Nutritional Profiling and Antioxidant Property of Three Wild Edible Mushrooms from North East India
Abstract
:1. Introduction
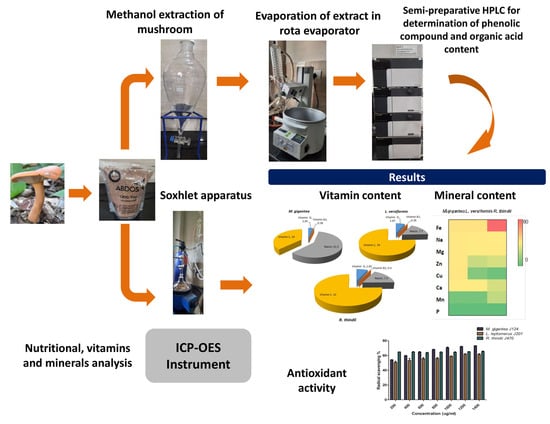
2. Results and Discussion
2.1. Nutritional Value
2.2. Vitamin Content
2.3. Mineral Content
2.4. Organic Acids
2.5. Phenolic Compound
2.6. Antioxidants
DPPH Scavenging Activity
3. Materials and Methods
3.1. Collection, Storage and Samples Preparation
3.2. Nutritional Value
3.3. Vitamin Content
3.4. Mineral Analysis
| View Direction | Radial | Axial |
| UV exposure time | 15 | 15 |
| UV RF Power | 1150 | 1150 |
| UV Neb Gas Flow | 0.5 | 0.5 |
| VIS Exposure Time | 5 | 5 |
| VIS RF Power | 1150 | 1150 |
| VIS Neb Gas Flow | 0.5 | 0.5 |
| Cool Gas Flow Rate | 12 | 12 |
| Aux Gas Flow Rate | 0.5 | 0.5 |
3.5. Phenolic Compounds by HPLC
3.6. Organic Acid by HPLC
3.7. Total Phenolic Content
3.8. Total Flavonoid Content
3.9. Antioxidants
DPPH Assay
- A sample = the absorbance of the extract/reference;
- A control = the absorbance of the DPPH solution without the addition of extract.
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Singh, Y.; Singh, B.; Li, J. Hydrocarbon Potential of the Paleogene Disang Group, Manipur Region, India-A Palyno-Logical Approach; Petroleum Geosciences, Indian Context; Springer International Publishing: Cham, Switzerland, 2015; pp. 191–204. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Tanti, B.; Lisha, G.; Chandra. Wild edible fungal resources are used by ethnic tribes of Nagaland, India. Indian J. Trad. Knowl. 2011, 10, 512–515. [Google Scholar]
- Kumar, R.; Ashwani, T.; Shailesh, P.; Rajib Kumar, B.; Devapod, B.; Jayasree, B. Macro-fungal diversity and nutrient content of some edible mushrooms of Nagaland, India. Nusant. Biosci. 2013, 5, 1–7. [Google Scholar] [CrossRef]
- Tapwal, A.; Kumar, R.; Pandey, S. Diversity and frequency of macrofungi associated with wet evergreen tropical forest in Assam, India. Biodiversitas 2013, 14, 73–78. [Google Scholar]
- Sarma, I. Wild edible mushrooms are used by some ethnic tribes of western assam. In Proceedings of the Bioscan, International Conference on Environment, Energy and Development (from Stockholm to Copenhagen and Beyond), Sambalpur University, Guwahati, India, 10–12 December 2010. [Google Scholar]
- Rn, B.; Kumar, R.; Pandey, S. Documentation of wild edible mushrooms from Meghalaya, Northeast India. Curr. Res. Environ. Appl. Mycol. 2016, 6, 238–247. [Google Scholar]
- Agrahar-Murugkar, D.; Subbulakshmi, G. Nutritional value of edible wild mushrooms collected from the Khasi hills of Meghalaya. Food Chem. 2005, 89, 599–603. [Google Scholar] [CrossRef]
- Salam, S.; Jamir, N. Wild Edible Fungi Sold in Local Markets of Ukhrul District of Manipur, India. Int. J. Sci. Res. Publ. 2018, 8, 208–210. [Google Scholar]
- Kumar, R.; Bisht, N.S.; Gaurav, K.M.; Kasmiri, K.; Rathindra, B. Micro- and macrofungal diversity in Langol herbal garden Manipur, India. Int. J. Curr. Life Sci. 2015, 1, 24–34. [Google Scholar]
- Ao, T.; Deb, C.R.; Khruomo, N. Wild edible mushrooms of Nagaland, India: A potential food resource. J. Exp. Biol. Agric. Sci. 2016, 4, 59–65. [Google Scholar] [CrossRef]
- Apshahana, K.; Sharma, A. Wild edible mushrooms traded by ethnic communities of Senapati and Kangpokpi district of Manipur, India. J. Pharmacogn. Phytochem. 2018, 7, 2303–2306. [Google Scholar]
- Roy Das, A.; Saha, A.K.; Das, P. Proximate composition and antimicrobial activity of three wild edible mushrooms consumed by ethnic inhabitants of Tripura in northeast India. Stud. Fungi 2017, 2, 17–25. [Google Scholar] [CrossRef]
- Das, K. Diversity and Conservation of Wild Mushrooms in Sikkim with Special Reference to Barsey Rhododendron Sanctuary. NeBIO 2010, 1, 1–13. [Google Scholar] [CrossRef]
- Manzi, P.; Gambelli, L.; Marconi, S.; Vivanti, V.; Pizzoferrato, L. Nutrients in edible mushrooms: An inter-species comparative study. Food Chem. 1999, 65, 477–482. [Google Scholar] [CrossRef]
- Breene, W.M. Nutritional and medicinal value of speciality mushrooms. J. Food Protect. 1990, 53, 883–894. [Google Scholar] [CrossRef]
- Sharma, S.K.; Gautam, N. Chemical and Bioactive Profiling, and Biological Activities of Coral Fungi from Northwestern Himalayas. Sci. Rep. 2017, 7, srep46570. [Google Scholar] [CrossRef]
- Vaz, J.A.; Barros, L.; Martins, A.; Morais, J.S.; Vasconcelos, M.H.; Ferreira, I.C. Phenolic profile of seventeen Portuguese wild mushrooms. LWT Food Sci. Technol. 2011, 44, 343–346. [Google Scholar] [CrossRef] [Green Version]
- Vaz, J.A.; Barros, L.; Martins, A.; Santos-Buelga, C.; Vasconcelos, M.H.; Ferreira, I.C. Chemical composition of wild edible mushrooms and antioxidant properties of their water soluble polysaccharidic and ethanolic fractions. Food Chem. 2011, 126, 610–616. [Google Scholar] [CrossRef]
- Guillamón, E.; García-Lafuente, A.; Lozano, M.; D’arrigo, M.; Rostagno, M.A.; Villares, A.; Martínez, J.A. Edible mushrooms: Role in the prevention of cardiovascular diseases. Fitoterapia 2010, 81, 715–723. [Google Scholar] [CrossRef]
- Mattila, P.; Könkö, K.; Eurola, M.; Pihlava, J.-M.; Astola, J.; Vahteristo, L.; Hietaniemi, V.; Kumpulainen, J.; Valtonen, M.; Piironen, V. Contents of Vitamins, Mineral Elements, and Some Phenolic Compounds in Cultivated Mushrooms. J. Agric. Food Chem. 2001, 49, 2343–2348. [Google Scholar] [CrossRef]
- Pegler, D.N.; Lodge, D.J.; Nakasone, K.K. The pantropical genus Macrocybe gen. nov. Mycologia 1998, 90, 494–504. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Tang, H.M.; Yu, H.; Zhang, Z.F. Comparison of nutrient components between wild and cultured fruiting bodies of Macrocybe gigantea. Edible Fungi China 2004, 24, 46–47. [Google Scholar]
- Li, Y.; Zhang, J.; Liu, H.G.; Jin, H.; Wang, Y.Z.; Li, T. Discrimination of storage periods for Macrocybe gigantea (Massee) Pegler & Lodge using UV spectral fingerprints. Czech J. Food Sci. 2015, 33, 441–448. [Google Scholar]
- Elkhateeb, W.; Elnahas, M.; Daba, G.M.; Wenhua, L.; Galappaththi, M.C.A. The coral mushrooms Ramaria and Clavaria. Stud. Fungi 2021, 6, 495–506. [Google Scholar] [CrossRef]
- Joshi, S.; Vishwakarma, M.; Mahar, R.; Bhatt, R. Medicinally important and edible species of genus Lactarius from Garhwal Himalaya, India. Mycosphere 2013, 4, 714–720. [Google Scholar] [CrossRef]
- Dai, Y.C.; Yang, Z.L. A revised checklist of medicinal fungi in China. Mycosystema 2008, 27, 801–824. [Google Scholar]
- Gezer, K.; Duru, M.; Kivrak, I.; Turkoglu, A.; Mercan, N.; Turkoglu, H.; Gulcan, S. Free-radical scavenging capacity and antimicrobial activity of wild edible mushroom from Turkey. Afr. J. Biotechnol. 2006, 5, 1924–1928. [Google Scholar]
- Barros, L.; Calhelha, R.C.; Vaz, J.A.; Ferreira, I.C.F.R.; Baptista, P.; Estevinho, L.M. Antimicrobial activity and bioactive compounds of Portuguese wild edible mushrooms methanolic extracts. Eur. Food Res. Technol. 2006, 225, 151–156. [Google Scholar] [CrossRef]
- Tala, M.F.; Qin, J.; Ndongo, J.T.; Laatsch, H. New Azulene-Type Sesquiterpenoids from the Fruiting Bodies of Lactarius deliciosus. Nat. Prod. Bioprospect. 2017, 7, 269–273. [Google Scholar] [CrossRef]
- Anke, H.; Bergendorff, O.; Sterner, O. Assays of the biological activities of guaiane sesquiterpenoids isolated from the fruit bodies of edible lactarius species. Food Chem. Toxicol. 1989, 27, 393–397. [Google Scholar] [CrossRef]
- Bergendorff, O.; Sterner, O. The sesquiterpenes of Lactarius deliciosus and Lactarius deterrimus. Phytochemistry 1988, 27, 97–100. [Google Scholar] [CrossRef]
- Kosani’c, M.; Rankovi’c, B.; Ranci´c, A.; Stanojkovi´c, T. Evaluation of metal concentration and antioxidant, antimicrobial, and anticancer potentials of two edible mushrooms Lactarius deliciosus and Macrolepiota procera. J. Food Drug Anal. 2016, 24, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.D.; Arumuganathan, T. Mushroom, their role in nature and society. In Frontiers in Mushroom Biotechnology; Rai, R.D., Upadhyay, R.C., Sharma, S.R., Eds.; NRCM: Himachal Pradesh, India, 2005; pp. 27–36. [Google Scholar]
- Ao, T.; Deb, C.R. Nutritional and antioxidant potential of some wild edible mushrooms of Nagaland, India. J. Food Sci. Technol. 2019, 56, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Crisan, E.V.; Sands, A. The Biology and Cultivation of Edible Mushrooms, Nutritional Value; Academic Press: New York, NY, USA, 1978; pp. 137–168. [Google Scholar]
- Gaur, T.; Rao, P.B.; Kushwaha, K. Nutritional and anti-nutritional components of some selected edible mushroom species. Indian J. Natl. Prod. Res. 2016, 7, 155–161. [Google Scholar]
- Mattila, P.H.; Piironen, V.I.; Uusi, R.; Koivistoinen, P.E. Vitamin D contents in edible mush. J. Agric. Food Chem. 1994, 42, 2449–2453. [Google Scholar] [CrossRef]
- Chang, S.T.; Buswell, J.A. Mushroom nutriceuticals. World J. Microbiol. Biotechnol. 1996, 12, 473–476. [Google Scholar] [CrossRef]
- Li, G.S.F.; Chang, S.T. Nutritive value of Volvariella volvacea. In Tropical Mushroom—Biological Nature and Cultivation Methods; Chang, S.T., Quimio, T.H., Eds.; The Chinese University Press: Hong Kong, 1982; pp. 199–219. [Google Scholar]
- Chang, S.T.; Hayes, W.A. The Biology and Cultivation of Edible Mushrooms; Academic Press Inc.: London, UK, 1978; p. 819. [Google Scholar]
- Chang, S.T.; Miles, G. Mushroom: Cultivation, Nutritional Value, Medicinal Effects and Environmental Impact, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004; p. 455. [Google Scholar]
- Van De Putte, K.; Nuytinck, J.; Das, K.; Verbeken, A. Exposing hidden diversity by concordant genealogies and morphology—A study of the Lactifluus volemus (Russulales) species complex in Sikkim Himalaya (India). Fungal Divers. 2012, 55, 171–194. [Google Scholar] [CrossRef]
- Rossi, W.; Das, K.; Hembrom, M.E.; Santamaria, S.; Parihar, A.; Ghosh, A.; Henkel, T.W.; Hofstetter, V.; Randrianjohany, E.; Vizzini, A.; et al. Fungal Biodiversity Profiles 91-100. Cryptogam. Mycol. 2020, 41, 69–107. [Google Scholar] [CrossRef]
- Moro, C.; Palacios, I.; Lozano, M.; D’Arrigo, M.; Guillamón, E.; Villares, A.; Martínez, J.A.; García-Lafuente, A. Anti-inflammatory activity of methanolic extracts from edible mushrooms in LPS activated RAW 264.7 macrophages. Food Chem. 2012, 130, 350–355. [Google Scholar] [CrossRef]
- George, W. Latimer. Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
- Sumaira, S.; Ghulam, M.; Hira, M.; Connie, M.W.; Yasir, J.; Muhammad, S. Proximate Composition and Micronutrient Mineral Profile of wild Ganoderma lucidum and Four Commercial Exotic Mushrooms by ICP-OES and LIBS. J. Food Nutr Res. 2016, 4, 703–708. [Google Scholar] [CrossRef]
- Seal, T. HPLC determination of phenolic acids, flavonoids and ascorbic acid in four different solvent extracts of zanthoxylum acanthopodium, a wild edible plant of meghalaya state of india. Int. J. Pharm. Pharm. Sci. 2016, 8, 103–109. [Google Scholar]
- Carvajal, A.E.S.; Koehnlein, E.A.; Soares, A.A.; Eler, G.J.; Nakashima, A.T.A.; Bracht, A.; Peralta, R.M. Bioactives of fruiting bodies and submerged culture mycelia of Agaricus brasiliensis (A. blazei) and their antioxidant properties. LWT-Food Sci. Technol. 2012, 46, 493–499. [Google Scholar] [CrossRef]
- Kaur, C.; Kapoor, H.C. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int. J. Food Sci. Technol. 2002, 37, 153–161. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Adebiyi, O.; Olayemi, F.O.; Ning-Hua, T.; Guang-Zhi, Z. In vitro antioxidant activity, total phenolic and flavonoid contents of ethanol extract of stem and leaf of Grewia carpinifolia. Beni-Suef Univ. J. Basic Appl. Sci. 2017, 6, 10–14. [Google Scholar] [CrossRef]
- Prasad, R.; Varshney, V.K.; Harsh, N.S.K.; Kumar, M. Antioxidant Capacity and Total Phenolics Content of the Fruiting Bodies and Submerged Cultured Mycelia of Sixteen Higher Basidiomycetes Mushrooms from India. Int. J. Med. Mushrooms 2015, 17, 933–941. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]



| Samples | Moisture (%) | Ash (%) | Fat (%) | Fiber (%) | Protein (%) | Carbohydrates (%) | Energy Value Kcal/100 g |
|---|---|---|---|---|---|---|---|
| M. gigantea J124 | 89.44 ± 0.16 | 9.90 ± 0.04 | 3.20 ± 0.04 | 5.90 ± 0.04 | 37.60 ± 0.09 | 32.00 ± 0.40 | 307 ± 0.40 |
| L. leptomerus J201 | 84.79 ± 0.22 | 6.80 ± 0.05 | 5.90 ± 0.05 | 14.10 ± 0.05 | 20.80 ± 0.05 | 43.00 ± 0.57 | 308 ± 0.57 |
| R. thindii J470 | 83.84 ± 0.26 | 7.90 ± 0.05 | 2.80 ± 0.05 | 6.40 ± 0.05 | 16.40 ± 0.05 | 57.20 ± 0.05 | 320 ± 0.57 |
| Mushroom | Vitamin D (µg/g) | Vitamin B1 (mg/100 g) | Vitamin B2 (mg/100 g) | Niacin (mg/100 g) | Folic Acid (mg/100 g) | Vitamin C (mg/100 g) |
|---|---|---|---|---|---|---|
| M. gigantea J124 | 2.85 ± 0.009 | BDL | 0.38 ± 0.009 | 51.50 ± 0.090 | BDL | 33.00 ± 0.400 |
| L. leptomerus J201 | 2.87 ± 0.057 | BDL | 0.36 ± 0.005 | 7.70 ± 0.057 | BDL | 34.00 ± 0.570 |
| R. thindii J470 | 2.83 ± 0.005 | BDL | 0.40 ± 0.005 | 7.30 ± 0.057 | BDL | 32.00 ± 0.288 |
| Minerals | M. gigantea J124 | L. leptomerus J201 | R. thindii J470 |
|---|---|---|---|
| Ca | 2.778 ± 0.153 | 1.699 ± 0.003 | 1.237 ± 0.111 |
| Cu | 5.771 ± 0.020 | 0.368 ± 0.115 | 0.149 ± 0.018 |
| Fe | 7.467 ± 0.199 | 6.645 ± 0.113 | 79.741 ± 0.194 |
| K | 210.380 ± 0.215 | 128.35 ± 0.200 | 133.99 ± 0.199 |
| Mg | 4.638 ± 0.064 | 1.663 ± 0.182 | 2.924 ± 0.123 |
| Mn | 0.225 ± 0.016 | 0.244 ± 0.001 | 1.023 ± 0.037 |
| Na | 4.488 ± 0.055 | 2.901 ± 0.085 | 2.827 ± 0.018 |
| P | 0.016 ± 0.0005 | n.d. | 0.004 ± 0.0005 |
| Zn | 4.149 ± 0.036 | 1.097 ± 0.041 | 1.252 ± 0.077 |
| Peak No. | Compounds | M. gigantea J124 | L. leptomerus J201 | R. thindii J470 |
|---|---|---|---|---|
| 1 | Organic acid (µg/mg) Oxalic acid | 213 ± 0.0009 | 38.985 ± 0.001 | 40.65 ± 0.0003 |
| 2 | Tartaric acid | 134.567 ± 0.00028 | 33.713 ± 0.001 | 37.217 ± 0.001 |
| 3 | Formic acid | 50.662 ± 0.00039 | 33.047 ± 0.0018 | 27.88 ± 0.0022 |
| 4 | Malic acid | 9.7675 ± 0.00021 | n.d. | n.d. |
| 5 | Malonic acid | 7.665 ± 0.00031 | n.d. | n.d. |
| 6 | Lactic acid | 6.77 ± 0.004 | 7.437 ± 0.001 | 9.255 ± 0.0005 |
| 7 | Acetic acid | n.d. | 2.685 ± 0.0019 | 1.649 ± 0.0004 |
| 8 | Citric acid | n.d. | 4.072 ± 0.0009 | 0.444 ± 0.0023 |
| 1 | Phenolic Compound (µg/g) Gallic acid | 51.399 ± 0.01 | 168.542 ± 0.01 | 253.827 ± 0.07 |
| 2 | Hydroxybenzoic acid | 585.56 ± 0.022 | 31.285 ± 0.016 | 1075.47 ± 0.014 |
| 3 | Epicatechin | n.d. | n.d. | 602.201 ± 0.011 |
| 4 | Quercetin | 94.699 ± 0.007 | 11.271 ± 0.019 | 92.127 ± 0.007 |
| TPC mg GAE/g dw | 29.23 ± 0.09 | 26.206 ± 0.077 | 30.99 ± 0.27 | |
| TFC mg QE/g dw | 6.646 ± 0.452 | 6.854 ± 0.517 | 9.187 ± 0.511 | |
| DPPH IC50 (µg/mL) | 550.4 | 689.0 | 242.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khumlianlal, J.; Sharma, K.C.; Singh, L.M.; Mukherjee, P.K.; Indira, S. Nutritional Profiling and Antioxidant Property of Three Wild Edible Mushrooms from North East India. Molecules 2022, 27, 5423. https://doi.org/10.3390/molecules27175423
Khumlianlal J, Sharma KC, Singh LM, Mukherjee PK, Indira S. Nutritional Profiling and Antioxidant Property of Three Wild Edible Mushrooms from North East India. Molecules. 2022; 27(17):5423. https://doi.org/10.3390/molecules27175423
Chicago/Turabian StyleKhumlianlal, Joshua, K. Chandradev Sharma, Leichombam Mohindro Singh, Pulok K. Mukherjee, and Sarangthem Indira. 2022. "Nutritional Profiling and Antioxidant Property of Three Wild Edible Mushrooms from North East India" Molecules 27, no. 17: 5423. https://doi.org/10.3390/molecules27175423