CdS Nanocubes Adorned by Graphitic C3N4 Nanoparticles for Hydrogenating Nitroaromatics: A Route of Visible-Light-Induced Heterogeneous Hollow Structural Photocatalysis
Abstract
:1. Introduction
2. Results
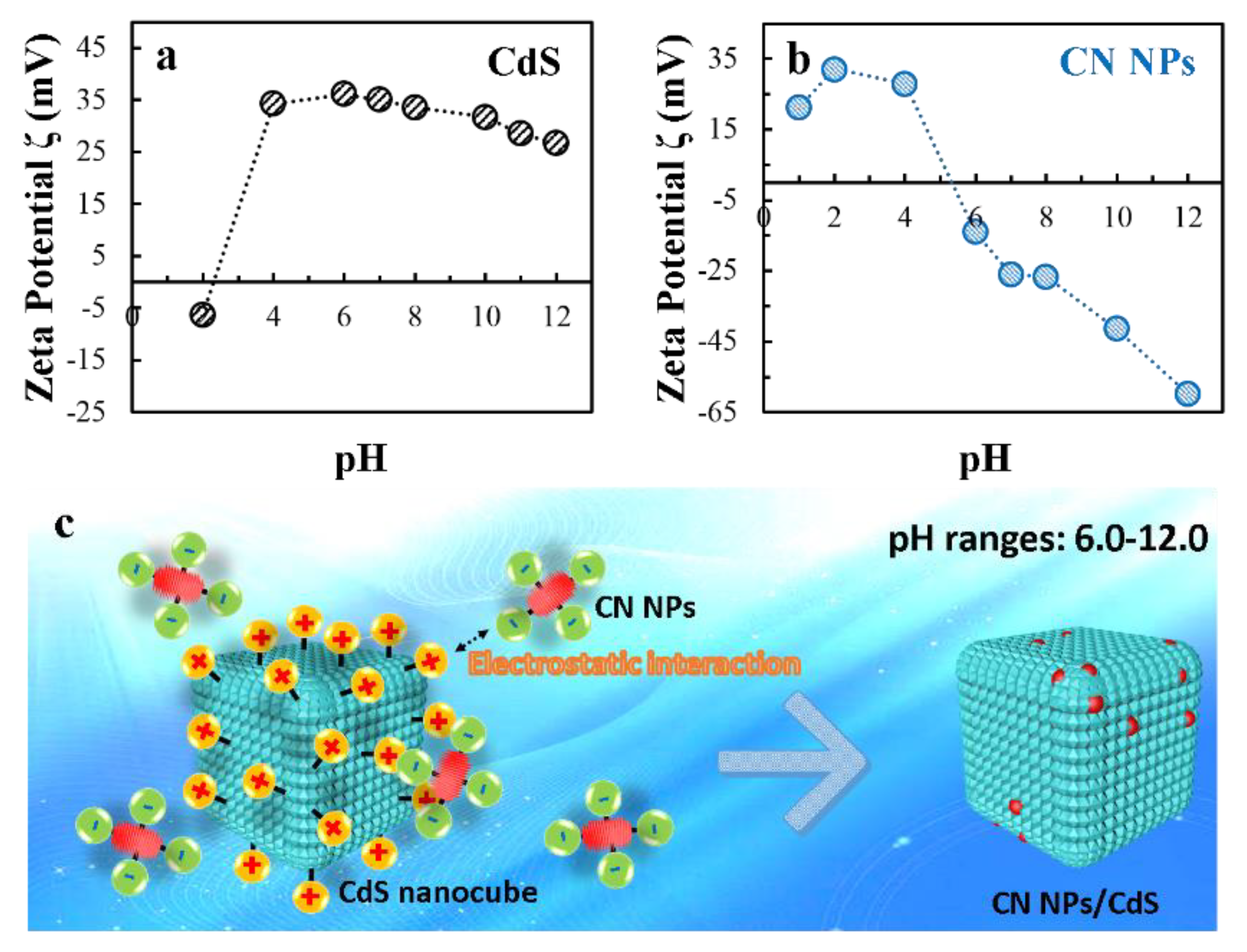
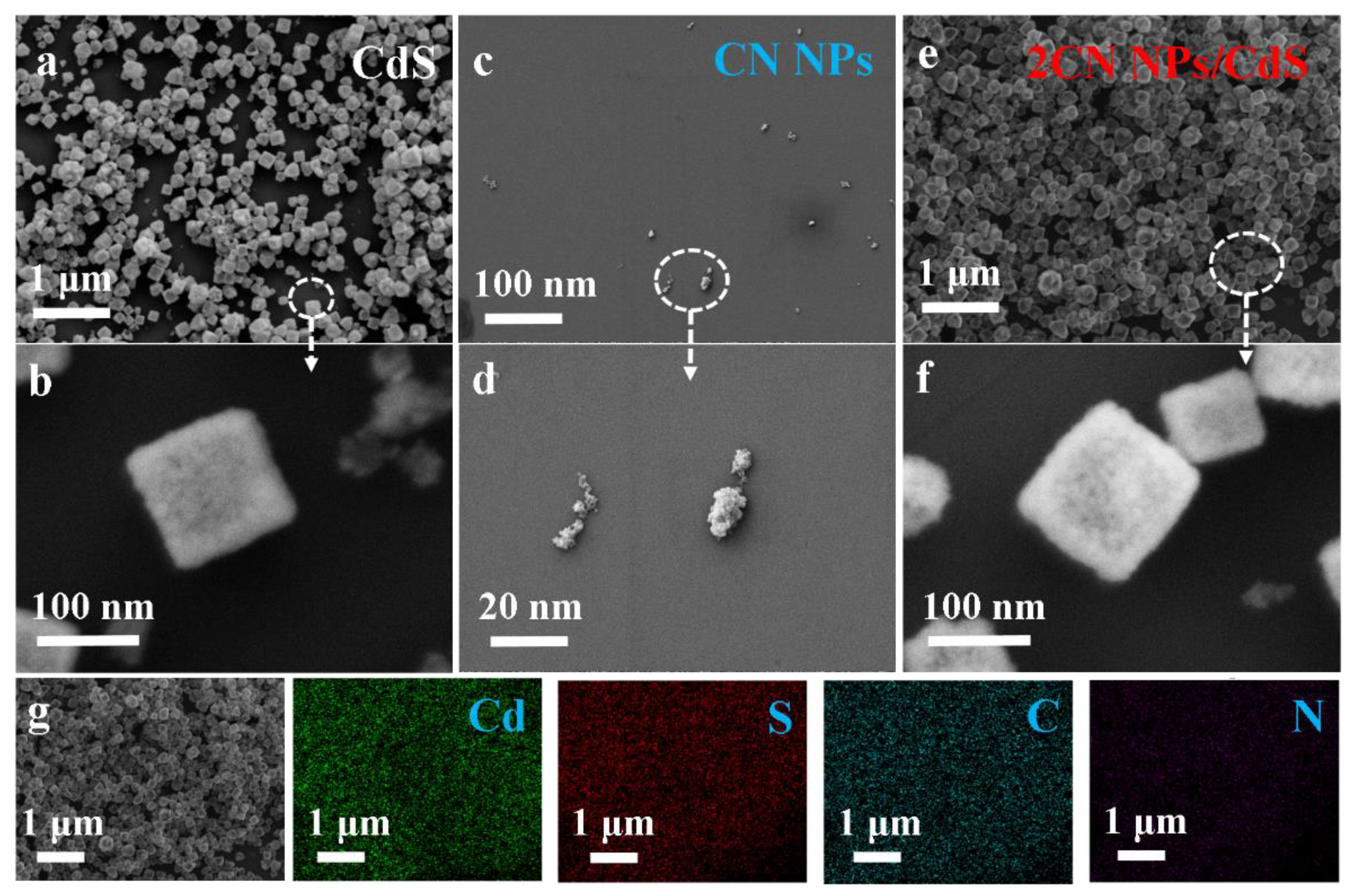
2.1. Characterizations of CN NPs/CdS NCs
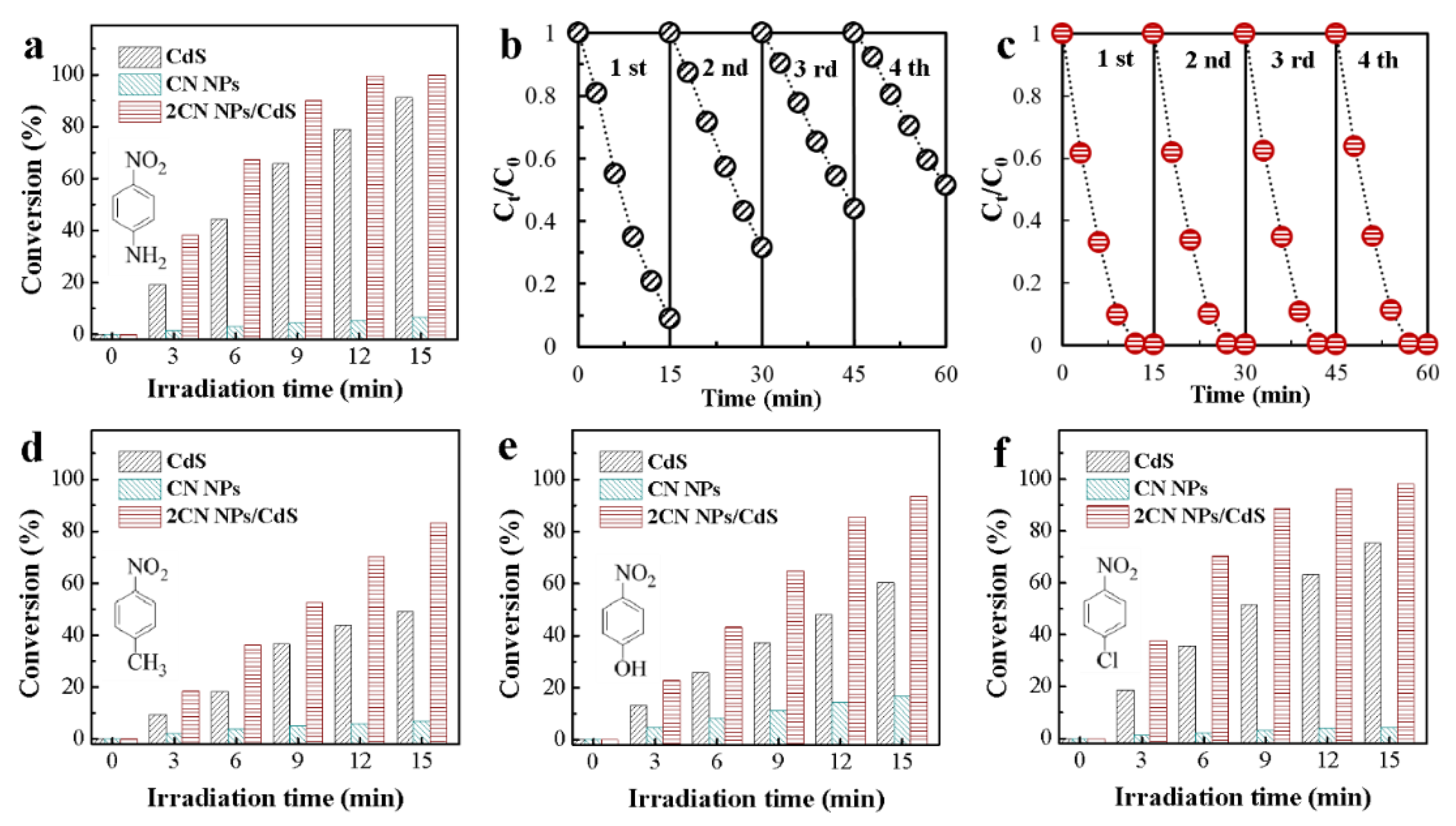
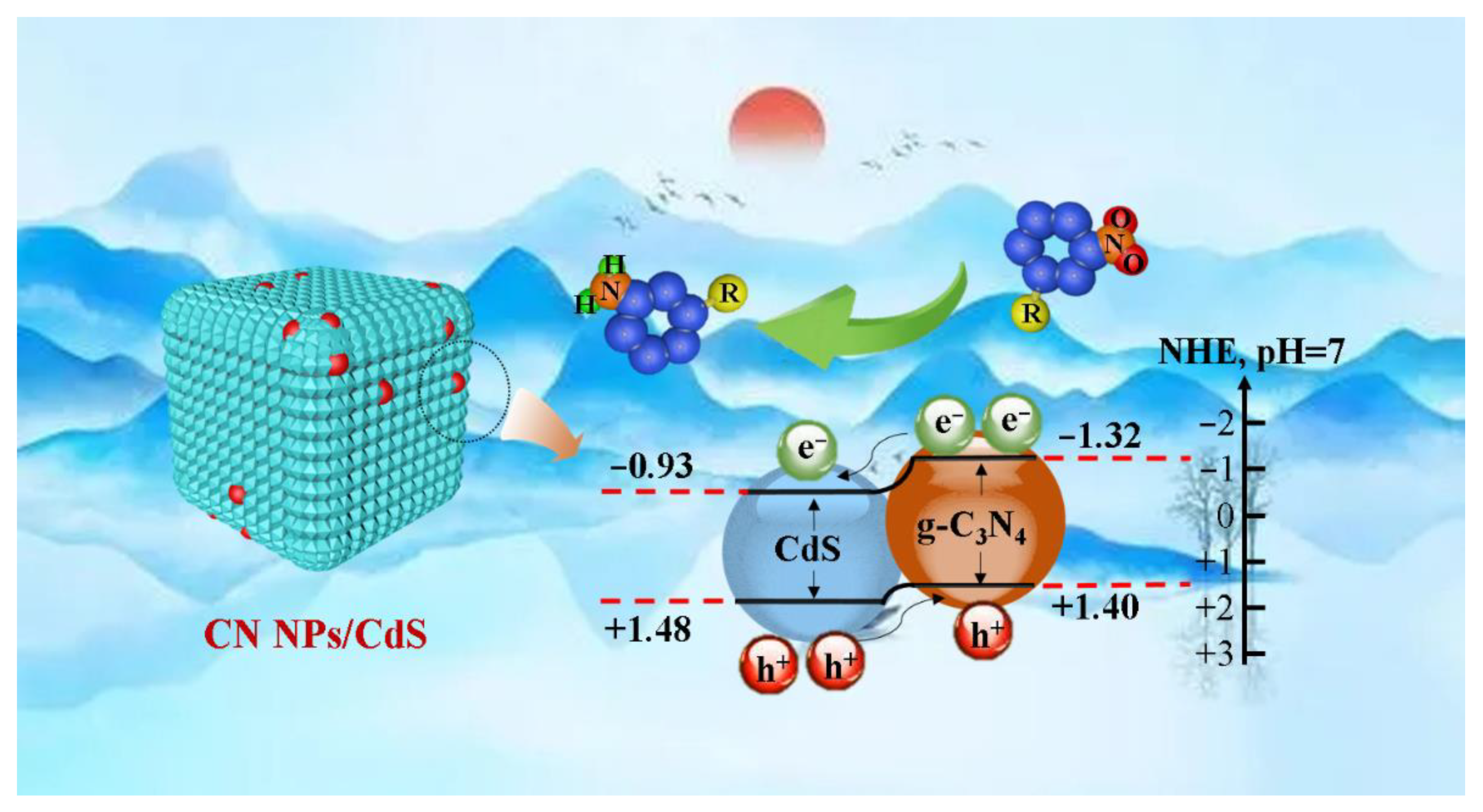
2.2. Photocatalytic Performances of CN NPs/CdS NCs
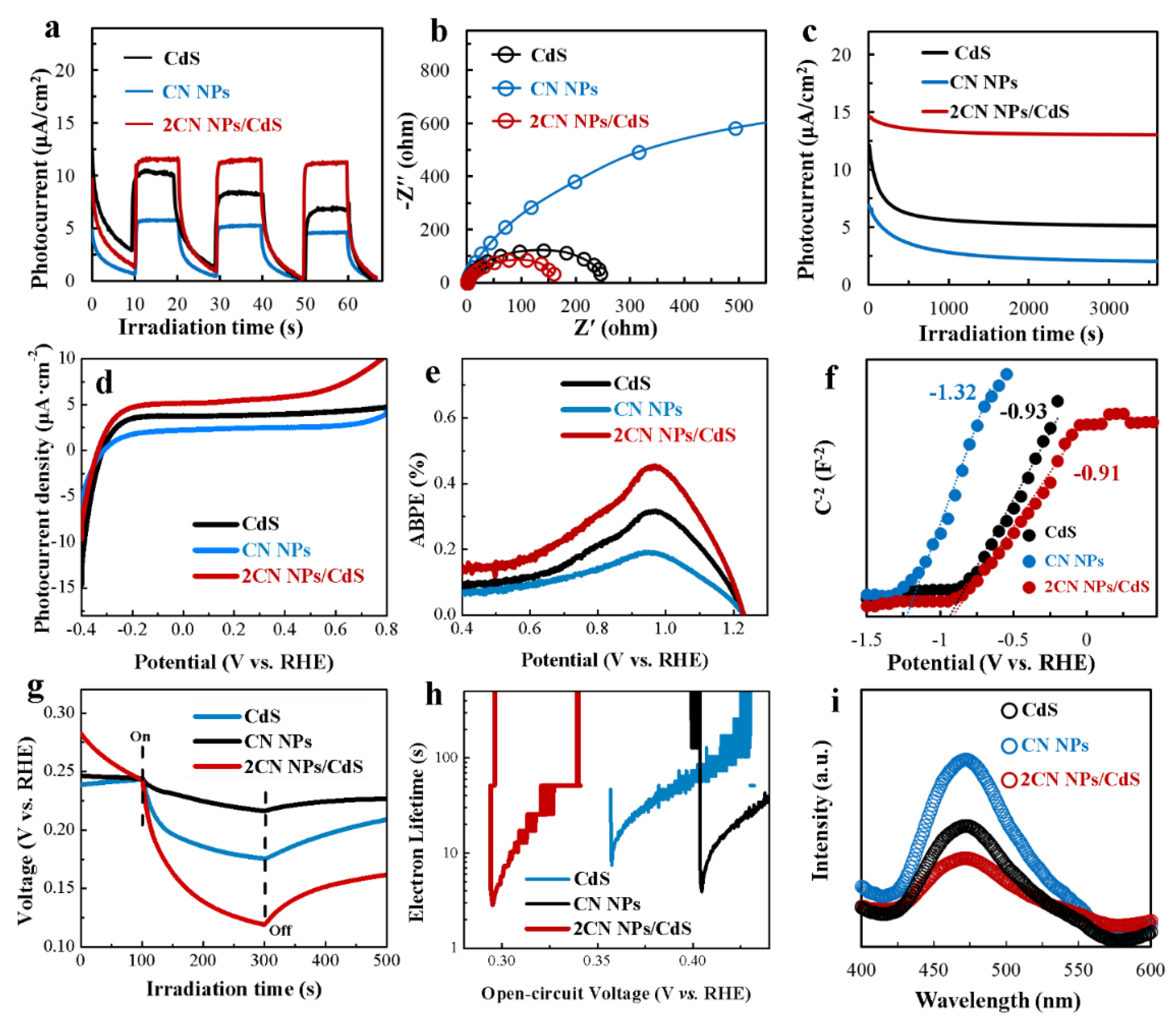
2.3. Photoelectrochemical (PEC) Measurements
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.2.1. Synthesis of CdS Nanocubes (CdS NCs)
3.2.2. Synthesis of g-C3N4 Nanoparticles (CN NPs)
3.2.3. Preparation of g-C3N4 Nanoparticles/CdS Nanocubes (CN NPs/CdS NCs)
3.3. Characterizations
3.4. Photoactivity Evolution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xu, C.; Anusuyadevi, P.R.; Aymonier, C.; Luque, R.; Marre, S. Nanostructured materials for photocatalysis. Chem. Soc. Rev. 2019, 48, 3868–3902. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Guo, L.; Xi, B.; Feng, Z.; Wu, F.; Lin, Y.; Liu, J.; Sun, D.; Feng, J.; Qian, Y.; et al. Embedding MnO@Mn3O4 nanoparticles in an N-doped-carbon framework derived from Mn-organic clusters for efficient lithium storage. Adv. Mater. 2018, 30, 201704244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, M.Y.; Yan, D.Y.; Mao, J.; Liu, H.; Hu, W.B.; Du, X.W.; Ling, T.; Qiao, S.Z. Engineering oxygen vacancy on NiO nanorod arrays for alkaline hydrogen evolution. Nano Energy 2018, 43, 103–109. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, C.; Lyu, M.; Lin, Y.; Cai, W.; Huang, P.; Tong, W.; Zou, Y.; Xie, Y. Ultrathin Co3S4 Nanosheets that Synergistically Engineer Spin States and Exposed Polyhedra that Promote Water Oxidation under Neutral Conditions. Angew. Chem. Int. Ed. 2015, 54, 11231–11235. [Google Scholar] [CrossRef]
- Lin, F.; Wang, H.; Wang, G. Facile synthesis of hollow polyhedral (cubic, octahedral and dodecahedral) NiO with enhanced lithium storage capabilities. Electrochimi. Acta 2016, 211, 207–216. [Google Scholar] [CrossRef]
- Liang, M.; Borjigin, T.; Zhang, Y.; Liu, B.; Liu, H.; Guo, H. Controlled assemble of hollow heterostructured g-C3N4@CeO2 with rich oxygen vacancies for enhanced photocatalytic CO2 reduction. Appl. Catal. B Environ. 2019, 243, 566–575. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, A.; Zhang, Y.; Shi, J.; Lin, J.; Liang, S.; Cao, G. Heterogeneous NiS/NiO multi-shelled hollow microspheres with enhanced electrochemical performances for hybrid-type asymmetric supercapacitors. J. Mater. Chem. A 2018, 6, 9153–9160. [Google Scholar] [CrossRef]
- Li, Q.; Xia, Y.; Yang, C.; Lv, K.; Lei, M.; Li, M. Building a direct Z-scheme heterojunction photocatalyst by ZnIn2S4 nanosheets and TiO2 hollowspheres for highly-efficient artificial photosynthesis. Chem. Eng. J. 2018, 349, 287–296. [Google Scholar] [CrossRef]
- Madhusudan, P.; Zhang, J.; Cheng, B.; Yu, J. Fabrication of CdMoO4@CdS core-shell hollow superstructures as high performance visible-light driven photocatalysts. Phys. Chem. Chem. Phys. 2015, 17, 15339–15347. [Google Scholar] [CrossRef]
- Kim, M.R.; Jang, D.J. One-step fabrication of well-defined hollow CdS nanoboxes. Chem. Commun. 2008, 41, 5218–5220. [Google Scholar] [CrossRef]
- Liu, X.; Sayed, M.; Bie, C.; Cheng, B.; Hu, B.; Yu, J.; Zhang, L. Hollow CdS-based photocatalysts. J. Mater. 2021, 7, 419–439. [Google Scholar] [CrossRef]
- Zou, Y.; Guo, C.; Cao, X.; Zhang, L.; Chen, T.; Guo, C.; Wang, J. Synthesis of CdS/CoP hollow nanocages with improved photocatalytic water splitting performance for hydrogen evolution. J. Environ. Chem. Eng. 2021, 9, 106270. [Google Scholar] [CrossRef]
- Xia, C.; Xue, C.; Bian, W.; Liu, J.; Wang, J.; Wei, Y.; Zhang, J. Hollow Co9S8/CdS nanocages as efficient photocatalysts for hydrogen evolution. ACS Appl. Nano Mater. 2021, 4, 2743–2751. [Google Scholar] [CrossRef]
- Wang, L.; Li, R.; Huang, M. Synthesis and adsorption properties of CdS-Au hybrid nanorings. Mater. Lett. 2021, 304, 130722. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, C.; Zhou, T.; Hu, J. Morphology-preserved transformation of CdS hollow structures toward photocatalytic H2 evolution. CrystEngComm 2020, 22, 1057–1062. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, H.; Zu, H.; Zhang, Z.; Han, J. Construction of TiO2/CdS heterojunction photocatslysts with enhanced visible light activity. Appl. Surf. Sci. 2018, 455, 729–735. [Google Scholar] [CrossRef]
- Wang, Z.; Hou, J.; Yang, C.; Jiao, S.; Zhu, H. Three-dimensional MoS2-CdS-gamma-TaON hollow composites for enhanced visible-light-driven hydrogen evolution. Chem. Commun. 2014, 50, 1731–1734. [Google Scholar] [CrossRef]
- Hu, Z.S.; Song, C.X.; Wang, D.B.; Wang, H.T.; Fu, X. Preparation and characterization of hollow spheres consisting of CdS/TiO2 composite. Rare Metal Mat. Eng. 2005, 34, 8–10. [Google Scholar]
- Peng, J.; Zheng, Z.; Tan, H.; Yang, J.; Zheng, D.; Song, Y.; Lu, F.; Chen, Y.; Gao, W. Rational design of ZnIn2S4/CdIn2S4/CdS with hollow heterostructure for the sensitive determination of carbohydrate antigen 19-9. Sensors Actuat. B Chem. 2022, 363, 131863. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, Z.; Cui, X.; Liu, H.; Tai, C.; Song, Y. Fabrication of hollow mesoporous CdS@TiO2@Au microspheres with high photocatalytic activity for hydrogen evolution from water under visible light. ACS Sustain. Chem. Eng. 2018, 6, 13766–13777. [Google Scholar] [CrossRef]
- Qiu, B.; Zhu, Q.; Du, M.; Fan, L.; Xing, M.; Zhang, J. Efficient solar light harvesting CdS/Co9S8 hollow cubes for Z-scheme photocatalytic water splitting. Angew. Chem. Int. Ed. 2017, 56, 2684–2688. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Zhang, J.; Bao, J.; Huang, X.; Mo, X. Facile synthesis of high-quality nano-sized CdS hollow spheres and their application in electrogenerated chemiluminescence sensing. J. Mater. Chem. 2007, 17, 1087–1093. [Google Scholar] [CrossRef]
- Liang, Z.-Y.; Wei, J.X.; Wang, X.; Yu, Y.; Xiao, F.X. Elegant Z-scheme-dictated g-C3N4 enwrapped WO3 superstructures: A multifarious platform for versatile photoredox catalysis. J. Mater. Chem. A 2017, 5, 15601–15612. [Google Scholar] [CrossRef]
- Liang, Z.; Wen, Q.; Wang, X.; Zhang, F.; Yu, Y. Chemically stable and reusable nano zero-valent iron/graphite-like carbon nitride nanohybrid for efficient photocatalytic treatment of Cr(VI) and rhodamine B under visible light. Appl. Surf. Sci. 2016, 386, 451–459. [Google Scholar] [CrossRef]
- Shi, H.; Li, Y.; Wang, X.; Yu, H.; Yu, J. Selective modification of ultra-thin g-C3N4 nanosheets on the (110) facet of Au/BiVO4 for boosting photocatalytic H2O2 production. Appl. Catal. B Environ. 2021, 297, 120414. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, M.; He, B.; Wang, R.; Wang, H.; Gong, Y. Facile synthesis of rod-like g-C3N4 by decorating Mo2C co-catalyst for enhanced visible-light photocatalytic activity. Appl. Surf. Sci. 2019, 470, 565–572. [Google Scholar] [CrossRef]
- Tzvetkov, G.; Tsvetkov, M.; Spassov, T. Ammonia-evaporation-induced construction of three-dimensional NiO/g-C3N4 composite with enhanced adsorption and visible light-driven photocatalytic performance. Superlattices Microst. 2018, 119, 122–133. [Google Scholar] [CrossRef]
- Jiang, X.H.; Xing, Q.J.; Luo, X.B.; Li, F.; Zou, J.-P.; Liu, S.S.; Li, X.; Wang, X.-K. Simultaneous photoreduction of Uranium (VI) and photooxidation of Arsenic (III) in aqueous solution over g-C3N4/TiO2 heterostructured catalysts under simulated sunlight irradiation. Appl. Catal. B Environ. 2018, 228, 29–38. [Google Scholar] [CrossRef]
- Jian, J.; Sun, J. A review of recent progress on silicon carbide for photoelectrochemical water splitting. Sol. RRL 2020, 4, 2000111. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Huang, D.; Zeng, G.; Huang, J.; Lai, C.; Zhou, C.; Wang, W.; Guo, H.; Xue, W.; et al. Boron nitride quantum dots decorated ultrathin porous g-C3N4: Intensified exciton dissociation and charge transfer for promoting visible-light-driven molecular oxygen activation. Appl. Catal. B Environ. 2019, 245, 87–99. [Google Scholar] [CrossRef]
- Reddy, K.R.; Reddy, C.V.; Nadagouda, M.N.; Shetti, N.P.; Jaesool, S.; Aminabhavi, T.M. Polymeric graphitic carbon nitride (g-C3N4)-based semiconducting nanostructured materials: Synthesis methods, properties and photocatalytic applications. J. Environ. Manag. 2019, 238, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Liang, S.; Li, M.; Yu, X.F.; Zhou, L.; Wang, Q.Q. Facile synthesis of Au nanocube-CdS core-shell nanocomposites with enhanced photocatalytic activity. Chin. Phys. Lett. 2014, 31, 064203. [Google Scholar] [CrossRef]
- Han, L.L.; Kulinich, S.A.; Zhang, Y.Y.; Zou, J.; Liu, H.; Wang, W.H.; Liu, H.; Li, H.B.; Yang, J.; Xin, H.L.; et al. Synergistic synthesis of quasi-monocrystal CdS nanoboxes with high-energy facets. J. Mater. Chem. A 2015, 3, 23106–23112. [Google Scholar] [CrossRef]
- Wu, C.; Jie, J.; Wang, L.; Yu, Y.; Peng, Q.; Zhang, X.; Cai, J.; Guo, H.; Wu, D.; Jiang, Y. Chlorine-doped n-type CdS nanowires with enhanced photoconductivity. Nanotechnology 2010, 21, 505203. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Guo, W.; Wang, H.; Zhu, B.; Yu, J.; Qiao, S.Z. Metal-Free 2D/2D Phosphorene/g-C3N4 Van der Waals Heterojunction for Highly Enhanced Visible-Light Photocatalytic H2 Production. Adv. Mater. 2018, 30, 1800128. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Tonda, S.; Kumar, S.; Gawli, Y.; Bhardwaj, M.; Ogale, S. g-C3N4 (2D)/CdS (1D)/rGO (2D) dual-interface nano-composite for excellent and stable visible light photocatalytic hydrogen generation. Int. J. Hydrog. Energy 2017, 42, 5971–5984. [Google Scholar] [CrossRef]
- Wei, R.B.; Huang, Z.L.; Gu, G.H.; Wang, Z.; Zeng, L.; Chen, Y.; Liu, Z.Q. Dual-cocatalysts decorated rimous CdS spheres advancing highly-efficient visible-light photocatalytic hydrogen production. Appl. Catal. B Environ. 2018, 231, 101–107. [Google Scholar] [CrossRef]
- Ma, S.; Deng, Y.; Xie, J.; He, K.; Liu, W.; Chen, X.; Li, X. Noble-metal-free Ni3C cocatalysts decorated CdS nanosheets for high efficiency visible-light-driven photocatalytic H2 evolution. Appl. Catal. B Environ. 2018, 227, 218–228. [Google Scholar] [CrossRef]
- Selvarajan, S.; Suganthi, A.; Rajarajan, M. Fabrication of g-C3N4/NiO heterostructured nanocomposite modified glassy carbon electrode for quercetin biosensor. Ultrason. Sonochem. 2018, 41, 651–660. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, X.; Zhang, H.; Yang, B.; Xiao, K.; Guo, T.; Zhang, J.; Shao, H.; Wang, Y.; Yu, G. MOF-derived nitrogen doped carbon modified g-C3N4 heterostructure composite with enhanced photocatalytic activity for bisphenol A degradation with peroxymonosulfate under visible light irradiation. Appl. Catal. B Environ. 2018, 233, 35–45. [Google Scholar] [CrossRef]
- Periasamy, P.; Krishnakumar, T.; Devarajan, V.P.; Sandhiya, M.; Sathish, M.; Chavali, M. Investigation of electrochemical supercapacitor performance of WO3-CdS nanocomposites in 1 M H2SO4 electrolyte prepared by microwave-assisted method. Mater. Lett. 2020, 274, 127998. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, D.; Tang, Z.; Hu, D.; Geng, H.; Zheng, H.; Zang, S.; Yu, Z.; Peng, P. Preparation of radial ZnSe-CdS nano-heterojunctions through atomic layer deposition method and their optoelectronic applications. J. Alloys Compd. 2019, 777, 102–108. [Google Scholar] [CrossRef]
- Liu, D.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Integration of 3D macroscopic graphene aerogel with OD-2D AgVO3-g-C3N4 heterojunction for highly efficient photocatalytic oxidation of nitric oxide. Appl. Catal. B Environ. 2019, 243, 576–584. [Google Scholar] [CrossRef]
- Tan, Y.; Shu, Z.; Zhou, J.; Li, T.; Wang, W.; Zhao, Z. One-step synthesis of nanostructured g-C3N4/TiO2 composite for highly enhanced visible-light photocatalytic H2 evolution. Appl. Catal. B Environ. 2018, 230, 260–268. [Google Scholar] [CrossRef]
- Jiang, K.Y.; Weng, Y.L.; Guo, S.Y.; Yu, Y.; Xiao, F.X. Self-assembly of metal/semiconductor heterostructures via ligand engineering: Unravelling the synergistic dual roles of metal nanocrystals toward plasmonic photoredox catalysis. Nanoscale 2017, 9, 16922–16936. [Google Scholar] [CrossRef]
- Xiao, F.X.; Miao, J.; Liu, B. Layer-by-Layer Self-assembly of CdS quantum dots/graphene nanosheets hybrid films for photoelectrochemical and photocatalytic applications. J. Am. Chem. Soc. 2014, 136, 1559–1569. [Google Scholar] [CrossRef]
- Garg, P.; Bhauriyal, P.; Mahata, A.; Rawat, K.S.; Pathak, B. Role of dimensionality for photocatalytic water splitting: CdS nanotube versus bulk structure. Chemphyschem 2019, 20, 383–391. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Gui, Y.; Liu, L. Catalytic transfer hydrogenation of nitrobenzene over Ti3C2/Pd nanohybrids boosted by electronic modification and hydrogen evolution inhibition. Appl. Surf. Sci. 2022, 592, 153334. [Google Scholar] [CrossRef]
- Ismail, A.A.; Albukhari, S.M.; Mahmoud, M.H.H. Highly efficient and accelerated photoreduction of nitrobenzene over visible-light-driven PtO@Cr2O3 nanocomposites. Surf. Interfaces 2021, 27, 101527. [Google Scholar] [CrossRef]
- Hu, W.; Wu, F.; Liu, W. Facile synthesis of Z-scheme Bi2O3/Bi2WO6 composite for highly effective visible-light-driven photocatalytic degradation of nitrobenzene. Chem. Phys. 2022, 552, 111377. [Google Scholar] [CrossRef]
- Mkhalid, I.A. Simple synthesis of NiCo2O4@rGO nanocomposites for conversion of nitrobenzene via its photoreduction to aniline using visible light. J. Mater. Res. Technol. 2021, 12, 1988–1998. [Google Scholar] [CrossRef]
- Ismail, A.A.; Albukhari, S.M.; Mahmoud, M.H.H. Mesoporous ZnCr2O4 photocatalyst with highly distributed PtO nanoparticles for visible-light-induced photoreduction of nitrobenzene. Opt. Mater. 2021, 122, 111676. [Google Scholar] [CrossRef]
- Lin, B.; Li, H.; An, H.; Hao, W.; Wei, J.; Dai, Y.; Ma, C.; Yang, G. Preparation of 2D/2D g-C3N4 nanosheet@ZnIn2S4 nanoleaf heterojunctions with well -designed high-speed charge transfer nanochannels towards high efficiency photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 220, 542–552. [Google Scholar] [CrossRef]
- Fang, X.X.; Wang, P.F.; Yi, W.; Chen, W.; Lou, S.C.; Liu, G.Q. Visible-light-mediated oxidative coupling of vinylarenes with bromocarboxylates leading to gamma-ketoesters. J. Org. Chem. 2019, 84, 15677–15684. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Z.-Y.; Chen, F.; Huang, R.-K.; Huang, W.-J.; Wang, Y.; Liang, R.-W.; Yan, G.-Y. CdS Nanocubes Adorned by Graphitic C3N4 Nanoparticles for Hydrogenating Nitroaromatics: A Route of Visible-Light-Induced Heterogeneous Hollow Structural Photocatalysis. Molecules 2022, 27, 5438. https://doi.org/10.3390/molecules27175438
Liang Z-Y, Chen F, Huang R-K, Huang W-J, Wang Y, Liang R-W, Yan G-Y. CdS Nanocubes Adorned by Graphitic C3N4 Nanoparticles for Hydrogenating Nitroaromatics: A Route of Visible-Light-Induced Heterogeneous Hollow Structural Photocatalysis. Molecules. 2022; 27(17):5438. https://doi.org/10.3390/molecules27175438
Chicago/Turabian StyleLiang, Zhi-Yu, Feng Chen, Ren-Kun Huang, Wang-Jun Huang, Ying Wang, Ruo-Wen Liang, and Gui-Yang Yan. 2022. "CdS Nanocubes Adorned by Graphitic C3N4 Nanoparticles for Hydrogenating Nitroaromatics: A Route of Visible-Light-Induced Heterogeneous Hollow Structural Photocatalysis" Molecules 27, no. 17: 5438. https://doi.org/10.3390/molecules27175438






