bio-Fabricated Monometallic Nanoparticles along with Their Anti-Bacterial, Anti-Inflammatory, Anti-Diabetic, Anti-Oxidative and Anti-Cancer Potentials. Colloid Interface Sci. Commun. 2020, 38, 100302. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Cha, S.J.; Yang, I.J.; Sreekanth, T.V.M.; Kim, K.J.; Shin, H.M. Antioxidant and Anti-Inflammatory Activities of Zinc Oxide Nanoparticles Synthesized Using Polygala Tenuifolia Root Extract. J. Photochem. Photobiol. B Biol. 2015, 146, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Dobrucka, R. Synthesis of MgO Nanoparticles Using Artemisia Abrotanum Herba Extract and Their Antioxidant and Photocatalytic Properties. Iran. J. Sci. Technol. Trans. A Sci. 2018, 42, 547–555. [Google Scholar] [CrossRef]
- Diplock, A.T. Will the ‘Good Fairies’ Please Prove to Us That Vitamin E Lessens Human Degenerative Disease? Free. Radic. Res. 1997, 27, 511–532. [Google Scholar] [CrossRef] [PubMed]
- Fontana, J.; Zima, M.; Vetvicka, V. Biological Markers of Oxidative Stress in Cardiovascular Diseases: After so Many Studies, What Do We Know? Immunol. Investig. 2018, 47, 823–843. [Google Scholar] [CrossRef]
- Ung, L.; Pattamatta, U.; Carnt, N.; Wilkinson-Berka, J.L.; Liew, G.; White, A.J.R. Oxidative Stress and Reactive Oxygen Species: A Review of Their Role in Ocular Disease. Clin. Sci. 2017, 131, 2865–2883. [Google Scholar] [CrossRef] [PubMed]
- Chavali, M.S.; Nikolova, M.P. Metal Oxide Nanoparticles and Their Applications in Nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef]
- Sarani, M.; Tosan, F.; Hasani, S.A.; Barani, M.; Adeli-Sardou, M.; Khosravani, M.; Niknam, S.; Jadidi Kouhbanani, M.A.-Y.-Y.; Beheshtkhoo, N. Study of in Vitro Cytotoxic Performance of Biosynthesized α-Bi2O3 NPs, Mn-Doped and Zn-Doped Bi2O3 NPs against MCF-7 and HUVEC Cell Lines. J. Mater. Res. Technol. 2022, 19, 140–150. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, S.; Garg, G.; Rizvi, S.I. Rapamycin Alleviates Oxidative Stress-Induced Damage in Rat Erythrocytes. Biochem. Cell Biol. 2016, 94, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, H.; Imaizumi, K.; Tyuma, I. Mechanism of Autocatalytic Oxidation of Oxyhemoglobin by Nitrite an Intermediate Detected by Electron Spin Resonance. Biochim. Et Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1982, 702, 237–241. [Google Scholar] [CrossRef]
- Abbasalipourkabir, R.; Moradi, H.; Zarei, S.; Asadi, S.; Salehzadeh, A.; Ghafourikhosroshahi, A.; Mortazavi, M.; Ziamajidi, N. Toxicity of Zinc Oxide Nanoparticles on Adult Male Wistar Rats. Food Chem. Toxicol. 2015, 84, 154–160. [Google Scholar] [CrossRef]
- Mullakkalparambil Velayudhan, J.; Mondal, D.; Raja, R.; Kumar, B.; Mandal, R.S.K.; Bhatt, S.; Singh, K.P.; Madhesh, K. Hepatoprotectant Potential of Sodium Alginate Coated Catechin Nanoparticles (SACC-NPs) in Rat Model. Inorg. Nano-Met. Chem. 2020, 50, 1334–1342. [Google Scholar] [CrossRef]
- Suriyakalaa, U.; Antony, J.J.; Suganya, S.; Siva, D.; Sukirtha, R.; Kamalakkannan, S.; Pichiah, P.B.T.; Achiraman, S. Hepatocurative Activity of Biosynthesized Silver Nanoparticles Fabricated Using Andrographis Paniculata. Colloids Surf. B Biointerfaces 2013, 102, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Avilkina, V.; Chauveau, C.; Ghali Mhenni, O. Sirtuin Function and Metabolism: Role in Pancreas, Liver, and Adipose Tissue and Their Crosstalk Impacting Bone Homeostasis. Bone 2022, 154, 116232. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zennadi, R. Oxidative Stress and Thrombosis during Aging: The Roles of Oxidative Stress in RBCs in Venous Thrombosis. IJMS 2020, 21, 4259. [Google Scholar] [CrossRef] [PubMed]
- Devaraja, S.; Girish, K.S.; Gowtham, Y.N.J.; Kemparaju, K. The Hag-Protease-II Is a Fibrin(Ogen)Ase from Hippasa Agelenoides Spider Venom Gland Extract: Purification, Characterization and Its Role in Hemostasis. Toxicon 2011, 57, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Devaraja, S.; Girish, K.S.; Devaraj, V.R.; Kemparaju, K. Factor Xa-like and Fibrin(Ogen)Olytic Activities of a Serine Protease from Hippasa Agelenoides Spider Venom Gland Extract. J. Thromb. Thrombolysis 2010, 29, 119–126. [Google Scholar] [CrossRef]
- Nandish, S.K.M.; Kengaiah, J.; Ramachandraiah, C.; Chandramma; Shivaiah, A.; Santhosh, S.M.; Thirunavukkarasu; San-naningaiah, D. Flaxseed Cysteine Protease Exhibits Strong Anticoagulant, Antiplatelet, and Clot-Dissolving Properties. Biochem. Moscow 2020, 85, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Nethravathi, P.C.; Manjula, M.V.; Devaraja, S.; Suresh, D. Ag and BiVO4 Decorated Reduced Graphene Oxide: A Potential Nano Hybrid Material for Photocatalytic, Sensing and Biomedical Applications. Inorg. Chem. Commun. 2022, 139, 109327. [Google Scholar] [CrossRef]
- Lingaraju, K.; Basavaraj, R.B.; Jayanna, K.; Bhavana, S.; Devaraja, S.; Kumar Swamy, H.M.; Nagaraju, G.; Nagabhushana, H.; Raja Naika, H. Biocompatible Fabrication of TiO2 Nanoparticles: Antimicrobial, Anticoagulant, Antiplatelet, Direct Hemolytic and Cytotoxicity Properties. Inorg. Chem. Commun. 2021, 127, 108505. [Google Scholar] [CrossRef]
- Srivastava, V.; Sharma, Y.C.; Sillanpää, M. Green Synthesis of Magnesium Oxide Nanoflower and Its Application for the Removal of Divalent Metallic Species from Synthetic Wastewater. Ceram. Int. 2015, 41, 6702–6709. [Google Scholar] [CrossRef]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” Nanotechnologies: Synthesis of Metal Nanoparticles Using Plants. Acta Nat. 2014, 6, 35–44. [Google Scholar] [CrossRef]
- Okoh, S.; Asekun, O.; Familoni, O.; Afolayan, A. Antioxidant and Free Radical Scavenging Capacity of Seed and Shell Essential Oils Extracted from Abrus precatorius (L.). Antioxidants 2014, 3, 278–287. [Google Scholar] [CrossRef]
- Srinivasa, C.; Kengaiah, J.; Nandish, S.K.M.; Ramachandraiah, C.; Hanumegowda, S.M.; Shivaiah, A.; Santhosh, S.; Sannaningaiah, D. Caesalpinia Crista Coat Extract Protects Red Blood Cell from Sodium Nitrite-Induced Oxidative Stress and Exhibits Antiplatelet Activity. Blood Coagul. Fibrinolysis 2020, 31, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Lenz, A.-G.; Costabel, U.; Shaltiel, S.; Levine, R.L. Determination of Carbonyl Groups in Oxidatively Modified Proteins by Reduction with Tritiated Sodium Borohydride. Anal. Biochem. 1989, 177, 419–425. [Google Scholar] [CrossRef]
- Zinellu, A.; Sotgia, S.; Usai, M.F.; Chessa, R.; Deiana, L.; Carru, C. Thiol Redox Status Evaluation in Red Blood Cells by Capillary Electrophoresis-Laser Induced Fluorescence Detection. Electrophoresis 2005, 26, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, M.S.; Hemshekhar, M.; Santhosh, M.S.; Paul, M.; Sunitha, K.; Thushara, R.M.; NaveenKumar, S.K.; Naveen, S.; Devaraja, S.; Rangappa, K.S.; et al. Tamarind Seed (Tamarindus Indica) Extract Ameliorates Adjuvant-Induced Arthritis via Regulating the Mediators of Cartilage/Bone Degeneration, Inflammation and Oxidative Stress. Sci. Rep. 2015, 5, 11117. [Google Scholar] [CrossRef] [PubMed]
- Beers, R.F.; Sizer, I.W. A Spectrophotometric Method for Measuring the Breakdown of Hydrogen Peroxide by Catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Ardlie, N.G.; Han, P. Enzymatic Basis for Platelet Aggregation and Release: The Significance of the ‘Platelet Atmosphere’ and the Relationship between Platelet Function and Blood Coagulation. Br. J. Haematol. 1974, 26, 331–356. [Google Scholar] [CrossRef]
- Gangaraju, S.; Manjappa, B.; Subbaiah, G.K.; Kempaiah, K.; Shashidharamurthy, R.; Plow, J.H.; Martin, S.S.; Shinde, M.; Sannaningaiah, D. Jackfruit (Artocarpus Heterophyllus) Seed Extract Exhibits Fibrino(Geno)Lytic Activity. Pharmacogn. J. 2015, 7, 171–177. [Google Scholar] [CrossRef]
- Denis, C.; Methia, N.; Frenette, P.S.; Rayburn, H.; Ullman-Culleré, M.; Hynes, R.O.; Wagner, D.D. A Mouse Model of Severe von Willebrand Disease: Defects in Hemostasis and Thrombosis. Proc. Natl. Acad. Sci. USA 1998, 95, 9524–9529. [Google Scholar] [CrossRef] [PubMed]
- Born, G.V.R.; Cross, M.J. The Aggregation of Blood Platelets. J. Physiol. 1963, 168, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Quick, A.J. Hemophilia: Quantitative Studies of the Coagulation Defect: A Modified Prothrombin-Consumption Test Using Erythrocytin. AMA Arch. Int. Med. 1956, 97, 524. [Google Scholar] [CrossRef] [PubMed]

| Biochemical Parameter | Group I Control | Group II DFC Alone | Group III DFC+Syl | Group IV DFC + TAFEMgONPs (50 mg/kg) | Group V DFC + TAFEMgONPs (75 mg/kg) | Group VI DFC + TAFEMgONPs (100 mg/kg) | Group VII TAFEMgONPs (100 mg/kg) |
|---|---|---|---|---|---|---|---|
| Albumin (g/dL) | 3.92 | 1.365 | 3.34 | 3.55 | 3.20 | 3.083 | 3.29 |
| Globulin (g/dL) | 4.48 | 2.73 | 4.26 | 4.06 | 4.49 | 4.683 | 4.71 |
| Total protein (g/dL) | 8.4 | 5.095 | 7.6 | 7.7 | 7.70 | 8.1 | 8.0 |
| Bilirubin (total) (g/dL) | 0.27 | 0.605 | 0.26 | 0.32 | 0.323 | 0.263 | 0.3 |
| Bilirubin (Direct) (g/dL) | 0.08 | 0.3 | 0.04 | 0.11 | 0.12 | 0.07 | 0.096 |
| Bilirubin (Indirect) (g/dL) | 0.19 | 0.305 | 0.22 | 0.2 | 0.203 | 0.193 | 0.203 |
| SGOT (U/L) | 176.4 | 218.0 | 180.6 | 165.8 | 167.23 | 174.3 | 176.06 |
| SGPT (U/L) | 164.5 | 245.6 | 178.6 | 156.1 | 159.4 | 160.13 | 157.26 |
| Alkaline phosphatase (U/L) | 115.6 | 200.0 | 149.6 | 131.23 | 120.23 | 123.6 | 109.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatappa, M.M.; Udagani, C.; Hanumegowda, S.M.; Pramod, S.N.; Venkataramaiah, S.; Rangappa, R.; Achur, R.; Alataway, A.; Dewidar, A.Z.; Al-Yafrsi, M.; et al. Effect of Biofunctional Green Synthesized MgO-Nanoparticles on Oxidative-Stress-Induced Tissue Damage and Thrombosis. Molecules 2022, 27, 5162. https://doi.org/10.3390/molecules27165162
Venkatappa MM, Udagani C, Hanumegowda SM, Pramod SN, Venkataramaiah S, Rangappa R, Achur R, Alataway A, Dewidar AZ, Al-Yafrsi M, et al. Effect of Biofunctional Green Synthesized MgO-Nanoparticles on Oxidative-Stress-Induced Tissue Damage and Thrombosis. Molecules. 2022; 27(16):5162. https://doi.org/10.3390/molecules27165162
Chicago/Turabian StyleVenkatappa, Manjula M., Chikkappa Udagani, Sujatha M. Hanumegowda, Siddanakoppalu N. Pramod, Shivakumar Venkataramaiah, Rajesh Rangappa, Rajeshwara Achur, Abed Alataway, Ahmed Z. Dewidar, Mohamed Al-Yafrsi, and et al. 2022. "Effect of Biofunctional Green Synthesized MgO-Nanoparticles on Oxidative-Stress-Induced Tissue Damage and Thrombosis" Molecules 27, no. 16: 5162. https://doi.org/10.3390/molecules27165162






