Chemical Contamination in Bread from Food Processing and Its Environmental Origin
Abstract
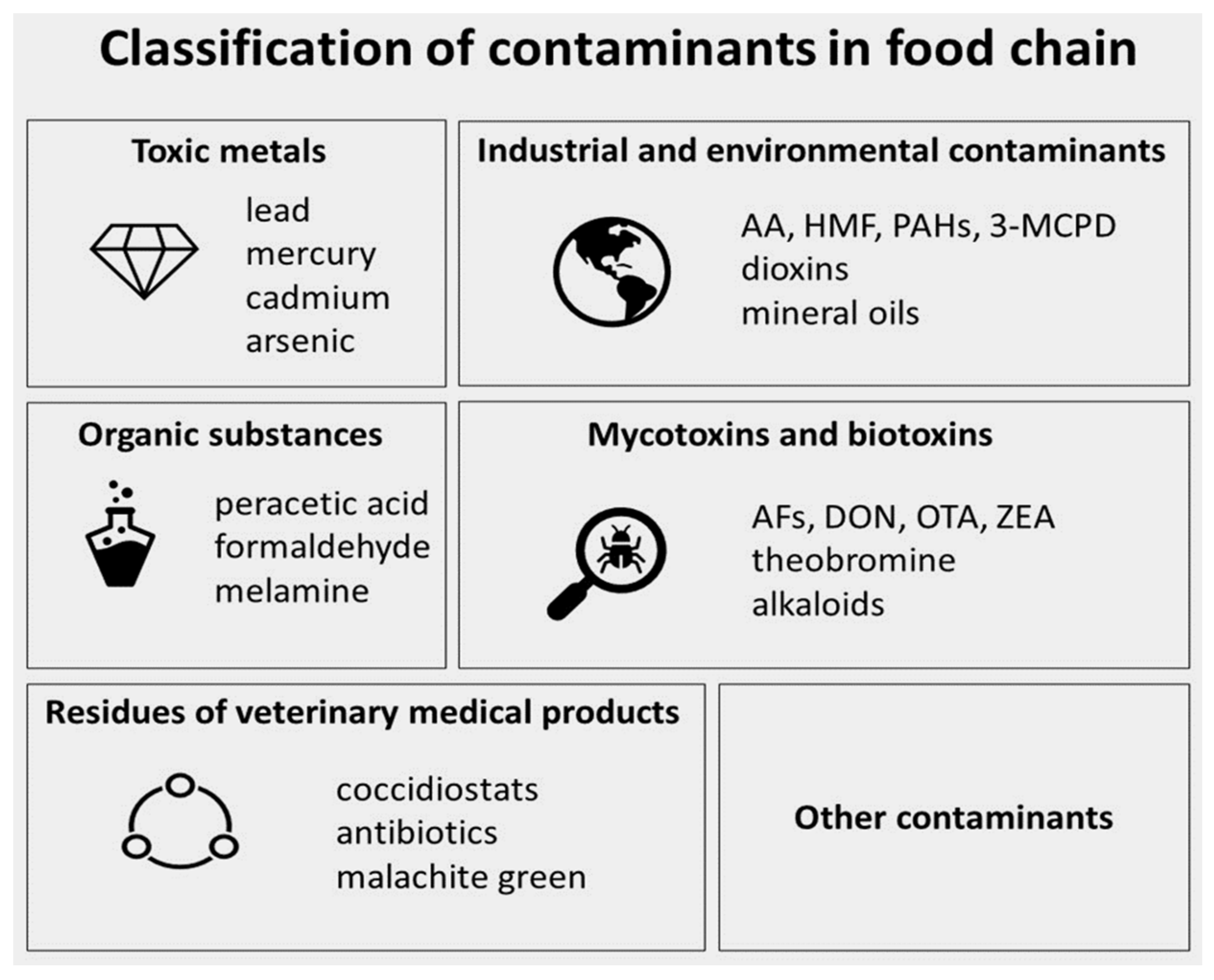
:1. Introduction
2. Methodology
3. Maillard Reaction as a Source of Bread Processing Contaminants
3.1. Acrylamide
3.2. Furan and Furan Derivatives
3.3. Polycyclic Aromatic Amines
3.4. Monochloropropanediols, Monochloropropanediols Esters, and Glycidyl Esters
4. Environment as a Source of Bread Chemical Contaminants
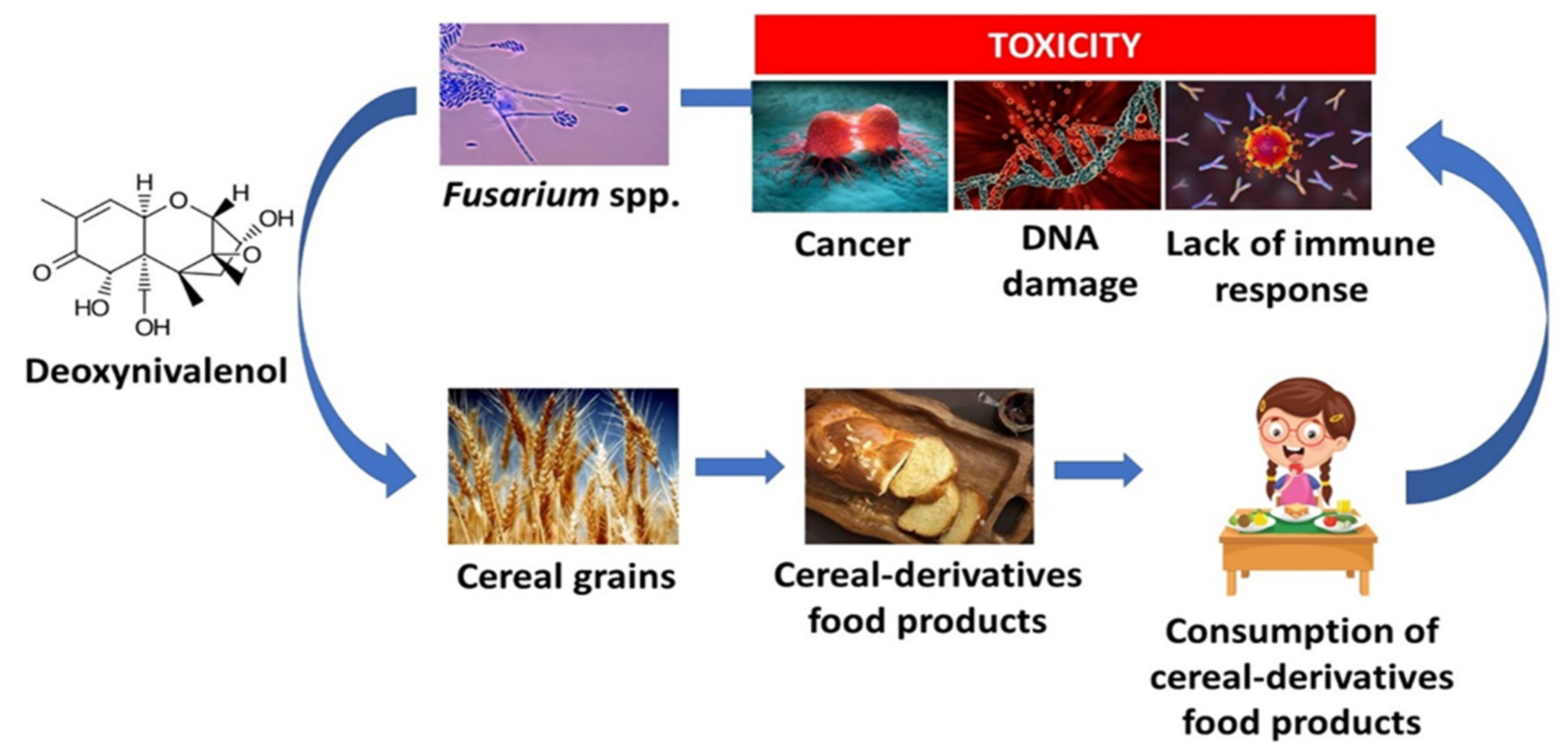
4.1. Mycotoxins
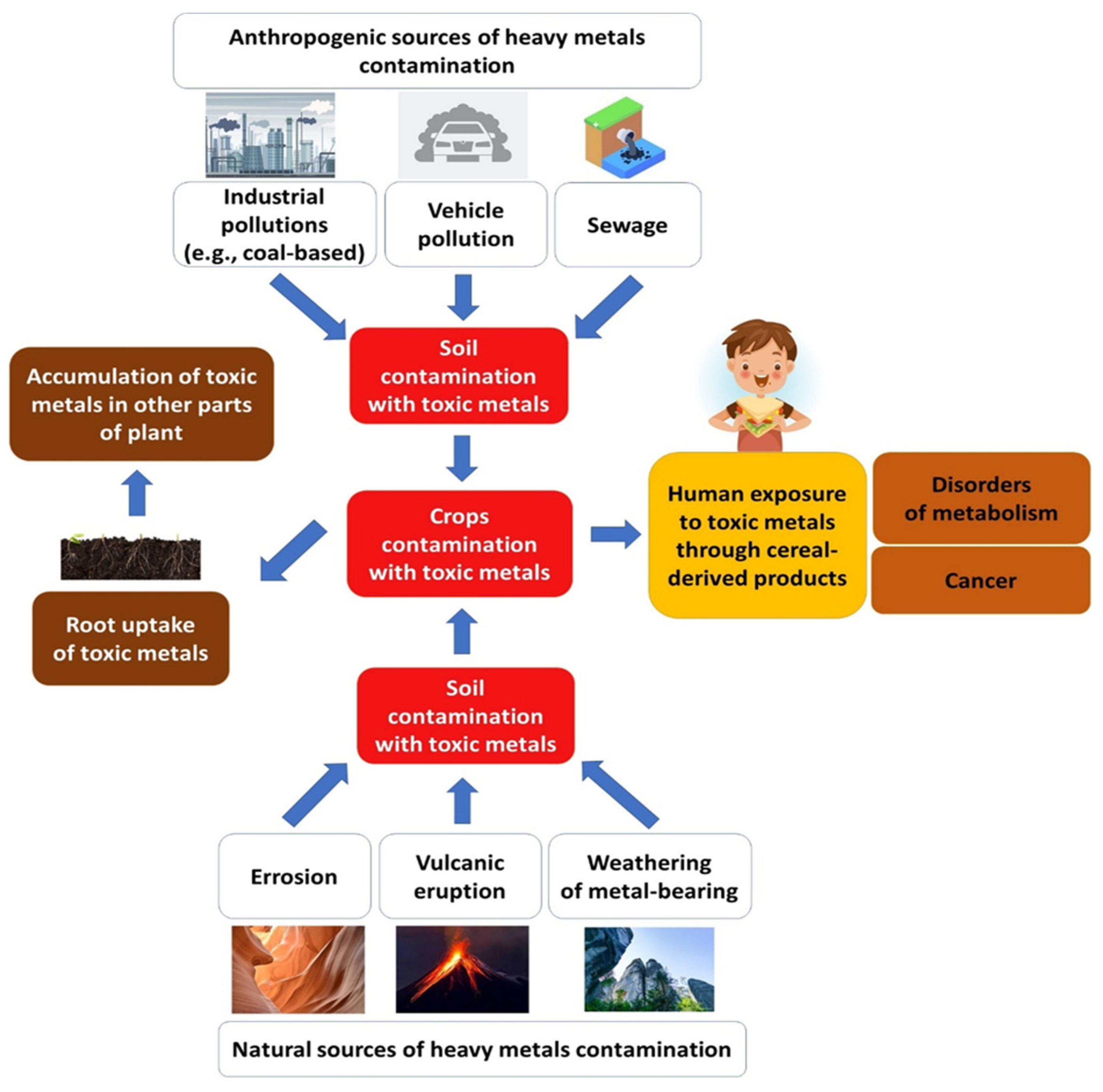
4.2. Toxic Metals
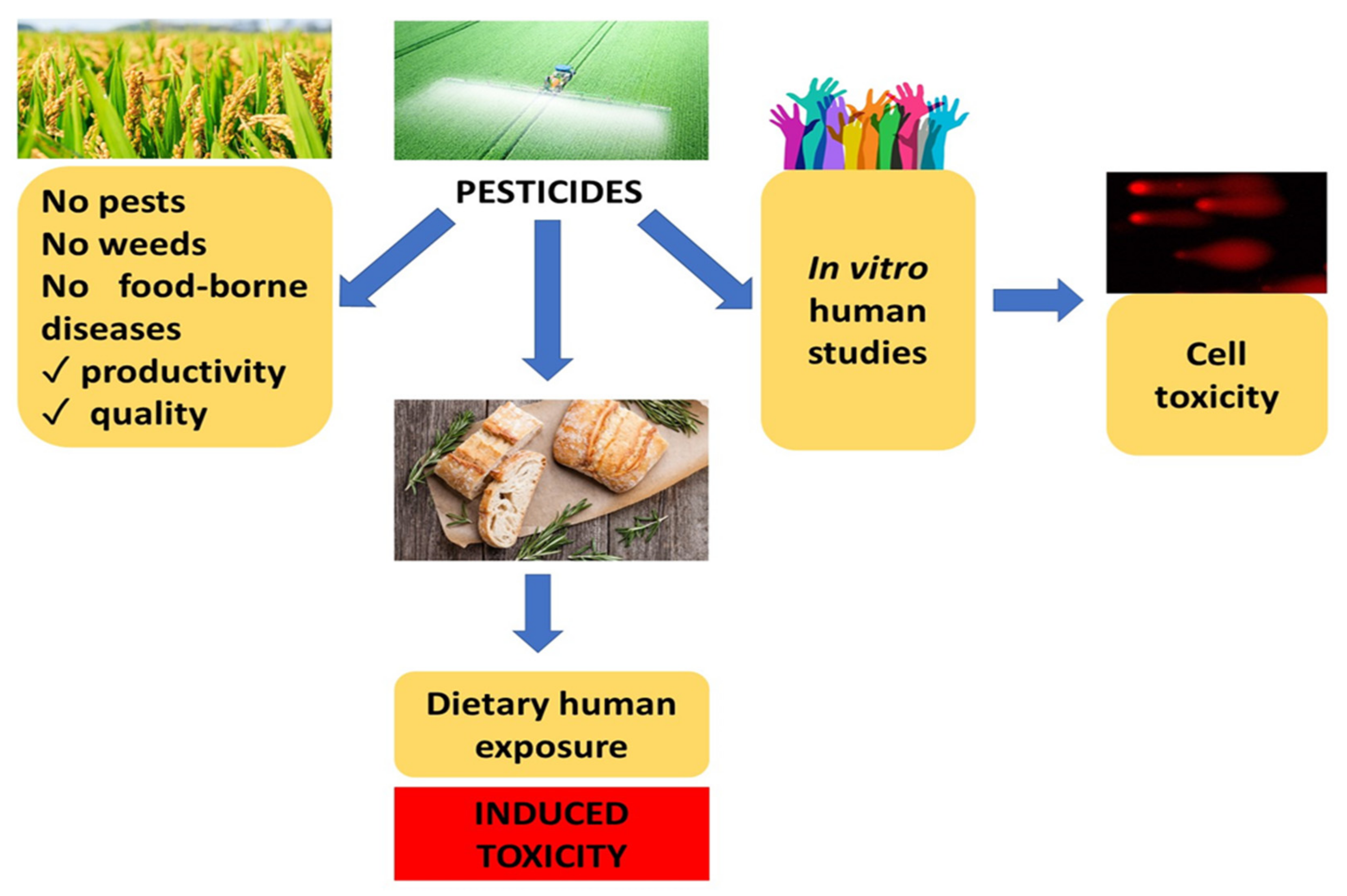
4.3. Pesticides
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Semla, M.; Goc, Z.; Martiniaková, M.; Omelka, R.; Formicki, G. Acrylamide: A common food toxin related to physiological functions and health. Physiol. Res. 2017, 66, 205–217. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Crop Prospects and Food Situation-Quarterly Global Report No. 4, December 2021; FAO: Rome, Italy, 2021; Available online: https://www.fao.org/3/cb7877en/cb7877en.pdf (accessed on 27 April 2022).
- Khaneghah, A.M.; Fakhri, Y.; Nematollahi, A.; Pirhadi, M. Potentially toxic elements (PTEs) in cereal-based foods: A systematic review and meta-analysis. Trends Food Sci. Technol. 2020, 96, 30–44. [Google Scholar] [CrossRef]
- Arata, G.J.; Martínez, M.; Elguezábal, C.; Rojas, D.; Cristos, D.; Dinolfo, M.I.; Arata, A.F. Effects of sowing date, nitrogen fertilization, and Fusarium graminearum in an Argentinean bread wheat: Integrated analysis of disease parameters, mycotoxin contamination, grain quality, and seed deterioration. J. Food Compost. Anal. 2022, 107, 104364. [Google Scholar] [CrossRef]
- CXS 193-1995; Codex Alimentarius, International Food Standards, General Standard for Contaminants and Toxins in Food and Feed. Food and Agriculture Organization (FAO): Rome, Italy; World Health Organization (WHO): Geneva, Switzerland, 2019. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B193-1995%252FCXS_193e.pdf (accessed on 27 April 2022).
- European Food Safety Authority (EFSA). Scientific Technical Assistance to RASFF on Chemical Contaminants: Risk Evaluation of Chemical Contaminants in Food in the Context of RASFF Notifications. EFSA Supporting Publ. 2019, 16, EN-1625. Available online: https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/sp.efsa.2019.EN-1625 (accessed on 27 April 2022).
- Bimpizas-Pinis, M.; Santagata, R.; Kaiser, S.; Liu, Y.; Lyu, Y. Additives in the food supply chain: Environmental assessment and circular economy implications. Environ. Sustain. Indic. 2022, 14, 100172. [Google Scholar] [CrossRef]
- CODEX STAN 192-1995; Codex Alimentarius, International Food Standards, General Standard for Food Additives Food and Agriculture Organization (FAO): Rome, Italy. World Health Organization (WHO): Geneva, Switzerland, 2019. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B192-1995%252FCXS_192e.pdf (accessed on 27 April 2022).
- Izzreen, M.N.Q.; Hansen, S.S.; Petersen, M.A. Volatile compounds in whole meal bread crust: The effects of yeast level and fermentation temperature. Food Chem. 2016, 210, 566–576. [Google Scholar] [CrossRef]
- Maillard, L.C. Action of amino acids on sugars. Formation of melanoidins in a methodical way. C. R. Acad. Sci. 1912, 154, 66–68. [Google Scholar]
- Favreau-Farhadi, N.; Pecukonis, L.; Barrett, A. The Inhibition of Maillard Browning by Different Concentrations of Rosmarinic Acid and Epigallocatechin-3-Gallate in Model, Bakery, and Fruit Systems. J. Food Sci. 2015, 80, C2140–C2146. [Google Scholar] [CrossRef]
- Jaeger, H.; Janositz, A.; Knorr, D. The Maillard reaction and its control during food processing. The potential of emerging technologies. Pathol. Biol. 2010, 58, 207–213. [Google Scholar] [CrossRef]
- Purlis, E.; Salvadori, V.O. Bread browning kinetics during baking. J. Food Eng. 2007, 80, 1107–1115. [Google Scholar] [CrossRef]
- Pozo-Bayón, M.A.; Guichard, E.; Cayot, N. Flavor Control in Baked Cereal Products. Food Rev. Int. 2006, 22, 335–379. [Google Scholar] [CrossRef]
- Mildner-Szkudlarz, S.; Siger, A.; Szwengiel, A.; Przygoński, K.; Wojtowicz, E.; Zawirska-Wojtasiak, R. Phenolic compounds reduce formation of N ε -(carboxymethyl)lysine and pyrazines formed by Maillard reactions in a model bread system. Food Chem. 2017, 231, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.W.; Chung, H.; Kim, Y.S. Effects of dicarbonyl trapping agents, antioxidants, and reducing agents on the formation of furan and other volatile components in canned-coffee model systems. Food Res. Int. 2015, 75, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.K.; Dubey, A.K.; Ameen, S.M.; Montalto, S.; Parisi, S. Analytical methods for the determination of Maillard reaction products in foods. An introduction. In Analytical Methods for the Assessment of Maillard Reactions in Foods, 1st ed.; Springer: Cham, Switzerland, 2018; pp. 1–14. [Google Scholar]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef]
- Kumar, J.; Das, S.; Teoh, S.L. Dietary Acrylamide and the Risks of Developing Cancer: Facts to Ponder. Front. Nutr. 2018, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Mencin, M.; Abramovič, H.; Vidrih, R.; Schreiner, M. Acrylamide levels in food products on the Slovenian market. Food Control 2020, 114, 107267. [Google Scholar] [CrossRef]
- Yang, Y.; Achaerandio, I.; Pujolà, M. Influence of the frying process and potato cultivar on acrylamide formation in French fries. Food Control 2016, 62, 216–223. [Google Scholar] [CrossRef]
- Başaran, B.; Anlar, P.; Oral, Z.F.Y.; Polat, Z.; Kaban, G. Risk assessment of acrylamide and 5-hydroxymethyl-2-furfural (5-HMF) exposure from bread consumption: Turkey. J. Food Compos. Anal. 2022, 107, 104409. [Google Scholar] [CrossRef]
- Esposito, F.; Velotto, S.; Rea, T.; Stasi, T.; Cirillo, T. Occurrence of Acrylamide in Italian Baked Products and Dietary Exposure Assessment. Molecules 2020, 25, 4156. [Google Scholar] [CrossRef]
- Mesìas, M.; Morales, F.J. Acrylamide in commercial potato crisps from Spanish market: Trends from 2004 to 2014 and assessment of the dietary exposure. Food Chem. Toxicol. 2015, 81, 104–110. [Google Scholar] [CrossRef]
- Wenzl, T.; Lachenmeier, D.W.; Gökmen, V. Analysis of heat-induced contaminants (acrylamide, chloropropanols and furan) in carbohydrate-rich food. Anal. Bioanal. Chem. 2007, 389, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Andačić, I.M.; Tot, A.; Ivešić, M.; Krivohlavek, A.; Thirumdas, R.; Barba, F.J.; Sabolović, M.B.; Kljusurić, J.G.; Brnčić, S.R. Exposure of the Croatian adult population to acrylamide through bread and bakery products. Food Chem. 2020, 322, 126771. [Google Scholar] [CrossRef] [PubMed]
- Komoike, Y.; Nomura-Komoike, K.; Matsuoka, M. Intake of acrylamide at the dietary relevant concentration causes splenic toxicity in adult zebrafish. Environ. Res. 2020, 189, 109977. [Google Scholar] [CrossRef] [PubMed]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Ekuban, F.A.; Zong, C.; Takikawa, M.; Morikawa, K.; Sakurai, T.; Ichihara, S.; Itoh, K.; Ohsako, S.; Ichihara, G. Genetic ablation of Nrf2 exacerbates neurotoxic effects of acrylamide in mice. Toxicology 2021, 456, 152785. [Google Scholar] [CrossRef]
- Prasad, S.N.; Muralidhara. Evidence of acrylamide induced oxidative stress and neurotoxicity in Drosophila melanogaster–Its amelioration with spice active enrichment: Relevance to neuropathy. Neurotoxicology 2012, 33, 1254–1264. [Google Scholar] [CrossRef]
- Santhanasabapathy, R.; Vasudevan, S.; Anupriya, K.; Pabitha, R.; Sudhandiran, G. Farnesol quells oxidative stress, reactive gliosis and inflammation during acrylamide-induced neurotoxicity: Behavioral and biochemical evidence. Neuroscience 2015, 308, 212–227. [Google Scholar] [CrossRef]
- Roberts, J.; Mehta, R.; Curran, I.; Raju, J. Dietary acrylamide exposure in F344 rats and colon tumor-bearing nude nu/nu mice: Dataset of gene expression of cancer pathway targets and methylation status of tumor suppressor genes in colon mucosae and tumors. Data Brief 2019, 27, 104763. [Google Scholar] [CrossRef]
- Dourson, M.; Hertzberg, R.; Allen, B.; Haber, L.; Parker, A.; Kroner, O.; Maier, A.; Kohrman, M. Evidence-based dose-response assessment for thyroid tumorigenesis from acrylamide. Regul. Toxicol. Pharm. 2008, 52, 264–289. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Monographs on the evaluation of carcinogenic risks of chemicals to humans. In Some Industrial Chemicals; IARC: Lyon, France, 1994; Volume 60. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on acrylamide in food. EFSA J. 2015, 13, 4104. Available online: https://efsa.onlinelibrary.wiley.com/doi/abs/10.2903/j.efsa.2015.4104 (accessed on 27 April 2022).
- The European Commission. Commission Regulation (EU) 2017/2158 of 20 November 2017 Establishing Mitigation Measures and Benchmark Levels for the Reduction of the Presence of Acrylamide in Food (Text with EEA Relevance.). Off. J. Eur. Union 2017, 60, 24–44. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32017R2158) (accessed on 27 April 2022).
- Food Drink Europe. Acrylamide toolbox. 2019. Available online: https://www.fooddrinkeurope.eu/wp-content/uploads/2021/05/FoodDrinkEurope_Acrylamide_Toolbox_2019.pdf (accessed on 27 April 2022).
- Belkova, B.; Chytilova, L.; Kocourek, V.; Slukova, M.; Mastovska, K.; Kyselka, J.; Hajslova, J. Influence of dough composition on the formation of processing contaminants in yeast-leavened wheat toasted bread. Food Chem. 2021, 338, 127715. [Google Scholar] [CrossRef] [PubMed]
- Roszko, M.Ł.; Szczepańska, M.; Szymczyk, K.; Rzepkowska, M. Dietary risk evaluation of acrylamide intake with bread in Poland, determined by two comparable cleanup procedures. Food Addit. Contam. B 2020, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Peng, Y.; Xi, J.; Zhao, Q.; Xu, D.; Jin, Z.; Xu, X. Effect of sourdough fermented with corn oil and lactic acid bacteria on bread flavor. LWT 2022, 155, 112935. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yang, Y.Y.; Li, H.Q.; Zhang, Q.D.; Xu, F.; Li, Z.J. Characterization of aroma-active compounds in steamed breads fermented with Chinese traditional sourdough. LWT 2021, 152, 112347. [Google Scholar] [CrossRef]
- Soceanu, A.; Dobrinas, S.; Popescu, V. Levels of Polycyclic Aromatic Hydrocarbons in Toasted Bread. Polycycl. Aromat. Comp. 2021. [Google Scholar] [CrossRef]
- González-Mulero, L.; Delgado-Andrade, C.; Morales, F.J.; Olombrada, E.; Mesias, M. Study of furanic compound content in common Spanish culinary preparations. Influence of the food preparation setting. J. Food Compos. Anal. 2022, 110, 104532. [Google Scholar] [CrossRef]
- Zelinkova, Z.; Giri, A.; Wenzl, T. Assessment of critical steps of a GC/MS based indirect analytical method for the determination of fatty acid esters of monochloropropanediols (MCPDEs) and of glycidol (GEs). Food Control 2017, 77, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Arisseto, A.P.; Silva, W.C.; Tivanello, R.G.; Sampaio, K.A.; Vicente, E. Recent advances in toxicity and analytical methods of monochloropropanediols and glycidyl fatty acid esters in foods. Curr. Opin. Food Sci. 2018, 24, 36–42. [Google Scholar] [CrossRef]
- Barrios-Rodríguez, Y.F.; Pedreschi, F.; Rosowski, J.; Gómez, J.P.; Figari, N.; Castillo, O.; Mariotti Celis, M.S. Is the dietary acrylamide exposure in Chile a public health problem? Food Addit. Contam. A 2021, 38, 1126–1135. [Google Scholar] [CrossRef]
- Pogurschi, E.N.; Zugravu, C.A.; Ranga, I.N.; Trifunschi, S.; Munteanu, M.F.; Popa, D.C.; Tudorache, M.; Custura, I. Determination of Acrylamide in Selected Foods from the Romanian Market. Foods 2021, 10, 2110. [Google Scholar] [CrossRef]
- Branciari, R.; Roila, R.; Ranucci, D.; Altissimi, M.; Mercuri, M.; Haouet, N. Estimation of acrylamide exposure in Italian schoolchildren consuming a canteen menu: Health concern in three age groups. Int. J. Food Sci. Nutr. 2020, 71, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Jesus, S.; Delgado, I.; Rego, A.; Brandão, C.; Galhano, R.; Castanheira, I. Determination of acrylamide in Portuguese bread by UPLC- MS/MS: Metrological and Chemometric tools. Acta Imeko 2018, 7, 96–101. [Google Scholar] [CrossRef]
- Pugajeva, I.; Zumbure, L.; Melngaile, A.; Bartkevics, V. Determination of acrylamide levels in selected foods in Latvia and assessment of the population intake. In Proceedings of the FoodBalt 2014: 9th Baltic Conference on Food Science and Technology “Food for Consumer Well-Being”, Jelgava, Latvia, 8–9 May 2014. [Google Scholar]
- Zhuang, H.; Zhang, T.; Liu, J.; Yuan, Y. Detection of Acrylamide Content in Traditional Chinese Food by High-Performance Liquid Chromatography Tandem Mass Spectrometry Method. Cyta-J. Food 2012, 10, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Hirvonen, T.; Jestoi, M.; Tapanainen, H.; Valsta, L.; Virtanen, S.M.; Sinkko, H.; Kronberg-Kippilä, C.; Kontto, J.; Virtamo, J.; Simell, O.; et al. Dietary acrylamide exposure among Finnish adults and children: The potential effect of reduction measures. Food Addit. Contam. A 2011, 28, 1483–1491. [Google Scholar] [CrossRef]
- Kim, C.T.; Hwang, E.-S.; Lee, H.J. An improved LC-MS/MS method for the quantitation of acrylamide in processed foods. Food Chem. 2007, 101, 401–409. [Google Scholar] [CrossRef]
- Saleh-Ghadimi, S.; Alizadeh, M.; Esfanjani, A.T.; Hezaveh, S.J.G.; Vayghan, H.J. Assessment of dietary exposure to 5-hydroxymethylfurfural from Traditional Iranian flat breads. Ital. J. Food Sci. 2014, 26, 169–175. [Google Scholar]
- Mildner-Szkudlarz, S.; Różańska, M.; Piechowska, P.; Waśkiewicz, A.; Zawirska-Wojtasiak, R. Effects of polyphenols on volatile profile and acrylamide formation in a model wheat bread system. Food Chem. 2019, 297, 125008. [Google Scholar] [CrossRef]
- Surdyk, N.; Rosén, J.; Andersson, R.; Åman, P. Effects of asparagine, fructose, and baking conditions on acrylamide content in yeast-leavened wheat bread. J. Agric. Food Chem. 2004, 52, 2047–2051. [Google Scholar] [CrossRef]
- Wang, S.; Yu, J.; Xin, Q.; Wang, S.; Copeland, L. Effects of starch damage and yeast fermentation on acrylamide formation in bread. Food Control B 2017, 73, 230–236. [Google Scholar] [CrossRef]
- Ames, J.M. The Maillard reaction. In Biochemistry of Food Proteins; Hudson, B.J.F., Ed.; Springer: Boston, MA, USA, 1992; pp. 99–153. [Google Scholar]
- Perez Locas, C.; Yaylayan, V.A. Isotope labeling studies on the formation of 5- (hydroxymethyl)-2-furaldehyde (HMF) from sucrose by pyrolysis-GC/MS. J. Agric. Food Chem. 2008, 56, 6717–6723. [Google Scholar] [CrossRef]
- Gökmen, V.; Kocadağlı, T.; Göncüoğlu, N.; Mogol, B.A. Model studies on the role of 5-hydroxymethyl-2-furfural in acrylamide formation from asparagine. Food Chem. 2012, 132, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Limacher, A.; Kerler, J.; Davidek, T.; Schmalzried, F.; Blank, I. Formation of furan and methylfuran by Maillard-type reactions in model systems and food. J. Agri. Food Chem. 2008, 56, 3639–3647. [Google Scholar] [CrossRef] [PubMed]
- Van Lancker, F.; Adams, A.; Owczarek-Fendor, A.; De Meulenaer, B.; De Kimpe, N. Mechanistic Insights into Furan Formation in Maillard Model Systems. J. Agri. Food Chem. 2011, 59, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Poinot, P.; Arvisenet, G.; Grua-Priol, J.; Colas, D.; Fillonneau, C.; Le Bail, A.; Prost, C. Influence of formulation and process on the aromatic profile and physical characteristics of bread. J. Cereal Sci. 2008, 48, 686–697. [Google Scholar] [CrossRef]
- Owczarek-Fendor, A.; De Meulenaer, B.; Scholl, G.; Adams, A.; Van Lancker, F.; Yogendrarajah, P.; Uytterhoeven, V.; Eppe, G.; De Pauw, E.; Scippo, M.-L.; et al. Importance of fat oxidation in starch-based emulsions in the generation of the process contaminant furan. J. Agric. Food Chem. 2010, 58, 9579–9586. [Google Scholar] [CrossRef]
- Høie, A.H.; Svendsen, C.; Brunborg, G.; Glatt, H.; Alexander, J.; Meinl, W.; Husøy, T. Genotoxicity of three food processing contaminants in transgenic mice expressing human sulfotransferases 1A1 and 1A2 as assessed by the in vivo alkaline single cell gel electrophoresis assay. Environ. Mol. Mutagen. 2015, 56, 709–714. [Google Scholar] [CrossRef]
- Pastoriza de la Cueva, S.; Álvarez, J.; Végvári, Á.; Montilla-Gómez, J.; Cruz-López, O.; Delgado-Andrade, C.; Rufián-Henares, J.A. Relationship between HMF intake and SMF formation in vivo: An animal and human study. Mol. Nutr. Food Res. 2017, 61, 1600773. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact With Food (AFC) Related to Flavouring Group Evaluation 13: Furfuryl and Furan Derivatives With and Without Additional Side-chain Substituents and Heteroatoms From Chemical Group 14. (Commission Regulation (EC) No 1565/2000 of 18 July 2000). EFSA J. 2005, 215, 1–73. Available online: http://www.efsa.europa.eu/en/efsajournal/pub/215 (accessed on 27 April 2022).
- Alizadeh, M.; Barati, M.; Saleh-Ghadimi, S.; Roshanravan, N.; Zeinalian, R.; Jabbari, M. Industrial furan and its biological effects on the body systems. J. Food Biochem. 2018, 42, e12597. [Google Scholar] [CrossRef]
- Moro, S.; Chipman, J.K.; Wegener, J.W.; Hamberger, C.; Dekant, W.; Mally, A. Furan in heat-treated foods: Formation, exposure, toxicity, and aspects of risk assessment. Mol. Nutr. Food Res. 2012, 56, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.A. Reactive metabolites in the biotransformation of molecules containing a furan ring. Chem. Res. Toxicol. 2013, 26, 6–25. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Monographs on the evaluation of carcinogenic risks of chemicals to humans. In Dry Cleaning, Some Chlorinated Solvents and Other Industrial Chemicals; IARC: Lyon, France, 1995; Volume 63. [Google Scholar]
- Gill, S.S.; Kavanagh, M.; Cherry, W.; Barker, M.; Weld, M.; Cooke, G.M. A 28-day gavage toxicity study in male Fischer 344 rats with 2-methylfuran. Toxicol. Pathol. 2014, 42, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Helou, C.; Gadonna-Widehem, P.; Robert, N.; Branlard, G.; Thebault, J.; Librere, S.; Jacquot, S.; Mardon, J.; Piquet-Pissaloux, A.; Chapron, S.; et al. The impact of raw materials and baking conditions on Maillard reaction products, thiamine, folate, phytic acid and minerals in white bread. Food Funct. 2016, 7, 2498–2507. [Google Scholar] [CrossRef]
- Leiva, G.E.; Naranjo, G.B.; Malec, L.S. A study of different indicators of Maillard reaction with whey proteins and different carbohydrates under adverse storage conditions. Food Chem. 2017, 215, 410–416. [Google Scholar] [CrossRef]
- Thakur, J.S. HMF as a Quality Indicator in Garcinia indica Fruit Juice Concentrate. Curr. Res. Nutr. Food Sci. J. 2018, 6, 227–233. [Google Scholar] [CrossRef]
- Zhao, Q.; Ou, J.; Huang, C.; Qiu, R.; Wang, Y.; Liu, F.; Zheng, J.; Ou, S. Absorption of 1-dicysteinethioacetal-5-hydroxymehthylfurfural (DCH) in rats, and its effect on oxidative stress and gut microbiota. J. Agric. Food Chem. 2018, 66, 11451–11458. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Risks for public health related to the presence of furan and methylfurans in food. EFSA J. 2017, 15, e05005. Available online: https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2017.5005 (accessed on 27 April 2022).
- Al-Rashdan, A.; Helaleh, M.I.H.; Nisar, A.; Ibtisam, A.; Al-Ballam, Z. Determination of the Levels of Polycyclic Aromatic Hydrocarbons in Toasted Bread Using Gas Chromatography Mass Spectrometry. Int. J. Anal. Chem. 2010, 2010, 821216. [Google Scholar] [CrossRef]
- Koszucka, A.; Nowak, A. Thermal processing food-related toxicants: A review. Crit. Rev. Food Sci. Nutr. 2018, 59, 3579–3596. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Xu, J.; Wu, A.; Zhao, Z.; Tong, M.; Luan, S. Chemical characterization of particulate organic matter from commercial restaurants: Alkyl PAHs as new tracers for cooking. Sci. Total Environ. 2021, 770, 145308. [Google Scholar] [CrossRef] [PubMed]
- Dost, K.; Deli, C. Determination of Polycyclic Aromatic Hydrocarbons in Edible Oils and Barbecued Food by HPLC/UV-Vis Detection. Food Chem. 2012, 133, 193–199. [Google Scholar] [CrossRef]
- Rose, M.; Holland, J.; Dowding, A.; Petch, S.R.G.; White, S.; Fernandes, A.; Mortimer, D. Investigation into the Formation of PAHs in Foods Prepared in the Home to Determine the Effects of Frying, Grilling, Barbecuing, Toasting and Roasting. Food Chem. Toxicol. 2015, 78, 1–9. [Google Scholar] [CrossRef]
- Kazerouni, N.; Sinha, R.; Hsu, C.; Greenberg, A.; Rothman, N. Analysis of 200 Food Items for Benzo[a]pyrene and Estimation of Its Intake in an Epidemiologic Study. Food Chem. Toxicol. 2001, 39, 423–436. [Google Scholar] [CrossRef]
- Farhadian, A.; Jinap, S.; Abas, F.; Sakar, Z.I. Determination of Polycyclic Aromatic Hydrocarbons in Grilled Meat. Food Control 2010, 21, 606–610. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on Polycyclic Aromatic Hydrocarbons in Food. EFSA J. 2008, 724, 1–114. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2008.724 (accessed on 27 April 2022).
- Knecht, A.L.; Goodale, B.C.; Truong, L.; Simonich, M.T.; Swanson, A.J.; Matzke, M.M.; Anderson, K.A.; Water, K.M.; Tanguay, R.L. Comparative developmental toxicity of environmentally relevant oxygenated PAHs. Toxicol. Appl. Pharm. 2013, 271, 266–275. [Google Scholar] [CrossRef]
- Timme-Laragy, A.R.; Van Tiem, L.A.; Linney, E.A.; Di Giulio, R.T. Antioxidant Responses and NRF2 in Synergistic Developmental Toxicity of PAHs in Zebrafish. Toxicol. Sci. 2009, 109, 217–227. [Google Scholar] [CrossRef]
- Dasgupta, S.; Cao, A.; Mauer, B.; Yan, B.; Uno, S.; McElroy, A. Genotoxicity of oxy-PAHs to Japanese medaka (Oryzias latipes) embryos assessed using the comet assay. Environ. Sci. Pollut. Res. 2014, 21, 13867–13876. [Google Scholar] [CrossRef]
- Wang, W.; Jariyasopit, N.; Schrlau, J.; Jia, Y.; Tao, S.; Yu, T.W.; Dashwood, R.H.; Zhang, W.; Wang, X.; Simonich, S.L.M. Concentration and photochemistry of PAHs, NPAHs, and OPAHs and toxicity of PM2. 5 during the Beijing Olympic Games. Environ. Sci. Technol. 2011, 45, 6887–6895. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; IARC: Lyon, France, 2002; Volume 82. [Google Scholar]
- International Agency for Research on Cancer (IARC). Monographs on the evaluation of carcinogenic risks of chemicals to humans. In Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures; IARC: Lyon, France, 2010; (Suppl. 7). [Google Scholar]
- International Agency for Research on Cancer (IARC). Monographs on the evaluation of carcinogenic risks of chemicals to humans. In Chemical Agents and Related Occupations; IARC: Lyon, France, 2012; Volume 100F. [Google Scholar]
- The Commission of the European Communities. Commission Recommendation of 4 February 2005 on the Further Investigation into the Levels of Polycyclic Aromatic Hydrocarbons in Certain Foods (Notified under Document Number C(2005) 256) (Text with EEA relevance) (2005/108/EC). Off. J. Eur. Union 2005, 48, 69–71. Available online: https://eur-lex.europa.eu/eli/reco/2005/108/oj (accessed on 27 April 2022).
- Chawda, S.; Tarafdar, A.; Sinha, A.; Mishra, B.K. Profiling and Health Risk Assessment of PAHs Content in Tandoori and Tawa Bread from India. Polycycl. Aromat. Comp. 2017, 40, 21–32. [Google Scholar] [CrossRef]
- Orecchio, S.; Papuzza, V. Levels, Fingerprint and Daily Intake of Polycyclic Aromatic Hydrocarbons (PAHs) in Bread Baked Using Wood as Fuel. J. Hazard. Mater. 2009, 164, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Oey, S.B.; van der Fels-Klerx, H.J.; Fogliano, V.; van Leeuwen, S.P.J. Mitigation Strategies for the Reduction of 2- and 3-MCPD Esters and Glycidyl Esters in the Vegetable Oil Processing Industry. Compr. Rev. Food Sci. Food Saf. 2019, 18, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Svejkovská, B.; Novotný, O.; Divinová, V.; Réblová, Z.; Doležal, M.; Velíšek, J. Esters of 3-Chloropropane-1,2-Diol in Foodstuffs. Czech J. Food Sci. 2004, 22, 190–196. [Google Scholar] [CrossRef]
- Zelinková, Z.; Svejkovská, B.; Velíšek, J.; Doležal, M. Fatty acid esters of 3-chloropropane-1,2-diol in edible oils. Food Addit. Contam. 2007, 23, 1290–1298. [Google Scholar] [CrossRef]
- Cao, R.; Wang, S.; Li, C.; Liu, W.; Zhou, H.; Yao, Y. Molecular Reaction Mechanism for the Formation of 3-Chloropropanediol Esters in Oils and Fats. J. Agric. Food Chem. 2019, 67, 2700–2708. [Google Scholar] [CrossRef]
- Craft, B.D.; Nagy, K.; Seefelder, W.; Dubois, M.; Destaillats, F. Glycidyl esters in refined palm (Elaeis guineensis) oil and related fractions. Part II: Practical recommendations for effective mitigation. Food Chem. 2012, 132, 73–79. [Google Scholar] [CrossRef]
- Destaillats, F.; Craft, B.D.; Dubois, M.; Nagy, K. Glycidyl esters in refined palm (Elaeis guineensis) oil and related fractions, Part I: Formation mechanism. Food Chem. 2012, 131, 1391–1398. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Monographs on the Evaluation of Carcinogenic Risks to Humans: Some Industrial Chemicals; IARC: Lyon, France, 2000; Volume 77. [Google Scholar]
- International Agency for Research on Cancer (IARC). Monographs on 3-Monochloro-1,2-Propanediol; IARC: Lyon, France, 2013; Volume 101. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Risks for human health related to the presence of 3- and 2-monochloropropanediol (MCPD), and their fatty acid esters, and glycidyl fatty acid esters in food. EFSA J. 2006, 14, e04426. Available online: https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2016.4426 (accessed on 27 April 2022).
- Hamlet, C.G.; Sadd, P.A. Chloropropanols and their esters in cereal products. Czech J. Food Sci. 2004, 22, 259–262. [Google Scholar] [CrossRef]
- Juraschek, L.M.; Kappenberg, A.; Amelung, W. Mycotoxins in soil and environment. Sci. Total Environ. 2022, 814, 152425. [Google Scholar] [CrossRef] [PubMed]
- Ingle, A.P.; Gupta, I.; Jogee, P.; Rai, M. Role of nanotechnology in the detection of mycotoxins: A smart approach. In Nanomycotoxicology, 1st ed.; Rai, M., Abd-Elsalam, K.A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 11–33. [Google Scholar]
- Bennett, J.W. Mycotoxins, mycotoxicoses, mycotoxicology andmycopathologia. Mycopathologia 1987, 100, 3–5. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture (USDA). Grain, Fungal Diseases and Mycotoxin Reference. Available online: https://www.ams.usda.gov/sites/default/files/media/FungalDiseaseandMycotoxinReference2017.pdf (accessed on 27 April 2022).
- Streit, E.; Naehrer, K.; Rodrigues, I.; Schatzmayr, G. Mycotoxin occurrence in feed and feed raw materials worldwide: Long-term analysis with special focus on Europe and Asia. J. Sci. Food Agric. 2013, 93, 2892–2899. [Google Scholar] [CrossRef]
- Kovalsky, P.; Kos, G.; Nährer, K.; Schwab, C.; Jenkins, T.; Schatzmayr, G.; Sulyok, M.; Krska, R. Co-occurrence of regulated, masked and emerging mycotoxins and secondary metabolites in finished feed and maize—an extensive survey. Toxins 2016, 8, 363. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Saladino, F.; Quiles, J.M.; Mañes, J.; Fernández-Franzón, M.; Luciano, F.B.; Meca, G. Dietary exposure to mycotoxins through the consumption of commercial bread loaf in Valencia, Spain. LWT 2017, 75, 697–701. [Google Scholar] [CrossRef]
- Degen, G.H. Mycotoxins in food: Occurrence, importance and health risk. Bundesgesundheitsblatt Gesundh. Gesundh. 2017, 60, 745–756. [Google Scholar] [CrossRef]
- De Boevre, M.; Mavungu, J.D.; Landshchoot, S.; Audenaert, K.; Eeckhout, M.; Maene, P. Natural occurrence of mycotoxins and their masked forms in food and feed products. World Mycotoxin J. 2012, 5, 207–219. [Google Scholar] [CrossRef]
- Sarmast, E.; Fallah, A.A.; Jafari, T.; Khaneghah, A.M. Occurrence and fate of mycotoxins in cereals and cereal-based products: A narrative review of systematic reviews and meta-analyses studies. Curr. Opin. Food Sci. 2021, 39, 68–75. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Zadeike, D.; Vaitkeviciene, R.; Bartkevics, V.; Bogdanova, E.; Bartkiene, E.; Lele, V.; Juodeikiene, G.; Cernauskas, D.; Valatkeviciene, Z. The expedient application of microbial fermentation after whole-wheat milling and fractionation to mitigate mycotoxins in wheat-based products. LWT 2021, 137, 110440. [Google Scholar] [CrossRef]
- Stanciu, O.; Juan, C.; Berrada, H.; Miere, D.; Loghin, F.; Mañes, J. Study on Trichothecene and Zearalenone Presence in Romanian Wheat Relative to Weather Conditions. Toxins 2019, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Eslamizad, S.; Yazdanpanah, H.; Hadian, Z.; Tsitsimpikou, C.; Goumenou, M.; AliAbadi, M.H.S.; Kamalabadi, M.; Tsatsakis, A. Exposure to multiple mycotoxins in domestic and imported rice commercially traded in Tehran and possible risk to public health. Toxicol. Rep. 2021, 8, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- Onyedum, S.C.; Adefolalu, F.S.; Muhammad, H.L.; Apeh, D.O.; Agada, M.S.; Imienwanrin, M.R.; Makun, H.A. Occurrence of major mycotoxins and their dietary exposure in North-Central Nigeria staples. Sci. Afr. 2020, 7, e00188. [Google Scholar] [CrossRef]
- Andrade, P.D.; Dias, J.V.; Souza, D.M.; Brito, A.P.; van Donkersgoed, G.; Pizzutti, I.R.; Caldas, E.D. Mycotoxins in cereals and cereal-based products: Incidence and probabilistic dietary risk assessment for the Brazilian population. Food Chem. Toxicol. 2020, 143, 111572. [Google Scholar] [CrossRef]
- Martins, C.; Torres, D.; Lopes, C.; Correia, D.; Goios, A.; Assunção, R.; Alvito, P.; Vidal, A.; De Boevre, M.; De Saeger, S.; et al. Deoxynivalenol exposure assessment through a modeling approach of food intake and biomonitoring data–A contribution to the risk assessment of an enteropathogenic mycotoxin. Food Res. Int. 2021, 140, 109863. [Google Scholar] [CrossRef]
- Zhang, Y.; Pei, F.; Fang, Y.; Li, P.; Zhao, Y.; Shen, F.; Zou, Y.; Hu, Q. Comparison of concentration and health risks of 9 Fusarium mycotoxins in commercial whole wheat flour and refined wheat flour by multi-IAC-HPLC. Food Chem. 2019, 275, 763–769. [Google Scholar] [CrossRef]
- Dahl, B.; Wilson, W.W. Risk premiums due to Fusarium Head Blight (FHB) in wheat and barley. Agric. Syst. 2018, 162, 145–153. [Google Scholar] [CrossRef]
- Wilson, W.; Dahl, B.; Nganje, W. Economic costs of Fusarium head blight, scab and deoxynivalenol. World Mycotoxin J. 2018, 11, 291–302. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing: Ames, IA, USA, 2006. [Google Scholar]
- Bullerman, L.B.; Bianchini, A. Stability of mycotoxins during food processing. Int. J. Food Microbiol. 2007, 119, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Ponts, N.; Pinson-Gadais, L.; Verdal-Bonnin, M.N.; Barreau, C.; Richard-Forget, F. Accumulation of deoxynivalenol and its 15-acetylated form is significantly modulated by oxidative stress in liquid cultures of Fusarium graminearum. FEMS Microbiol. Lett. 2006, 258, 102–107. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of Carcinogenic Risk to Humans. In Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; IARC: Lyon, France, 1993; Volume 56. [Google Scholar]
- World Health Organization (WHO); Joint FAO/WHO Expert Committee on Food Additives (JECFA). Evaluation of Certain Contaminants in Food: Eighty-Third Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series 1002; WHO: Geneva, Switzerland, 2017; Available online: https://apps.who.int/iris/handle/10665/254893 (accessed on 27 April 2022).
- The Commission of the European Communities. Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs (Text with EEA Relevance). Off. J. Eur. Union 2006, 49, 558–577. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R1881from=EN (accessed on 27 April 2022).
- Chen, Z.; Chen, H.; Li, X.; Yuan, Q.; Su, J.; Yang, L.; Ning, L.; Lei, H. Fumonisin B 1 damages the barrier functions of porcine intestinal epithelial cells in vitro. J. Biochem. Mol. Toxicol. 2019, 787, 147405. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Jia, B.; Liu, N.; Yu, D.; Zhang, S.; Wu, A. Fumonisin B1 triggers carcinogenesis via HDAC/PI3K/Akt signalling pathway in human esophageal epithelial cells. Sci. Total Environ. 2021, 787, 147405. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Xian, R.; Lin, F.; Li, X.; Li, X.; Qiang, F.; Li, X. Fumonisin B1 induces hepatotoxicity in mice through the activation of oxidative stress, apoptosis and fibrosis. Chemosphere 2022, 296, 133910. [Google Scholar] [CrossRef]
- Krupashree, K.; Rachitha, P.; Khanum, F. Apocynin ameliorates fumonisin b1 induced hepatotoxicity via NADPH oxidase inhibition and quantification of sphingosine and sphinganine. Pharmacol. Res. Mod. Chin. Med. 2022, 2, 100036. [Google Scholar] [CrossRef]
- Awad, W.A.; Zentek, J. The feed contaminant deoxynivalenol affects the intestinal barrier permeability through inhibition of protein synthesis. Arch. Toxicol. 2014, 89, 961–965. [Google Scholar] [CrossRef]
- Faeste, C.K.; Pierre, F.; Ivanova, L.; Sayyari, A.; Massotte, D. Behavioural and metabolomic changes from chronic dietary exposure to low-level deoxynivalenol reveal impact on mouse well-being. Arch. Toxicol. 2019, 93, 2087–2102. [Google Scholar] [CrossRef]
- Salahipour, M.H.; Hasanzadeh, S.; Malekinejad, H.; Razi, M.; Farrokhi-Ardebili, F. Deoxynivalenol reduces quality parameters and increases DNA damage in mice spermatozoa. Andrologia 2019, 51, e13238. [Google Scholar] [CrossRef]
- World Health Organization (WHO); Joint FAO/WHO Expert Committee on Food Additives (JECFA). Evaluation of Certain Contaminants in Food: Seventy-Second Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series 959; WHO: Rome, Italy, 2011; Available online: https://apps.who.int/iris/bitstream/handle/10665/44514/WHO_TRS_959_eng.pdf?sequence=1isAllowed=y (accessed on 27 April 2022).
- World Health Organization (WHO); Joint FAO/WHO Expert Committee on Food Additives (JECFA). Evaluation of Certain Mycotoxins in Food: Fifty-Sixth Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series 906; WHO: Geneva, Switzerland, 2002; Available online: https://apps.who.int/iris/bitstream/handle/10665/42448/WHO_TRS_906.pdf?sequence=1isAllowed=y (accessed on 27 April 2022).
- Ruíz-Arias, M.A.; Bernal-Hernández, Y.Y.; Medina-Díaz, I.M.; González-Arias, C.A.; Barrón-Vivanco, B.S.; Herrera-Moreno, M.; Sordo, J.F.; Rojas-García, A.E. Genotoxic effects of the ochratoxin A (OTA), its main metabolite (OTα) per se and in combination with fumonisin B1 in HepG2 cells and human lymphocytes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2022, 878, 503482. [Google Scholar] [CrossRef]
- Schwerdt, G.; Kopf, M.; Gekle, M. The Impact of the Nephrotoxin Ochratoxin A on Human Renal Cells Studied by a Novel Co-Culture Model Is Influenced by the Presence of Fibroblasts. Toxins 2021, 13, 219. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.A.; Vaidya, V.S.; Bonventre, J.V. Biomarkers of nephrotoxic acute kidney injury. Toxicology 2008, 245, 182–193. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risk assessment of ochratoxin A in food. EFSA J. 2020, 18, 6113. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2020.6113 (accessed on 27 April 2022).
- Kowalska, K.; Habrowska-Górczyńska, D.; Urbanek, K.; Domińska, K.; Piastowska-Ciesielska, A. Estrogen Receptor α Is Crucial in Zearalenone-Induced Invasion and Migration of Prostate Cancer Cells. Toxins 2018, 10, 98. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011, 9, 2197. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2011.2197 (accessed on 27 April 2022). [CrossRef]
- The European Commission. Commission Recommendation of 27 March 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products (Text with EEA relevance) (2013/165/EU). Off. J. Eur. Union 2013, 56, 12–15. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32013H0165from=EN (accessed on 27 April 2022).
- Istituto Superiore di Sanità (ISS); Italian National Agency for New Technologies; Energy and Sustainable Economic Development (ENEA); French Agency for Food, Environmental and Occupational Health Safety (ANSES). In vivo toxicity and genotoxicity of beauvericin and enniatins. Combined approach to study in vivo toxicity and genotoxicity of mycotoxins beauvericin (BEA) and enniatin B (ENNB). EFSA Supporting Publ. 2018, 15, 1406E. Available online: https://efsa.onlinelibrary.wiley.com/doi/abs/10.2903/sp.efsa.2018.EN-1406 (accessed on 27 April 2022).
- Ali, H.; Khan, E. What are heavy metals? Long-standing controversy over the scientific use of the term “heavy metals”-proposal of a comprehensive definition. Toxicol. Environ. Chem. 2018, 100, 6–19. [Google Scholar] [CrossRef]
- Barakat, M.A. New Trends in Removing Heavy Metals from Industrial Wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Fu, J.; Zhou, Q.; Liu, J.; Liu, W.; Wang, T.; Zhang, Q.; Jiang, G. High Levels of Heavy Metals in Rice (Oryza Sativa L.) from a Typical E-Waste Recycling Area in Southeast China and Its Potential Risk to Human Health. Chemosphere 2008, 71, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.H.; Sibly, R.M.; Hopkin, S.P.; Peakall, D.B. Principles of Ecotoxicology, 4th ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Naghipour, D.; Amouei, A.; Nazmara, S.A. A Comparative Evaluation of Heavy Metals in the Different Breads in Iran: A Case Study of Rasht City. Health Scope 2014, 3, e18175. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Basaran, B. Comparison of heavy metal levels and health risk assessment of different bread types marketed in Turkey. J. Food Compos. Anal. 2022, 108, 104443. [Google Scholar] [CrossRef]
- Wieczorek-Dąbrowska, M.; Tomza-Marciniak, A.; Pilarczyk, B.; Balicka-Ramisz, A. Roe and red deer as bioindicators of heavy metals contamination in north-western Poland. Chem. Ecol. 2013, 29, 100–110. [Google Scholar] [CrossRef]
- Guo, G.; Lei, M.; Chen, T.; Yang, J. Evaluation of different amendments and foliar fertilizer for immobilization of heavy metals in contaminated soils. J. Soil. Sediment. 2018, 18, 239–247. [Google Scholar] [CrossRef]
- Zioła-Frankowska, A.; Karaś, K.; Mikołajczak, K.; Kurzyca, I.; Kowalski, A.; Frankowski, M. Identification of metal(loid)s compounds in fresh and pre-baked bread with evaluation of risk health assessment. J. Cereal Sci. 2021, 97, 103164. [Google Scholar] [CrossRef]
- Woldetsadik, D.; Llorent-Martínez, E.J.; Ortega-Barrales, P.; Haile, A.; Hailu, H.; Madani, N.; Warner, N.S.; Fleming, D.E.B. Contents of Metal(loid)s in a Traditional Ethiopian Flat Bread (Injera), Dietary Intake, and Health Risk Assessment in Addis Ababa, Ethiopia. Biol. Trace Elem. Res. 2020, 198, 732–743. [Google Scholar] [CrossRef]
- Ashot, D.P.; Sergey, A.H.; Radik, M.B.; Arthur, S.S.; Mantovani, A. Risk assessment of dietary exposure to potentially toxic trace elements in emerging countries: A pilot study on intake via flour-based products in Yerevan, Armenia. Food Chem. Toxicol. 2020, 146, 111768. [Google Scholar] [CrossRef]
- Nawaz, H.; Anwar-ul-Haq, M.; Akhtar, J.; Arfan, M. Cadmium, chromium, nickel, and nitrate accumulation in wheat (Triticum aestivum L.) using wastewater irrigation and health risks assessment. Ecotoxicol. Environ. Saf. 2021, 208, 111685. [Google Scholar] [CrossRef]
- Wang, L.; Yin, X.; Gao, S.; Jiang, T.; Ma, C. In vitro oral bioaccessibility investigation and human health risk assessment of heavy metals in wheat grains grown near the mines in North China. Chemosphere 2020, 252, 126522. [Google Scholar] [CrossRef]
- Busari, M.B.; Hamzah, R.U.; Muhammad, H.L.; Yusuf, R.S.; Adeniyi, J.O.; Ibrahim, Y.O.; Adakole, J.O. Phenolics-rich extracts of Nauclea latifolia fruit ameliorates lead acetate-induced haematology and lung tissues toxicity in male Wistar rats. Sci. Afr. 2021, 11, e00686. [Google Scholar] [CrossRef]
- Melak, D.; Ferreccio, C.; Kalman, D.; Parra, R.; Acevedo, J.; Pérez, L.; Cortes, S.; Smith, H.A.; Yuan, Y.; Liaw, J.; et al. Arsenic methylation and lung and bladder cancer in a case-control study in northern Chile. Toxicol. Appl. Pharmacol. 2014, 274, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.; Santander, A.; Chavarría, L.; Cardozo, R.; Savio, F.; Sobrevia, L.; Nicolson, G.L. Functional consequences of lead and mercury exposomes in the heart. Mol. Asp. Med. 2021, 101048. [Google Scholar] [CrossRef]
- Tan, Q.; Ma, J.; Zhou, M.; Wang, D.; Wang, B.; Nie, X.; Mu, G.; Zhang, X.; Chen, W. Heavy metals exposure, lipid peroxidation and heart rate variability alteration: Association and mediation analyses in urban adults. Ecotoxicol. Environ. Saf. 2020, 205, 111149. [Google Scholar] [CrossRef]
- Melila, M.; Rajaram, R.; Ganeshkumar, A.; Kpemissi, M.; Pakoussi, T.; Agbere, S.; Lazar, I.M.; Lazar, G.; Amouzou, K.; Paray, B.A.; et al. Assessment of renal and hepatic dysfunction by co-exposure to toxic metals (Cd, Pb) and fluoride in people living nearby an industrial zone. J. Trace Elem. Med. Biol. 2022, 69, 126890. [Google Scholar] [CrossRef]
- Liu, J.; Ren, L.; Wei, J.; Zhang, J.; Zhu, Y.; Li, X.; Jing, L.; Duan, J.; Zhou, X.; Sun, Z. Fine particle matter disrupts the blood-testis barrier by activating TGF-beta3/p38 MAPK pathway and decreasing testosterone secretion in rat. Environ. Toxicol. 2018, 33, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.; Kawu, M.U.; Shittu, M.; Saleh, A.; Jolayemi, K.O.; Ibrahim, N.B.; Oyetunde, J.S.; Okoronkwo, M.O. Comparative effects of methanol leaf extract of Moringa oleifera and ascorbic acid on haematological and histopathological changes induced by subchronic lead toxicity in male wistar rats. Pharmacol. Res. Mod. Chin. Med. 2022, 2, 100031. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). ATSDR’s Substance Priority List. Available online: https://www.atsdr.cdc.gov/spl/index.html#2019spl (accessed on 27 April 2022).
- International Agency for Research on Cancer (IARC). IARC Monographs on the Identification of Carcinogenic Hazards to Humans. List of Classifications. Agents Classified by the IARC Monographs; Volume 1–129. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 27 April 2022).
- World Health Organization (WHO). 10 Chemicals of Public Health Concern; World Health Organization (WHO): Geneva, Switzerland, 2020; Available online: https://www.who.int/news-room/photo-story/photo-story-detail/10-chemicals-of-public-health-concern (accessed on 27 April 2022).
- The European Commission. Commission Recommendation of 4 April 2014 on the Reduction of the Presence of Cadmium in Foodstuffs (Text with EEA Relevance) (2014/193/EU). Off. J. Eur. Union 2014, 57, 80–81. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014H0193from=EN (accessed on 27 April 2022).
- The European Commission. Commission Recommendation (EU) 2015/1381 of 10 August 2015 on the Monitoring of Arsenic in Food. Off. J. Eur. Union 2015, 58, 9–10. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015H1381from=EN (accessed on 27 April 2022).
- World Health Organization (WHO). Exposure of Children to Chemical Hazards in Food; Fact Sheet 4.4. December 2009. Code: RPG4_Food_Ex1; World Health Organization (WHO): Geneva, Switzerland, 2009; Available online: https://www.euro.who.int/__data/assets/pdf_file/0004/97042/4.4.-Exposure-of-children-to-chemical-hazards-in-food-EDITED_layouted.pdf (accessed on 27 April 2022).
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific opinion on lead in food. EFSA J. 2010, 8, 1570. Available online: https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2010.1570 (accessed on 27 April 2022).
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Cadmium dietary exposure in the European population. EFSA J. 2012, 10, 2551. Available online: https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2012.2551 (accessed on 27 April 2022). [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risk for public health related to the presence of mercury and methyl mercury in food. EFSA J. 2012, 10, 2985. Available online: https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2012.2985 (accessed on 27 April 2022).
- Farooq, M.; Ullah, A.; Usman, M.; Siddique, K.H.M. Application of zinc and biochar help to mitigate cadmium stress in bread wheat raised from seeds with high intrinsic zinc. Chemosphere 2020, 260, 127652. [Google Scholar] [CrossRef] [PubMed]
- Rebekić, A.; Lončarić, Z. Genotypic difference in cadmium effect on agronomic traits and grain zinc and iron concentration in winter wheat. Emir. J. Food Agric. 2016, 28, 772–778. [Google Scholar] [CrossRef]
- Groppa, M.D.; Rosales, E.P.; Iannone, M.F.; Benavides, M.P. Nitric oxide, polyamines and Cd-induced phytotoxicity in wheat roots. Phytochemistry 2008, 69, 2609–2615. [Google Scholar] [CrossRef]
- Khazaal, S.; El Darra, N.; Kobeissi, A.; Jammoul, R.; Jammoul, A. Risk assessment of pesticide residues from foods of plant origin in Lebanon. Food Chem. 2022, 374, 131676. [Google Scholar] [CrossRef]
- Weber, J.B. Properties and Behavior of Pesticides in Soil. In Mechanisms of Pesticide Movement into Ground Water, 1st ed.; Honeycutt, R.C., Schabacker, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 15–42. [Google Scholar]
- Dar, M.A.; Kaushik, G.; Chiu, J.F.V. Pollution status and biodegradation of organophosphate pesticides in the environment. In Abatement of Environmental Pollutants, 1st ed.; Singh, P., Kumar, A., Borthakur, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 2, pp. 25–66. [Google Scholar]
- Fatunsin, O.T.; Oyeyiola, A.O.; Moshood, M.O.; Akanbi, L.M.; Fadahunsi, D.E. Dietary Risk Assessment of Organophosphate and Carbamate Pesticide Residues in Commonly Eaten Food Crops. Sci. Afr. 2020, 8, e00442. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef]
- Choudhury, B.H.; Das, B.K.; Baruah, A.A.L.H. Monitoring of Pesticide Residues in Market Basket Vegetables of Jorhat District of Assam, India. Int. J. Adv. Res. Technol. 2013, 2, 250–261. [Google Scholar]
- Witczak, A.; Abdel-Gawad, H. Assessment of health risk from organochlorine pesticides residues in high-fat spreadable foods produced in Poland. J. Environ. Sci. Health B 2014, 49, 917–928. [Google Scholar] [CrossRef]
- Poirier, L.; Brun, L.; Jacquet, P.; Lepolard, C.; Armstrong, N.; Torre, C.; Daudé, D.; Ghigo, E.; Chabrière, E. Enzymatic degradation of organophosphorus insecticides decreases toxicity in planarians and enhances survival. Sci. Rep. 2017, 7, 15194. [Google Scholar] [CrossRef]
- Reiler, E.; Jørs, E.; Bælum, J.; Huici, O.; Alvarez Caero, M.M.; Cedergreen, N. The influence of tomato processing on residues of organochlorine and organophosphate insecticides and their associated dietary risk. Sci. Total Environ. 2015, 527–528, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Blaznik, U.; Yngve, A.; Eržen, I.; Ribič, C.H. Consumption of fruits and vegetables and probabilistic assessment of the cumulative acute exposure to organophosphorus and carbamate pesticides of schoolchildren in Slovenia. Public Health Nutr. 2016, 19, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Okediran, O.; Dauda, M.S.; Kolawole, S.A. Assessment of Pesticide Residues in Fresh Vegetables from Three Major Markets in Lagos Using QuEChERS Method and GC-MS. Int. Res. J. Pure Appl. Chem. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Kortenkamp, A. Ten years of mixing cocktails: A review of combination effects of endocrine-disrupting chemicals. Environ. Health Perspect. 2007, 115 (Suppl. 1), 98–105. [Google Scholar] [CrossRef] [PubMed]
- Coremen, M.; Turkyilmaz, I.B.; Us, H.; Us, A.S.; Celik, S.; Ozel, A.; Bulan, O.K.; Yanardag, R. Lupeol inhibits pesticides induced hepatotoxicity via reducing oxidative stress and inflammatory markers in the rats. Food Chem. Toxicol. 2022, 164, 113068. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.T.; Rushin, A.; Boyda, J.; Souders, C.L.; Martyniuk, C.J. Dieldrin-induced neurotoxicity involves impaired mitochondrial bioenergetics and an endoplasmic reticulum stress response in rat dopaminergic cells. Neurotoxicology 2017, 63, 1–12. [Google Scholar] [CrossRef]
- Wee, S.Y.; Aris, A.Z.; Yusoff, F.M.; Praveena, S.M. Occurrence and risk assessment of multiclass endocrine disrupting compounds in an urban tropical river and a proposed risk management and monitoring framework. Sci. Total Environ. 2019, 671, 431–442. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahmad, M. Deciphering the toxic effects of organochlorine pesticide, dicofol on human RBCs and lymphocytes. Pestic. Biochem. Phys. 2017, 143, 127–134. [Google Scholar] [CrossRef]
- Parada, H.; Sun, X.; Tse, C.K.; Engel, L.S.; Olshan, A.F.; Troester, M.A. Plasma levels of dichlorodiphenyldichloroethene (DDE) and dichlorodiphenyltrichloroethane (DDT) and survival following breast cancer in the Carolina Breast Cancer Study. Environ. Int. 2019, 125, 161–171. [Google Scholar] [CrossRef]
- Yin, S.; Wei, J.; Wei, Y.; Jin, L.; Wang, L.; Zhang, X.; Jia, X.; Ren, A. Organochlorine pesticides exposure may disturb homocysteine metabolism in pregnant women. Sci. Total Environ. 2019, 708, 135146. [Google Scholar] [CrossRef]
- Palaniswamy, S.; Abass, K.; Rysä, J.; Odland, J.Ø.; Grimalt, J.O.; Rautio, A.; Järvelin, M.-R. Non-occupational exposure to pesticides and health markers in general population in Northern Finland: Differences between sexes. Environ. Int. 2021, 156, 106766. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiyama, T.; Ito, Y.; Oya, N.; Nomasa, K.; Sato, H.; Minato, K.; Kitamori, K.; Oshima, S.; Minematsu, A.; Niwa, K.; et al. Quantitative analysis of organophosphate pesticides and dialkylphosphates in duplicate diet samples to identify potential sources of measured urinary dialkylphosphates in Japanese women. Environ. Pollut. 2022, 298, 118799. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. In Evaluation of Five Organophosphate Insecticides and Herbicides; IARC: Lyon, France, 2015; Volume 112. [Google Scholar]
- The European Parliament and the Council of the European Union. Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC (Text with EEA relevance). Off. J. Eur. Union 2005, 48, 1–16. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32005R0396from=PL (accessed on 27 April 2022).
- The Council of the European Communities. Council Directive of 24 July 1986 on the fixing of maximum levels for pesticide residues in and on cereals (86/362/EEC). Off. J. Eur. Union 1986, 29, 37–42. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31986L0362from=EN (accessed on 27 April 2022).
- Tao, Y.; Jia, C.; Jing, J.; Zhang, J.; Yu, P.; He, M.; Wu, J.; Chen, L.; Zhao, E. Occurrence and dietary risk assessment of 37 pesticides in wheat fields in the suburbs of Beijing, China. Food Chem. 2021, 350, 129245. [Google Scholar] [CrossRef]
- Galani, Y.J.H.; Houbraken, M.; Wumbei, A.; Djeugap, J.F.; Fotio, D.; Gong, Y.Y.; Spanoghe, P. Monitoring and dietary risk assessment of 81 pesticide residues in 11 local agricultural products from the 3 largest cities of Cameroon. Food Control 2020, 118, 107416. [Google Scholar] [CrossRef]
- Ingenbleek, L.; Hu, R.; Pereira, L.L.; Paineau, A.; Colet, I.; Koné, A.Z.; Adegboye, A.; Hossou, S.E.; Dembélé, Y.; Oyedele, A.D.; et al. Sub-Saharan Africa total diet study in Benin, Cameroon, Mali and Nigeria: Pesticides occurrence in foods. Food Chem. X 2019, 2, 100034. [Google Scholar] [CrossRef] [PubMed]
- Chun, O.K.; Kang, H.G. Estimation of risks of pesticide exposure, by food intake, to Koreans. Food Chem. Toxicol. 2003, 41, 1063–1076. [Google Scholar] [CrossRef]
- Crépet, A.; Luong, T.M.; Baines, J.; Boon, P.E.; Ennis, J.; Kennedy, M.; Massarelli, I.; Miller, D.; Nako, S.; Reuss, R.; et al. An international probabilistic risk assessment of acute dietary exposure to pesticide residues in relation to codex maximum residue limits for pesticides in food. Food Control 2021, 121, 107563. [Google Scholar] [CrossRef]
- Torović, L.; Vuković, G.; Dimitrov, N. Pesticide residues in fruit juice in Serbia: Occurrence and health risk estimates. J. Food Compos. Anal. 2021, 99, 103889. [Google Scholar] [CrossRef]
- Ramírez-Jiménez, A.; García-Villanova, B.; Guerra-Hernández, E. Hydroxymethylfurfural and methylfurfural content of selected bakery products. Food Res. Int. 2000, 33, 833–838. [Google Scholar] [CrossRef]
- Gülcan, Ü.; Uslu, C.C.; Mutlu, C.; Arslan-Tontul, S.; Erbaş, M. Impact of inert and inhibitor baking atmosphere on HMF and acrylamide formation in bread. Food Chem. 2020, 332, 127434. [Google Scholar] [CrossRef] [PubMed]
- Ciecierska, M.; Obiedziński, M.W. Polycyclic aromatic hydrocarbons in the bakery chain. Food Chem. 2013, 141, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.F.; Das, B.S. Determination of Nanogram/Kilogram Levels of Polycyclic Aromatic Hydrocarbons in Foods by HPLC with Fluorescence Detection. Int. J. Environ. Anal. Chem. 1986, 24, 113–131. [Google Scholar] [CrossRef]
- Chung, S.W.C.; Kwong, K.P.; Yau, J.C.W.; Wong, A.M.C.; Xiao, Y. Chloropropanols levels in foodstuffs marketed in Hong Kong. J. Food Compost. Anal. 2008, 21, 569–573. [Google Scholar] [CrossRef]
- Vaclavikova, M.; Malachova, A.; Veprikova, Z.; Dzuman, Z.; Zachariasova, M.; Hajslova, J. “Emerging” mycotoxins in cereals processing chains: Changes of enniatins during beer and bread making. Food Chem. 2013, 136, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Xiao, Y.; Lyu, W.; Li, M.; Wang, W.; Tang, B.; Wang, X.; Yang, H. Probabilistic Risk Assessment of Combined Exposure to Deoxynivalenol and Emerging Alternaria Toxins in Cereal-Based Food Products for Infants and Young Children in China. Toxins 2022, 14, 509. [Google Scholar] [CrossRef]
- Zhao, J.; Cheng, T.; Xu, W.; Han, X.; Zhang, J.; Zhang, H.; Wang, C.; Fanning, S.; Lia, F. Natural co-occurrence of multi-mycotoxins in unprocessed wheat grains from China. Food Control 2021, 130, 108321. [Google Scholar] [CrossRef]
- Nachi, I.; Fhoula, I.; Smida, I.; Taher, I.B.; Chouaibi, M.; Jaunbergs, J.; Bartkevics, V.; Hassouna, M. Assessment of lactic acid bacteria application for the reduction of acrylamide formation in bread. LWT 2018, 92, 435–441. [Google Scholar] [CrossRef]
- Albedwawi, A.S.; Al Sakkaf, R.; Osaili, T.M.; Yusuf, A.; Olaimat, A.; Liu, S.-Q.; Palmisano, G.; Shah, N.P.; Ayyash, M.M. Investigating acrylamide mitigation by potential probiotics Bifidobacterium breve and Lactiplantibacillus plantarum: Optimization, in vitro gastrointestinal conditions, and mechanism. LWT 2022, 163, 113553. [Google Scholar] [CrossRef]
- Taheur, F.B.; Mansour, C.; Kouidhi, B.; Chaieb, K. Use of lactic acid bacteria for the inhibition of Aspergillus flavus and Aspergillus carbonarius growth and mycotoxin production. Toxicon 2019, 166, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ming, Q.; Cai, R.; Yue, T.; Yuan, Y.; Gao, Z.; Wang, Z. Biosorption of Cd2+ and Pb2+ from apple juice by the magnetic nanoparticles functionalized lactic acid bacteria cells. Food Control 2020, 109, 106916. [Google Scholar] [CrossRef]
- Yuan, S.; Li, C.; Yu, H.; Xie, Y.; Guo, Y.; Yao, W. Screening of lactic acid bacteria for degrading organophosphorus pesticides and their potential protective effects against pesticide toxicity. LWT 2021, 147, 111672. [Google Scholar] [CrossRef]


| Food Processing Contaminant | Sample | Content [μg/kg] | Reference | |
|---|---|---|---|---|
| Mean | Min-Max | |||
| AA | Multigrain bread | 79 | 79 | [22] |
| White bread | 87 | 87 | ||
| Whole wheat bread | 77 | 77 | ||
| Wholemeal bread | 84 | 84 | ||
| Rye bread | 83 | 83 | ||
| Toasted wheat bread | 22 | 10–34 | [38] | |
| Wheat bread | 21 | <20–30 | [26] | |
| Rye bread | 31 | <20–42 | ||
| Cornbread | 27 | <20–34 | ||
| Mixed bread | 25 | <20–39 | ||
| Bread | 57 | 31–90 | [23] | |
| Bread rolls | 52 | 42–67 | ||
| Friselle | 358 | 306–454 | ||
| Wholemeal bread | 61 | 44–88 | ||
| Wholemeal friselle | 384 | 328–450 | ||
| Toast bread | 134 | 45–246 | [20] | |
| Non-wheat bread | 43 | 4–163 | [39] | |
| Wheat bread | 27 | 6–65 | ||
| 5-HMF | Multigrain bread | 16,000 | 16,000 | [22] |
| Wholemeal bread | 23,000 | 23,000 | ||
| Whole wheat bread | 10,000 | 10,000 | ||
| Rye bread | 17,000 | 17,000 | ||
| White bread | 37,000 | 37,000 | ||
| 2-Pentylfuran | Bread crust | 270 | 258–282 | [40] |
| Chinese sourdough steamed bread | 902 | 180–1625 | [41] | |
| Furfural | Bread crust | 216 | 183–249 | [40] |
| Naphthalene | Chinese sourdough steamed bread | 65 | 21–109 | [41] |
| PAHs | Toasted bread | 1.5 | 0–3 | [42] |
| 3-MCPD | White bread | 5 | 5 | [43] |
| Bread | 120 | 120 | [44] | |
| 3-MCPDE | White bread | 1 | 0–2 | [43] |
| Bread and bread rolls | 29 | 23–26 | [45] | |
| 2-MCPD | White bread | <10 | <10 | [43] |
| Bread | 30 | 30 | [44] | |
| 2-MCPDE | White bread | 1 | 1–2 | [43] |
| Bread and bread rolls | 14 | 10–20 | [45] | |
| Glycidol | Bread | 650 | 650 | [44] |
| GE | White bread | 3 | 3 | [43] |
| Bread and bread rolls | 8 | 8 | [45] | |
| Food Processing Contaminant | Country | Sample | Average Dietary Exposure | Reference |
|---|---|---|---|---|
| AA | Turkey | Multigrain bread | 0.22 μg/kg BW/day | [22] |
| Spain | White bread | 0.31 μg/day | [43] | |
| Chile | Bread | 0.22 μg/kg BW/day | [46] | |
| Romania | 14 μg/kg | [47] | ||
| Croatia | Wheat bread | 0.16 μg/kg BW/day | [26] | |
| Italy | Bread | 100 μg/kg | [48] | |
| Slovenia | Toast bread | 0.21 μg/kg BW/day | [20] | |
| Poland | Wheat bread | 0.31 μg/kg BW/day | [39] | |
| Portugal | Bread | 787 μg/kg | [49] | |
| Latvia | Wheat bread | 0.89 μg/person/day | [50] | |
| China | Bread | 35 μg/kg | [51] | |
| Finland | Rye bread | 51 μg/kg | [52] | |
| Korea | Bread | 33 μg/kg | [53] | |
| HMF | Turkey | Multigrain bread | 87,000 μg/kg BW/day | [22] |
| 5-HMF | Iran | Flat bread | 12,000 μg/kg BW/day | [54] |
| PAHs | Romania | Toasted bread | 0.005 μg/kg BW/day | [42] |
| 3-MCPD | Spain | White bread | 0.30 μg/day | [43] |
| 2-MCPD | 0.31 μg/day | |||
| 3-MCPDEs | 0.04 μg/day | |||
| 2-MCPDEs | 0.06 μg/day | |||
| GE | 0.19 μg/day |
| Mycotoxin | Sample | Content [μg/kg] | Reference | |
|---|---|---|---|---|
| Mean | Min-Max | |||
| AFB1 | Wheat | 1 | 1 | [117] |
| White bread | 5.6 | 4.2–7.1 | [113] | |
| Whole wheat bread | 6.1 | 6.1 | ||
| Multigrain, oatmeal, corn, kamut, rye, lactose and gluten-free | 5.2 | 5.2 | ||
| AFB2 | White bread | 3.6 | 3.1–4.2 | |
| Whole wheat bread | 2.2 | 0.5–3.2 | ||
| Crustless white bread | 4.1 | 1.0–5.3 | ||
| Crustless whole wheat bread | 1.8 | 0.8–3.5 | ||
| AFG1 | White bread | 2.9 | 2.9 | |
| Multigrain, oatmeal, corn, kamut, rye, lactose and gluten-free | 2.5 | 2.5 | ||
| Enniatin A (ENA) | Wheat wholemeal grains | 15.7 | 3.0–28.4 | [118] |
| Enniatin A1 (ENA1) | Whole wheat bread | 2.4 | 2.2–2.6 | [113] |
| Multigrain, oatmeal, corn, kamut, rye, lactose and gluten-free | 2.6 | 2.6 | ||
| Enniatin B (ENB) | Wheat wholemeal grains | 408.7 | 30.1–787.3 | [118] |
| White bread | 9.8 | 2.0–18.7 | [113] | |
| Whole wheat bread | 16.5 | 1.3–41.1 | ||
| Multigrain, oatmeal, corn, kamut, rye, lactose and gluten-free | 16.9 | 0.4–54.0 | ||
| Crustless white bread | 14.8 | 1.4–8.7 | ||
| Crustless whole wheat bread | 10.6 | 1.0–31.0 | ||
| Enniatin B1 (ENB1) | Wheat wholemeal grains | 130.3 | 4.8–255.8 | [118] |
| White bread | 2.9 | 0.2–6.0 | [113] | |
| Whole wheat bread | 6.5 | 1.5–14.8 | ||
| Multigrain, oatmeal, corn, kamut, rye, lactose and gluten-free | 6.3 | 0.2–14.0 | ||
| Crustless white bread | 4.6 | 0.4–13.0 | ||
| Crustless whole wheat bread | 5.1 | 2.4–13.0 | ||
| DON | Bread | 41.0 | 39.4–42.6 | [3] |
| Wheat | 369 | 369 | [117] | |
| 44.3 | 1.1–955 | [119] | ||
| 15-ADON | Wheat wholemeal grains | 33.6 | 10.9–55.8 | [118] |
| Bread | 5.6 | 3.8–7.3 | [3] | |
| Wheat | 18.6 | 8.9–30 | [119] | |
| 3-ADON | Bread | 8.4 | 6.9–9.9 | [3] |
| Wheat | 7.5 | 2.7–12 | [119] | |
| FUMs | 117 | 117 | [117] | |
| OTA | Bread | 2.7 | 2.5–2.9 | [3] |
| Wheat | 3 | 3 | [117] | |
| T-2 | Bread | 4.6 | 3.3–5.4 | [3] |
| Wheat | 25 | 25 | [117] | |
| HT-2 | Bread | 19.3 | 11.5–27.1 | [3] |
| Wheat | 51.7 | 24.7–98.5 | [119] | |
| NIV | Bread | 50.8 | 17.5–84.0 | [3] |
| Wheat | 55.9 | 40.0–64.3 | [119] | |
| ZEA | Bread | 11.2 | 9.6–12.9 | [3] |
| Wheat | 34 | 34 | [117] | |
| 90.7 | 11.7–300 | [119] | ||
| White bread | 56.8 | 36–80.0 | [113] | |
| Whole wheat bread | 48.8 | 29.0–100.0 | ||
| Multigrain, oatmeal, corn, kamut, rye, lactose and gluten-free | 178.6 | 27.0–905.0 | ||
| Crustless white bread | 96.8 | 40.0–214.0 | ||
| Crustless whole wheat bread | 67.0 | 30.0–135.0 | ||
| Mycotoxin | Country | Sample | Average Dietary Exposure | Reference |
|---|---|---|---|---|
| AFB1 | Iran | Rice | 10 ng/kg BW/day | [120] |
| AFT | 16 ng/kg BW/day | |||
| AFs | Nigeria | Sorghum | 0.08 mg/kg BW/day | [121] |
| AFB1 | Spain | White bread | 1.06 ng/kg BW/day | [113] |
| AFB2 | ||||
| AFG1 | ||||
| DON | Iran | Rice | 242.71 ng/kg BW/day | [120] |
| Brazil | Wheat flour | 0.05 μg/kg BW/day | [122] | |
| Portugal | 0.24 µg/kg BW/day | [123] | ||
| China | Whole wheat | 0.65 μg/kg BW/day | [124] | |
| 3-ADON | 0.02 μg/kg BW/day | |||
| 15-ADON | 0.008 μg/kg BW/day | |||
| ENA1 | Spain | White bread | 1.06 ng/kg BW/day | [113] |
| ENB | ||||
| ENB1 | ||||
| FB1 | Iran | Rice | 118 ng/kg BW/day | [120] |
| FUMs | Nigeria | Sorghum | 33.58 mg/kg BW/day | [121] |
| FB1 | Brazil | Wheat flour | 0.07 μg/kg BW/day | [122] |
| FB2 | ||||
| FB3 | ||||
| OTA | Iran | Rice | 0.7 ng/kg BW/day | [120] |
| Nigeria | Sorghum | 13.22 μg/kg BW/day | [121] | |
| Brazil | Wheat flour | 0.01 μg/kg BW/day | [122] | |
| China | Whole wheat flour | 0.003 μg/kg BW/day | [124] | |
| Spain | White bread | 2.60 ng/kg BW/day | [113] |
| Toxic Metal | Sample | Content [μg/kg] | Reference | |
|---|---|---|---|---|
| Mean | Min-Max | |||
| Al | Multigrain bread | 9670 | 7110–12,500 | [156] |
| Wholemeal bread | 10,900 | 7660–16,800 | ||
| Whole wheat bread | 7730 | 6140–12,10 | ||
| Rye bread | 18,100 | 7210–123,000 | ||
| White bread | 7720 | 5600–13,500 | ||
| Various types of bread samples | 3620 | 2060–6560 | [159] | |
| Homemade bread | 296,500 | 249,000–344,000 | [158] | |
| As | Multigrain bread | 9.7 | 4.8–31.6 | [156] |
| Wholemeal bread | 15.3 | 11.4–25.7 | ||
| Whole wheat bread | 17.3 | 7.9–48.6 | ||
| Rye bread | 21.6 | 13–28.3 | ||
| White bread | 16.4 | 7.8–56.8 | ||
| Various types of bread samples | 5.1 | 2.9–16.2 | [159] | |
| Homemade bread | 67.5 | 54–81 | [160] | |
| Boron (B) | Various types of bread samples | 2760 | 90–6850 | [159] |
| Calcium (C)a | 440,000 | 310,000–1,920,000 | ||
| Cd | Multigrain bread | 15.9 | 13.4–49.5 | [156] |
| Wholemeal bread | 17.0 | 11.4–20.6 | ||
| Whole wheat bread | 12.6 | 3.3–19.3 | ||
| Rye bread | 11.8 | 4–16.7 | ||
| White bread | 11.7 | 3. 9–17.8 | ||
| Various types of bread samples | 35.9 | 12.7–53.8 | [159] | |
| White bread | 5.8 | 5.8 | [161] | |
| Co | Multigrain bread | 20.9 | <0.06–69.1 | [156] |
| Wholemeal bread | 21.6 | 7.7–30 | ||
| Whole wheat bread | 2.8 | <0.06–47.6 | ||
| Rye bread | 6.5 | <0.06–25 | ||
| White bread | 1.6 | <0.06–22.3 | ||
| Cr | Multigrain bread | 72.4 | 38.5–535 | |
| Wholemeal bread | 65.2 | 45.1–126 | ||
| Whole wheat bread | 47.3 | 37.2–70.0 | ||
| Rye bread | 89.1 | 75.2–280 | ||
| White bread | 50 | 21.5–174 | ||
| Various types of bread samples | 62.9 | 36.8–266.1 | [159] | |
| Homemade bread | 425 | 370–510 | [160] | |
| Cu | Multigrain bread | 4040 | 2220–6640 | [156] |
| Wholemeal bread | 3900 | 3300–4210 | ||
| Whole wheat bread | 3530 | 2910–4920 | ||
| Rye bread | 3060 | 2710–3890 | ||
| White bread | 2890 | 2640–4590 | ||
| Various types of bread samples | 1660 | 930–3850 | [159] | |
| White bread | 0.002 | 0.002 | [161] | |
| Fe | Various types of bread samples | 15,130 | 7420–39,200 | [159] |
| Hg | Multigrain bread | 0.29 | <0.26–0.61 | [156] |
| Wholemeal bread | 0.28 | 0.16–0.94 | ||
| Whole wheat bread | <0.26 | <0.26–0.38 | ||
| Rye bread | 0.08 | <0.26–0.69 | ||
| White bread | <0.26 | <0.26–0.71 | ||
| Various types of bread samples | 2.63 | 0.93–8.63 | [159] | |
| White bread | 8.6 | 8.6 | [161] | |
| Potassium (K) | Various types of bread samples | 3,310,000 | 1,840,000–4,750,000 | [159] |
| Mg | 50,000,000 | 240,000–1,420,000 | ||
| Mn | Multigrain bread | 28,900 | 8850–42,000 | [156] |
| Wholemeal bread | 25,900 | 20,700–32,800 | ||
| Whole wheat bread | 23,900 | 16,700–43,700 | ||
| Rye bread | 17,700 | 16,400–24,300 | ||
| White bread | 15,500 | 11,600–43,100 | ||
| Various types of bread samples | 7280 | 2800–15,830 | [159] | |
| Molybdeum (Mo) | White bread | 0.03 | 0.03 | [161] |
| Sodium (Na) | Various types of bread samples | 6,780,000 | 5,940,000–8,770,000 | [159] |
| Ni | Multigrain bread | 597 | 89.8–2720 | [156] |
| Wholemeal bread | 281 | 249–427 | ||
| Whole wheat bread | 269 | 203–498 | ||
| Rye bread | 339 | 198–699 | ||
| White bread | 228 | 141–642 | ||
| Various types of bread samples | 120 | 10–410 | [159] | |
| White bread | 0.02 | 0.02 | [161] | |
| Homemade bread | 1415 | 1330–1500 | [160] | |
| Pb | Multigrain bread | 22.6 | 4.6–83.0 | [156] |
| Wholemeal bread | 11.2 | 1. 5–68.4 | ||
| Whole wheat bread | 11.2 | <0.1–149 | ||
| Rye bread | 46.2 | 20–121 | ||
| White bread | 27.9 | <0.14–97.6 | ||
| Various types of bread samples | 38.5 | 29.5–98.6 | [159] | |
| White bread | 0.1 | 0.1 | [161] | |
| Homemade bread | 160 | 140–180 | [160] | |
| Selenium (Se) | Various types of bread samples | 15.1 | 4.8–53.1 | [159] |
| Zn | 8890 | 5940–15,120 | ||
| Toxic Metal | Country | Sample | Average Dietary Exposure | Reference |
|---|---|---|---|---|
| Al | Turkey | Multigrain bread | 25.8 μg/kg BW/day | [156] |
| Poland | Various types of bread samples | 9.84 μg/kg BW/day | [159] | |
| As | Turkey | Multigrain bread | 0.06 μg/kg BW/day | [156] |
| Poland | Various types of bread samples | 0.148 μg/kg BW/day | [159] | |
| Cd | Turkey | Multigrain bread | 0.03 μg/kg BW/day | [156] |
| Pakistan | Wastewater irrigated wheat | 1.04 μg/kg/day | [162] | |
| Poland | Various types of bread samples | 0.081 μg/kg BW/day | [159] | |
| China | Wheat grain | 0.45 μg/kg BW/day | [163] | |
| Co | Turkey | Multigrain bread | 0.3 μg/kg BW/day | [156] |
| Cr | Turkey | 0.19 μg/kg BW/day | ||
| Pakistan | Wastewater irrigated wheat | 1.17 μg/kg/day | [162] | |
| Poland | Various types of bread samples | 0.399 μg/kg BW/day | [159] | |
| Cu | Turkey | Multigrain bread | 8.39 μg/kg BW/day | [156] |
| China | Wheat grain | 11.52 μg/kg BW/day | [163] | |
| Hg | Turkey | Multigrain bread | <0.01 μg/kg BW/day | [156] |
| Poland | Various types of bread samples | 0.013 μg/kg BW/day | [159] | |
| Mn | Turkey | Multigrain bread | 53.4 μg/kg BW/day | [156] |
| Ni | Turkey | 0.74 μg/kg BW/day | ||
| Pakistan | Wastewater irrigated wheat | 0.96 μg/kg/day | [162] | |
| Poland | Various types of bread samples | 0.615 μg/kg BW/day | [159] | |
| Pb | Turkey | Multigrain bread | 0.09 μg/kg BW/day | [156] |
| Poland | Various types of bread samples | 0.024 μg/kg BW/day | [159] | |
| China | Wheat grain | 0.13 μg/kg BW/day | [163] | |
| Zn | 60.45 μg/kg BW/day |
| Pesticide | Sample | Content | Reference | |
|---|---|---|---|---|
| Mean | Min-Max | |||
| 3-hydroxylcarbofuran | Wheat | <0.30 ng/g | <0.30 ng/g | [186] |
| Bifenthrin | Whole wheat flour | 7.9 μg/kg | <1.0–76.1 μg/kg | [206] |
| Carbaryl (sevin) | Wheat | <0.30 ng/g | <0.30 ng/g | [186] |
| Agricultural products | 0.0189 mg/kg | 0.0064–0.0471 mg/kg | [207] | |
| Carbendazim | Whole wheat flour | 212.8 μg/kg | <1.0–4279.7 μg/kg | [206] |
| Carbofuran | Wheat | <0.25 ng/g | <0.25 ng/g | [186] |
| Agricultural products | 0.0966 mg/kg | 0.0017–0.3562 mg/kg | [207] | |
| Cyhalothrin | Whole wheat flour | 5.8 μg/kg | <3.0–48.3 μg/kg | [206] |
| Dichlorvos | <0.1 μg/kg | <0.1–3.1 μg/kg | ||
| Wheat | <0.26 ng/g | <0.26 ng/g | [186] | |
| Bread | 1.2 µg/kg | 1.1–1.3 µg/kg | [208] | |
| Disulfoton | Wheat | <0.20 µg/kg | <0.20 µg/kg | |
| Dursban (chlorpyrifos) | Agricultural products | 0.0016 mg/kg | 0.0016 mg/kg | [207] |
| Wheat | <0.50 µg/kg | <0.50 µg/kg | [208] | |
| Guthion (azinphos-methyl) | 4.0 µg/kg | 4.0 µg/kg | ||
| Lambda cyhalothrin | Bread | 0.15 µg/kg | 0.0–0.3 µg/kg | |
| Malathion | Agricultural products | 0.0285 mg/kg | 0.0027–0.0739 mg/kg | [207] |
| Methiocarb | Wheat | <0.30 ng/g | <0.30 ng/g | [188] |
| Agricultural products | 0.0068 mg/kg | 0.0064–0.007 mg/kg | [207] | |
| Methyl parathion | Wheat | <0.20 ng/g | <0.20 ng/g | [186] |
| Mocap (ethoprophos) | <0.16 ng/g | <0.16 ng/g | ||
| Agricultural products | 0.0051 mg/kg | 0.0009–0.0127 mg/kg | [207] | |
| Nicosulfuron | Whole wheat flour | 1.9 μg/kg | <1.0–19.0 μg/kg | [206] |
| Parathion | <5.0 μg/kg | <5.0 μg/kg | ||
| Propiconazole | 1.1 μg/kg | <1.0–16.8 μg/kg | ||
| Propoxur | Wheat | <0.24 ng/g | <0.24 ng/g | [186] |
| Agricultural products | 0.022 mg/kg | 0.0056–0.0523 mg/kg | [207] | |
| Tebuconazole | Whole wheat flour | 4.4 μg/kg | <1.0–45.4 μg/kg | [206] |
| Agricultural products | 0.0314 mg/kg | 0.0032–0.0979 mg/kg | [207] | |
| Tokuthion (prothiofos) | Wheat | <0.15 ng/g | <0.15 ng/g | [186] |
| Pesticide | Country /Continent | Sample | Average Dietary Exposure | Reference |
|---|---|---|---|---|
| Carbaryl (sevin) | Nigeria | Cereal | 2.05 ng/kg/day | [186] |
| Korea | Agricultural products | 1114.81 µg/person/day | [209] | |
| Carbofuran | Brazil | Fruits, vegetables, grains and cereals | 0.10 µg/kg | [210] |
| Canada | 0.08 µg/kg | |||
| Czech Republic | 0.08 µg/kg | |||
| Italy | 0.01 µg/kg | |||
| USA | 0.08 µg/kg | |||
| Serbia | Fruit juice | 76.7 mg/kg BW | [211] | |
| Nigeria | Cereal | 3.41 ng/kg/day | [186] | |
| Korea | Agricultural products | 185.9 µg/person/day | [209] | |
| Dichlorvos | Australia | Fruits, vegetables, grains and cereals | 0.01 µg/kg | [210] |
| Brazil | 0.2 µg/kg | |||
| Canada | 0.2 µg/kg | |||
| Czech Republic | 0.08 µg/kg | |||
| Italy | 0.05 µg/kg | |||
| USA | 0.5 µg/kg | |||
| Nigeria | Cereal | 4.75 ng/kg/day | [186] | |
| Korea | Agricultural products | 91.37 µg/person/day | [209] | |
| Disulfoton | Nigeria | Cereal | 1.37 ng/kg/day | [186] |
| Korea | Agricultural products | 223.53 µg/person/day | [209] | |
| Dursban (chlorpyrifos) | Nigeria | Cereal | 5.97 ng/kg/day | [186] |
| Korea | Agricultural products | 263.77 µg/person/day | [209] | |
| Malathion | 338.1 µg/person/day | |||
| Methiocarb | Nigeria | Cereal | 2.05 ng/kg/day | [186] |
| Korea | Agricultural products | 56.63 µg/person/day | [209] | |
| Methyl parathion | Nigeria | Cereal | 1.37 ng/kg/day | [190] |
| Mocap (ethoprophos) | 1.09 ng/kg/day | |||
| Tokuthion (prothiofos) | 4.18 ng/kg/day | |||
| Guthion (azinphos-methyl) | 14.34 ng/kg/day | |||
| Phorate | Australia | Fruits, vegetables, grains and cereals | 0.15 µg/kg | [210] |
| Brazil | 0.33 µg/kg | |||
| Czech Republic | 0.14 µg/kg | |||
| Italy | 0.07 µg/kg | |||
| USA | 0.21 µg/kg | |||
| Propoxur | Nigeria | Cereal | 1.64 ng/kg/day | [186] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maher, A.; Nowak, A. Chemical Contamination in Bread from Food Processing and Its Environmental Origin. Molecules 2022, 27, 5406. https://doi.org/10.3390/molecules27175406
Maher A, Nowak A. Chemical Contamination in Bread from Food Processing and Its Environmental Origin. Molecules. 2022; 27(17):5406. https://doi.org/10.3390/molecules27175406
Chicago/Turabian StyleMaher, Agnieszka, and Adriana Nowak. 2022. "Chemical Contamination in Bread from Food Processing and Its Environmental Origin" Molecules 27, no. 17: 5406. https://doi.org/10.3390/molecules27175406
APA StyleMaher, A., & Nowak, A. (2022). Chemical Contamination in Bread from Food Processing and Its Environmental Origin. Molecules, 27(17), 5406. https://doi.org/10.3390/molecules27175406






