Screen Printable Sol-Gel Materials for High-Throughput Borosilicate Glass Film Production
Abstract
:1. Introduction
1.1. Screen Printing of Functional Layers
1.2. Liquid Dopant Sources for Silicon Solar Cells
1.3. Scope of this Work
2. Materials and Methods
2.1. Synthesis of Sol-Gel Derived Screen Printing Pastes
2.2. Rheological Characterization and Printing Tests
2.3. Spectroscopic Thin-Film Analysis
2.4. Evaluation of Dopant Properties
3. Results
3.1. Rheology and Suitability for Screen Printing
3.2. Thin-Film Properties after Thermal Conversion
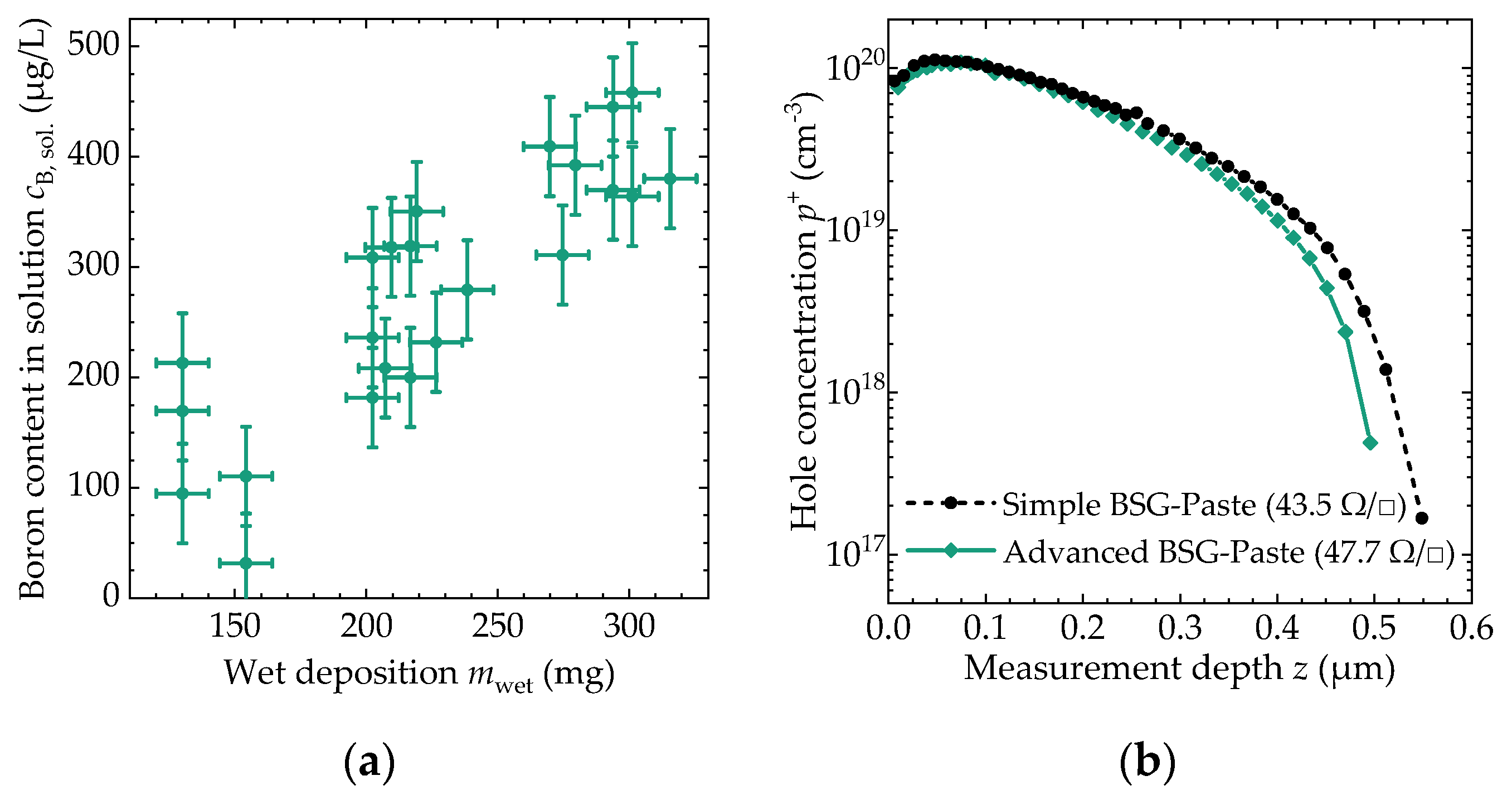
3.3. Achievable Dopant Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pettit, R.B.; Brinker, C.J. Use of sol-gel thin films in solar energy applications. Sol. Energy Mater. 1986, 14, 269–287. [Google Scholar] [CrossRef]
- Balaji, N.; Park, C.; Raja, J.; Ju, M.; Venkatesan, M.R.; Lee, H.; Yi, J. Low Surface Recombination Velocity on p-Type Cz–Si Surface by Sol–Gel Deposition of Al2O3 Films for Solar Cell Applications. J. Nanosci. Nanotech. 2015, 15, 5123–5128. [Google Scholar] [CrossRef] [PubMed]
- Huyeng, J.D.; Efinger, R.; Keding, R.J.; Doll, O.; Clement, F. Screen-Printed Borosilicate Glass Derived from Sol–Gel Materials for Back-Contact Back-Junction Solar Cells. Sol. RRL 2020, 4, 2000271. [Google Scholar] [CrossRef]
- Nold, S.; Voigt, N.; Friedrich, L.; Weber, D.; Haedrich, I.; Mittag, M.; Wirth, H.; Thaidigsmann, B.; Brucker, I.; Hofmann, M.; et al. Cost Modeling of Silicon Solar Cell Production Innovation along the PV Value Chain. In Proceedings of the 27th EU PVSEC, Frankfurt, Germany, 24–28 September 2012; pp. 1084–1090. [Google Scholar]
- Lammert, M.D.; Schwartz, R.J. The interdigitated back contact solar cell: A silicon solar cell for use in concentrated sunlight. IEEE Trans. Electron Devices 1977, 24, 337–342. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Kawasaki, H.; Yoshida, W.; Irie, T.; Konishi, K.; Nakano, K.; Uto, T.; Adachi, D.; Kanematsu, M.; Uzu, H.; et al. Silicon heterojunction solar cell with interdigitated back contacts for a photoconversion efficiency over 26%. Nat. Energy 2017, 2, 17032. [Google Scholar] [CrossRef]
- Ralph, E.L. Recent Advancements in Low Cost Solar Cell Processing. In Proceedings of the 11th IEEE Photovoltaic Specialists Conference, Scottsdale, AZ, USA, 6–8 May 1975; Institute of Electrical and Electronics Engineers, Inc.: New York, NY, USA; pp. 315–316. [Google Scholar]
- Schubert, G.; Fischer, B.; Fath, P. Formation and nature of AG thick film front contacts on crystalline silicon solar cells. In PV in Europe; From PV technology to energy solutions: Proceedings of the international conference held in Rome, Italy, 7–11 October 2002; PV Conference; PV in Europe Conference and Exhibition, Rome, Italy, 7–11 October; Bal, J.-L., Ed.; WIP-Munich: München, Germany; ETA-Florence: Florence, Italy, 2002; pp. 343–346. ISBN 3-936338-12-4. [Google Scholar]
- Fischer, M.; Woodhouse, M.; Herritsch, S.; Trube, J. International Technology Roadmap for Photovoltaic (ITRPV): Results 2019, 11th ed.; Frankfurt am Main: Frankfurt, Germany, 2020. [Google Scholar]
- Green, M.A. Solar Cells: Operating Principles, Technology and System Applications; University of New South Wales: Kensington, CA, USA, 1998; ISBN 0 85823 580 3. [Google Scholar]
- Cuevas, A. A good recipe to make silicon solar cells. In Proceedings of the 22nd IEEE Photovoltaic Specialists Conference Las Vegas, Las Vegas, NV, USA, 7–11 October 1991; pp. 466–470. [Google Scholar]
- Arai, E.; Nakamura, H.; Terenuma, Y. Interface Reactions of B2O3-Si System and Boron Diffusion into Silicon. J. Electrochem. Soc. 1973, 1973, 980. [Google Scholar] [CrossRef]
- Kessler, M.A.; Ohrdes, T.; Wolpensinger, B.; Bock, R.; Harder, N.-P. Characterisation and implications of the boron rich layer resulting from open-tube liquid source BBR3 boron diffusion processes. In Proceedings of the 34th IEEE Photovoltaic Specialists Conference, Philadelphia, PA, USA, 7–12 June 2009; IEEE: Piscataway, NJ, USA; pp. 1556–1561. [Google Scholar]
- Terheiden, B. CVD Boron Containing Glasses—An Attractive Alternative Diffusion Source for High Quality Emitters and Simplified Processing—A Review. Energy Procedia 2016, 92, 486–492. [Google Scholar] [CrossRef]
- Keding, R.; Woehl, R.; Stüwe, D.; Fallisch, A.; Hofmann, A.; Rentsch, J.; Biro, D. Diffusion and characterization of doped patterns in silicon from prepatterned boron- and phosphorus-doped silicate glasses. In Proceedings of the 26th EU PVSEC Proceedings, Hamburg, Germany, 5–9 September 2011; pp. 1385–1389, ISBN 3-936338-27-2. [Google Scholar]
- Singha, B.; Solanki, C.S. Boron-rich layer properties formed by boron spin on dopant diffusion in n -type silicon. Mater. Sci. Semicond. Processing 2017, 57, 83–89. [Google Scholar] [CrossRef]
- Perova, T.S.; Nolan-Jones, M.; McGilp, J.; Gamble, H.S. Borosilicate glass nanolayer as a spin-on dopant source: FTIR and spectroscopic ellipsometry investigations. J. Mater. Sci: Mater. Electron. 2016, 27, 6292–6304. [Google Scholar] [CrossRef]
- Kiaee, Z.; Reichel, C.; Feldmann, F.; Jahn, M.; Huyeng, J.D.; Keding, R.; Hermle, M.; Clement, F. Printed Dopant Sources for Locally-Doped SiOx/Poly-Si Passivating Contacts. In Proceedings of the 35th EUPVSEC, Brussels, Belgium, 24–28 September 2018. [Google Scholar]
- Scardera, G.; Inns, D.; Wang, G.; Dugan, S.; Dee, J.; Dang, T.; Bendimerad, K.; Lemmi, F.; Antoniadis, H. All-screen-printed Dopant Paste Interdigitated Back Contact Solar Cell. Energy Procedia 2015, 77, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Lachiq, A.; Slaoui, A.; Georgopoulos, L.; Ventura, L.; Monna, R.; Muller, J.C. Simultaneous dopant diffusion and surface passivation in a single rapid thermal cycle. Prog. Photovolt Res. Appl. 1996, 4, 329–339. [Google Scholar] [CrossRef]
- Sommer, D. Simulation, Entwicklung Und Charakterisierung Von IBC-Solarzellen Mit Neuartigen Emitterschichten. Ph.D. Thesis, University of Constance, Constance, Germany, 2017. [Google Scholar]
- Mezger, T. Das Rheologie-Handbuch: Für Anwender von Rotations- und Oszillations-Rheometern, 4th ed.; Vincentz Network: Hannover, Germany, 2012; ISBN 9783866308633. [Google Scholar]
- Tolstoj, V.P.; Černyšova, I.V.; Skryševskij, V.A. Handbook of Infrared Spectroscopy of Ultrathin Films; Wiley-Interscience: Hoboken, NJ, USA, 2003; ISBN 0-471-35404-X. [Google Scholar]
- Tenney, A.S.; Wong, J. Vibrational Spectra of Vapor-Deposited Binary Borosilicate Glasses. J. Chem. Phys. 1972, 56, 5516–5523. [Google Scholar] [CrossRef]
- Nolan, M.; Perova, T.S.; Moore, R.A.; Beitia, C.E.; McGilp, J.F.; Gamble, H.S. Spectroscopic Investigations of Borosilicate Glass and Its Application as a Dopant Source for Shallow Junctions. J. Electrochem. Soc. 2000, 147, 3100. [Google Scholar] [CrossRef]
- Peak, D.; Luther, G.W.; Sparks, D.L. ATR-FTIR spectroscopic studies of boric acid adsorption on hydrous ferric oxide. Geochim. Et Cosmochim. Acta 2003, 67, 2551–2560. [Google Scholar] [CrossRef]
- Kaşgöz, A.; Misono, T.; Abe, Y. Sol–gel preparation of borosilicates. J. Non-Cryst. Solids 1999, 243, 168–174. [Google Scholar] [CrossRef]
- Nogami, M.; Moriya, Y. Glass formation of the SiO2-B2O3 system by the gel process from metal alkoxides. J. Non-Cryst. Solids 1982, 48, 359–366. [Google Scholar] [CrossRef]
- Sakka, S. Viscosity and Spinnability of Gelling Solutions. In Handbook of Sol-Gel Science and Technology; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1453–1485. ISBN 978-3-319-32099-1. [Google Scholar]
- Irwin, A.D.; Holmgren, J.S.; Zerda, T.W.; Jonas, J. Spectroscopic investigations of borosiloxane bond formation in the sol-gel process. J. Non-Cryst. Solids 1987, 89, 191–205. [Google Scholar] [CrossRef]
- Feldmann, F.; Bivour, M.; Reichel, C.; Hermle, M.; Glunz, S.W. A passivated rear contact for high-efficiency n-type Si solar cells enabling high Voc’s and FF>82%. In Proceedings of the 28th EU PVSEC and Exhibition, Paris, France, 30 September–4 October 2013; pp. 988–992. [Google Scholar]

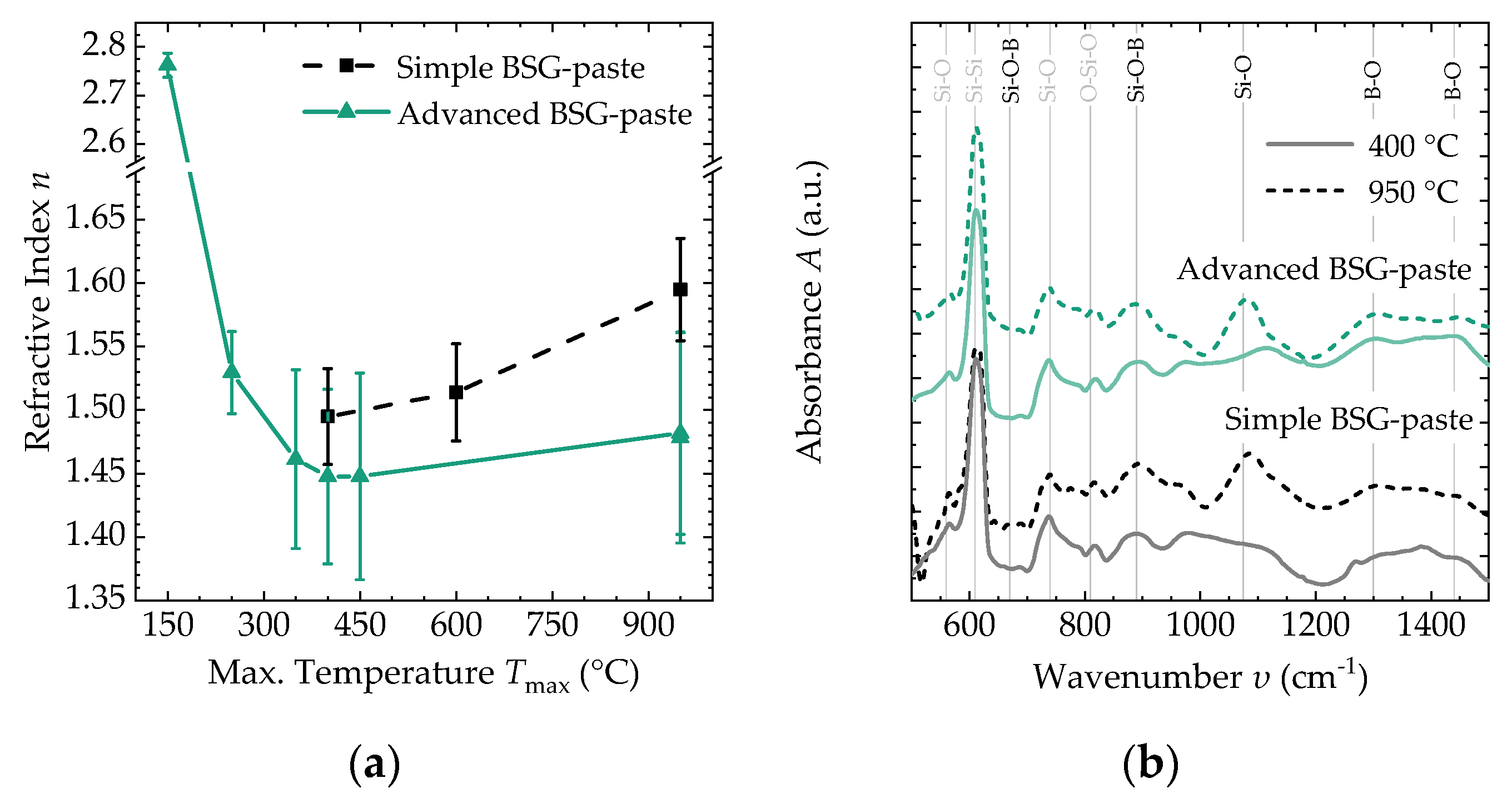
| 400 °C | 950 °C | |
|---|---|---|
| Simple BSG-paste | 1.51 ± 0.03 | 1.57 ± 0.04 |
| Advanced BSG-paste | 1.45 ± 0.07 | 1.48 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huyeng, J.D.; Efinger, R.; Bruge, D.; Doll, O.; Keding, R.J.; Clement, F. Screen Printable Sol-Gel Materials for High-Throughput Borosilicate Glass Film Production. Molecules 2022, 27, 5408. https://doi.org/10.3390/molecules27175408
Huyeng JD, Efinger R, Bruge D, Doll O, Keding RJ, Clement F. Screen Printable Sol-Gel Materials for High-Throughput Borosilicate Glass Film Production. Molecules. 2022; 27(17):5408. https://doi.org/10.3390/molecules27175408
Chicago/Turabian StyleHuyeng, Jonas D., Raphael Efinger, David Bruge, Oliver Doll, Roman J. Keding, and Florian Clement. 2022. "Screen Printable Sol-Gel Materials for High-Throughput Borosilicate Glass Film Production" Molecules 27, no. 17: 5408. https://doi.org/10.3390/molecules27175408






