Pro-Apoptotic Effect of Zeolitic Imidazolate Framework-8 (ZIF-8)-Loaded Dihydromyricetin on HepG2 Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of DHM@ZIF-8
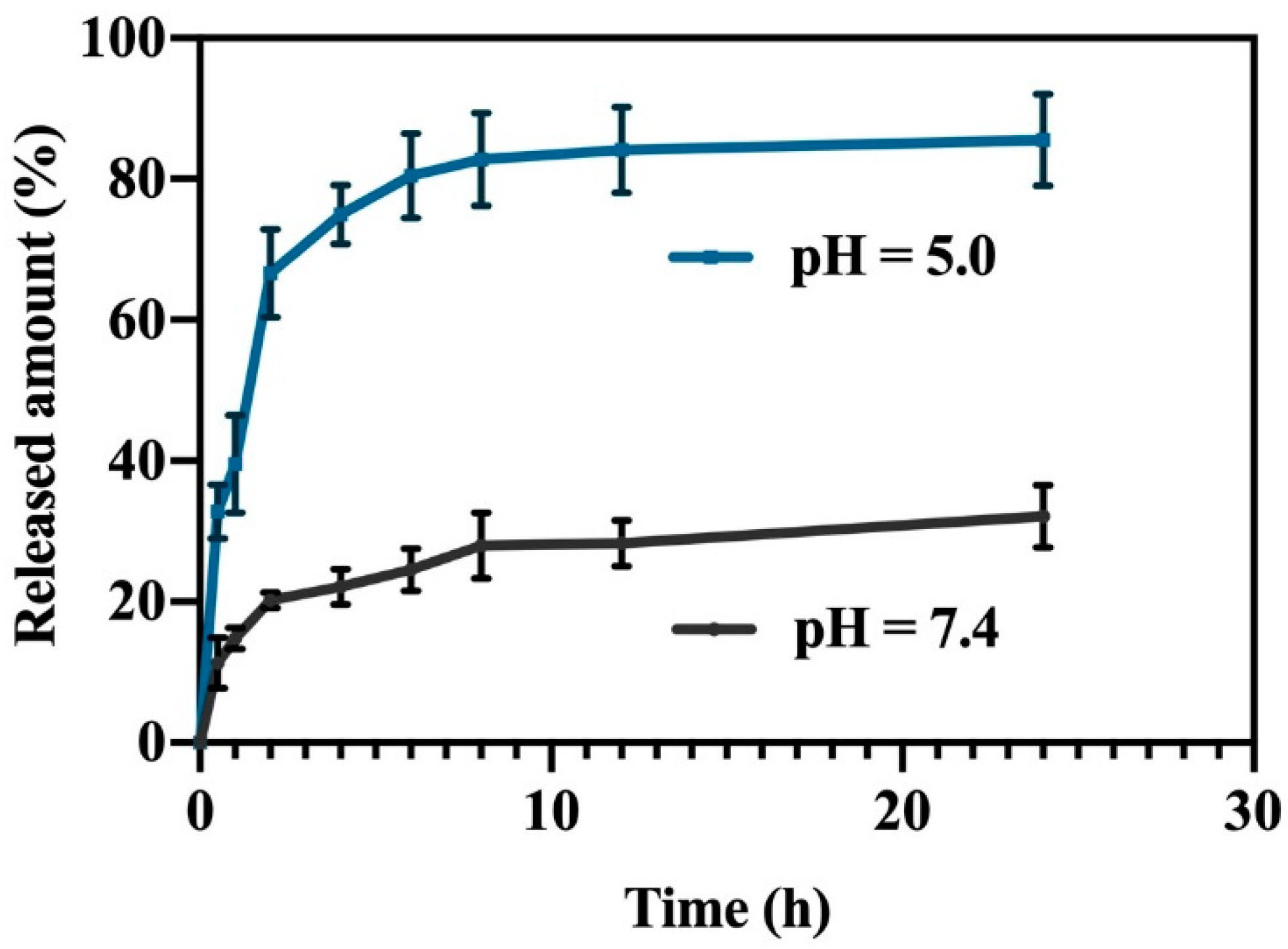
2.2. Drug Loading Rate and Drug Releasing Rate
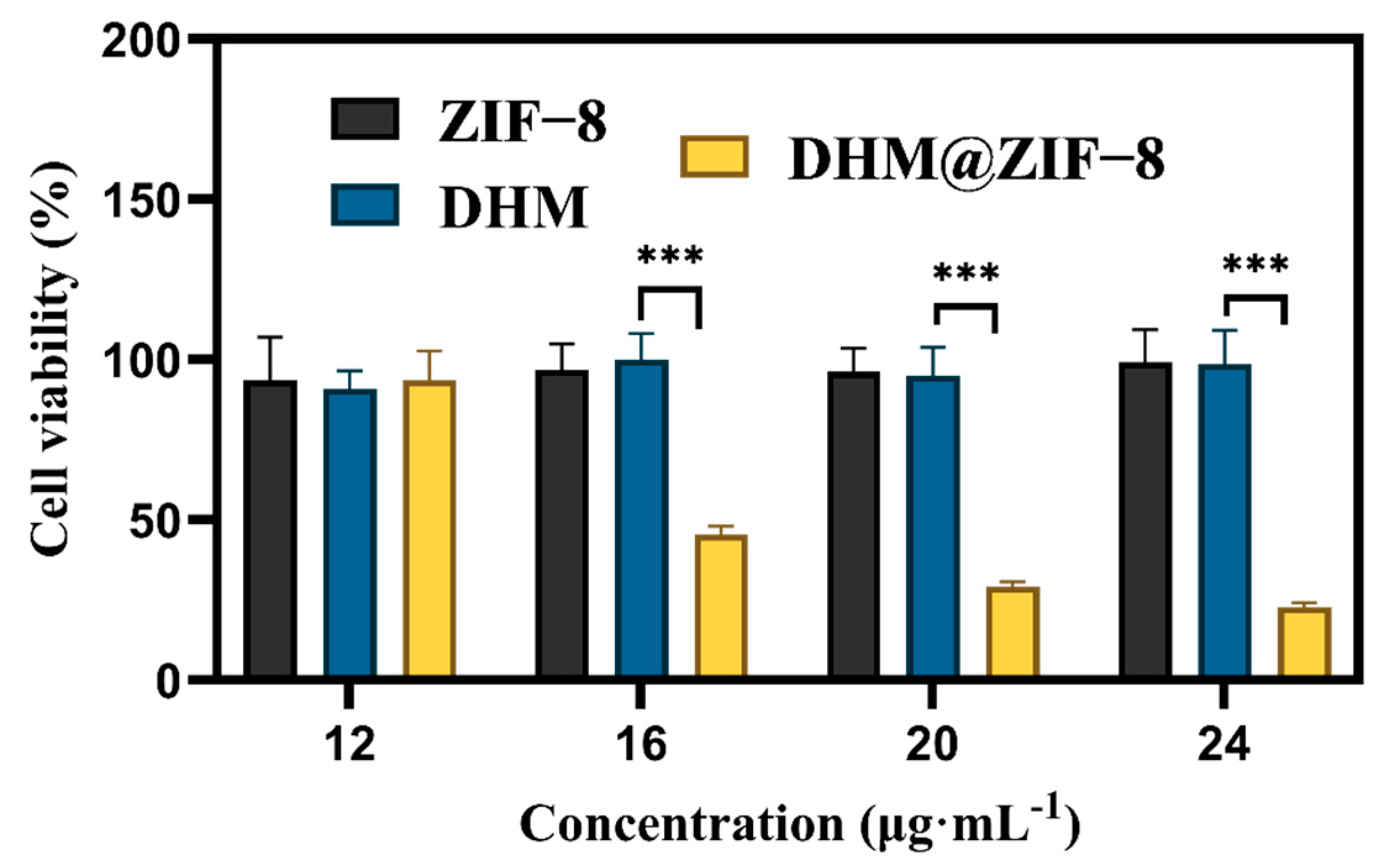
2.3. Cytotoxicity Assay
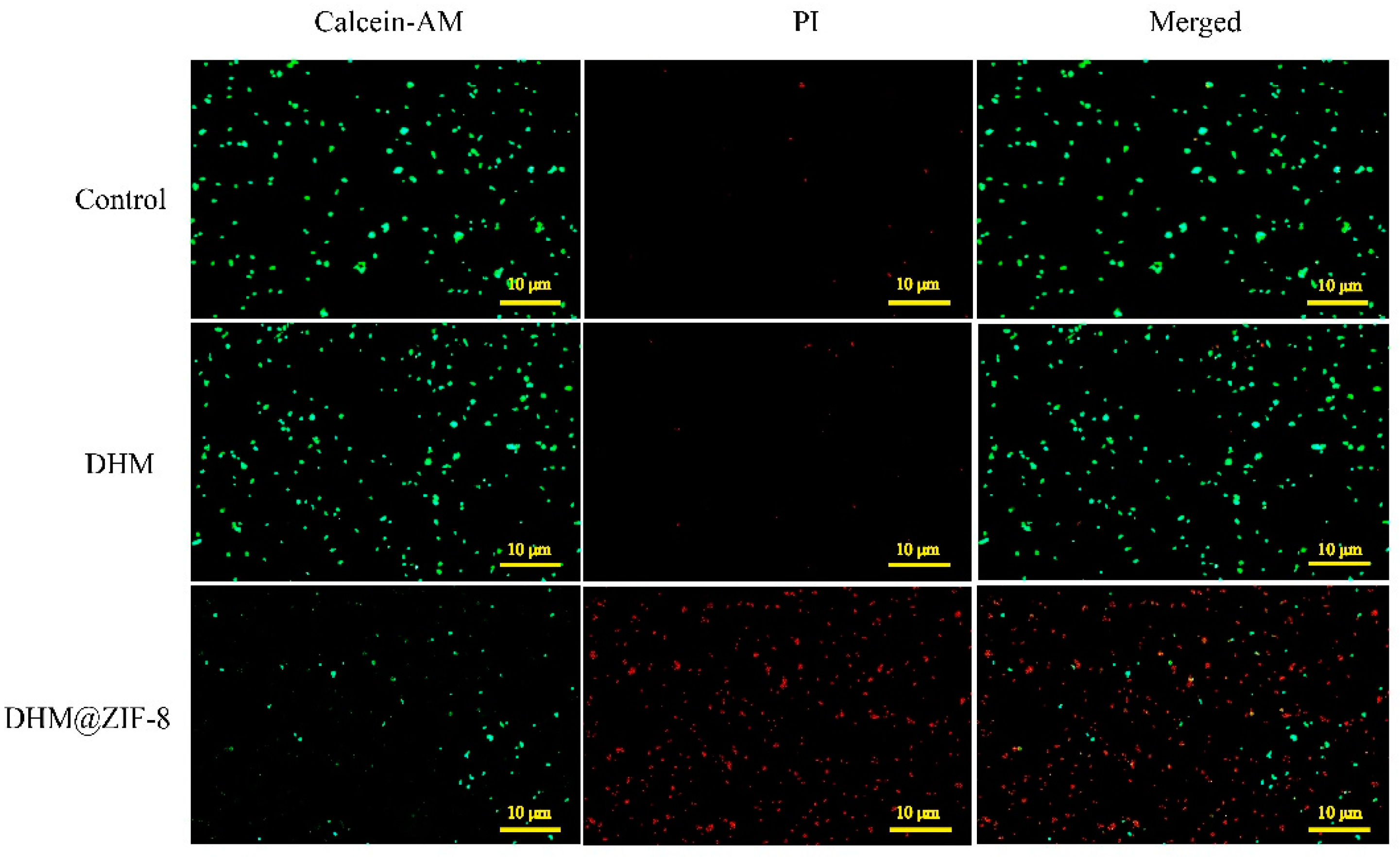
2.4. Live/Dead Cell Staining
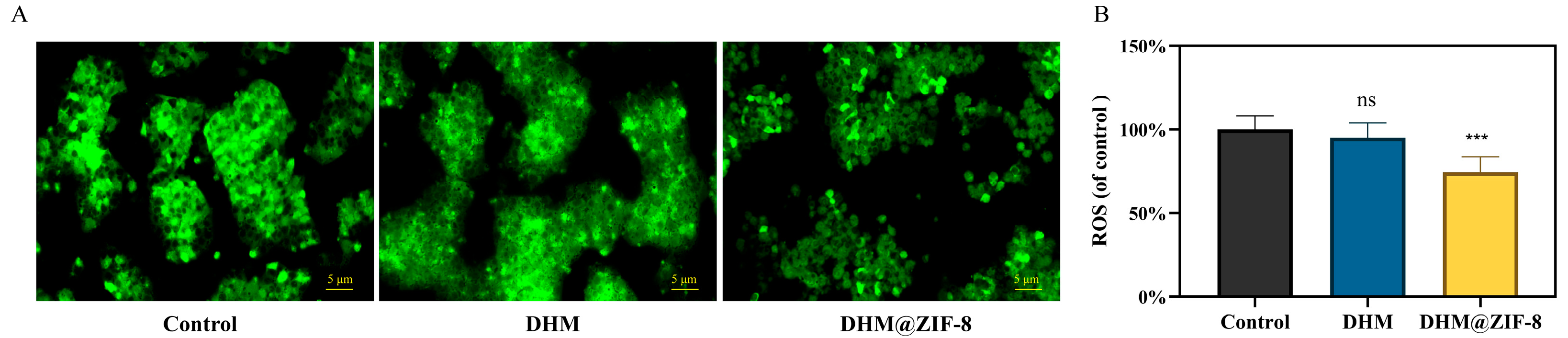
2.5. Determination of ROS Content
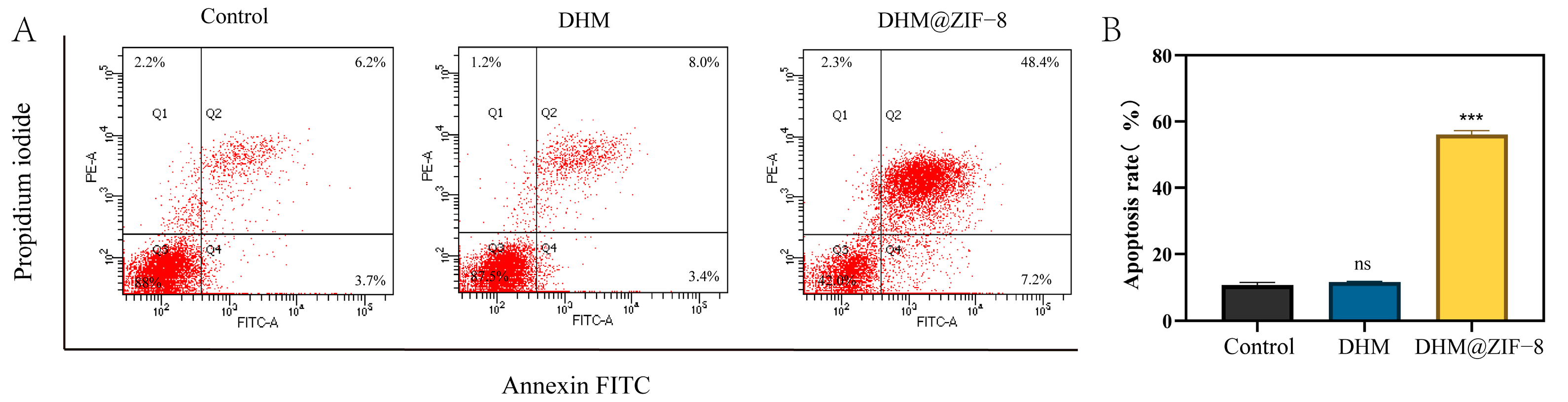
2.6. Cell Apoptosis
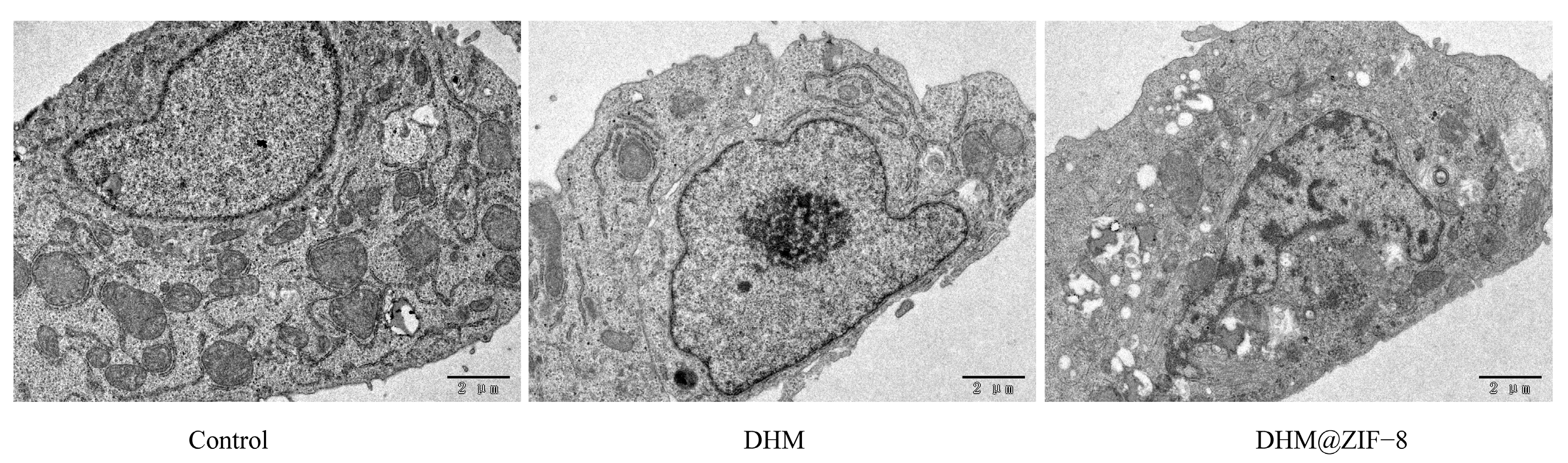
2.7. Apoptotic Cell Morphology Observed Using the Transmission Electron Microscope
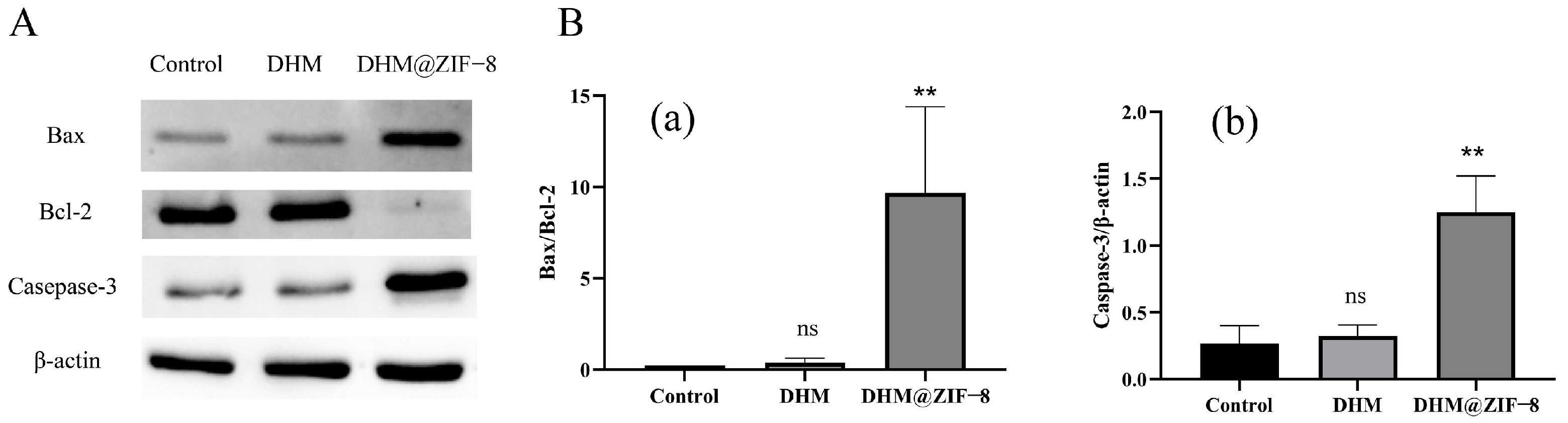
2.8. Western Blot
3. Materials and Methods
3.1. Materials and Reagents
3.2. Cell Lines
3.3. Synthesis of DHM@ZIF-8
3.3.1. Synthesis of ZIF-8
3.3.2. Synthesis of DHM@ZIF-8
3.4. Characterization of DHM@ZIF-8
3.5. Determination of Drug-Loading Rate and Drug-Releasing Rate
3.6. Role of In Vitro Anti-Hepatocellular Carcinoma Cells HepG2
3.6.1. Cytotoxicity Assay
3.6.2. Living/Dead Cell Staining
3.7. Determination of ROS Content
3.8. Cell Apoptosis
3.9. Observation Using the Transmission Electron Microscope
3.10. Apoptosis-Related Proteins Detected Using Western Blot
3.11. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xu, W.; Li, K.; Song, C.; Wang, X.; Li, Y.; Xu, B.; Liang, X.; Deng, W.; Wang, J.; Liu, J. Knockdown of lncRNA LINC01234 Suppresses the Tumorigenesis of Liver Cancer via Sponging miR-513a-5p. Front. Oncol. 2020, 10, 571565. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Mo, M.; Tan, H.J.J.; Tan, C.; Zeng, X.; Zhang, G.; Huang, D.; Liang, J.; Liu, S.; Qiu, X. LINC02499, a novel liver-specific long non-coding RNA with potential diagnostic and prognostic value, inhibits hepatocellular carcinoma cell proliferation, migration, and invasion. Hepatol. Res. 2020, 50, 726–740. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Jiang, M.; Jiang, H.-M.; Ye, Z.J.; Huang, Y.S.; Li, X.S.; Qin, B.Y.; Zhou, R.S.; Pan, H.F.; Zheng, D.Y. The Roles of circRNAs in Liver Cancer Immunity. Front. Oncol. 2021, 10, 598464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, H.; Chen, S.; Xu, Y.; Yao, A.; Liao, Q.; Han, L.; Zou, Z.; Zhang, X. Dihydromyricetin induces mitochondria-mediated apoptosis in HepG2 cells through down-regulation of the Akt/Bad pathway. Nutr. Res. 2017, 38, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, Q.; Ren, H.; Ma, S.; Lu, C.; Liu, B.; Liu, J.; Liang, J.; Li, M.; Zhu, R. Dihydromyricetin Enhances the Chemo-Sensitivity of Nedaplatin via Regulation of the p53/Bcl-2 Pathway in Hepatocellular Carcinoma Cells. PLoS ONE 2015, 10, e0124994. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Liu, B.; Xia, J.; Chen, N.; Chen, X.; Cao, Y.; Zhang, C.; Lu, C.; Li, M.; et al. Dihydromyricetin promotes hepatocellular carcinoma regression via a p53 activation-dependent mechanism. Sci. Rep. 2014, 4, 4628. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Xia, T.; Bi, Y.; Liu, B.; Fu, J.; Zhu, R. Molecular mechanisms and therapeutic implications of dihydromyricetin in liver disease. Biomed. Pharmacother. 2021, 142, 111927. [Google Scholar] [CrossRef]
- Zhou, D.Z.; Sun, H.Y.; Yue, J.Q.; Peng, Y.; Chen, Y.M.; Zhong, Z.J. Dihydromyricetin induces apoptosis and cytoprotective autophagy through ROS-NF-κB signalling in human melanoma cells. Free Radic. Res. 2017, 51, 517–528. [Google Scholar] [CrossRef]
- Xiang, J.; Lv, Q.; Yi, F.; Song, Y.; Le, L.; Jiang, B.; Xu, L.; Xiao, P. Dietary Supplementation of Vine Tea Ameliorates Glucose and Lipid Metabolic Disorder via Akt Signaling Pathway in Diabetic Rats. Molecules 2019, 24, 1866. [Google Scholar] [CrossRef]
- Xiao, T.; Wei, Y.; Cui, M.; Li, X.; Ruan, H.; Zhang, L.; Bao, J.; Ren, S.; Gao, D.; Wang, M.; et al. Effect of dihydromyricetin on SARS-CoV-2 viral replication and pulmonary inflammation and fibrosis. Phytomedicine 2021, 91, 153704. [Google Scholar]
- Shevelev, A.B.; La Porta, N.; Isakova, E.P.; Martens, S.; Biryukova, Y.K.; Belous, A.S.; Sivokhin, D.A.; Trubnikova, E.V.; Zylkova, M.V.; Belyakova, A.V.; et al. In Vivo Antimicrobial and Wound-Healing Activity of Resveratrol, Dihydroquercetin, and Dihydromyricetin against Staphylococcus aureus, Pseudomonas aeruginosa, and Candida albicans. Pathogens 2020, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Jiang, B.; Wang, H.; Ouyang, W.; Chen, X.; Wang, T.; Dong, D.; Yi, S.; Yi, J.; Huang, Y.; et al. Dihydromyricetin induced lncRNA MALAT1-TFEB-dependent autophagic cell death in cuta-neous squamous cell carcinoma. J. Cancer 2019, 10, 4245–4255. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Guo, S.; Fang, T.; Feng, D.; Zhang, X.; Zhang, Q.; Liu, J.; Liu, B.; Li, M.; Zhu, R. Dihydromyricetin induces autophagy in HepG2 cells involved in inhibition of mTOR and regulating its upstream pathways. Food Chem. Toxicol. 2014, 66, 7–13. [Google Scholar] [CrossRef]
- Jiang, L.; Ye, W.C.; Li, Z.; Yang, Y.; Dai, W.; Li, M. Anticancer effects of dihydromyricetin on the proliferation, migration, apoptosis and in vivo tu-morigenicity of human hepatocellular carcinoma Hep3B cells. BMC Complement. Med. Ther. 2021, 21, 194. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.J.; Lee, W.J.; Chang, J.H.; Chow, J.M.; Chung, C.L.; Hung, W.Y.; Chien, M.H. Suppression of reactive oxygen species-mediated ERK and JNK activation sensitizes dihy-dromyricetin-induced mitochondrial apoptosis in human non-small cell lung cancer. Environ. Toxicol. 2017, 32, 1426–1438. [Google Scholar] [CrossRef]
- Wang, F.; Chen, X.; Yuan, D.; Yi, Y.; Luo, Y. Golgi reassembly and stacking protein 65 downregulation is required for the anti-cancer effect of dihydromyricetin on human ovarian cancer cells. PLoS ONE 2019, 14, e0225450. [Google Scholar] [CrossRef]
- Liu, D.; Mao, Y.; Ding, L.; Zeng, X.-A. Dihydromyricetin: A review on identification and quantification methods, biological activities, chemical stability, metabolism and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2019, 91, 586–597. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Luo, H.; Sun, L.; Xu, M.; Yu, J.; Zhou, Q.; Meng, G.; Yang, S. Recent Update on the Pharmacological Effects and Mechanisms of Dihydromyricetin. Front. Pharmacol. 2018, 9, 1204. [Google Scholar] [CrossRef]
- Dong, K.; Zhang, Y.; Zhang, L.; Wang, Z.; Ren, J.; Qu, X. Facile preparation of metal−organic frameworks-based hydrophobic anticancer drug delivery nanoplatform for targeted and enhanced cancer treatment. Talanta 2019, 194, 703–708. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. Zeolitic Imidazolate Frameworks (ZIF-8) for Biomedical Applications: A Review. Curr. Med. Chem. 2021, 28, 7023–7075. [Google Scholar] [CrossRef]
- Mi, X.; Hu, M.; Dong, M.; Yang, Z.; Zhan, X.; Chang, X.; Lu, J.; Chen, X. Folic Acid Decorated Zeolitic Imidazolate Framework (ZIF-8) Loaded with Baicalin as a Nano-Drug Delivery System for Breast Cancer Therapy. Int. J. Nanomed. 2021, 16, 8337–8352. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.-L.; Liu, H.-X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics 2021, 11, 4839–4857. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Tan, X.; Liang, J.; Wu, S.; Liu, J.; Zhang, Q.; Zhu, R. A reduction in reactive oxygen species contributes to dihydromyricetin-induced apoptosis in human hepatocellular carcinoma cells. Sci. Rep. 2014, 4, 7041. [Google Scholar] [CrossRef]
- Dutta, S.; Mahalanobish, S.; Saha, S.; Ghosh, S.; Sil, P.C. Natural products: An upcoming therapeutic approach to cancer. Food Chem. Toxicol. 2019, 128, 240–255. [Google Scholar] [CrossRef]
- Del Poeta, G.; Venditti, A.; Del Principe, M.I.; Maurillo, L.; Buccisano, F.; Tamburini, A.; Cox, M.C.; Franchi, A.; Bruno, A.; Mazzone, C.; et al. Amount of spontaneous apoptosis detected by Bax/Bcl-2 ratio predicts outcome in acute myeloid leukemia (AML). Blood 2003, 101, 2125–2131. [Google Scholar] [CrossRef]
- Vucicevic, K.; Jakovljevic, V.; Colovic, N.; Tosic, N.; Kostic, T.; Glumac, I.; Pavlovic, S.; Karan-Djurasevic, T.; Colovic, M. Association of Bax Expression and Bcl2/Bax Ratio with Clinical and Molecular Prognostic Markers in Chronic Lymphocytic Leukemia. J. Med. Biochem. 2016, 35, 150–157. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, X.; Lu, J.; Dong, M.; Lou, Y.; Zhan, X.; Chen, X. Pro-Apoptotic Effect of Zeolitic Imidazolate Framework-8 (ZIF-8)-Loaded Dihydromyricetin on HepG2 Cells. Molecules 2022, 27, 5484. https://doi.org/10.3390/molecules27175484
Mi X, Lu J, Dong M, Lou Y, Zhan X, Chen X. Pro-Apoptotic Effect of Zeolitic Imidazolate Framework-8 (ZIF-8)-Loaded Dihydromyricetin on HepG2 Cells. Molecules. 2022; 27(17):5484. https://doi.org/10.3390/molecules27175484
Chicago/Turabian StyleMi, Xiao, Juan Lu, Mingran Dong, Yang Lou, Xia Zhan, and Xi Chen. 2022. "Pro-Apoptotic Effect of Zeolitic Imidazolate Framework-8 (ZIF-8)-Loaded Dihydromyricetin on HepG2 Cells" Molecules 27, no. 17: 5484. https://doi.org/10.3390/molecules27175484
APA StyleMi, X., Lu, J., Dong, M., Lou, Y., Zhan, X., & Chen, X. (2022). Pro-Apoptotic Effect of Zeolitic Imidazolate Framework-8 (ZIF-8)-Loaded Dihydromyricetin on HepG2 Cells. Molecules, 27(17), 5484. https://doi.org/10.3390/molecules27175484








