A Novel Class of Cyclometalated Platinum(II) Complexes for Solution-Processable OLEDs
Abstract
:1. Introduction
2. Results and Discussion
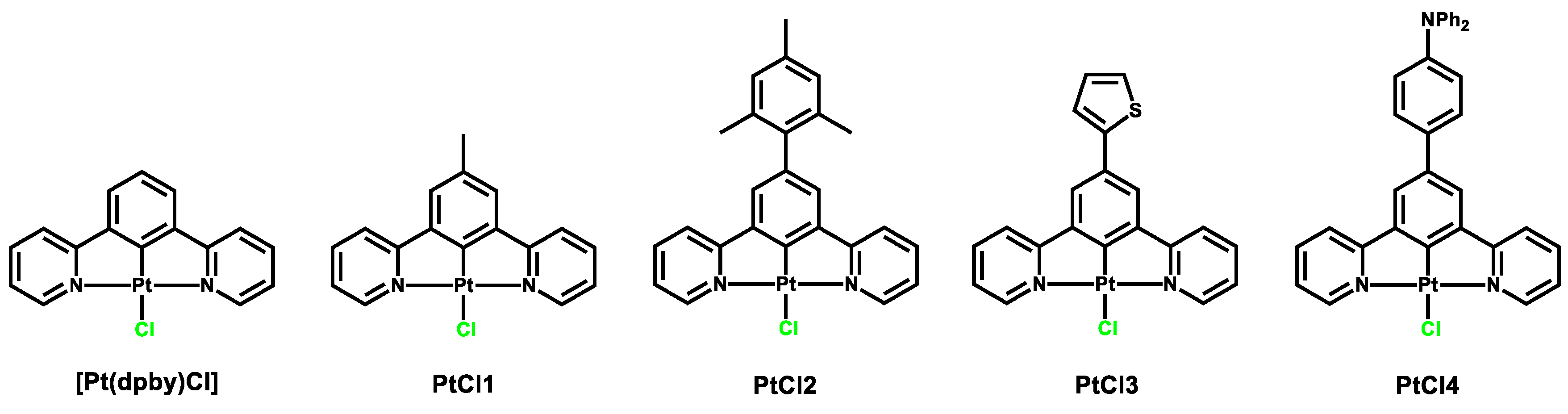
2.1. Photophysical Properties
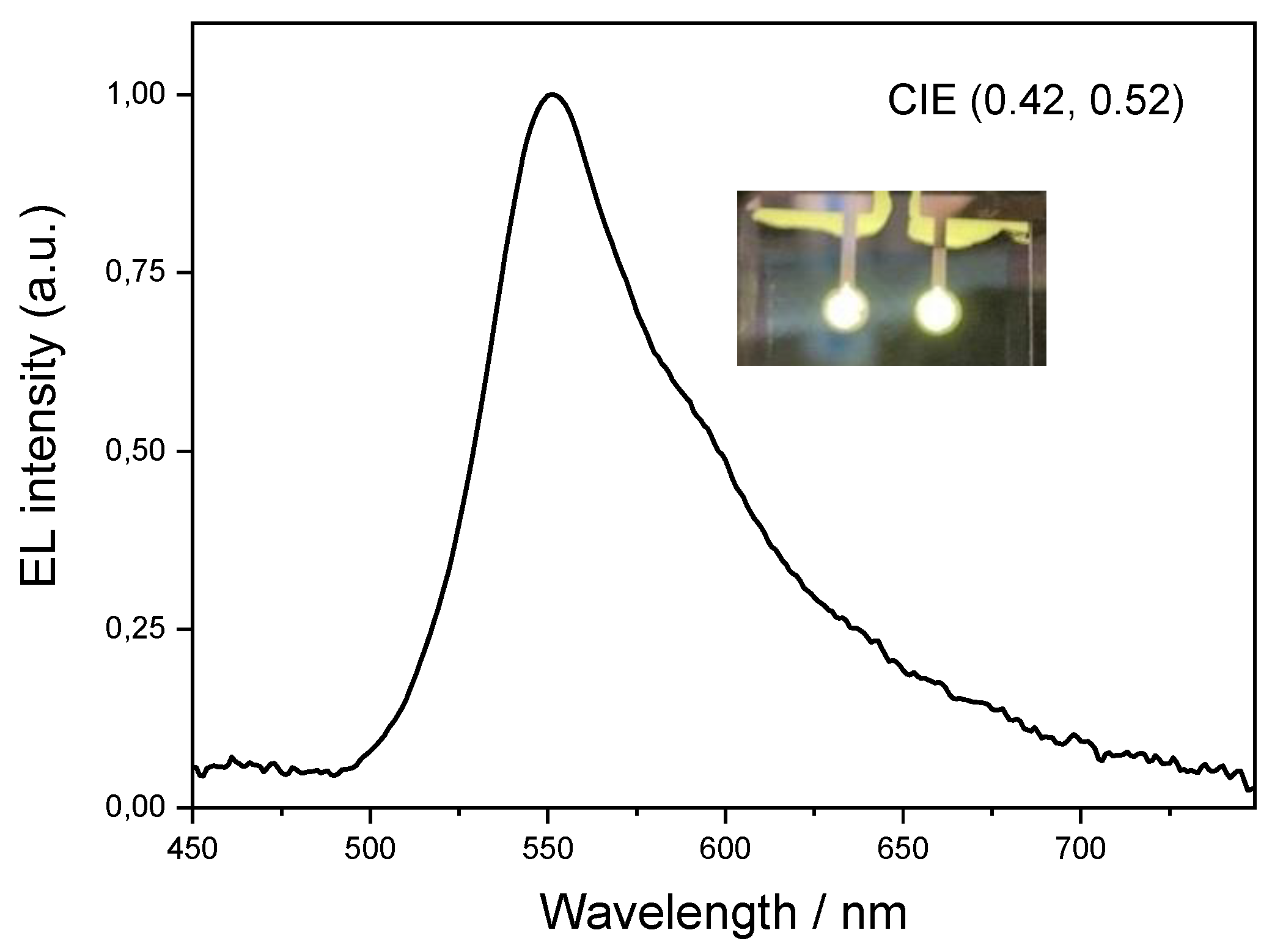
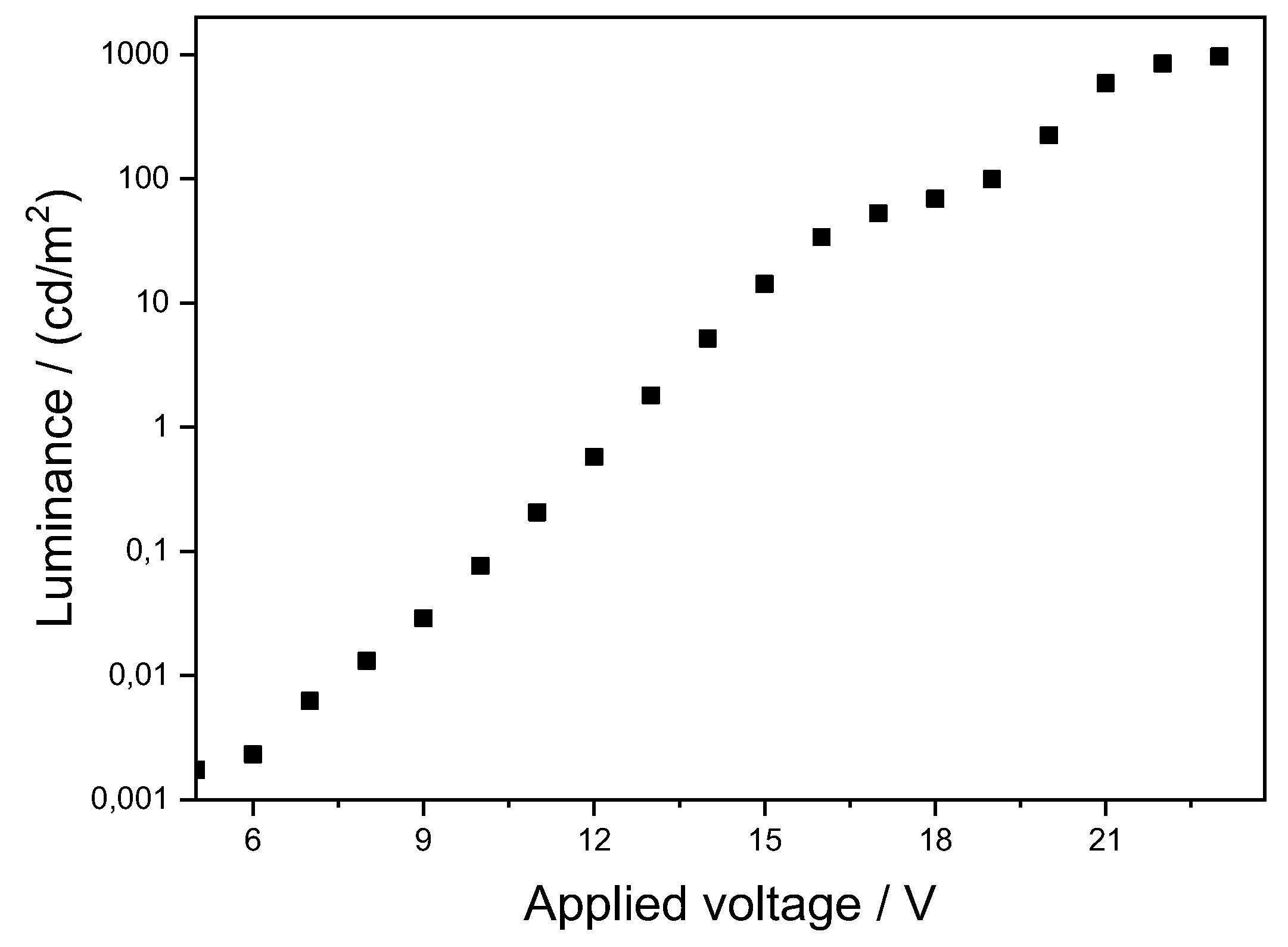
2.2. OLED Device Produced with Pt3
3. Materials and Methods
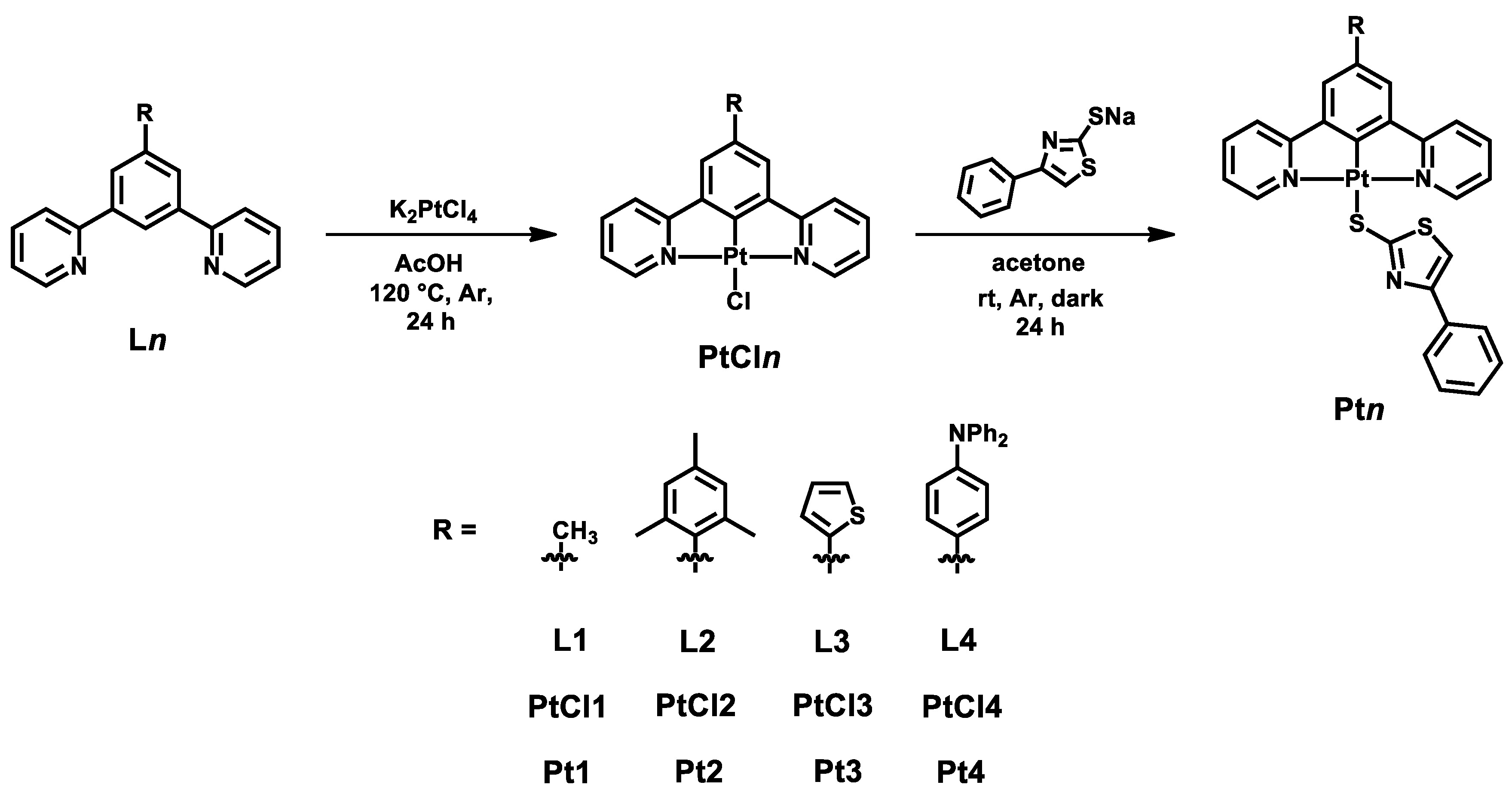
General Synthesis of Complexes Pt1–Pt4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Puttock, E.V.; Melissa, T.W.; Williams, J.A.G. The luminescence properties of multinuclear platinum complexes. Coord. Chem Rev. 2018, 367, 127–162. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Law, A.S.-Y. Luminescent d8 metal complexes of platinum(II) and gold(III): From photophysics to photofunctional materials and probes. Coord. Chem. Rev. 2020, 414, 213298. [Google Scholar] [CrossRef]
- Tang, M.-C.; Chan, A.K.-W.; Chan, M.-Y.; Yam, V.W.-W. Platinum and Gold Complexes for OLEDs. Top. Curr. Chem. 2016, 374, 46. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.G.; Develay, S.; Rochester, D.L.; Murphy, L. Optimising the luminescence of platinum (II) complexes and their application in organic light emitting devices (OLEDs). Coord. Chem. Rev. 2008, 252, 2596–2611. [Google Scholar] [CrossRef]
- Kalinowski, J.; Fattori, V.; Cocchi, M.; Williams, J.A.G. Light-emitting devices based on organometallic platinum complexes as emitters. Coord. Chem. Rev. 2011, 255, 2401–2425. [Google Scholar] [CrossRef]
- Baggaley, E.; Weinstein, J.A.; Williams, J.A.G. Lighting the way to see inside the live cell with luminescent transition metal complexes. Coord. Che. Rev. 2012, 256, 1762–1785. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, C.; Li, F. Phosphorescent heavy-metal complexes for bioimaging. Chem. Soc. Rev. 2011, 40, 2508–2524. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.K.-W.; Choi, A.W.-T.; Law, W.H.-T. Applications of luminescent inorganic and organometallic transition metal complexes as biomolecular and cellular probes. Dalton Trans. 2012, 41, 6021–6047. [Google Scholar] [CrossRef]
- Farley, S.J.; Rochester, D.L.; Thompson, A.L.; Howard, J.A.K.; Williams, J.A.G. Controlling Emission Energy, Self-Quenching, and Excimer Formation in Highly Luminescent N^C^N-Coordinated Platinum(II) Complexes. Inorg. Chem. 2005, 44, 9690–9703. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.; Brulatti, P.; Fattori, V.; Cocchi, M.; Williams, J.A.G. Blue-Shifting the Monomer and Excimer Phosphorescence of Tridentate Cyclometallated Platinum(II) Complexes for Optimal White-Light OLEDs. Chem. Commun. 2012, 48, 5817–5819. [Google Scholar] [CrossRef]
- Rossi, E.; Colombo, A.; Dragonetti, C.; Roberto, D.; Demartin, F.; Cocchi, M.; Brulatti, P.; Fattori, V.; Williams, J.A.G. From Red to near Infra-Red OLEDs: The Remarkable Effect of Changing from X = -Cl to -NCS in a Cyclometallated [Pt(N^C^N)X] Complex (N^C^N = 5-Mesityl-1,3-Di-(2-Pyridyl)Benzene). Chem. Commun. 2012, 48, 3182–3184. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Murphy, L.; Brothwood, P.L.; Colombo, A.; Dragonetti, C.; Roberto, D.; Ugo, R.; Cocchi, M.; Williams, J.A.G. Cyclometallated Platinum(II) Complexes of 1,3-Di(2-Pyridyl)Benzenes: Tuning Excimer Emission from Red to near-Infrared for NIR-OLEDs. J. Mater. Chem. 2011, 21, 15501–15510. [Google Scholar] [CrossRef]
- Nisic, F.; Colombo, A.; Dragonetti, C.; Roberto, D.; Valore, A.; Malicka, J.M.; Cocchi, M.; Freeman, G.R.; Williams, J.A.G. Platinum(II) Complexes with Cyclometallated 5-π-Delocalized-Donor-1,3- Di(2-Pyridyl)Benzene Ligands as Efficient Phosphors for NIR-OLEDs. J. Mater. Chem. C 2014, 2, 1791–1800. [Google Scholar] [CrossRef]
- Rao, Y.L.; Schoenmakers, D.; Chang, Y.L.; Lu, J.S.; Lu, Z.H.; Kang, Y.; Wang, S. Bluish-Green BMes2-Functionalized PtII Complexes for High Efficiency PhOLEDs: Impact of the BMes2 Location on Emission Color. Eur. J. Chem. 2012, 18, 11306–11316. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, M.; Kalinowski, J.; Fattori, V.; Williams, J.A.G.; Murphy, L. Color-Variable Highly Efficient Organic Electrophosphorescent Diodes Manipulating Molecular Exciton and Excimer Emissions. Appl. Phys. Lett. 2009, 94, 073309. [Google Scholar] [CrossRef]
- Cocchi, M.; Virgili, D.; Fattori, V.; Rochester, D.L.; Williams, J.A.G. N∧C∧N-Coordinated Platinum(II) Complexes as Phosphorescent Emitters in High-Performance Organic Light-Emitting Devices. Adv. Funct. Mater. 2007, 17, 285–289. [Google Scholar] [CrossRef]
- Doherty, R.E.; Sazanovich, I.V.; McKenzie, L.K.; Stasheuski, A.S.; Coyle, R.; Baggaley, E.; Bottomley, S.; Weinstein, J.A.; Bryant, H.E. Photodynamic Killing of Cancer Cells by a Platinum(II) Complex with Cyclometallating Ligand. Sci. Rep. 2016, 6, 22668. [Google Scholar] [CrossRef] [PubMed]
- Chatzisideri, T.; Thysiadis, S.; Katsamakas, S.; Dalezis, P.; Sigala, I.; Lazarides, T.; Nikolakaki, E.; Trafalis, D.; Gederaas, O.A.; Lindgren, M.; et al. Synthesis and Biological Evaluation of a Platinum(II)-c(RGDyK) Conjugate for Integrin-Targeted Photodynamic Therapy. Eur. J. Med. Chem. 2017, 141, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Baggaley, E.; Sazanovich, I.V.; Williams, J.A.G.; Haycock, J.W.; Botchway, S.W.; Weinstein, J.A. Two-Photon Phosphorescence Lifetime Imaging of Cells and Tissues Using a Long-Lived Cyclometallated Npyridyl^Cphenyl^N Pyridyl Pt(II) Complex. RSC Adv. 2014, 4, 35003–35008. [Google Scholar] [CrossRef]
- Botchway, S.W.; Charnley, M.; Haycock, J.W.; Parker, A.W.; Rochester, D.L.; Weinstein, J.A.; Williams, J.A.G. Time-Resolved and Two-Photon Emission Imaging Microscopy of Live Cells with Inert Platinum Complexes. Proc. Natl. Acad. Sci. USA 2008, 105, 16071–16076. [Google Scholar] [CrossRef]
- Raza, A.; Colley, H.E.; Baggaley, E.; Sazanovich, I.V.; Green, N.H.; Weinstein, J.A.; Botchway, S.W.; MacNeil, S.; Haycock, J.W. Oxygen Mapping of Melanoma Spheroids Using Small Molecule Platinum Probe and Phosphorescence Lifetime Imaging Microscopy. Sci. Rep. 2017, 7, 10743. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.; Fiorini, F.; Septiadi, D.; Dragonetti, C.; Nisic, F.; Valore, A.; Roberto, D.; Mauro, M.; de Cola, L. Neutral N^C^N Terdentate Luminescent Pt(II) Complexes: Their Synthesis, Photophysical Properties, and Bio-Imaging Applications. Dalton Trans. 2015, 44, 8478–8487. [Google Scholar] [CrossRef] [PubMed]
- Baggaley, E.; Botchway, S.W.; Haycock, J.W.; Morris, H.; Sazanovich, I.V.; Williams, J.A.G.; Weinstein, J.A. Long-Lived Metal Complexes Open up Microsecond Lifetime Imaging Microscopy under Multiphoton Excitation: From FLIM to PLIM and Beyond. Chem. Sci. 2014, 5, 879–886. [Google Scholar] [CrossRef]
- Mróz, W.; Botta, C.; Giovanella, U.; Rossi, E.; Colombo, A.; Dragonetti, C.; Roberto, D.; Ugo, R.; Valore, A.; Williams, J.A.G. Cyclometallated Platinum(II) Complexes of 1,3-Di(2-Pyridyl)Benzenes for Solution-Processable WOLEDs Exploiting Monomer and Excimer Phosphorescence. J. Mater. Chem. 2011, 21, 8653–8661. [Google Scholar] [CrossRef]
- Shi, H.; Clarkson, G.J.; Sadler, P.J. Dual Action Photosensitive Platinum(II) Anticancer Prodrugs with Photoreleasable Azide Ligands. Inorg. Chim. Acta 2019, 489, 230–235. [Google Scholar] [CrossRef]
- Scarpaci, A.; Monnereau, C.; Hergué, N.; Blart, E.; Legoupy, S.; Odobel, F.; Gorfo, A.; Pérez-Moreno, J.; Clays, K.; Asselberghs, I. Preparation and Characterization of Second Order Non-Linear Optical Properties of New “Push-Pull” Platinum Complexes. Dalton Trans. 2009, 4538–4546. [Google Scholar] [CrossRef] [PubMed]
- Lien, C.Y.; Hsu, Y.F.; Liu, Y.H.; Peng, S.M.; Shinmyozu, T.; Yang, J.S. Steric Engineering of Cyclometalated Pt(II) Complexes toward High-Contrast Monomer-Excimer-Based Mechanochromic and Vapochromic Luminescence. Inorg. Chem. 2020, 59, 11584–11594. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Liu, Y.H.; Peng, S.M.; Shinmyozu, T.; Yang, J.S. Excimer-Monomer Photoluminescence Mechanochromism and Vapochromism of Pentiptycene-Containing Cyclometalated Platinum(II) Complexes. Inorg. Chem. 2017, 56, 4978–4989. [Google Scholar] [CrossRef]
- Rossi, E.; Colombo, A.; Dragonetti, C.; Roberto, D.; Ugo, R.; Valore, A.; Falciola, L.; Brulatti, P.; Cocchi, M.; Williams, J.A.G. Novel N^C^N-Cyclometallated Platinum Complexes with Acetylide Co-Ligands as Efficient Phosphors for OLEDs. J. Mater. Chem. 2012, 22, 10650–10655. [Google Scholar] [CrossRef]
- Ai, Y.; Chan, M.H.-Y.; Chan, A.K.-W.; Ng, M.; Li, Y.; Yam, V.W.-W. A platinum(II) molecular hinge with motions visualized by phosphorescence changes. Proc. Natl. Acad. Sci. USA 2019, 116, 13856–13861. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, Y.; Chan, M.H.Y.; Yam, V.W.W. Phosphorescent Cyclometalated Platinum(II) Enantiomers with Circularly Polarized Luminescence Properties and Their Assembly Behaviors. J. Am. Chem. Soc. 2021, 143, 21676–21684. [Google Scholar] [CrossRef] [PubMed]
- Garoni, E.; Boixel, J.; Dorcet, V.; Roisnel, T.; Roberto, D.; Jacquemin, D.; Guerchais, V. Controlling the Emission in Flexibly-Linked (N^C^N)Platinum Dyads. Dalton Trans. 2018, 47, 224–232. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Yamawaki, K.; Kimura, T.; Kuwabara, J.; Yasuda, T.; Nishimura, Y.; Kanbara, T. Multi-Molecular Emission of a Cationic Pt(II) Complex through Hydrogen Bonding Interactions. Dalton Trans. 2018, 47, 4087–4092. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, J.; Yamaguchi, K.; Yamawaki, K.; Yasuda, T.; Nishimura, Y.; Kanbara, T. Modulation of the Emission Mode of a Pt(II) Complex via Intermolecular Interactions. Inorg. Chem. 2017, 56, 8726–8729. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, W.; Che, C.M. Luminescent Pincer-Type Cyclometalated Platinum(II) Complexes with Auxiliary Isocyanide Ligands: Phase-Transfer Preparation, Solvatomorphism, and Self-Aggregation. Organometallics 2013, 32, 350–353. [Google Scholar] [CrossRef]
- Wang, Z.; Turner, E.; Mahoney, V.; Madakuni, S.; Groy, T.; Li, J. Facile Synthesis and Characterization of Phosphorescent Pt(N^C^N)X Complexes. Inorg. Chem. 2010, 49, 11276–11286. [Google Scholar] [CrossRef] [PubMed]
- Tarran, W.A.; Freeman, G.R.; Murphy, L.; Benham, A.M.; Kataky, R.; Williams, J.A.G. Platinum(II) Complexes of N^C^N- Coordinating 1,3-Bis(2-Pyridyl)Benzene Ligands: Thiolate Coligands Lead to Strong Red Luminescence from Charge-Transfer States. Inorg. Chem. 2014, 53, 5738–5749. [Google Scholar] [CrossRef]
- Zheng, Q.; Borsley, S.; Tu, T.; Cockroft, S.L. Reversible Stimuli-Responsive Chromism of a Cyclometallated Platinum(Ii) Complex. Chem. Commun. 2020, 56, 14705–14708. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Borsley, S.; Nichol, G.S.; Duarte, F.; Cockroft, S.L. The Energetic Significance of Metallophilic Interactions. Angew. Chem. Int. Ed. 2019, 58, 12617–12623. [Google Scholar] [CrossRef]
- Fagnani, F.; Colombo, A.; Dragonetti, C.; Roberto, D.; Marinotto, D. The Intriguing Effect of Thiolates as Co-Ligands in Platinum(II) Complexes Bearing a Cyclometalated 1,3-Di(2-Pyridyl)Benzene. Inorg. Chim. Acta 2022, 532, 120744. [Google Scholar] [CrossRef]
- Dragonetti, C.; Fagnani, F.; Marinotto, D.; di Biase, A.; Roberto, D.; Cocchi, M.; Fantacci, S.; Colombo, A. First Member of an Appealing Class of Cyclometalated 1,3-Di-(2-Pyridyl)Benzene Platinum(II) Complexes for Solution-Processable OLEDs. J. Mater. Chem. C 2020, 8, 7873–7881. [Google Scholar] [CrossRef]
- Dragonetti, C.; Colombo, A.; Fagnani, F.; de Soricellis, G.; Cocchi, M.; Carboni, B.; Guerchais, V.; Marinotto, D. Introduction of a Triphenylamine Substituent on Pyridyl Rings as a Springboard for a New Appealing Brightly Luminescent 1,3-Di-(2-Pyridyl)Benzene Platinum(II) Complex Family. Dalton Trans. 2022. [Google Scholar] [CrossRef]
- Rochester, D.L.; Develay, S.; Záliš, S.; Williams, J.A.G. Localised to Intraligand Charge-Transfer States in Cyclometalated Platinum Complexes: An Experimental and Theoretical Study into the Influence of Electron-Rich Pendants and Modulation of Excited States by Ion Binding. Dalton Trans. 2009, 10, 1728–1741. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Kobayashi, A.; Kaneko, S.; Takehira, K.; Yoshihara, T.; Ishida, H.; Shiina, Y.; Oishic, S.; Tobita, S. Reevaluation of absolute luminescence quantum yields of standard solutions using a spectrometer with an integrating sphere and a back-thinned CCD detector. Phys. Chem. Chem. Phys. 2009, 11, 9850–9860. [Google Scholar] [CrossRef]

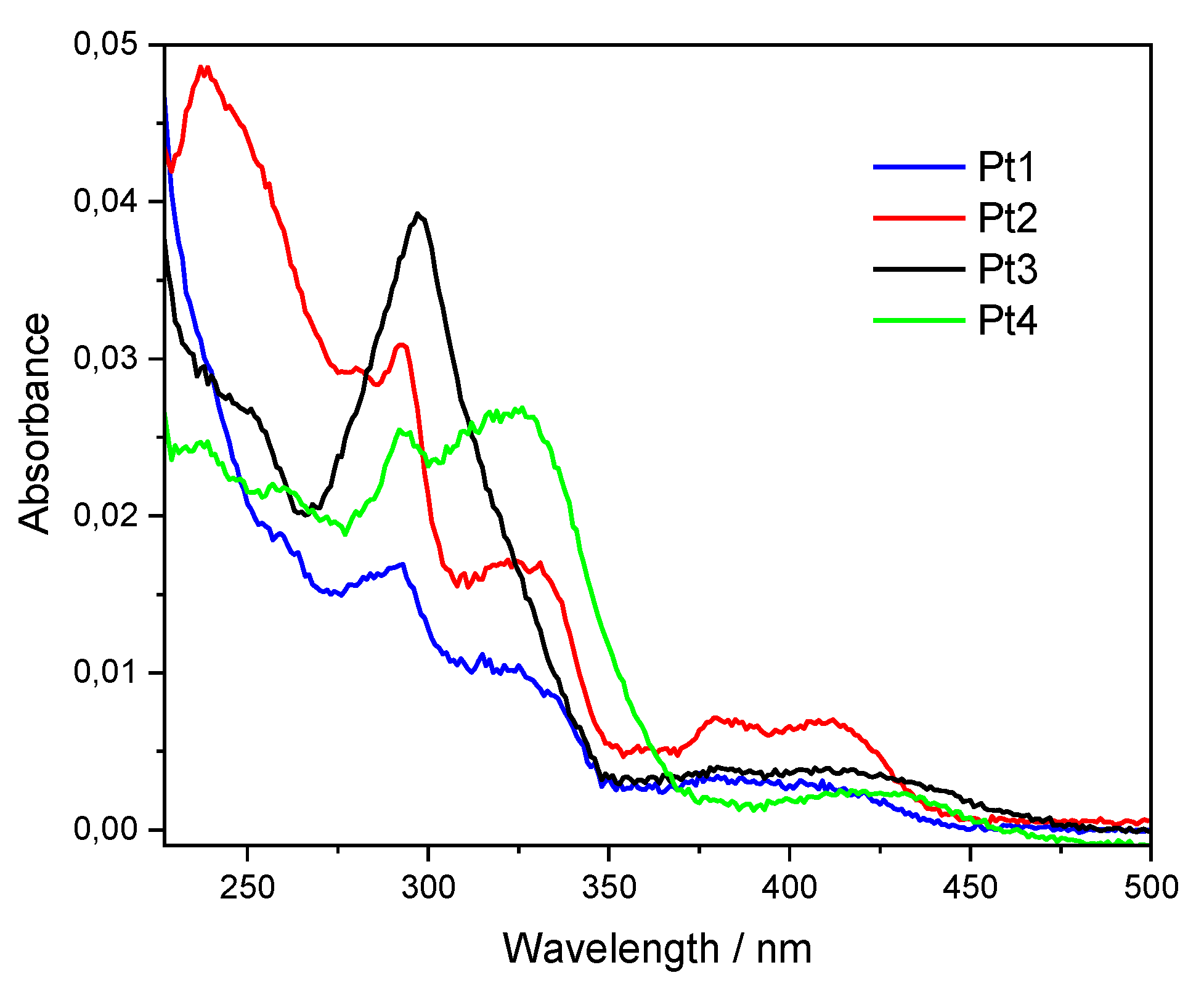
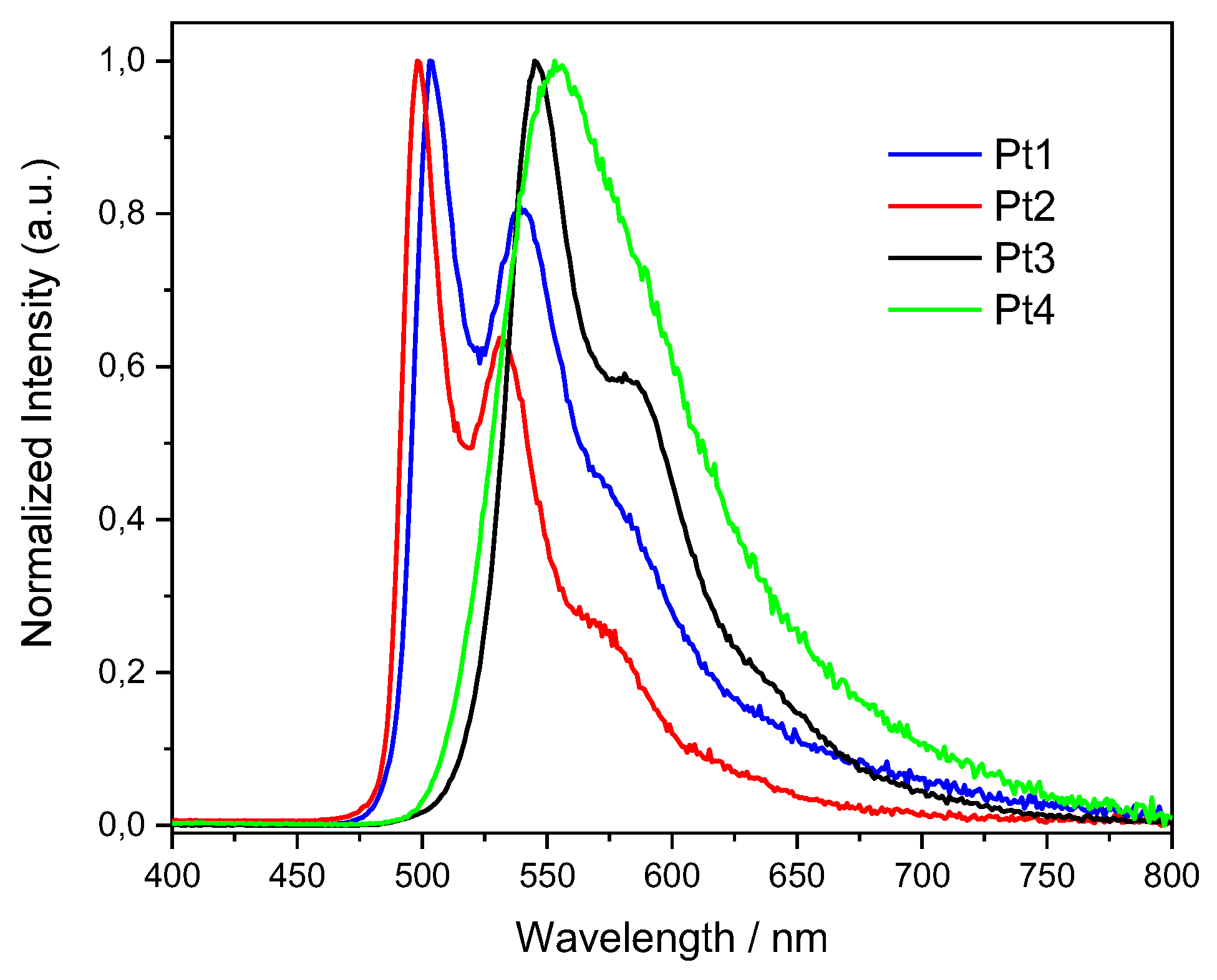
| Complex | λmax, em/nm | Φlum before FPT/% | Φlum after FPT/% | τ/µs |
|---|---|---|---|---|
| PtCl11 | 505 | - | 68 | 7.8 |
| PtCl21 | 501 | - | 62 | 7.9 |
| PtCl31 | 548 | - | 54 | 20.5 |
| PtCl42 | 557 | - | 29 | 9.0 |
| Pt13 | 503 | 2.5 | 65 | 7.9 |
| Pt23 | 498 | 3.5 | 55 | 7.7 |
| Pt33 | 545 | 3.0 | 89 | 19.1 |
| Pt43 | 554 | 2.5 | 72 | 13.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roberto, D.; Colombo, A.; Dragonetti, C.; Fagnani, F.; Cocchi, M.; Marinotto, D. A Novel Class of Cyclometalated Platinum(II) Complexes for Solution-Processable OLEDs. Molecules 2022, 27, 5171. https://doi.org/10.3390/molecules27165171
Roberto D, Colombo A, Dragonetti C, Fagnani F, Cocchi M, Marinotto D. A Novel Class of Cyclometalated Platinum(II) Complexes for Solution-Processable OLEDs. Molecules. 2022; 27(16):5171. https://doi.org/10.3390/molecules27165171
Chicago/Turabian StyleRoberto, Dominique, Alessia Colombo, Claudia Dragonetti, Francesco Fagnani, Massimo Cocchi, and Daniele Marinotto. 2022. "A Novel Class of Cyclometalated Platinum(II) Complexes for Solution-Processable OLEDs" Molecules 27, no. 16: 5171. https://doi.org/10.3390/molecules27165171
APA StyleRoberto, D., Colombo, A., Dragonetti, C., Fagnani, F., Cocchi, M., & Marinotto, D. (2022). A Novel Class of Cyclometalated Platinum(II) Complexes for Solution-Processable OLEDs. Molecules, 27(16), 5171. https://doi.org/10.3390/molecules27165171









