Biological Applications of Ball-Milled Synthesized Biochar-Zinc Oxide Nanocomposite Using Zea mays L.
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material
2.2. Preparation of the Biochar
2.3. Preparation of the MB-ZnO Nanocomposites
2.4. Characterization of the Nanocomposites
2.5. Biocompatibility Assays
2.6. Protein Kinase Inhibition Assay (PK)
2.7. Alpha-Amylase (AA) Inhibition Assay
2.8. Antileishmanial Potential of MB-ZnO
2.9. Anti-Inflammatory Activity
2.10. Antioxidant Assays
2.10.1. Total Antioxidant Capacity Determination (TAC)
2.10.2. Total Reducing Power Determination (TRP)
2.10.3. Free Radical Scavenging Assay (FRSA)
2.11. Antifungal Activity
2.12. Statistical Analysis
3. Results and Discussion
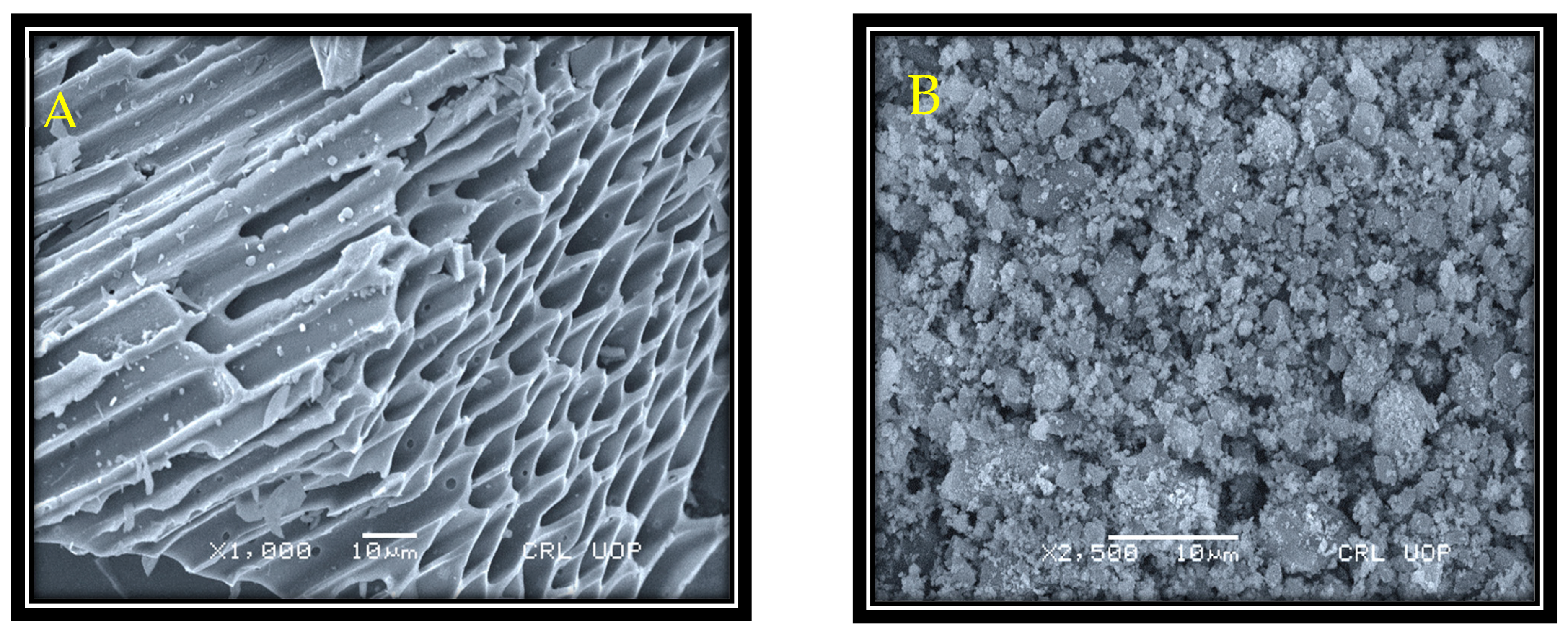
3.1. SEM and EDX Study
3.2. X-ray Powder Diffraction Analysis
3.3. FTIR Spectroscopy
3.4. Thermogravimetric Analysis (TGA)
3.5. UV Analysis
3.6. Antioxidant Potential of MB-ZnO Nanocomposites
3.7. Anti-Inflammatory Activity
3.8. Enzyme Inhibition Potential
3.9. Biocompatibility Potential Assay
3.10. Antileishmanial Activity
3.11. Antifungal Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Khan, S.A.; Bello, B.A.; Khan, J.A.; Anwar, Y.; Mirza, M.B.; Qadri, F.; Farooq, A.; Adam, I.K.; Asiri, A.M.; Khan, S.B. Albizia chevalier based Ag nanoparticles: Anti-proliferation, bactericidal and pollutants degradation performance. J. Photochem. Photobiol. B Biol. 2018, 182, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Saqib, S.; Nazeer, A.; Ali, M.; Zaman, W.; Younas, M.; Shahzad, A.; Sunera; Nisar, M. Catalytic potential of endophytes facilitates synthesis of biometallic zinc oxide nanoparticles for agricultural application. BioMetals 2022, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Khatami, M.; Varma, R.S.; Zafarnia, N.; Yaghoobi, H.; Sarani, M.; Kumar, V.G. Applications of green synthesized Ag, ZnO and Ag/ZnO nanoparticles for making clinical antimicrobial wound-healing bandages. Sustain. Chem. Pharm. 2018, 10, 9–15. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Ahmad, R.; Mahmood, T.; Ali, B.; Khalil, A.T.; Kanwal, S.; Shah, S.A.; Alam, M.M.; Badshah, H.; et al. Nanomedicines for developing cancer nanotherapeutics: From benchtop to bedside and beyond. Appl. Microbiol. Biotechnol. 2018, 102, 9449–9470. [Google Scholar] [CrossRef]
- Wu, Q.; Nouara, A.; Li, Y.; Zhang, M.; Wang, W.; Tang, M.; Ye, B.; Ding, J.; Wang, D. Comparison of toxicities from three metal oxide nanoparticles at environmental relevant concentrations in nematode Caenorhabditis elegans. Chemosphere 2013, 90, 1123–1131. [Google Scholar] [CrossRef]
- Asghar, M.; Habib, S.; Zaman, W.; Hussain, S.; Ali, H.; Saqib, S. Synthesis and characterization of microbial mediated cadmium oxide nanoparticles. Microsc. Res. Tech. 2020, 83, 1574–1584. [Google Scholar] [CrossRef]
- Khatoon, U.T.; Mantravadi, K.M.; Rao, G.V.S.N. Strategies to synthesise copper oxide nanoparticles and their bio applications—A review. Mater. Sci. Technol. 2018, 34, 2214–2222. [Google Scholar] [CrossRef]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Román, L.E.; Huachani, J.; Uribe, C.; Solis, J.L.; Gómez, M.; Costa, S.; Costa, S. Blocking erythemally weighted UV radiation using cotton fabrics functionalized with ZnO nanoparticles in situ. Appl. Surf. Sci. 2019, 469, 204–212. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Chen, K.-C.; Tsai, T.-Y.; Hsueh, T.-J. Fabrication of gas sensor based on p-type ZnO nanoparticles and n-type ZnO nanowires. Sens. Actuators B Chem. 2013, 182, 190–196. [Google Scholar] [CrossRef]
- Yodyingyong, S.; Zhang, Q.; Park, K.; Dandeneau, C.S.; Zhou, X.; Triampo, D.; Cao, G. ZnO nanoparticles and nanowire array hybrid photoanodes for dye-sensitized solar cells. Appl. Phys. Lett. 2010, 96, 073115. [Google Scholar] [CrossRef]
- Arya, S.; Lehana, P.K.; Rana, S.B. Synthesis of Zinc Oxide Nanoparticles and Their Morphological, Optical, and Electrical Characterizations. J. Electron. Mater. 2017, 46, 4604–4611. [Google Scholar] [CrossRef]
- Shrestha, P.K.; Chun, Y.T.; Chu, D. A high-resolution optically addressed spatial light modulator based on ZnO nanoparticles. Light. Sci. Appl. 2015, 4, e259. [Google Scholar] [CrossRef]
- Bhuyan, T.; Mishra, K.; Khanuja, M.; Prasad, R.; Varma, A. Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater. Sci. Semicond. Process. 2015, 32, 55–61. [Google Scholar] [CrossRef]
- Moustafa, H.; Youssef, A.M.; Darwish, N.A.; Abou-Kandil, A.I. Eco-friendly polymer composites for green packaging: Future vision and challenges. Compos. Part B Eng. 2019, 172, 16–25. [Google Scholar] [CrossRef]
- Nagajyothi, P.; Cha, S.J.; Yang, I.J.; Sreekanth, T.; Kim, K.J.; Shin, H.M. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J. Photochem. Photobiol. B Biol. 2015, 146, 10–17. [Google Scholar] [CrossRef]
- Azam, Z.; Ayaz, A.; Younas, M.; Qureshi, Z.; Arshad, B.; Zaman, W.; Ullah, F.; Nasar, M.Q.; Bahadur, S.; Irfan, M.M.; et al. Microbial synthesized cadmium oxide nanoparticles induce oxidative stress and protein leakage in bacterial cells. Microb. Pathog. 2020, 144, 104188. [Google Scholar] [CrossRef]
- Samat, N.A.; Nor, R. Sol–gel synthesis of zinc oxide nanoparticles using Citrus aurantifolia extracts. Ceram. Int. 2013, 39, S545–S548. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, J. Electrodeposition of silver nanoparticles on a zinc oxide film: Improvement of amperometric sensing sensitivity and stability for hydrogen peroxide determination. Microchim. Acta 2010, 169, 361–365. [Google Scholar] [CrossRef]
- Javanbakht, S.; Pooresmaeil, M.; Namazi, H. Green one-pot synthesis of carboxymethylcellulose/Zn-based metal-organic framework/graphene oxide bio-nanocomposite as a nanocarrier for drug delivery system. Carbohydr. Polym. 2019, 208, 294–301. [Google Scholar] [CrossRef]
- Namazi, H.; Belali, S. Starch-g-lactic acid/montmorillonite nanocomposite: Synthesis, characterization and controlled drug release study. Starch-Stärke 2015, 68, 177–187. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Nahrawy, A.M.; Abou Hammad, A.B. Sol-gel synthesis and characterizations of hybrid chitosan-PEG/calcium silicate nanocomposite modified with ZnO-NPs and (E102) for optical and antibacterial applications. Int. J. Biol. Macromol. 2017, 97, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Pudełko, A.; Postawa, P.; Stachowiak, T.; Malińska, K.; Dróżdż, D. Waste derived biochar as an alternative filler in biocomposites-Mechanical, thermal and morphological properties of biochar added biocomposites. J. Clean. Prod. 2021, 278, 123850. [Google Scholar] [CrossRef]
- Saqib, S.; Zaman, W.; Ullah, F.; Majeed, I.; Ayaz, A.; Munis, M.F.H. Organometallic assembling of chitosan-Iron oxide nanoparticles with their antifungal evaluation against Rhizopus oryzae. Appl. Organomet. Chem. 2019, 33, e5190. [Google Scholar] [CrossRef]
- Chen, M.; Bao, C.; Hu, D.; Jin, X.; Huang, Q. Facile and low-cost fabrication of ZnO/biochar nanocomposites from jute fibers for efficient and stable photodegradation of methylene blue dye. J. Anal. Appl. Pyrolysis 2019, 139, 319–332. [Google Scholar] [CrossRef]
- Gonçalves, M.G.; da Silva Veiga, P.A.; Fornari, M.R.; Peralta-Zamora, P.; Mangrich, A.S.; Silvestri, S. Relationship of the physicochemical properties of novel ZnO/biochar composites to their efficiencies in the degradation of sulfamethoxazole and methyl orange. Sci. Total Environ. 2020, 748, 141381. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Wang, D. RETRACTED: Corn stover pretreatment by metal oxides for improving lignin removal and reducing sugar degradation and water usage. Bioresour. Technol. 2018, 263, 232–241. [Google Scholar] [CrossRef]
- Li, W.; Dang, Q.; Brown, R.C.; Laird, D.; Wright, M.M. The impacts of biomass properties on pyrolysis yields, economic and environmental performance of the pyrolysis-bioenergy-biochar platform to carbon negative energy. Bioresour. Technol. 2017, 241, 959–968. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; Wan, Z.; Sun, Y.; Tsang, D.C.; Gupta, J.; Gao, B.; Cao, X.; Tang, J.; Ok, Y.S. Ball milling as a mechanochemical technology for fabrication of novel biochar nanomaterials. Bioresour. Technol. 2020, 312, 123613. [Google Scholar] [CrossRef]
- Shi, J.; Wang, J.; Liang, L.; Xu, Z.; Chen, Y.; Chen, S.; Xu, M.; Wang, X.; Wang, S. Carbothermal synthesis of biochar-supported metallic silver for enhanced photocatalytic removal of methylene blue and antimicrobial efficacy. J. Hazard. Mater. 2021, 401, 123382. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, Y.; Gao, B.; Cao, X. N-doped biochar synthesized by a facile ball-milling method for enhanced sorption of CO2 and reactive red. Chem. Eng. J. 2019, 368, 564–572. [Google Scholar] [CrossRef]
- Zheng, Y.; Wan, Y.; Chen, J.; Chen, H.; Gao, B. MgO modified biochar produced through ball milling: A dual-functional adsorbent for removal of different contaminants. Chemosphere 2020, 243, 125344. [Google Scholar] [CrossRef] [PubMed]
- Damonte, L.; Zélis, L.M.; Soucase, B.M.; Fenollosa, M.H. Nanoparticles of ZnO obtained by mechanical milling. Powder Technol. 2004, 148, 15–19. [Google Scholar] [CrossRef]
- Štrbac, D.; Aggelopoulos, C.A.; Štrbac, G.; Dimitropoulos, M.; Novaković, M.; Ivetić, T.; Yannopoulos, S.N. Photocatalytic degradation of Naproxen and methylene blue: Comparison between ZnO, TiO2 and their mixture. Process Saf. Environ. Prot. 2018, 113, 174–183. [Google Scholar] [CrossRef]
- Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Shinwari, Z.K.; Hassan, D.; Maaza, M. Sageretia thea (Osbeck.) modulated biosynthesis of NiO nanoparticles and their in vitro pharmacognostic, antioxidant and cytotoxic potential. Artif. Cells Nanomed. Biotechnol. 2018, 46, 838–852. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Ambreen, S.; Javed, R.; Tabassum, S.; Haq, I.U.; Zia, M. ZnO nanostructure fabrication in different solvents transforms physio-chemical, biological and photodegradable properties. Mater. Sci. Eng. C 2017, 74, 137–145. [Google Scholar] [CrossRef]
- Malagoli, D. A full-length protocol to test hemolytic activity of palytoxin on human erythrocytes. Invertebr. Surviv. J. 2007, 4, 92–94. [Google Scholar]
- Fatima, H.; Khan, K.; Zia, M.; Ur-Rehman, T.; Mirza, B.; Haq, I.-U. Extraction optimization of medicinally important metabolites from Datura innoxia Mill.: An in vitro biological and phytochemical investigation. BMC Complement. Altern. Med. 2015, 15, 376. [Google Scholar] [CrossRef] [Green Version]
- Ebrahiminezhad, A.; Zare-Hoseinabadi, A.; Sarmah, A.K.; Taghizadeh, S.; Ghasemi, Y.; Berenjian, A. Plant-Mediated Synthesis and Applications of Iron Nanoparticles. Mol. Biotechnol. 2018, 60, 154–168. [Google Scholar] [CrossRef]
- Ahmad, A.; Syed, F.; Imran, M.; Khan, A.U.; Tahir, K.; Khan, Z.U.H.; Yuan, Q. Photosynthesis and antileishmanial activity of gold nanoparticles by Maytenus royleanus. J. Food Biochem. 2016, 40, 420–427. [Google Scholar] [CrossRef]
- Sakat, S.; Tupe, P.; Hule, A.; Juvekar, A. Anti-inflammatory potential of flavonoid fraction of Tamarindus indica Linn (seeds). Planta Med. 2010, 76, SL_20. [Google Scholar] [CrossRef]
- Ali, M.; Iqbal, R.; Safdar, M.; Murtaza, S.; Mustafa, M.; Sajjad, M.; Bukhari, S.A.; Huma, T. Antioxidant and antibacterial activities of Artemisia absinthium and Citrus paradisi extracts repress viability of aggressive liver cancer cell line. Mol. Biol. Rep. 2021, 48, 7703–7710. [Google Scholar] [CrossRef] [PubMed]
- Javed, R.; Usman, M.; Tabassum, S.; Zia, M. Effect of capping agents: Structural, optical and biological properties of ZnO nanoparticles. Appl. Surf. Sci. 2016, 386, 319–326. [Google Scholar] [CrossRef]
- Baqi, A.; Samiullah; Tareen, R.B.; Mengal, A.; Khan, N.; Behlil, F.; Achakzai, A.K.K.; Anwer, M.; Rehman, A.U.; Faheem, M. Determination of antioxidants in two medicinally important plants, Haloxylon griffithii and Convolvulus leiocalycinus, of Balochistan. Pure Appl. Biol. 2018, 7, 296–308. [Google Scholar] [CrossRef]
- Kamal, A.; Saleem, M.H.; Alshaya, H.; Okla, M.K.; Chaudhary, H.J.; Munis, M.F.H. Ball-milled synthesis of maize biochar-ZnO nanocomposite (MB-ZnO) and estimation of its photocatalytic ability against different organic and inorganic pollutants. J. Saudi Chem. Soc. 2022, 26, 101445. [Google Scholar] [CrossRef]
- Yu, F.; Tian, F.; Zou, H.; Ye, Z.; Peng, C.; Huang, J.; Zheng, Y.; Zhang, Y.; Yang, Y.; Wei, X.; et al. ZnO/biochar nanocomposites via solvent free ball milling for enhanced adsorption and photocatalytic degradation of methylene blue. J. Hazard. Mater. 2021, 415, 125511. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, M.A.; Zeng, J.; Jagdale, P.; Castellino, M.; Sacco, A.; Farkhondehfal, M.A.; Pirri, C.F. Biochar/Zinc Oxide Composites as Effective Catalysts for Electrochemical CO2 Reduction. ACS Sustain. Chem. Eng. 2021, 9, 5445–5453. [Google Scholar] [CrossRef]
- Bakr, A.; Sayed, N.; Salama, T.; Ali, I.O.; Gayed, R.; Negm, N. Potential of Mg–Zn–Al layered double hydroxide (LDH)/montmorillonite nanocomposite in remediation of wastewater containing manganese ions. Res. Chem. Intermed. 2018, 44, 389–405. [Google Scholar] [CrossRef]
- Arfin, T.; Rangari, S.N. Graphene oxide–ZnO nanocomposite modified electrode for the detection of phenol. Anal. Methods 2018, 10, 347–358. [Google Scholar] [CrossRef]
- Chu, C.-C.; White, K.L.; Liu, P.; Zhang, X.; Sue, H.-J. Electrical conductivity and thermal stability of polypropylene containing well-dispersed multi-walled carbon nanotubes disentangled with exfoliated nanoplatelets. Carbon 2012, 50, 4711–4721. [Google Scholar] [CrossRef]
- Batra, N.; Tomar, M.; Gupta, V. Realization of an efficient cholesterol biosensor using ZnO nanostructured thin film. Analyst 2012, 137, 5854–5859. [Google Scholar] [CrossRef]
- Gholami, P.; Dinpazhoh, L.; Khataee, A.; Orooji, Y. Sonocatalytic activity of biochar-supported ZnO nanorods in degradation of gemifloxacin: Synergy study, effect of parameters and phytotoxicity evaluation. Ultrason. Sonochem. 2019, 55, 44–56. [Google Scholar] [CrossRef]
- Pietro, M.; Paola, C. Thermal analysis for the evaluation of the organic matter evolution during municipal solid waste aerobic composting process. Thermochim. Acta 2004, 413, 209–214. [Google Scholar] [CrossRef]
- Law, Z.X. A Comparative Study on the Photocatalytic Degradation of Organic Dye in the Presence of Biochar Composites Using Various Oxidants. Ph.D. Thesis, Universiti Tunku Abdul Rahman, Kampar, Malaysia, 2020. [Google Scholar]
- Teo, E.Y.L.; Muniandy, L.; Ng, E.-P.; Adam, F.; Mohamed, A.R.; Jose, R.; Chong, K.F. High surface area activated carbon from rice husk as a high performance supercapacitor electrode. Electrochim. Acta 2016, 192, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Moharram, A.H.; Mansour, S.A.; Hussein, M.A.; Rashad, M. Direct Precipitation and Characterization of ZnO Nanoparticles. J. Nanomater. 2014, 2014, 716210. [Google Scholar] [CrossRef] [Green Version]
- Ahn, K.-S.; Yan, Y.; Lee, S.-H.; Deutsch, T.; Turner, J.; Tracy, C.E.; Perkins, C.L.; Al-Jassim, M. Photoelectrochemical Properties of N-Incorporated ZnO Films Deposited by Reactive RF Magnetron Sputtering. J. Electrochem. Soc. 2007, 154, B956–B959. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, T.-R.; Zhou, B.; Cui, Y.-T.; Yan, H.; Liu, Z.; Schmitt, F.; Lee, J.; Moore, R.; Chen, Y.; et al. Direct observation of the transition from indirect to direct bandgap in atomically thin epitaxial MoSe2. Nat. Nanotechnol. 2014, 9, 111–115. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Ul-Haq, I.; Ullah, N.; Bibi, G.; Kanwal, S.; Ahmad, M.S.; Mirza, B. Antioxidant and Cytotoxic Activities and Phytochemical Analysis of Euphorbia wallichii Root Extract and its Fractions. Iran. J. Pharm. Res. 2012, 11, 241–249. [Google Scholar]
- Tamer, T.M.; Alsehli, M.H.; Omer, A.M.; Afifi, T.H.; Sabet, M.M.; Mahy-Eldin, M.S.; Hassan, M.A. Development of polyvinyl alcohol/kaolin sponges stimulated by marjoram as hemostatic, antibacterial, and antioxidant dressings for wound healing promotion. Int. J. Mol. Sci. 2021, 22, 13050. [Google Scholar] [CrossRef]
- Shanmugam, R.; Subramaniam, R.; Kathirason, S.G.; Ali, D.; Balusamy, S.R.; Gurusamy, A.; Arunachalam, K.; Sellami, H. Curcumin-Chitosan Nanocomposite Formulation Containing Pongamia pinnata-Mediated Silver Nanoparticles, Wound Pathogen Control, and Anti-Inflammatory Potential. BioMed Res. Int. 2021, 2021, 3091587. [Google Scholar] [CrossRef]
- Yao, G.; Sebisubi, F.M.; Voo, L.Y.C.; Ho, C.C.; Tan, G.T.; Chang, L.C. Citrinin derivatives from the soil filamentous fungus Penicillium sp. H9318. J. Braz. Chem. Soc. 2011, 22, 1125–1129. [Google Scholar] [CrossRef] [Green Version]
- Oyedemi, S.O.; Oyedemi, B.O.; Ijeh, I.I.; Ohanyerem, P.E.; Coopoosamy, R.M.; Aiyegoro, O.A. Alpha-Amylase Inhibition and Antioxidative Capacity of Some Antidiabetic Plants Used by the Traditional Healers in Southeastern Nigeria. Sci. World J. 2017, 2017, 3592491. [Google Scholar] [CrossRef] [Green Version]
- Dobrovolskaia, M.A.; Clogston, J.; Neun, B.W.; Hall, J.B.; Patri, A.K.; McNeil, S.E. Method for Analysis of Nanoparticle Hemolytic Properties in Vitro. Nano Lett. 2008, 8, 2180–2187. [Google Scholar] [CrossRef]
- Dash, B.C.; Réthoré, G.; Monaghan, M.; Fitzgerald, K.; Gallagher, W.; Pandit, A. The influence of size and charge of chitosan/polyglutamic acid hollow spheres on cellular internalization, viability and blood compatibility. Biomaterials 2010, 31, 8188–8197. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, J.; Abbasi, B.A.; Ahmad, R.; Mahmood, T.; Kanwal, S.; Ali, B.; Khalil, A.T.; Shah, A.; Alam, M.M.; Badshah, H. Ursolic acid a promising candidate in the therapeutics of breast cancer: Current status and future implications. Biomed. Pharmacother. 2018, 108, 752–756. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, M.; Wu, M.; Zhang, H.; Song, Q.; Yu, S. Synthesis of biochar-based Cu2O nanoparticles and their antibacterial activity against Escherichia coli. Inorg. Nano-Met. Chem. 2019, 49, 12–16. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Yang, M.-Y.; Sun, Z.-H.; Tan, C.-X.; Weng, J.-Q.; Wu, H.-K.; Liu, X.-H. Synthesis and Antifungal Activity of 1,3,4-Thiadiazole Derivatives Containing Pyridine Group. Lett. Drug Des. Discov. 2014, 11, 1107–1111. [Google Scholar] [CrossRef]
- Hoseinzadeh, A.; Habibi-Yangjeh, A.; Davari, M. Antifungal activity of magnetically separable Fe3O4/ZnO/AgBr nanocomposites prepared by a facile microwave-assisted method. Prog. Nat. Sci. Mater. Int. 2016, 26, 334–340. [Google Scholar] [CrossRef] [Green Version]
- Omar, K.; Meena, B.I.; Muhammed, S.A. Study on the activity of ZnO-SnO2 nanocomposite against bacteria and fungi. Physicochem. Probl. Miner. Process. 2016, 52, 754–766. [Google Scholar]
- Thaya, R.; Malaikozhundan, B.; Vijayakumar, S.; Sivakamavalli, J.; Jeyasekar, R.; Shanthi, S.; Vaseeharan, B.; Ramasamy, P.; Sonawane, A. Chitosan coated Ag/ZnO nanocomposite and their antibiofilm, antifungal and cytotoxic effects on murine macrophages. Microb. Pathog. 2016, 100, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Vu, V.T.; Nguyen, T.H.; Nguyen, T.A.; Tran, V.K.; Nguyen-Tri, P. Antibacterial activity of TiO2- and ZnO-decorated with silver nanoparticles. J. Compos. Sci. 2019, 3, 61. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L.; Dias, N.; Carvalho, J.; Fernandes, S.; Santos, C.; Lima, N. Synthesis, characterization and antifungal activity of chemically and fungal-produced silver nanoparticles against Trichophyton rubrum. J. Appl. Microbiol. 2014, 117, 1601–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pomastowski, P.; Król-Górniak, A.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanocomposites—Extracellular synthesis, physicochemical characterization and antibacterial potential. Materials 2020, 13, 4347. [Google Scholar] [CrossRef]
- Jadhav, S.; Gaikwad, S.; Nimse, M.; Rajbhoj, A. Copper oxide nanoparticles: Synthesis, characterization and their antibacterial activity. J. Clust. Sci. 2011, 22, 121–129. [Google Scholar] [CrossRef]
- Omer, A.; Ziora, Z.; Tamer, T.; Khalifa, R.; Hassan, M.; Mohy-Eldin, M.; Blaskovich, M. Formulation of Quaternized Aminated Chitosan Nanoparticles for Efficient Encapsulation and Slow Release of Curcumin. Molecules 2021, 26, 449. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamal, A.; Haroon, U.; Manghwar, H.; Alamer, K.H.; Alsudays, I.M.; Althobaiti, A.T.; Iqbal, A.; Akbar, M.; Farhana; Anar, M.; et al. Biological Applications of Ball-Milled Synthesized Biochar-Zinc Oxide Nanocomposite Using Zea mays L. Molecules 2022, 27, 5333. https://doi.org/10.3390/molecules27165333
Kamal A, Haroon U, Manghwar H, Alamer KH, Alsudays IM, Althobaiti AT, Iqbal A, Akbar M, Farhana, Anar M, et al. Biological Applications of Ball-Milled Synthesized Biochar-Zinc Oxide Nanocomposite Using Zea mays L. Molecules. 2022; 27(16):5333. https://doi.org/10.3390/molecules27165333
Chicago/Turabian StyleKamal, Asif, Urooj Haroon, Hakim Manghwar, Khalid H. Alamer, Ibtisam M. Alsudays, Ashwaq T. Althobaiti, Anila Iqbal, Mahnoor Akbar, Farhana, Maryam Anar, and et al. 2022. "Biological Applications of Ball-Milled Synthesized Biochar-Zinc Oxide Nanocomposite Using Zea mays L." Molecules 27, no. 16: 5333. https://doi.org/10.3390/molecules27165333
APA StyleKamal, A., Haroon, U., Manghwar, H., Alamer, K. H., Alsudays, I. M., Althobaiti, A. T., Iqbal, A., Akbar, M., Farhana, Anar, M., Nazish, M., Chaudhary, H. J., & Munis, M. F. H. (2022). Biological Applications of Ball-Milled Synthesized Biochar-Zinc Oxide Nanocomposite Using Zea mays L. Molecules, 27(16), 5333. https://doi.org/10.3390/molecules27165333







