Comparison between 1,2-Dihydropyridine and 1,4-Dihydropyridine on Hydride-Donating Ability and Activity
Abstract
:1. Introduction
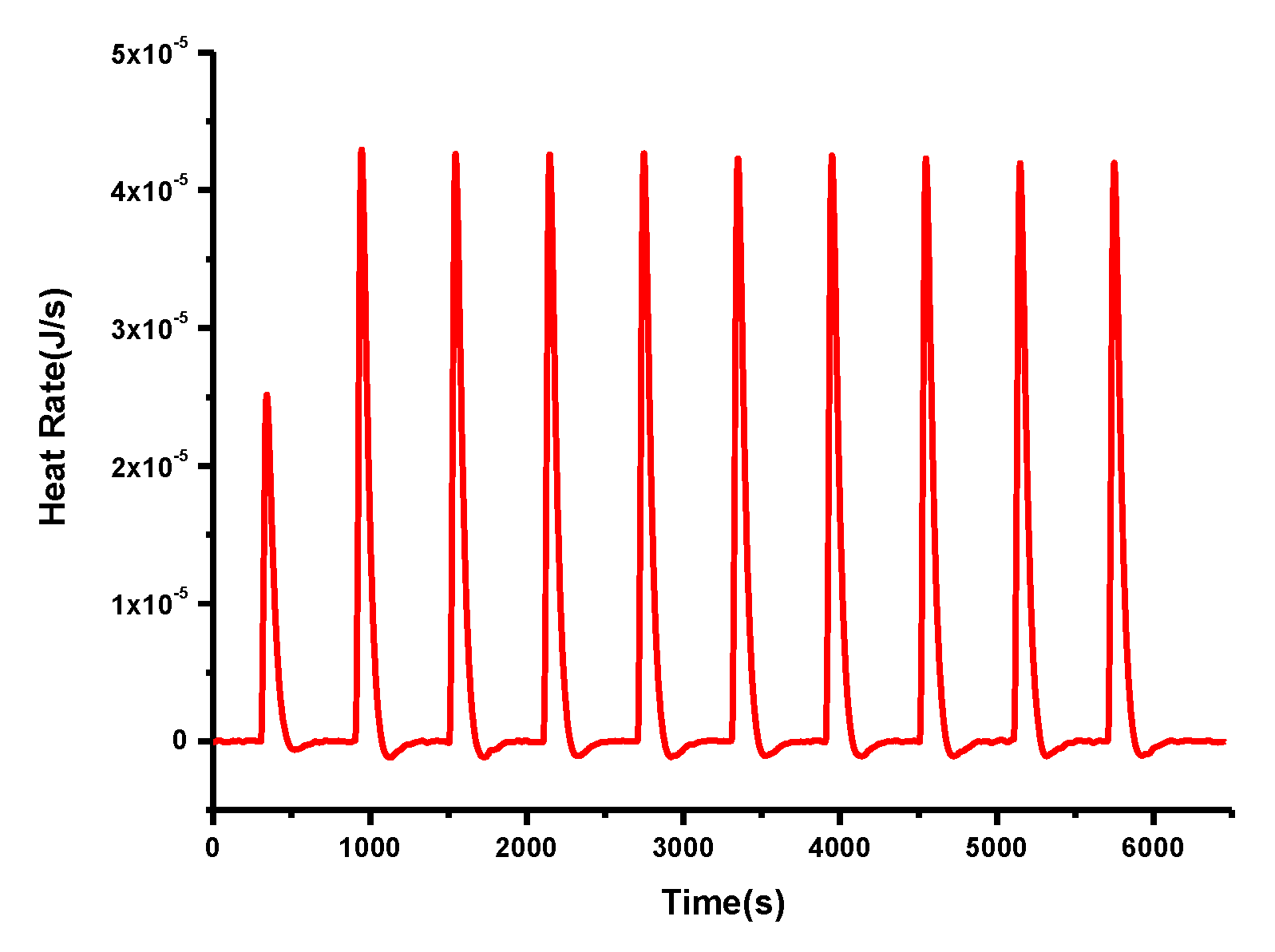
2. Results
3. Discussion
3.1. Analysis of Thermodynamic Driving Forces of 1,2/4-PNAH, 1,2/4-HEH, and 1,2/4-PYH as Hydride Donors in Acetonitrile at 298 K
3.2. Analysis of Kinetic Intrinsic Barriers of 1,2/4-PNAH, 1,2/4-HEH, and 1,2/4-PYH as Hydride Donors in Acetonitrile at 298 K
3.3. Analysis of Thermo-Kinetic Parameters of 1,2/4-PNAH, 1,2/4-HEH, and 1,2/4-PYH as Hydride Donors in Acetonitrile at 298 K
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, Z.; He, J.H.; Zhang, M.; Shi, Z.J.; Tang, H.; Zhou, X.Y.; Tian, J.J.; Wang, X.C. Borane-Catalyzed C3-Alkylation of Pyridines with Imines, Aldehydes, or Ketones as Electrophiles. J. Am. Chem. Soc. 2022, 144, 4810–4818. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, J.-S.; Wu, W.-B.; Zhang, Z.-H.; Mu, B.-S. Recent Advances in Synthesis of Chiral 1,2-Dihydropyridines. Acta Chim. Sin. 2021, 79, 685–693. [Google Scholar] [CrossRef]
- Zhu, X.Q.; Zhang, J.Y.; Cheng, J.P. Negative Kinetic Temperature Effect on the Hydride Transfer from NADH Analogue BNAH to the Radical Cation of N-Benzylphenothiazine in Acetonitrile. J. Org. Chem. 2006, 71, 7007–7015. [Google Scholar] [CrossRef]
- Zhu, X.Q.; Li, H.R.; Li, Q.; Ai, T.; Lu, J.Y.; Yang, Y.; Cheng, J.P. Determination of the C4-H Bond Dissociation Energies of NADH Models and Their Radical Cations in Acetonitrile. Chem. Eur. J. 2003, 9, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, X.Q. Theoretical Prediction of Activation Free Energies of Various Hydride Self-Exchange Reactions in Acetonitrile at 298 K. ACS Omega 2018, 3, 872–885. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Suenobu, T.; Patz, M.; Hirasaka, T.; Itoh, S.; Fujitsuka, M.; Ito, O. Selective One-Electron and Two-Electron Reduction of C60 with NADH and NAD Dimer Analogues via Photoinduced Electron Transfer. J. Am. Chem. Soc. 1998, 120, 8060–8068. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Inada, O.; Suenobu, T. Mechanisms of Electron-Transfer Oxidation of NADH Analogues and Chemiluminescence. Detection of the Keto and Enol Radical Cations. J. Am. Chem. Soc. 2003, 125, 4808–4816. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Fujioka, N.; Kotani, H.; Ohkubo, K.; Lee, Y.M.; Nam, W. Mechanistic Insights into Hydride-Transfer and Electron-Transfer Reactions by a Manganese(IV)-Oxo Porphyrin Complex. J. Am. Chem. Soc. 2009, 131, 17127–17134. [Google Scholar] [CrossRef]
- Peterson, R.L.; Himes, R.A.; Kotani, H.; Suenobu, T.; Tian, L.; Siegler, M.A.; Solomon, E.I.; Fukuzumi, S.; Karlin, K.D. Cupric superoxo-mediated intermolecular C-H activation chemistry. J. Am. Chem. Soc. 2011, 133, 1702–1705. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.D.; Cheng, J.P. Diazaphosphinanes as hydride, hydrogen atom, proton or electron donors under transition-metal-free conditions: Thermodynamics, kinetics, and synthetic applications. Chem. Sci. 2020, 11, 3672–3679. [Google Scholar] [CrossRef]
- Vala, R.M.; Patel, D.M.; Sharma, M.G.; Patel, H.M. Impact of an aryl bulky group on a one-pot reaction of aldehyde with malononitrile and N-substituted 2-cyanoacetamide. RSC Adv. 2019, 9, 28886–28893. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.G.; Vala, R.M.; Patel, H.M. Pyridine-2-carboxylic acid as an effectual catalyst for rapid multi-component synthesis of pyrazolo[3,4-b]quinolinones. RSC Adv. 2020, 10, 35499–35504. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.G.; Vala, R.M.; Patel, D.M.; Lagunes, I.; Fernandes, M.X.; Padrón, J.M.; Ramkumar, V.; Gardas, R.L.; Patel, H.M. Anti-Proliferative 1,4-Dihydropyridine and Pyridine Derivatives Synthesized through a Catalyst-Free, One-Pot Multi-Component Reaction. ChemistrySelect 2018, 3, 12163–12168. [Google Scholar] [CrossRef]
- Patel, D.M.; Sharma, M.G.; Vala, R.M.; Lagunes, I.; Puerta, A.; Padron, J.M.; Rajani, D.P.; Patel, H.M. Hydroxyl alkyl ammonium ionic liquid assisted green and one-pot regioselective access to functionalized pyrazolodihydropyridine core and their pharmacological evaluation. Bioorg. Chem. 2019, 86, 137–150. [Google Scholar] [CrossRef]
- Sharma, M.G.; Rajani, D.P.; Patel, H.M. Green approach for synthesis of bioactive Hantzsch 1,4-dihydropyridine derivatives based on thiophene moiety via multicomponent reaction. R Soc. Open Sci. 2017, 4, 170006. [Google Scholar] [CrossRef]
- A Khedkar, S.; B Auti, P. 1, 4-Dihydropyridines: A Class of Pharmacologically Important Molecules. Med. Chem. 2014, 14, 282–290. [Google Scholar] [CrossRef]
- Yang, J.D.; Chen, B.L.; Zhu, X.Q. New Insight into the Mechanism of NADH Model Oxidation by Metal Ions in Nonalkaline Media. J. Phys. Chem. B 2018, 122, 6888–6898. [Google Scholar] [CrossRef]
- Sharma, V.K.; Singh, S.K. Synthesis, utility and medicinal importance of 1,2- & 1,4-dihydropyridines. RSC Adv. 2017, 7, 2682–2732. [Google Scholar] [CrossRef]
- Sharma, M.G.; Pandya, J.; Patel, D.M.; Vala, R.M.; Ramkumar, V.; Subramanian, R.; Gupta, V.K.; Gardas, R.L.; Dhanasekaran, A.; Patel, H.M. One-Pot Assembly for Synthesis of 1,4-Dihydropyridine Scaffold and Their Biological Applications. Polycycl. Aromat. Compd. 2019, 41, 1495–1505. [Google Scholar] [CrossRef]
- Zhu, X.Q.; Cao, L.; Liu, Y.; Yang, Y.; Lu, J.Y.; Wang, J.S.; Cheng, J.P. Thermodynamics and kinetics of the hydride-transfer cycles for 1-aryl-1,4-dihydronicotinamide and its 1,2-dihydroisomer. Chem. Eur. J. 2003, 9, 3937–3945. [Google Scholar] [CrossRef]
- Xia, K.; Shen, G.B.; Zhu, X.Q. Thermodynamics of various F420 coenzyme models as sources of electrons, hydride ions, hydrogen atoms and protons in acetonitrile. Org. Biomol. Chem. 2015, 13, 6255–6268. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.B.; Xia, K.; Li, X.T.; Li, J.L.; Fu, Y.H.; Yuan, L.; Zhu, X.Q. Prediction of Kinetic Isotope Effects for Various Hydride Transfer Reactions Using a New Kinetic Model. J. Phys. Chem. A 2016, 120, 1779–1799. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Q.; Zhang, M.T.; Yu, A.; Wang, C.H.; Cheng, J.P. Hydride, Hydrogen Atom, Proton, and Electron Transfer Driving Forces of Various Five-Membered Heterocyclic Organic Hydrides and Their Reaction Intermediates in Acetonitrile. J. Am. Chem. Soc. 2008, 130, 2501–2516. [Google Scholar] [CrossRef]
- Yuasa, J.; Yamada, S.; Fukuzumi, S. A Mechanistic Dichotomy in Scandium Ion-Promoted Hydride Transfer of an NADH Analogue: Delicate Balance between One-Step Hydride-Transfer and Electron-Transfer Pathways. J. Am. Chem. Soc. 2006, 128, 14938–14948. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.H.; Shen, G.B.; Wang, K.; Zhu, X.Q. Comparison of Thermodynamic, Kinetic Forces for Three NADH Analogues to Release Hydride Ion or Hydrogen Atom in Acetonitrile. ChemistrySelect 2021, 6, 8007–8010. [Google Scholar] [CrossRef]
- Fu, Y.H.; Wang, K.; Shen, G.B.; Zhu, X.Q. Quantitative comparison of the actual antioxidant activity of Vitamin C, Vitamin E, and NADH. J. Phys. Org. Chem. 2022, 35, e4358. [Google Scholar] [CrossRef]
- Fu, Y.-H.; Shen, G.-B.; Li, Y.; Yuan, L.; Li, J.-L.; Li, L.; Fu, A.-K.; Chen, J.-T.; Chen, B.-L.; Zhu, L.; et al. Realization of Quantitative Estimation for Reaction Rate Constants Using only One Physical Parameter for Each Reactant. ChemistrySelect 2017, 2, 904–925. [Google Scholar] [CrossRef]
- Bobbitt, J.M. Oxoammonium Salts. 6. 4-Acetylamino-2,2,6,6-tetramethylpiperidine-1-oxoammonium Perchlorate: A Stable and Convenient Reagent for the Oxidation of Alcohols. Silica Gel Catalysis. J. Org. Chem. 1998, 63, 9367–9374. [Google Scholar] [CrossRef]
- Comins, D.L.; Abdullah, A.H. Synthesis of l-Acyl-1,4-dihydropyridines via Copper Hydride Reduction of 1-Acylpyridinium Salts. J. Org. Chem. 1984, 49, 3392–3394. [Google Scholar] [CrossRef]
- Zhu, X.Q.; Deng, F.H.; Yang, J.D.; Li, X.T.; Chen, Q.; Lei, N.P.; Meng, F.K.; Zhao, X.P.; Han, S.H.; Hao, E.J.; et al. A classical but new kinetic equation for hydride transfer reactions. Org. Biomol. Chem. 2013, 11, 6071–6089. [Google Scholar] [CrossRef]
- Zhu, X.Q.; Tan, Y.; Cao, C.T. Thermodynamic Diagnosis of the Properties and Mechanism of Dihydropyridine-Type Compounds as Hydride Source in Acetonitrile with “Molecule ID Card”. J. Phys. Chem. B 2010, 114, 2058–2075. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, Y.; Zhu, X.Q. Thermodynamic Parameters of Elementary Steps for 3,5-Disubstituted 1,4-Dihydropyridines To Release Hydride Anions in Acetonitrile. ACS Omega 2018, 3, 13598–13608. [Google Scholar] [CrossRef] [PubMed]
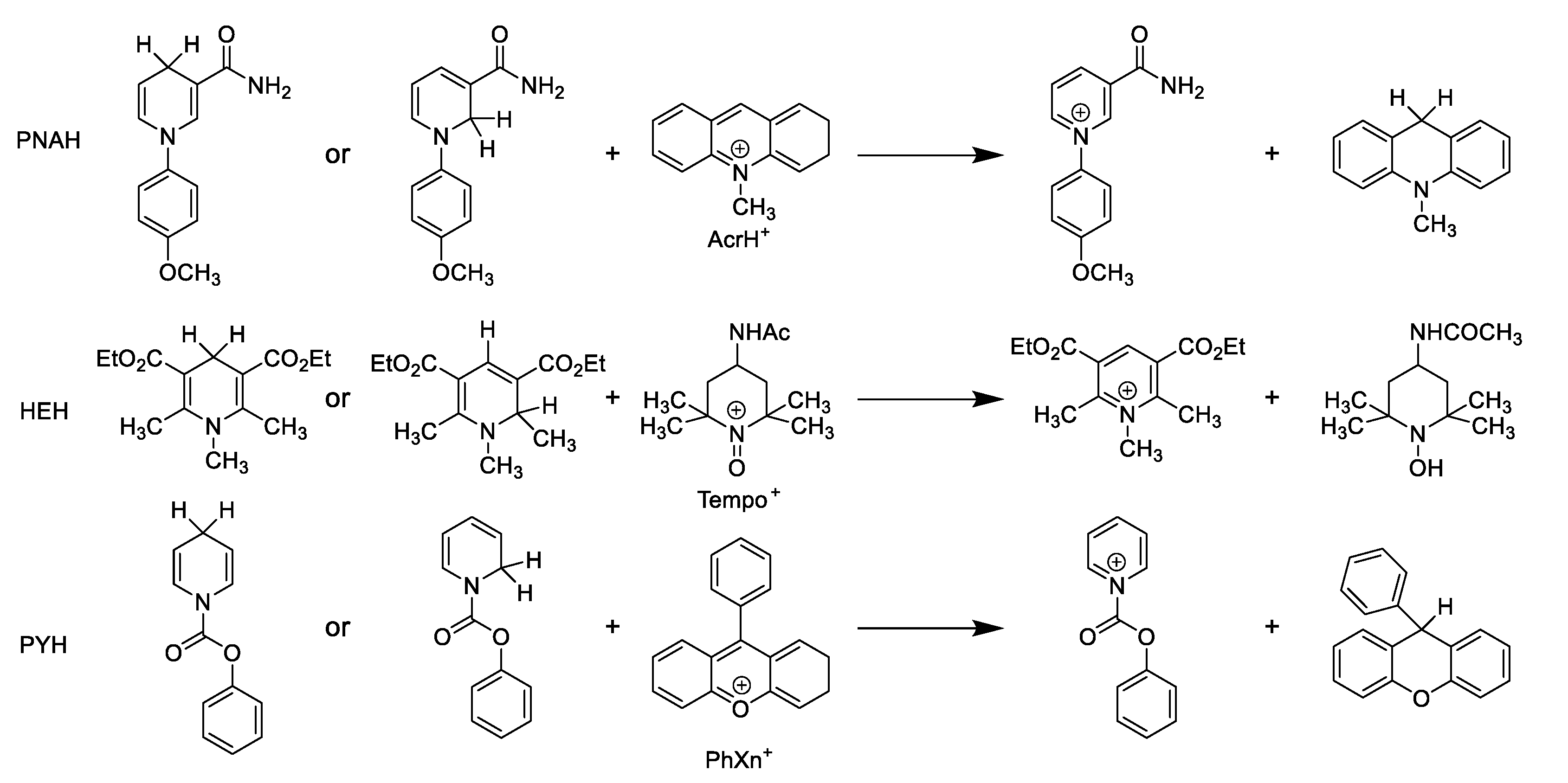

| PNAH + AcrH+ | HEH + TEMPO+ | PYH + PhXn+ | ||||
|---|---|---|---|---|---|---|
| 1,2-PNAH | 1,4-PNAH | 1,2-HEH | 1,4-HEH | 1,2-PYH | 1,4-PYH | |
| k2a | 10.06 | 12.30 | 1.44 × 105 | 2.34 × 103 | 9.50 × 10−1 | 4.76 × 101 |
| ΔG≠ b | 16.05 | 15.96 | 10.41 | 12.85 | 17.47 | 15.16 |
| ΔG° c | −15.70 | −14.30 | −37.30 | −35.70 | −21.70 | −19.00 |
| Compounds | ΔG°(Y+) a | ΔG≠o(Y+) b |
|---|---|---|
| AcrH+ | −76.2 | −28.16 |
| TEMPO+ | −100.7 | −37.13 |
| PhXn+ | −91.6 | −34.01 |
| 1,2-PNAH | 1,4-PNAH | 1,2-HEH | 1,4-HEH | 1.2-PYH | 1,4-PYH | |
|---|---|---|---|---|---|---|
| ΔG°(XH) | 60.50 | 61.90 | 63.40 | 65.00 | 69.90 | 72.60 |
| ΔG≠XH/X | 27.92 | 26.34 | 31.68 | 34.96 | 33.06 | 25.74 |
| ΔG≠°(XH) | 44.21 | 44.12 | 47.54 | 49.98 | 51.48 | 49.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.-Y.; Zhu, X.-Q. Comparison between 1,2-Dihydropyridine and 1,4-Dihydropyridine on Hydride-Donating Ability and Activity. Molecules 2022, 27, 5382. https://doi.org/10.3390/molecules27175382
Zhang J-Y, Zhu X-Q. Comparison between 1,2-Dihydropyridine and 1,4-Dihydropyridine on Hydride-Donating Ability and Activity. Molecules. 2022; 27(17):5382. https://doi.org/10.3390/molecules27175382
Chicago/Turabian StyleZhang, Jin-Ye, and Xiao-Qing Zhu. 2022. "Comparison between 1,2-Dihydropyridine and 1,4-Dihydropyridine on Hydride-Donating Ability and Activity" Molecules 27, no. 17: 5382. https://doi.org/10.3390/molecules27175382






