N-Formylsaccharin: A Sweet(able) Formylating Agent in Mechanochemistry
Abstract
:1. Introduction
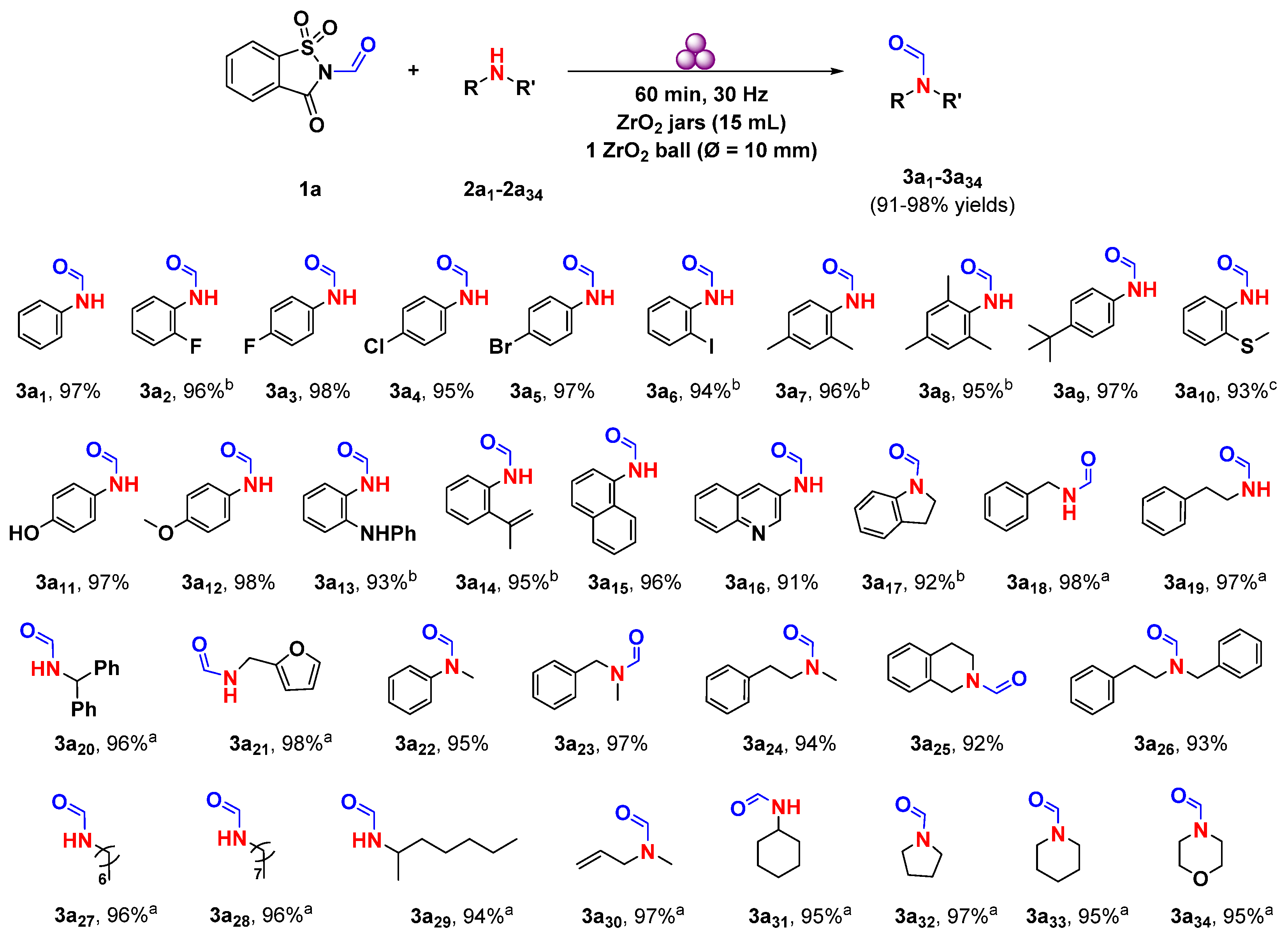
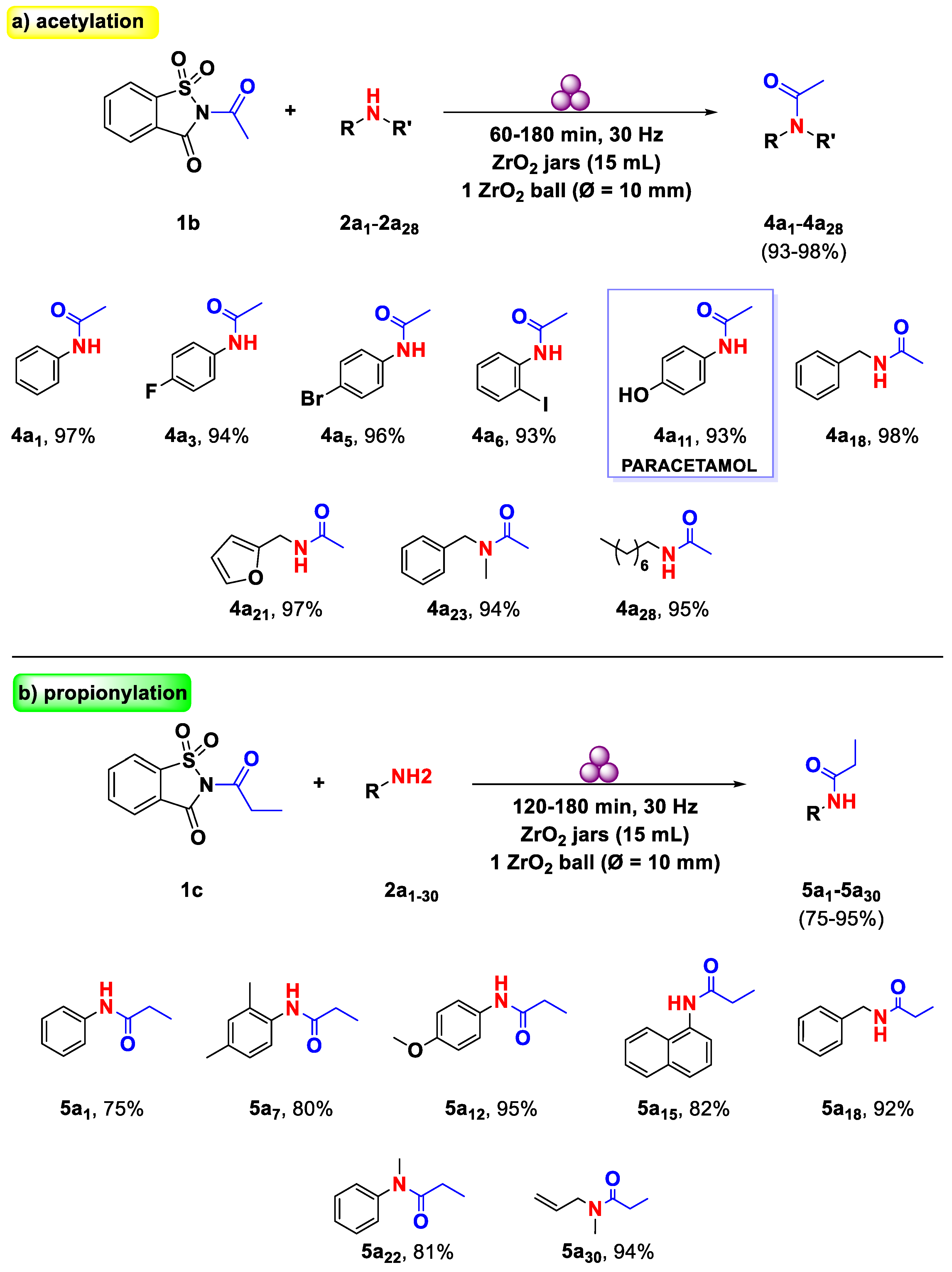
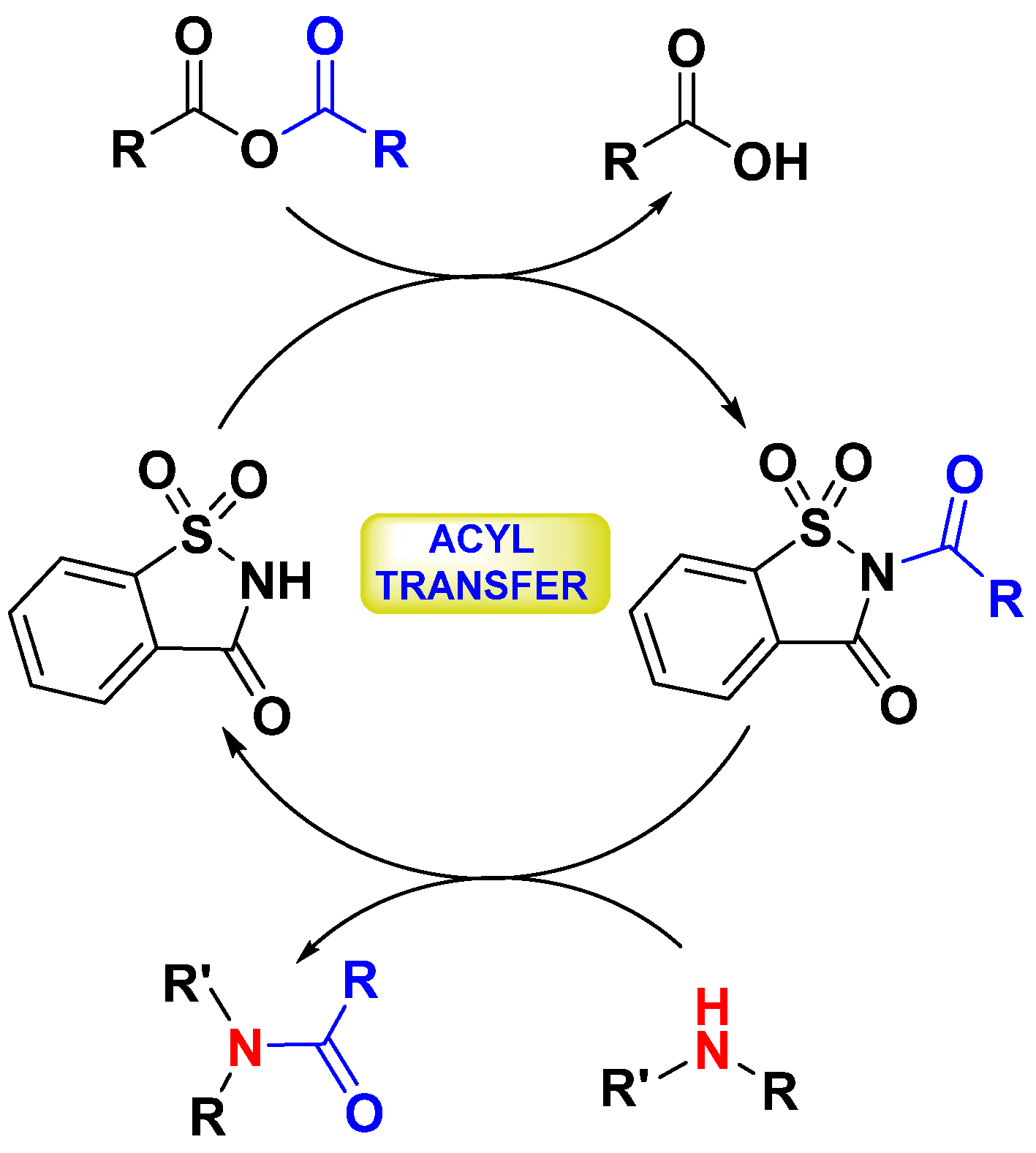
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. General Procedure for N-Formamides Synthesis from Primary and Secondary Amines
3.3. General Procedure for N-Acetamides Pynthesis from Primary and Secondary Amines
3.4. General Procedure for N-Propionamide Pynthesis from Aniline
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wu, K.M. A New Classification of Prodrugs: Regulatory Perspectives. Pharmaceuticals 2009, 2, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Stella, V.J.; Borchardt, R.T.; Hageman, M.J.; Oliyai, R.; Maag, H.; Tilley, J.W. Prodrugs: Challenges and Rewards; Springer Science & Business Media: Berlin, Germany, 2007; ISBN 978-0-387-49785-3. [Google Scholar] [CrossRef]
- Warwick, C. Paracetamol and Fever Management. J. R. Soc. Promot. Health 2008, 128, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Ebell, M.H.; Call, M.; Shinholser, J.A. Effectiveness of Oseltamivir in Adults: A meta-analysis of Published and Un-published Clinical Trials. Fam. Pract. 2013, 30, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Gerack, C.J.; McElwee-White, L. Formylation of Amines. Molecules 2014, 19, 7689–7713. [Google Scholar] [CrossRef] [PubMed]
- LaCrone, M.E.; Buening, N.; Paul, N. A Retrospective Review of an Inhaler to Nebulizer Therapeutic Interchange Program Across a Health System. J. Pharm. Pract. 2022, 08971900221101761. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.H.; Donohue, J.F.; Ohar, J.A. Review of Drug Safety and Efficacy of Arformoterol in Chronic Obstructive Pulmonary Disease. Expert Opin. Drug Saf. 2015, 14, 463–472. [Google Scholar] [CrossRef]
- Bodkin, J.A.; Humphries, E.J.; McLeod, M.D. The Total Synthesis of (-)-Tetrahydrolipstatin. Aust. J. Chem. 2003, 56, 795–803. [Google Scholar] [CrossRef]
- Massarotti, A.; Brunelli, F.; Aprile, S.; Giustiniano, M.; Tron, G.C. Medicinal Chemistry of Isocyanides. Chem. Rev. 2021, 121, 10742–10788. [Google Scholar] [CrossRef]
- Ugi, I.; Fetzer, U.; Eholzer, U.; Knupfer, H.; Offermann, K. Isonitrile Syntheses. Angew. Chem. Int. Ed. 1965, 4, 472–484. [Google Scholar] [CrossRef]
- Ugi, I.; Meyr, R. Darstellung von Isonitrilen aus monosubstituierten Formamiden durch Wasserabspaltun. Chem. Ber. 1960, 93, 239–248. [Google Scholar] [CrossRef]
- Weber, W.P.; Gokel, G.W.; Ugi, I.K. Phase Transfer Catalysis in the Hofmann Carbylamine Reaction. Angew. Chem. Int. Ed. 1972, 11, 530–531. [Google Scholar] [CrossRef]
- Waibel, K.A.; Nickisch, R.; Möhl, N.; Seim, R.; Meier, M.A.R. A More Sustainable and Highly Practicable Synthesis of Aliphatic Isocyanides. Green Chem. 2020, 22, 933–941. [Google Scholar] [CrossRef]
- Porcheddu, A.; Giacomelli, G.; Salaris, M. Microwave-Assisted Synthesis of Isonitriles: A General Simple Methodology. J. Org. Chem. 2005, 70, 2361–2363. [Google Scholar] [CrossRef] [PubMed]
- Basoccu, F.; Cuccu, F.; Casti, F.; Mocci, R.; Fattuoni, C.; Porcheddu, A. A Trustworthy Mechanochemical Route to Isocyanides. Beilstein Arch. 2022, 18, 732–737. [Google Scholar] [CrossRef]
- Margetić, D.; Štrukil, V. Recent Advances in Mechanochemical Organic Synthesis; Nandeshwarappa, V.Š.E.-B.P., Ed.; IntechOpen: Rijeka, Croatia, 2020; ISBN 978-1-78985-944-7. [Google Scholar]
- Mocci, R.; Murgia, S.; De Luca, L.; Colacino, E.; Delogu, F.; Porcheddu, A. Ball-Milling and Cheap Reagents Breathe Green Life into the One Hundred-Year-Old Hofmann Reaction. Org. Chem. Front. 2018, 5, 531–538. [Google Scholar] [CrossRef]
- Oxley, P.; Peak, D.A.; Short, W.F. Amidines. Part, X. Preparation of amidines from substituted amides, a sulphonyl chloride, and an amine. J. Chem. Soc. 1948, 37, 1618–1619. [Google Scholar] [CrossRef]
- Kotachi, S.; Tsuji, Y.; Kondo, T.; Watanabe, Y. Ruthenium catalysed N,N′-Diarylurea Synthesis from N-Aryl substituted Formamides and Aminoarenes. J. Chem. Soc. Chem. Commun. 1990, 7, 549–550. [Google Scholar] [CrossRef]
- Lane, E.M.; Hazari, N.; Bernskoetter, W.H. Iron-catalyzed urea synthesis: Dehydrogenative coupling of methanol and amines. Chem. Sci. 2018, 9, 4003–4008. [Google Scholar] [CrossRef]
- Lyons, J.E. Conversion of Formamides to Isocyanates. U.S. Patent US3960914A, 1976. Available online: https://worldwide.espacenet.com/patent/search?q=pn%3DUS3960914A (accessed on 18 August 2022).
- Bonin, M.A.; Giguère, D.; Roy, R. N-Arylimidazole Synthesis by Cross-Cycloaddition of Isocyanides Using a Novel Catalytic System. Tetrahedron 2007, 63, 4912–4917. [Google Scholar] [CrossRef]
- Lygin, A.V.; de Meijere, A. Isocyanides in the Synthesis of Nitrogen Heterocycles. Angew. Chem. Int. Ed. 2010, 49, 9094–9124. [Google Scholar] [CrossRef]
- Kuhlmann, R.; Künnemann, K.U.; Hinderink, L.; Behr, A.; Vorholt, A.J. CO2 Based Synthesis of Various Formamides in Miniplant Scale: A Two-Step Process Design. ACS Sustain. Chem. Eng. 2019, 7, 4924–4931. [Google Scholar] [CrossRef]
- Ghorbani-Vaghei, R.; Veisi, H.; Amiri, M. Poly(N,N′-dichloro-N-ethyl-benzene-1,3-disulfonamide, N,N,N′,N′-tetrachlorobenzene-1,3-disulfonamide, poly(N,N′-dibromo-N-ethyl-benzene-1,3-disulfonamide, and N,N,N′,N′-tetrabromobenzene-1,3-disulfonamide catalyzed Formylation of Amines and Alcohols Using Ethyl Formate under Microwave Irradiation. J. Iran. Chem. Soc. 2009, 6, 761–768. [Google Scholar]
- Takahashi, K.; Shibagaki, M.; Matsushita, H. Formylation of Amines by Dimethylformamide in the Presence of Hydrous Zirconium Oxide. Agric. Biol. Chem. 1988, 52, 853–854. [Google Scholar] [CrossRef]
- Karami, B.; Farahi, M.; Pam, F. A Green Protocol for The N-Formylation of Amines Using Molybdate Sulfuric Acid as a Reusable Solid Catalyst. Tetrahedron Lett. 2014, 55, 6292–6296. [Google Scholar] [CrossRef]
- Chandra Shekhar, A.; Ravi Kumar, A.; Sathaiah, G.; Luke Paul, V.; Sridhar, M.; Shanthan Rao, P. Facile N-formylation of Amines using Lewis’s Acids as Novel Catalysts. Tetrahedron Lett. 2009, 50, 7099–7101. [Google Scholar] [CrossRef]
- Kajanus, J.; Jacobson, I.; Åstrand, A.; Olsson, R.I.; Gran, U.; Björe, A.; Fjellström, O.; Davidsson, Ö.; Emtenäs, H.; Dahlén, A.; et al. Isoindolinone Compounds Active as Kv1.5 Blockers Identified Using a Multicomponent Reaction Approach. Bioorg. Med. Chem. Lett. 2016, 26, 2023–2029. [Google Scholar] [CrossRef]
- Lei, M.; Ma, L.; Hu, L. A Convenient One-Pot Synthesis of Formamide Derivatives Using Thiamine Hydrochloride as a Novel Catalyst. Tetrahedron Lett. 2010, 51, 4186–4188. [Google Scholar] [CrossRef]
- Zhang, F.; Li, L.; Ma, J.; Gong, H. MoS 2 -Catalyzed Transamidation Reaction. Sci. Rep. 2019, 9, 2536. [Google Scholar] [CrossRef]
- Brahmachari, G.; Laskar, S. A Very Simple and Highly Efficient Procedure for N-Formylation of Primary and Secondary Amines at Room Temperature Under Solvent-Free Conditions. Tetrahedron Lett. 2010, 51, 2319–2322. [Google Scholar] [CrossRef]
- De Luca, L.; Giacomelli, G.; Porcheddu, A.; Salaris, M. A New, Simple Procedure for The Synthesis Of Formyl Amides. Synlett 2004, 14, 2570–2572. [Google Scholar] [CrossRef]
- Cochet, T.; Bellosta, V.; Greiner, A.; Roche, D.; Cossy, J. N-Formylsaccharin: A New Formylating Agent. Synlett 2011, 13, 1920–1922. [Google Scholar] [CrossRef]
- Uzzaman, M.; Shawon, J.; Siddique, Z.A. Molecular Docking, Dynamics Simulation and ADMET Prediction of Acetaminophen and Its Modified Derivatives Based on Quantum Calculations. SN Appl. Sci. 2019, 1, 1437. [Google Scholar] [CrossRef]
- Vardanyan, R.S.; Hruby, V.J. Fentanyl-Related Compounds and Derivatives: Current Status and Future Pro-spects For Pharmaceutical Applications. Future Med. Chem. 2014, 6, 385–412. [Google Scholar] [CrossRef] [PubMed]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and Techniques for Solvent Selection: Green Solvent Selection Guides. Sustain. Chem. Process. 2016, 4, 7. [Google Scholar] [CrossRef]
- Welton, T. Solvents and Sustainable Chemistry. Proc. R. Soc. 2015, 471, 20150502. [Google Scholar] [CrossRef]
- Hessel, V.; Tran, N.N.; Asrami, M.R.; Tran, Q.D.; Van Duc Long, N.; Escribà-Gelonch, M.; Tejada, J.O.; Linke, S.; Sundmacher, K. Sustainability of Green Solvents—Review and Perspective. Green Chem. 2022, 24, 410–437. [Google Scholar] [CrossRef]
- Mendis, N.P.; Wang, J.; Lakerveld, R. Simultaneous Solvent Selection and Process Design for Continuous Reaction–Extraction–Crystallization Systems. Ind. Eng. Chem. Res. 2022, 61, 11504–11517. [Google Scholar] [CrossRef]
- Harris, K. How grinding evolves. Nat. Chem. 2013, 5, 12–14. [Google Scholar] [CrossRef]
- Gomollón-Bel, F. Ten Chemical Innovations That Will Change Our World: IUPAC identifies emerging technologies in Chemistry with potential to make our planet more sustainable. Chem. Int. 2019, 41, 12–17. [Google Scholar] [CrossRef]
- Do, J.-L.; Friščić, T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Andersen, J.; Mack, J. Mechanochemistry and Organic Synthesis: From Mystical to Practical. Green Chem. 2018, 20, 1435–1443. [Google Scholar] [CrossRef]
- Bolm, C.; Hernández, J.G. Mechanochemistry of Gaseous Reactants. Angew. Chem. Int. Ed. 2019, 58, 3285–3299. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Ito, H. Mechanochemical Cross-Coupling Reactions. Trends Chem. 2020, 2, 1066–1081. [Google Scholar] [CrossRef]
- Espro, C.; Rodríguez-Padrón, D. Re-Thinking Organic Synthesis: Mechanochemistry as a Greener Approach. Curr. Opin. Green Sustain. Chem. 2021, 30, 100478. [Google Scholar] [CrossRef]
- Ardila-Fierro, K.J.; Hernández, J.G. Sustainability Assessment of Mechanochemistry by Using the Twelve Principles of Green Chemistry. ChemSusChem 2021, 14, 2145–2162. [Google Scholar] [CrossRef]
- Jones, A.C.; Leitch, J.A.; Raby-Buck, S.E.; Browne, D.L. Mechanochemical Techniques for the Activation and Use of Zero-Valent Metals in Synthesis. Nat. Synth. 2022, 1–13. [Google Scholar] [CrossRef]
- Cuccu, F.; De Luca, L.; Delogu, F.; Colacino, E.; Solin, N.; Mocci, R.; Porcheddu, A. Mechanochemistry: New Tools to Navigate the Uncharted Territory of “Impossible” Reactions. ChemSusChem 2022, e202200362. [Google Scholar] [CrossRef]
- O’Neill, R.T.; Boulatov, R. The Many Flavours of Mechanochemistry and Its Plausible Conceptual Underpinnings. Nat. Rev. Chem. 2021, 5, 148–167. [Google Scholar] [CrossRef]
- Sanna, A.L.; Carta, M.; Pia, G.; Garroni, S.; Porcheddu, A.; Delogu, F. Chemical effects induced by the mechanical processing of granite powder. Sci. Rep. 2022, 12, 9445. [Google Scholar] [CrossRef]
- Carta, M.; Delogu, F.; Porcheddu, A. A Phenomenological Kinetic Equation for Mechanochemical Reactions Involving Highly Deformable Molecular Solids. Phys. Chem. Chem. Phys. 2021, 23, 14178–14194. [Google Scholar] [CrossRef]
- Hutchings, B.P.; Crawford, D.E.; Gao, L.; Hu, P.; James, S.L. Feedback Kinetics in Mechanochemistry: The Importance of Cohesive States. Angew. Chem. Int. Ed. 2017, 129, 15454–15458. [Google Scholar] [CrossRef]
- Konishi, H.; Fukuda, M.; Ueda, T.; Manabe, K. Palladium-catalyzed External-CO-Free Reductive Carbonylation of Bromoarenes. Org. Synth 2017, 94, 66–76. [Google Scholar] [CrossRef]
- Choi, S.-Y.; Lee, S.-G.; Yoon, Y.-J.; Kim, K.-W. Reaction of N-Hydroxymethylsaccharin with Aliphatic Carboxylic Acid Derivatives: Synthesis of N-Acylsaccharins and N-(Saccharinylmethyl) Aliphatic Carboxylates. J. Heterocycl. Chem. 1989, 26, 1073–1076. [Google Scholar] [CrossRef]
- Abraham, R.J.; Griffiths, L.; Perez, M. 1H NMR spectra. Part 30: 1H chemical shifts in amides and the magnetic anisotropy, electric field and steric effects of the amide group. Magn. Reason. Chem. 2013, 51, 143–155. [Google Scholar] [CrossRef]
- Hosseini-Sarvari, M.; Sharghi, H. ZnO as a New Catalyst for N-Formylation of Amines under Solvent-Free Conditions. J. Org. Chem. 2006, 71, 6652–6654. [Google Scholar] [CrossRef]
- Lygin, A.V.; Meijere, A.D. ortho-Lithiophenyl Isocyanide: A Versatile Precursor for 3H-Quinazolin-4-ones and 3H-Quinazolin-4-thiones. Org. Lett. 2009, 11, 389–392. [Google Scholar] [CrossRef]
- Vougioukalakis, G.C.; Grubbs, R.H. Synthesis and Activity of Ruthenium Olefin Metathesis Catalysts Coordinated with Thiazol-2-ylidene Ligands. J. Am. Chem. Soc. 2008, 130, 2234–2245. [Google Scholar] [CrossRef]
- Gim, H.J.; Kang, B.; Jeon, R. Synthesis and biological activity of 5-(4-[2-(Methyl-p-substituted phenylamino)ethoxy]benzyl)thiazolidine-2,4-diones. Arch. Pharmacal Res. 2007, 30, 1055–1061. [Google Scholar] [CrossRef]
- Di Nunno, L.; Scilimati, A. Decomposition of arylazides by thf/-butyllithium-II-isolation of 1-aryl-4,5-dihydro-5-hydroxy-1h-1,2,3-triazoles. Tetrahedron 1986, 42, 3913–3920. [Google Scholar] [CrossRef]
- Kametani, T.; Suzuki, Y.; Takeda, H.; Kasai, H.; Yamazaki, K.; Takada, N.; Honda, T. Novel Construction of Quinoline Ring System by Electrocyclic Reaction of Azahexatriene Derivatives. J. Pharm. Soc. Jpn. 1987, 107, 107–110. [Google Scholar] [CrossRef] [Green Version]
- Suchý, M.; Elmehriki, A.A.H.; Hudson, R.H.E. A remarkably simple protocol for the N-formylation of amino acid esters and primary amines. Org. Lett. 2011, 13, 3952–3955. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Ushitora, H. Trapping of carbamic acid species with (trimethylsilyl)diazomethane. Tetrahedron 2006, 62, 226–235. [Google Scholar] [CrossRef]
- Available online: https://scifinder-n.cas.org/searchDetail/substance/62b1d814fe25af742cd24d7d/substanceDetails (accessed on 18 August 2022).
- Rahman, M.; Kundu, D.; Hajra, A.; Majee, A. Formylation without catalyst and solvent at 80 °C. Tetrahedron Lett. 2010, 51, 2896–2899. [Google Scholar] [CrossRef]
- Saidi, O.; Bamford, M.J.; Blacker, A.J.; Lynch, J.; Marsden, S.P.; Plucinski, P.; Watson, R.J.; Williams, J.M.J. Iridium-catalyzed formylation of amines with paraformaldehyde. Tetrahedron Lett. 2010, 51, 5804–5806. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Yao, G.; Lan, X.; Zhao, X. The conversion of secondary into tertiary amides using benzotriazole methodology. J. Org. Chem. 1993, 58, 2086–2093. [Google Scholar] [CrossRef]
- Kulkarni, A.; Gianatassio, R.; Török, B. Pd/C-Catalyzed Reductive Formylation of Indoles and Quinolines Using Formic Acid. Synthesis 2011, 8, 1227–1232. [Google Scholar]
- Lee, H.-L.; Aubé, J. Intramolecular and intermolecular Schmidt reactions of alkyl azides with aldehydes. Tetrahedron 2007, 63, 9007–9015. [Google Scholar] [CrossRef]
- Tumma, H.; Nagaraju, N.; Reddy, K.V. A facile method for the N-formylation of primary and secondary amines by liquid phase oxidation of methanol in the presence of hydrogen peroxide over basic copper hydroxyl salts. J. Mol. Cat. A Chem. 2009, 310, 121–129. [Google Scholar] [CrossRef]
- Available online: https://scifinder-n.cas.org/searchDetail/substance/62b1e864fe25af742cd26e84/substanceDetails (accessed on 18 August 2022).
- Furuya, Y.; Ishihara, K.; Yamamoto, H. Cyanuric Chloride as a Mild and Active Beckmann Rearrangement Catalyst. J. Am. Chem. Soc. 2005, 127, 11240–11241. [Google Scholar] [CrossRef]
- Augustine, J.K.; Kumar, R.; Bombrun, A.; Mandal, A.B. An efficient catalytic method for the Beckmann rearrangement of ketoximes to amides and aldoximes to nitriles mediated by propylphosphonic anhydride (T3P®). Tetrahedron Lett. 2011, 52, 1074–1077. [Google Scholar] [CrossRef]
- Ramalingan, C.; Park, Y.-T. Mercury-Catalyzed Rearrangement of Ketoximes into Amides and Lactams in Acetonitrile. J. Org. Chem. 2007, 72, 4536–4538. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, A.V.; Aksenov, N.A.; Nadein, O.N.; Aksenova, I.V. Nitroethane in Polyphosphoric Acid: A New Reagent for Acetamidation and Amination of Aromatic Compounds. Synlett 2010, 17, 2628–2630. [Google Scholar] [CrossRef]
- Dhake, K.P.; Qureshi, Z.S.; Singhal, R.S.; Bhanage, B.M. Candida antarctica lipase B-catalyzed synthesis of acetamides using [BMIm(PF6)] as a reaction medium. Tetrahedron Lett. 2009, 50, 2811–2814. [Google Scholar] [CrossRef]
- Roger, J.; Doucet, H. Palladium-Catalysed Direct 5-Arylation of Furfurylamine or 2-(Aminoalkyl)thiophene Derivatives. Eur. J. Org. Chem. 2010, 2010, 4412–4425. [Google Scholar] [CrossRef]
- Srivastava, V.P.; Patel, R.; Garima, L.; Yadav, D.S. Cyclopropenium ion catalysed Beckmann rearrangement. Chem. Comm. 2010, 46, 5808–5810. [Google Scholar] [CrossRef]
- Gowda, S.; Gowda, B.T. 1H and 13C NMR Spectral Studies on N-(j,k-Dichlorophenyl)- and N-(j,k-Dimethylphenyl)-acetamides and Substituted Acetamides. Naturforsch. A 2007, 62, 84–90. [Google Scholar] [CrossRef]
- Available online: https://scifinder-n.cas.org/searchDetail/substance/62d1700f0b6f646c3b6f55bc/substanceDetails (accessed on 18 August 2022).
- Starkov, P.; Sheppard, T.D. Borate esters as convenient reagents for direct amidation of carboxylic acids and transamidation of primary amides. Org. Biomol. Chem. 2011, 9, 1320–1323. [Google Scholar] [CrossRef]
- Shaffer, C.L.; Harriman, S.; Koen, Y.M.; Hanzlik, R.P. Formation of cyclopropanone during cytochrome P450-catalyzed N-dealkylation of a cyclopropylamine. J. Am. Chem. Soc. 2002, 124, 8268–8274. [Google Scholar] [CrossRef]
- Chaminade, X.; Duñach, E.; Esteves, A.P.; Medeiros, M.J.; Neves, C.S.; Olivero, S. Electrosynthesis of nitrogen heterocycles using environmentally friendly methodologies Electrochim. Acta 2009, 54, 5120–5126. [Google Scholar]



| Entry | Bases | R-NH2/Base Ratio | Yields a |
|---|---|---|---|
| 1 | None | - | 52% |
| 2 | Li2CO3 (anhydrous) | 1:1 | 35% |
| 3 | Li2CO3 (wet) b | 1:1 | 32% |
| 4 | Li2CO3 (anhydrous) | 1:3 | 29% |
| 5 | Li2CO3 (wet) b | 1:3 | 29% |
| 6 | K2CO3 (anhydrous) | 1:1 | 56% |
| 7 | K2CO3 (wet) b | 1:1 | 40% |
| 8 | K2CO3 (anhydrous) | 1:3 | 35% |
| 9 | K2CO3 (wet) b | 1:3 | 32% |
| 10 | Na2CO3 (anhydrous) | 1:1 | 53% |
| 11 | Na2CO3 (wet) b | 1:1 | 42% |
| 12 | Na2CO3 (anhydrous) | 1:3 | 39% |
| 13 | Na2CO3 (wet) b | 1:3 | 40% |
| 14 | Cs2CO3 (anhydrous) | 1:1 | 46% |
| 15 | Cs2CO3 (wet) b | 1:1 | 43% |
| 16 | Cs2CO3 (anhydrous) | 1:3 | 45% |
| 17 | Cs2CO3 (wet) b | 1:3 | 45% |
| 18 | MgO | 1:1 | 26% |
| 19 | CaO | 1:1 | 15% |
| 20 | N-methylimidazole | 1:1 | 36% |
| 21 | Imidazole | 1:1 | 33% |
| 22 | t-BuOK | 1:1 | 23% |
| Procedure | E-Factor |
|---|---|
| Solvent-based [32] | >374 |
| Mechanochemistry | 26.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuccu, F.; Basoccu, F.; Fattuoni, C.; Porcheddu, A. N-Formylsaccharin: A Sweet(able) Formylating Agent in Mechanochemistry. Molecules 2022, 27, 5450. https://doi.org/10.3390/molecules27175450
Cuccu F, Basoccu F, Fattuoni C, Porcheddu A. N-Formylsaccharin: A Sweet(able) Formylating Agent in Mechanochemistry. Molecules. 2022; 27(17):5450. https://doi.org/10.3390/molecules27175450
Chicago/Turabian StyleCuccu, Federico, Francesco Basoccu, Claudia Fattuoni, and Andrea Porcheddu. 2022. "N-Formylsaccharin: A Sweet(able) Formylating Agent in Mechanochemistry" Molecules 27, no. 17: 5450. https://doi.org/10.3390/molecules27175450







