Gene Ontology (GO)-Driven Inference of Candidate Proteomic Markers Associated with Muscle Atrophy Conditions
Abstract
1. Introduction
2. Results
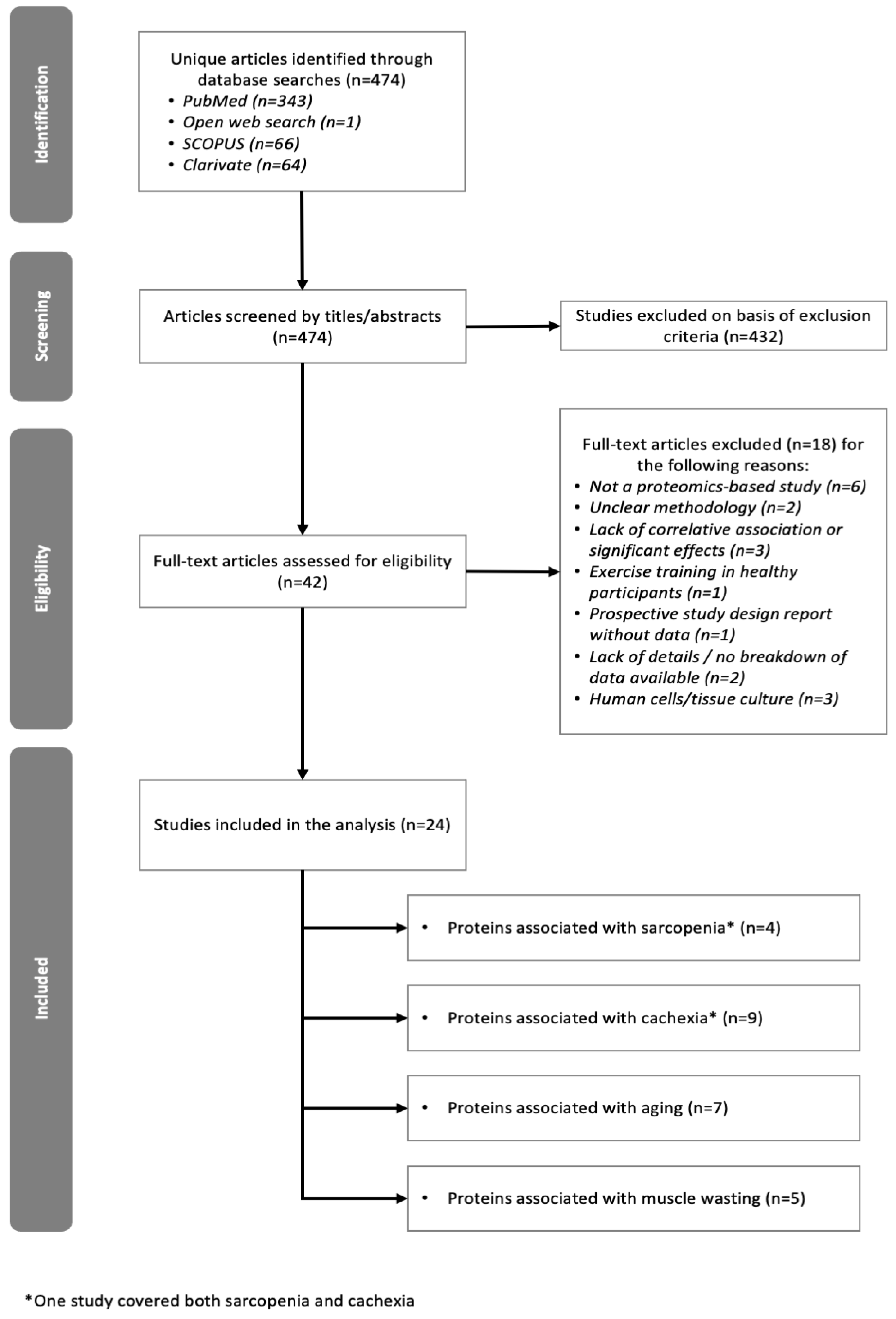
2.1. Overview of Studies Included
2.2. Analysis at the Molecular Level
2.3. Differences and Convergent Molecular Pathways across Cachexia, Sarcopenia, Aging, and Muscle-Wasting Conditions
2.4. Analysis of the Biological Processes and Functions
2.4.1. Biological Processes and Functions Affected in Cachexia
2.4.2. Biological Processes and Functions Affected in Sarcopenia
2.4.3. Biological Processes and Functions Affected by Aging
2.4.4. Biological Processes and Functions Affected by Muscle Wasting
3. Methods
3.1. Study Selection and Inclusion Criteria
3.2. Exclusion Criteria
3.3. Data Collection
3.4. Gene Ontology Analysis
4. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Frontera, W.R.; Ochala, J. Skeletal Muscle: A Brief Review of Structure and Function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of Muscle Atrophy and Hypertrophy: Implications in Health and Disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef]
- Giordani, L.; He, G.J.; Negroni, E.; Sakai, H.; Law, J.Y.C.; Siu, M.M.; Wan, R.; Corneau, A.; Tajbakhsh, S.; Cheung, T.H.; et al. High-Dimensional Single-Cell Cartography Reveals Novel Skeletal Muscle-Resident Cell Populations. Mol. Cell 2019, 74, 609–621. [Google Scholar] [CrossRef]
- Lee, J.H.; Jun, H. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front. Physiol. 2019, 10, 42. [Google Scholar] [CrossRef]
- Das, S.K.; Eder, S.; Schauer, S.; Diwoky, C.; Temmel, H.; Guertl, B.; Gorkiewicz, G.; Tamilarasan, K.P.; Kumari, P.; Trauner, M.; et al. Adipose Triglyceride Lipase Contributes to Cancer-Associated Cachexia. Science 2011, 333, 233–238. [Google Scholar] [CrossRef]
- Vainshtein, A.; Grumati, P.; Sandri, M.; Bonaldo, P. Skeletal Muscle, Autophagy, and Physical Activity: The Ménage À Trois of Metabolic Regulation in Health and Disease. J. Mol. Med. 2014, 92, 127–137. [Google Scholar] [CrossRef]
- Berardi, E. Muscular Dystrophies and Cancer Cachexia: Similarities in Chronic Skeletal Muscle Degeneration. J. Funct. Morphol. Kinesiol. 2017, 2, 39. [Google Scholar] [CrossRef]
- Chumlea, W.C.; Cesari, M.; Evans, W.J.; Ferrucci, L.; Fielding, R.A.; Pahor, M.; Studenski, S.; Vellas, B.; The Task, F.M. International Working Group on Sarcopenia. J. Nutr. Health Aging 2011, 15, 450–455. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and Classification of Cancer Cachexia: An International Consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Furrer, R.; Handschin, C. Muscle Wasting Diseases: Novel Targets and Treatments. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 315–339. [Google Scholar] [CrossRef]
- Clemson, L.; Stark, S.; Pighills, A.C.; Torgerson, D.J.; Sherrington, C.; Lamb, S.E. Environmental Interventions for Preventing Falls in Older People Living in the Community. Cochrane Database Syst. Rev. 2019, 2019, CD013258. [Google Scholar] [CrossRef]
- Yedigaryan, L.; Sampaolesi, M. Therapeutic Implications of miRNAs for Muscle-Wasting Conditions. Cells 2021, 10, 3035. [Google Scholar] [CrossRef]
- Jennings, M.J.; Kagiava, A.; Vendredy, L.; Spaulding, E.L.; Stavrou, M.; Hathazi, D.; Grüneboom, A.; De Winter, V.; Gess, B.; Schara, U.; et al. NCAM1 and GDF15 are Biomarkers of Charcot-Marie-Tooth Disease in Patients and Mice. Brain 2022, awac055, online ahead of print. [Google Scholar] [CrossRef]
- Picca, A.; Coelho-Junior, H.J.; Calvani, R.; Marzetti, E.; Vetrano, D.L. Biomarkers Shared by Frailty and Sarcopenia in Older Adults: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2022, 73, 101530. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Cesari, M.; Landi, F.; Bernabei, R.; Coelho-Júnior, H.J.; Marzetti, E. Biomarkers of Physical Frailty and Sarcopenia: Coming Up to the Place? Int. J. Mol. Sci. 2020, 21, 5635. [Google Scholar] [CrossRef]
- Tavares, P.; Gonçalves, D.M.; Santos, L.L.; Ferreira, R. Revisiting the Clinical Usefulness of C-Reactive Protein in the Set of Cancer Cachexia. Porto Biomed. J. 2021, 6, e123. [Google Scholar] [CrossRef]
- Van der Burgt Yuri, E.M. Protein Biomarker Discovery is Still Relevant and has Entered a New Phase. EBioMedicine 2019, 43, 15. [Google Scholar] [CrossRef]
- Anderson, N.L.; Ptolemy, A.S.; Rifai, N. The Riddle of Protein Diagnostics: Future Bleak Or Bright? Clin. Chem. 2013, 59, 194–197. [Google Scholar] [CrossRef]
- Argilés, J.M.; López-Soriano, J.; Almendro, V.; Busquets, S.; López-Soriano, F.J. Cross-Talk between Skeletal Muscle and Adipose Tissue: A Link with Obesity? Med. Res. Rev. 2005, 25, 49–65. [Google Scholar] [CrossRef]
- Ebhardt, H.A.; Degen, S.; Tadini, V.; Schilb, A.; Johns, N.; Greig, C.A.; Fearon, K.C.H.; Aebersold, R.; Jacobi, C. Comprehensive Proteome Analysis of Human Skeletal Muscle in Cachexia and Sarcopenia: A Pilot Study. J. Cachexia Sarcopenia Muscle 2017, 8, 567–582. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhang, Y.; Mamtawla, G.; Wan, S.; Gao, X.; Zhang, L.; Li, G.; Wang, X. Iron Overload is Related to Muscle Wasting in Patients with Cachexia of Gastric Cancer: Using Quantitative Proteome Analysis. Med. Oncol. 2020, 37, 113. [Google Scholar] [CrossRef] [PubMed]
- Aniort, J.; Stella, A.; Philipponnet, C.; Poyet, A.; Polge, C.; Claustre, A.; Combaret, L.; Béchet, D.; Attaix, D.; Boisgard, S.; et al. Muscle Wasting in Patients with End-stage Renal Disease or Early-stage Lung Cancer: Common Mechanisms at Work. J. Cachexia Sarcopenia Muscle 2019, 10, 323–337. [Google Scholar] [CrossRef]
- Costa, R.G.F.; Caro, P.L.; Matos-Neto, E.M.; Lima, J.D.C.C.; Radloff, K.; Alves, M.J.; Camargo, R.G.; Pessoa, A.F.M.; Simoes, E.; Gama, P.; et al. Cancer Cachexia Induces Morphological and Inflammatory Changes in the Intestinal Mucosa. J. Cachexia Sarcopenia Muscle 2019, 10, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, A.; Shahda, S.; Kays, J.K.; Perkins, S.M.; Cheng, L.; Schloss, K.N.H.; Schloss, D.E.I.; Koniaris, L.G.; Zimmers, T.A. Identification of Potential Serum Protein Biomarkers and Pathways for Pancreatic Cancer Cachexia using an Aptamer-Based Discovery Platform. Cancers 2020, 12, 3787. [Google Scholar] [CrossRef] [PubMed]
- Neto, N.I.P.; De Pinho Murari, A.S.; Oyama, L.M.; Otoch, J.P.; Alcântara, P.S.M.; Tokeshi, F.; Figuerêdo, R.G.; Alves, M.J.; Lima, J.D.C.C.; Matos-Neto, E.M.D.; et al. Peritumoural Adipose Tissue Pro-Inflammatory Cytokines are Associated with Tumoural Growth Factors in Cancer Cachexia Patients. J. Cachexia Sarcopenia Muscle 2018, 9, 1101–1108. [Google Scholar] [CrossRef]
- Muqaku, B.; Eisinger, M.; Meier, S.M.; Tahir, A.; Pukrop, T.; Haferkamp, S.; Slany, A.; Reichle, A.; Gerner, C. Multi-Omics Analysis of Serum Samples Demonstrates Reprogramming of Organ Functions Via Systemic Calcium Mobilization and Platelet Activation in Metastatic Melanoma. Mol. Cell Proteom. 2017, 16, 86–99. [Google Scholar] [CrossRef]
- Skipworth, R.J.E.; Stewart, G.D.; Bhana, M.; Christie, J.; Sturgeon, C.M.; Guttridge, D.C.; Cronshaw, A.D.; Fearon, K.C.H.; Ross, J.A. Mass Spectrometric Detection of Candidate Protein Biomarkers of Cancer Cachexia in Human Urine. Int. J. Oncol. 2010, 36, 973–982. [Google Scholar] [CrossRef][Green Version]
- Arner, P.; Henjes, F.; Schwenk, J.M.; Darmanis, S.; Dahlman, I.; Iresjö, B.; Naredi, P.; Agustsson, T.; Lundholm, K.; Nilsson, P.; et al. Circulating Carnosine Dipeptidase 1 Associates with Weight Loss and Poor Prognosis in Gastrointestinal Cancer. PLoS ONE 2015, 10, e0123566. [Google Scholar] [CrossRef]
- Gueugneau, M.; Coudy-Gandilhon, C.; Chambon, C.; Verney, J.; Taillandier, D.; Combaret, L.; Polge, C.; Walrand, S.; Roche, F.; Barthélémy, J.; et al. Muscle Proteomic and Transcriptomic Profiling of Healthy Aging and Metabolic Syndrome in Men. Int. J. Mol. Sci. 2021, 22, 4205. [Google Scholar] [CrossRef]
- Bergen, H.R.; Farr, J.N.; Vanderboom, P.M.; Atkinson, E.J.; White, T.A.; Singh, R.J.; Khosla, S.; Lebrasseur, N.K. Myostatin as a Mediator of Sarcopenia Versus Homeostatic Regulator of Muscle Mass: Insights using a New Mass Spectrometry-Based Assay. Skelet. Muscle 2015, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- L’Hôte, C.; Cordier, B.; Labasse, A.; Boileau, C.; Costes, B.; Henrotin, Y. Identification of New Biomarkers for Sarcopenia and Characterization of Cathepsin D Biomarker. JCSM Rapid Commun. 2021, 4, 122–132. [Google Scholar] [CrossRef]
- Lin, C.; Liao, C.; Huang, C.; Tung, Y.; Chang, H.; Hsu, M.; Huang, C. Proteomics Analysis to Identify and Characterize the Biomarkers and Physical Activities of Non-Frail and Frail Older Adults. Int. J. Med. Sci. 2017, 14, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Brocca, L.; Mcphee, J.S.; Longa, E.; Canepari, M.; Seynnes, O.; De Vito, G.; Pellegrino, M.A.; Narici, M.; Bottinelli, R. Structure and Function of Human Muscle Fibres and Muscle Proteome in Physically Active Older Men. J. Physiol. 2017, 595, 4823–4844. [Google Scholar] [CrossRef]
- Ubaida-Mohien, C.; Gonzalez-Freire, M.; Lyashkov, A.; Moaddel, R.; Chia, C.W.; Simonsick, E.M.; Sen, R.; Ferrucci, L. Physical Activity Associated Proteomics of Skeletal Muscle: Being Physically Active in Daily Life may Protect Skeletal Muscle from Aging. Front. Physiol. 2019, 10, 312. [Google Scholar] [CrossRef]
- Lourenço Dos Santos, S.; Baraibar, M.A.; Lundberg, S.; Eeg-Olofsson, O.; Larsson, L.; Friguet, B. Oxidative Proteome Alterations during Skeletal Muscle Ageing. Redox Biol. 2015, 5, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Gueugneau, M.; Coudy-Gandilhon, C.; Gourbeyre, O.; Chambon, C.; Combaret, L.; Polge, C.; Taillandier, D.; Attaix, D.; Friguet, B.; Maier, A.B.; et al. Proteomics of Muscle Chronological Ageing in Post-Menopausal Women. BMC Genom. 2014, 15, 1165. [Google Scholar] [CrossRef] [PubMed]
- Théron, L.; Gueugneau, M.; Coudy, C.; Viala, D.; Bijlsma, A.; Butler-Browne, G.; Maier, A.; Béchet, D.; Chambon, C. Label-Free Quantitative Protein Profiling of Vastus Lateralis Muscle during Human Aging. Mol. Cell Proteom. 2014, 13, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Staunton, L.; Zweyer, M.; Swandulla, D.; Ohlendieck, K. Mass Spectrometry-Based Proteomic Analysis of Middle-Aged Vs. Aged Vastus Lateralis Reveals Increased Levels of Carbonic Anhydrase Isoform 3 in Senescent Human Skeletal Muscle. Int. J. Mol. Med. 2012, 30, 723–733. [Google Scholar] [CrossRef]
- Rittweger, J.; Albracht, K.; Flück, M.; Ruoss, S.; Brocca, L.; Longa, E.; Moriggi, M.; Seynnes, O.; Di Giulio, I.; Tenori, L.; et al. Sarcolab Pilot Study into Skeletal Muscle’s Adaptation to Long-Term Spaceflight. NPJ Microgravity 2018, 4, 18. [Google Scholar] [CrossRef]
- Capri, M.; Morsiani, C.; Santoro, A.; Moriggi, M.; Conte, M.; Martucci, M.; Bellavista, E.; Fabbri, C.; Giampieri, E.; Albracht, K.; et al. Recovery from 6-month Spaceflight at the International Space Station: Muscle-related Stress into a Proinflammatory Setting. FASEB J. 2019, 33, 5168–5180. [Google Scholar] [CrossRef] [PubMed]
- Husi, H.; MacDonald, A.; Skipworth, R.J.E.; Miller, J.; Cronshaw, A.; Greig, C.; Fearon, K.C.H.; Ross, J.A. Urinary Diagnostic Proteomic Markers for Dynapenia in Cancer Patients. Biomed. Rep. 2018, 8, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Lakhdar, R.; Drost, E.M.; MacNee, W.; Bastos, R.; Rabinovich, R.A. 2D-DIGE Proteomic Analysis of Vastus Lateralis from COPD Patients with Low and Normal Fat Free Mass Index and Healthy Controls. Respir. Res. 2017, 18, 81. [Google Scholar] [CrossRef] [PubMed]
- Husi, H.; MacDonald, A.; Skipworth, R.J.E.; Miller, J.; Cronshaw, A.; Fearon, K.C.H.; Ross, J.A. Proteomic Identification of Potential Markers of Myosteatosis in Human Urine. Biomed. Rep. 2018, 8, 557–564. [Google Scholar] [CrossRef]
- Wiedmer, P.; Jung, T.; Castro, J.P.; Pomatto, L.C.D.; Sun, P.Y.; Davies, K.J.A.; Grune, T. Sarcopenia—Molecular Mechanisms and Open Questions. Ageing Res. Rev. 2021, 65, 101200. [Google Scholar] [CrossRef]
- Argilés, J.M.; Campos, N.; Lopez-Pedrosa, J.; Rueda, R.; Rodriguez-Mañas, L. Skeletal Muscle Regulates Metabolism Via Interorgan Crosstalk: Roles in Health and Disease. J. Am. Med. Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef]
- Van Gassel Robert, J.J.; Baggerman, M.R.; van de Poll Marcel, C.G. Metabolic Aspects of Muscle Wasting during Critical Illness. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 96–101. [Google Scholar] [CrossRef]
- Renzini, A.; Riera, C.S.; Minic, I.; D’Ercole, C.; Lozanoska-Ochser, B.; Cedola, A.; Gigli, G.; Moresi, V.; Madaro, L. Metabolic Remodeling in Skeletal Muscle Atrophy as a Therapeutic Target. Metabolites 2021, 11, 517. [Google Scholar] [CrossRef]
- Gingrich, A.; Volkert, D.; Kiesswetter, E.; Thomanek, M.; Bach, S.; Sieber, C.C.; Zopf, Y. Prevalence and Overlap of Sarcopenia, Frailty, Cachexia and Malnutrition in Older Medical Inpatients. BMC Geriatr. 2019, 19, 120. [Google Scholar] [CrossRef]
- Ali, S.; Garcia, J.M. Sarcopenia, Cachexia and Aging: Diagnosis, Mechanisms and Therapeutic Options—A Mini-Review. Gerontology 2014, 60, 294–305. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cachexia and Sarcopenia: Mechanisms and Potential Targets for Intervention. Curr. Opin. Pharmacol. 2015, 22, 100–106. [Google Scholar] [CrossRef]
- Rolland, Y.; Van Kan, G.A.; Gillette-Guyonnet, S.; Vellas, B. Cachexia Versus Sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.H.; Glass, D.; Guttridge, D. Cancer Cachexia: Mediators, Signaling, and Metabolic Pathways. Cell Metab. 2012, 16, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Porporato, P.E. Understanding Cachexia as a Cancer Metabolism Syndrome. Oncogenesis 2016, 5, e200. [Google Scholar] [CrossRef] [PubMed]
- Loumaye, A.; Thissen, J. Biomarkers of Cancer Cachexia. Clin. Biochem. 2017, 50, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zhao, K.; Jose, I.; Hoogenraad, N.J.; Osellame, L.D. Biomarkers for Cancer Cachexia: A Mini Review. Int. J. Mol. Sci. 2021, 22, 4501. [Google Scholar] [CrossRef]
- Schiliro, C.; Firestein, B.L. Mechanisms of Metabolic Reprogramming in Cancer Cells Supporting Enhanced Growth and Proliferation. Cells 2021, 10, 1056. [Google Scholar] [CrossRef]
- Doru, P. The Systemic Hallmarks of Cancer. J. Cancer Metastatis Treat. 2020, 6, 29. [Google Scholar] [CrossRef]
- Pavlides, S.; Whitaker-Menezes, D.; Castello-Cros, R.; Flomenberg, N.; Witkiewicz, A.K.; Frank, P.G.; Casimiro, M.C.; Wang, C.; Fortina, P.; Addya, S.; et al. The Reverse Warburg Effect: Aerobic Glycolysis in Cancer Associated Fibroblasts and the Tumor Stroma. Cell Cycle 2009, 8, 3984–4001. [Google Scholar] [CrossRef]
- San-Millán, I.; Brooks, G.A. Reexamining Cancer Metabolism: Lactate Production for Carcinogenesis could be the Purpose and Explanation of the Warburg Effect. Carcinogenesis 2017, 38, 119–133. [Google Scholar] [CrossRef]
- Benny, S.; Mishra, R.; Manojkumar, M.K.; Aneesh, T.P. From Warburg Effect to Reverse Warburg Effect; the New Horizons of Anti-Cancer Therapy. Med. Hypotheses 2020, 144, 110216. [Google Scholar] [CrossRef]
- Mannelli, M.; Gamberi, T.; Magherini, F.; Fiaschi, T. A Metabolic Change Towards Fermentation Drives Cancer Cachexia in Myotubes. Biomedicines 2021, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Marceca, G.P.; Londhe, P.; Calore, F. Management of Cancer Cachexia: Attempting to Develop New Pharmacological Agents for New Effective Therapeutic Options. Front. Oncol. 2020, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Pisitkun, T.; Johnstone, R.; Knepper, M.A. Discovery of Urinary Biomarkers. Mol. Cell Proteom. 2006, 5, 1760–1771. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Edwards, S.; Smeuninx, B.; McKendry, J.; Nishimura, Y.; Perkins, M.; Philp, A.; Joanisse, S.; Breen, L. Immobilization Leads to Alterations in Intracellular Phosphagen and Creatine Transporter Content in Human Skeletal Muscle. Am. J. Physiol. Cell Physiol. 2020, 319, C34–C44. [Google Scholar] [CrossRef]
- Murgia, M.; Toniolo, L.; Nagaraj, N.; Ciciliot, S.; Vindigni, V.; Schiaffino, S.; Reggiani, C.; Mann, M. Single Muscle Fiber Proteomics Reveals Fiber-Type-Specific Features of Human Muscle Aging. Cell Rep. 2017, 19, 2396–2409. [Google Scholar] [CrossRef]
- Kramer, I.F.; Snijders, T.; Smeets, J.S.J.; Leenders, M.; van Kranenburg, J.; den Hoed, M.; Verdijk, L.B.; Poeze, M.; van Loon Luc, J.C. Extensive Type II Muscle Fiber Atrophy in Elderly Female Hip Fracture Patients. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1369–1375. [Google Scholar] [CrossRef]
- Tanganelli, F.; Meinke, P.; Hofmeister, F.; Jarmusch, S.; Baber, L.; Mehaffey, S.; Hintze, S.; Ferrari, U.; Neuerburg, C.; Kammerlander, C.; et al. Type-2 Muscle Fiber Atrophy is Associated with Sarcopenia in Elderly Men with Hip Fracture. Exp. Gerontol. 2021, 144, 111171. [Google Scholar] [CrossRef]
- Curtis, J.M.; Hahn, W.S.; Long, E.K.; Burrill, J.S.; Arriaga, E.A.; Bernlohr, D.A. Protein Carbonylation and Metabolic Control Systems. Trends Endocrinol. Metab. 2012, 23, 399–406. [Google Scholar] [CrossRef]
- Gonos, E.S.; Kapetanou, M.; Sereikaite, J.; Bartosz, G.; Naparło, K.; Grzesik, M.; Sadowska-Bartosz, I. Origin and Pathophysiology of Protein Carbonylation, Nitration and Chlorination in Age-related Brain Diseases and Aging. Aging 2018, 10, 868–901. [Google Scholar] [CrossRef]
- Heck, T.G.; Scomazzon, S.P.; Ludwig, M.S.; de Bittencourt, H.P.I., Jr. Role of heat shock proteins in skeletal muscle. In Skeletal Muscle: From Myogenesis to Clinical Relations; Cseri, J., Ed.; IntechOpen: London, UK, 2012; pp. 105–122. [Google Scholar]
- Calderwood, S.K.; Murshid, A.; Prince, T. The Shock of Aging: Molecular Chaperones and the Heat Shock Response in Longevity and Aging—A Mini-Review. Gerontology 2009, 55, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Baraibar, M.A.; Hyzewicz, J.; Rogowska-Wrzesinska, A.; Bulteau, A.; Prip-Buus, C.; Butler-Browne, G.; Friguet, B. Impaired Energy Metabolism of Senescent Muscle Satellite Cells is Associated with Oxidative Modifications of Glycolytic Enzymes. Aging 2016, 8, 3375–3388. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Caldwell, M.E.; Greene, N.P. Muscle Metabolism and Atrophy: Let’s Talk about Sex. Biol. Sex Differ. 2019, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.J.; Liu, H.; Garcia, J.M. Sex Differences in Muscle Wasting. In Sex and Gender Factors Affecting Metabolic Homeostasis, Diabetes and Obesity. Advances in Experimental Medicine and Biology; Mauvais-Jarvis, F., Ed.; Springer International Publishing: Cham, Switzerland, 2017; Volume 1043, pp. 153–197. [Google Scholar] [CrossRef]
- Laakkonen, E.K.; Soliymani, R.; Karvinen, S.; Kaprio, J.; Kujala, U.M.; Baumann, M.; Sipilä, S.; Kovanen, V.; Lalowski, M. Estrogenic Regulation of Skeletal Muscle Proteome: A Study of Premenopausal Women and Postmenopausal MZ Cotwins Discordant for Hormonal Therapy. Aging Cell 2017, 16, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.C.; Laakkonen, E.K.; Lowe, D.A. Aging of the Musculoskeletal System: How the Loss of Estrogen Impacts Muscle Strength. Bones 2019, 123, 137–144. [Google Scholar] [CrossRef]
- Miller, M.S.; Bedrin, N.G.; Callahan, D.M.; Previs, M.J.; Jennings, M.E., II; Ades, P.A.; Maughan, D.W.; Palmer, B.M.; Toth, M.J. Age-Related Slowing of Myosin Actin Cross-Bridge Kinetics is Sex Specific and Predicts Decrements in Whole Skeletal Muscle Performance in Humans. J. Appl. Physiol. 2013, 115, 1004–1014. [Google Scholar] [CrossRef]
- Takada, S.; Masaki, Y.; Kinugawa, S.; Matsumoto, J.; Furihata, T.; Mizushima, W.; Kadoguchi, T.; Fukushima, A.; Homma, T.; Takahashi, M.; et al. Dipeptidyl Peptidase-4 Inhibitor Improved Exercise Capacity and Mitochondrial Biogenesis in Mice with Heart Failure Via Activation of Glucagon-Like Peptide-1 Receptor Signalling. Cardiovasc. Res. 2016, 111, 338–347. [Google Scholar] [CrossRef]
- Ishii, S.; Nagai, Y.; Kato, H.; Fukuda, H.; Tanaka, Y. Effect of the Dipeptidyl Peptidase-4 Inhibitor Sitagliptin on Muscle Mass and the Muscle/Fat Ratio in Patients with Type 2 Diabetes. J. Clin. Med. Res. 2020, 12, 122–126. [Google Scholar] [CrossRef]
- Kalaitzoglou, E.; Fowlkes, J.L.; Popescu, I.; Thrailkill, K.M. Diabetes Pharmacotherapy and Effects on the Musculoskeletal System. Diabetes Metab. Res. Rev. 2019, 35, e3100. [Google Scholar] [CrossRef]
- Izumi, K.; Iwamoto, H.; Yaegashi, H.; Nohara, T.; Shigehara, K.; Kadono, Y.; Nanjo, S.; Yamada, T.; Ohtsubo, K.; Yano, S.; et al. Androgen Replacement Therapy for Cancer-related Symptoms in Male: Result of Prospective Randomized Trial (ARTFORM Study). J. Cachexia Sarcopenia Muscle 2021, 12, 831–842. [Google Scholar] [CrossRef]
- Rich-Edwards, J.W.; Kaiser, U.B.; Chen, G.L.; Manson, J.E.; Goldstein, J.M. Sex and Gender Differences Research Design for Basic, Clinical, and Population Studies: Essentials for Investigators. Endocr. Rev. 2018, 39, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Buehring, B.; Siglinsky, E.; Krueger, D.; Evans, W.; Hellerstein, M.; Yamada, Y.; Binkley, N. Comparison of Muscle/Lean Mass Measurement Methods: Correlation with Functional and Biochemical Testing. Osteoporos Int. 2017, 29, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Huot, J.R.; Pin, F.; Bonetto, A. Muscle Weakness Caused by Cancer and Chemotherapy is Associated with Loss of Motor Unit Connectivity. Am. J. Cancer Res. 2021, 11, 2990–3001. [Google Scholar] [PubMed]
- Jarmusch, S.; Baber, L.; Bidlingmaier, M.; Ferrari, U.; Hofmeister, F.; Hintze, S.; Mehaffey, S.; Meinke, P.; Neuerburg, C.; Schoser, B.; et al. Influence of IGF-I Serum Concentration on Muscular Regeneration Capacity in Patients with Sarcopenia. BMC Musculoskelet. Disord. 2021, 22, 807. [Google Scholar] [CrossRef] [PubMed]
- Gubert, C.; Hannan, A.J. Exercise Mimetics: Harnessing the Therapeutic Effects of Physical Activity. Nat. Rev. Drug Discov. 2021, 20, 862–879. [Google Scholar] [CrossRef]
- Narkar, V.A.; Downes, M.; Yu, R.T.; Embler, E.; Wang, Y.; Banayo, E.; Mihaylova, M.M.; Nelson, M.C.; Zou, Y.; Juguilon, H.; et al. AMPK and PPARδ Agonists are Exercise Mimetics. Cell 2008, 134, 405–415. [Google Scholar] [CrossRef]
- Chen, Z.; Li, B.; Zhan, R.; Rao, L.; Bursac, N. Exercise Mimetics and JAK Inhibition Attenuate IFN-Γ-Induced Wasting in Engineered Human Skeletal Muscle. Sci. Adv. 2021, 7, eabd9502. [Google Scholar] [CrossRef]
- Burgess, L.C.; Taylor, P.; Wainwright, T.W.; Bahadori, S.; Swain, I.D. Adherence to Neuromuscular Electrical Stimulation Interventions for Muscle Impairment in Hip and Knee Osteoarthritis: A Systematic Review. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2021, 14, 11795441211028746. [Google Scholar] [CrossRef]
- Sanchis-Gomar, F.; Lopez-Lopez, S.; Romero-Morales, C.; Maffulli, N.; Lippi, G.; Pareja-Galeano, H. Neuromuscular Electrical Stimulation: A New Therapeutic Option for Chronic Diseases Based on Contraction-Induced Myokine Secretion. Front. Physiol. 2019, 10, 1463. [Google Scholar] [CrossRef]
- Haraguchi, M.; Ichinose, K.; Miyaaki, H.; Hanada, M.; Fukushima, M.; Sasaki, R.; Miuma, S.; Hara, T.; Kugiyama, T.; Soyama, A.; et al. Comparative Study of the Effect of Neuromuscular Electrical Stimulation and Oral Administration of Branched-Chain Amino Acid on Preventing Sarcopenia in Patients After Living-Donor Liver Transplantation: Study Protocol for an Open-Label Randomized Controlled Trial. Trials 2021, 22, 137. [Google Scholar] [CrossRef]


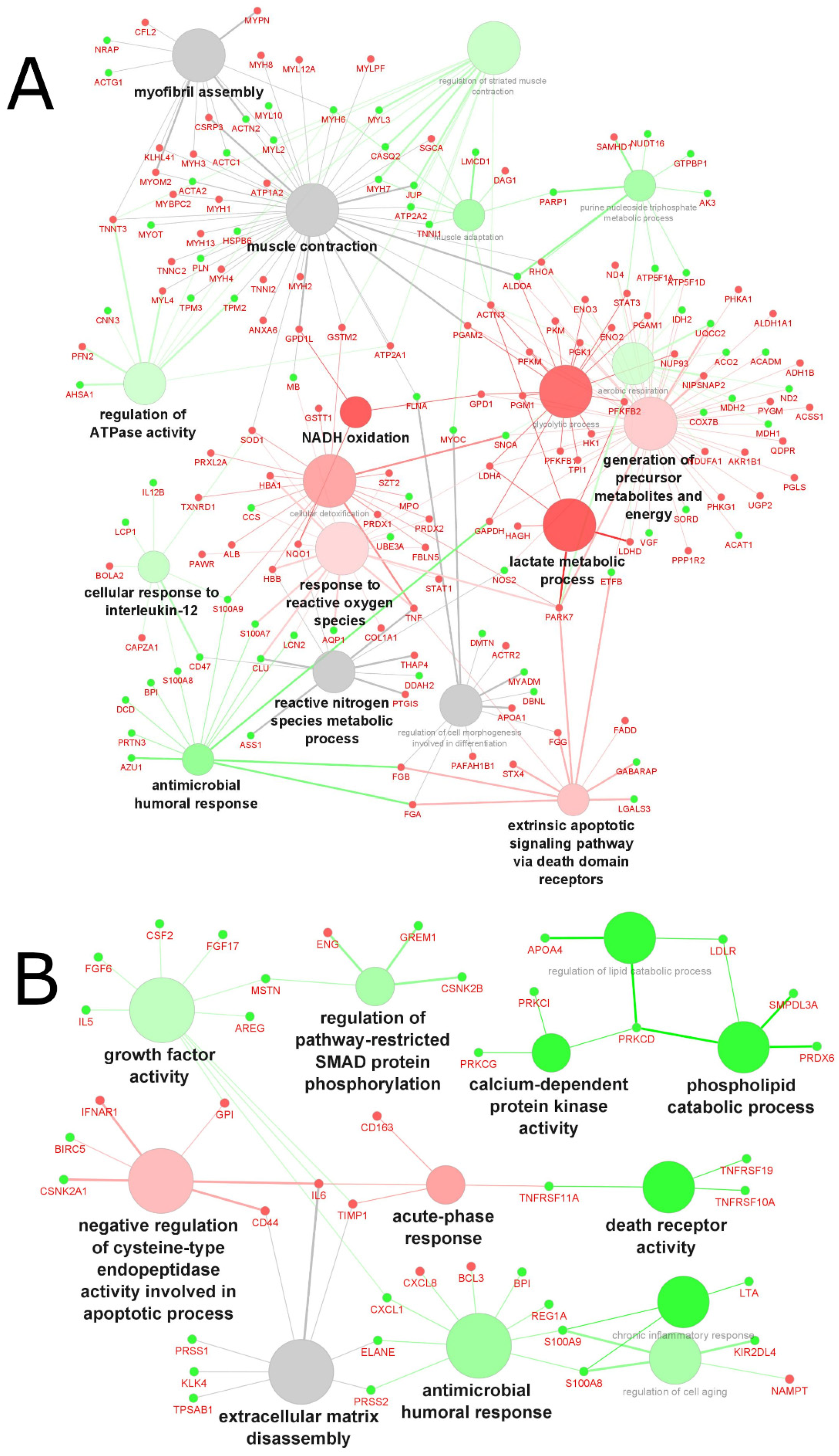
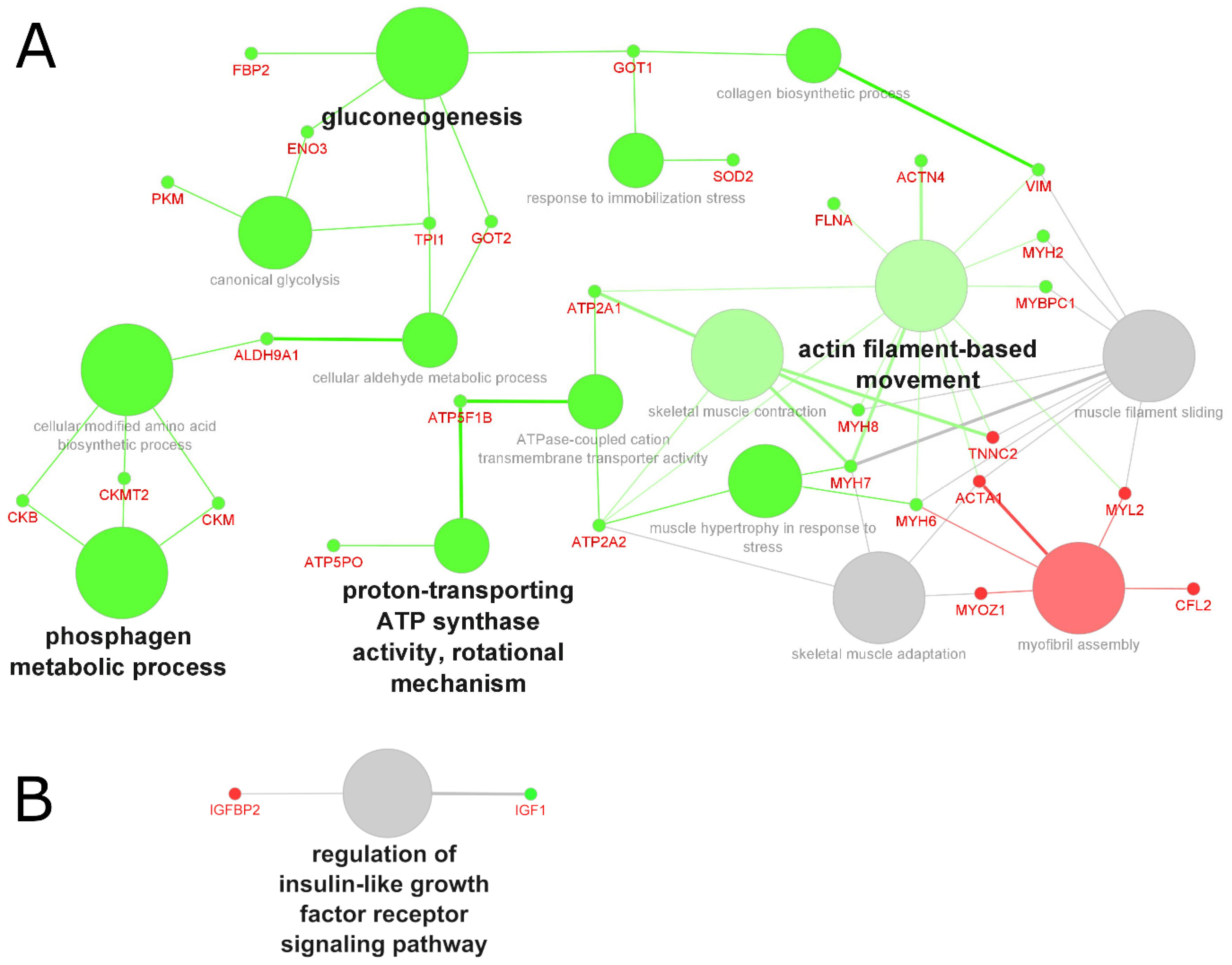
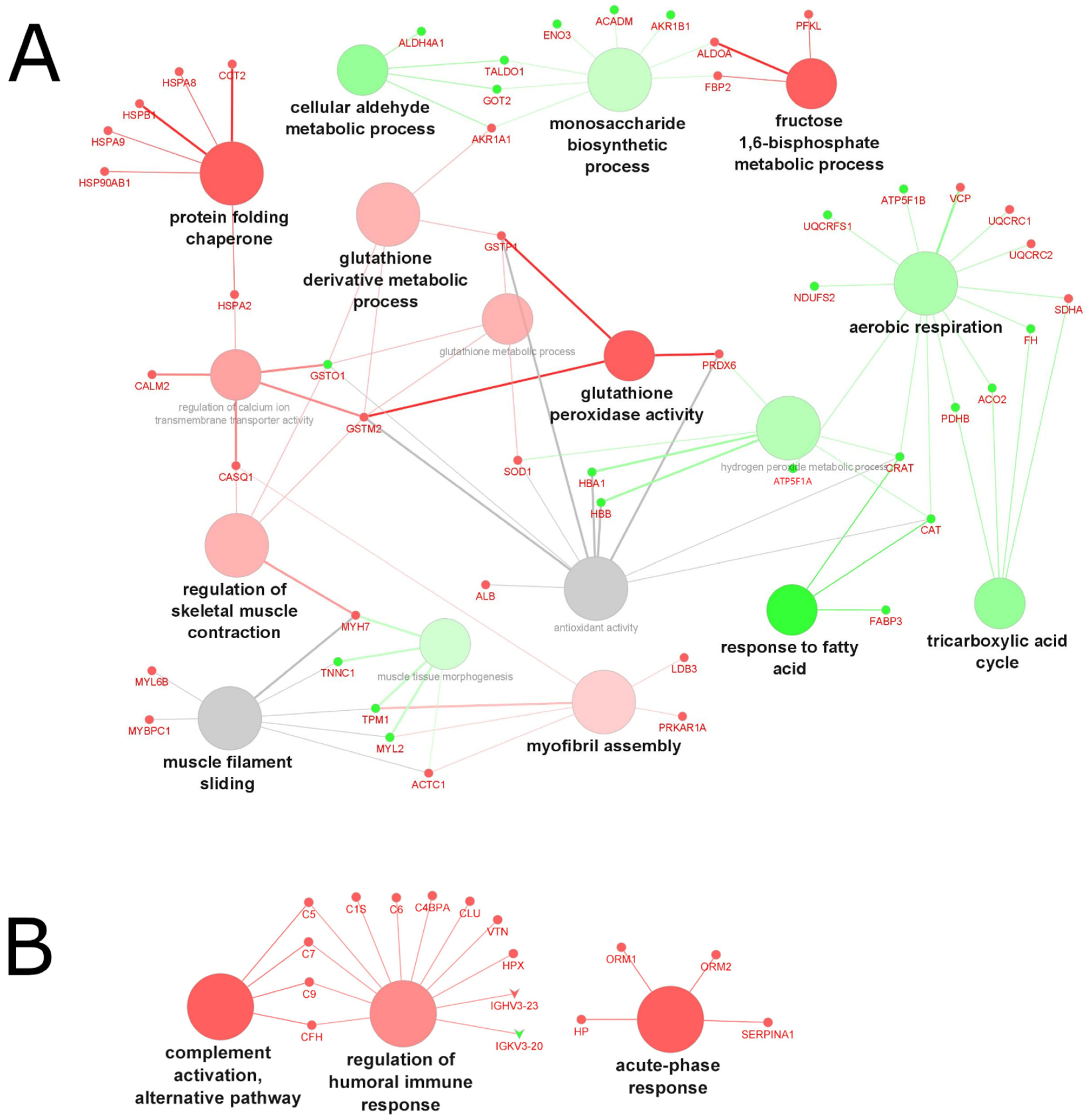
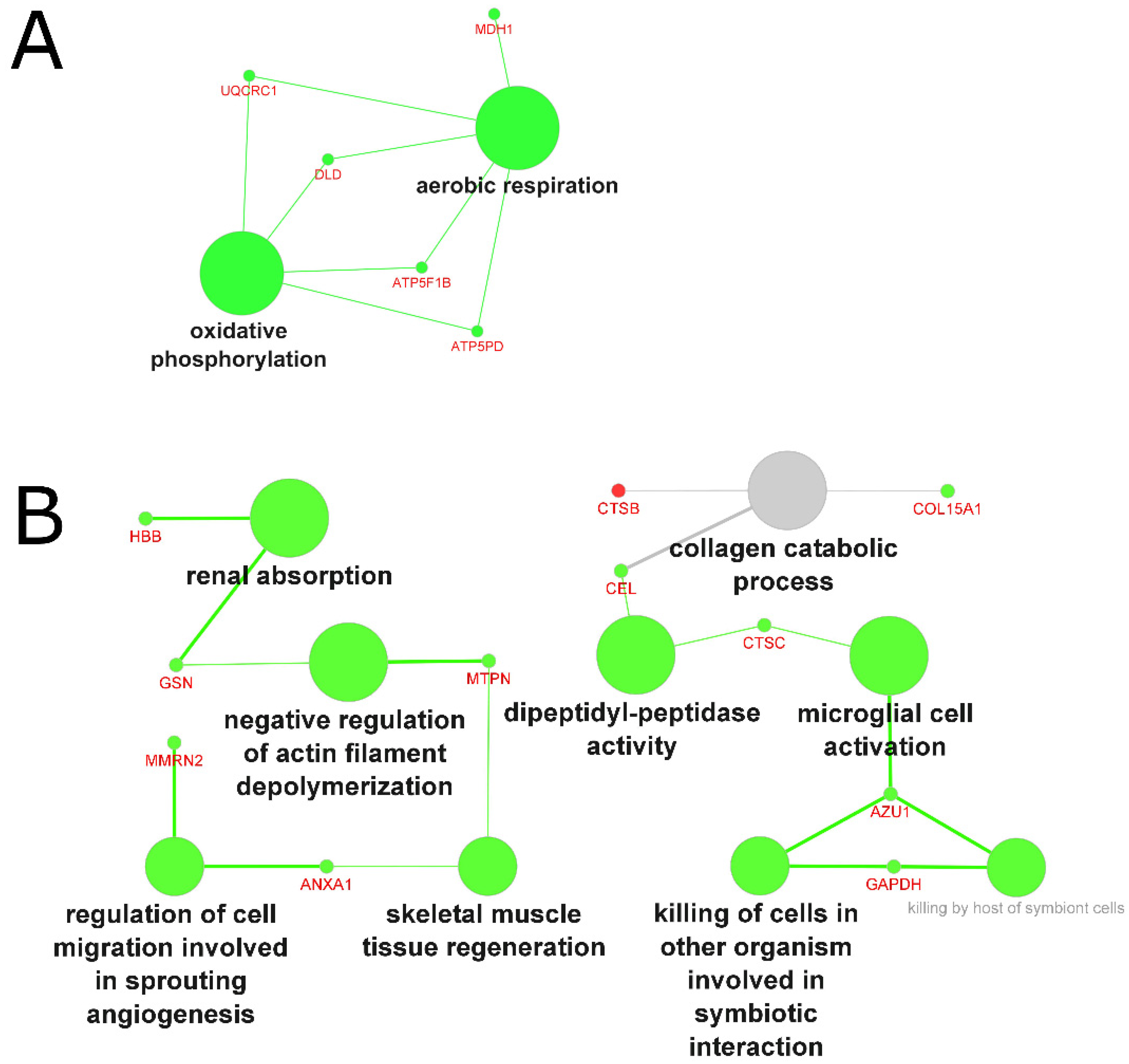
| Study | Muscle Atrophy Condition | N | Groups | Tissue Analyzed |
|---|---|---|---|---|
| Ebhardt et al. (2017) [21] | Cachexia | 19 | Cachectic (n = 5) Non-cachectic (n = 14) | Quadriceps muscle biopsy |
| Zhou et al. (2020) [22] | Cachexia | 23 | Cachexia with sarcopenia (n = 13) Normal weight (n = 10) | Abdominal muscle biopsies |
| Aniort et al. (2019) [23] | Cachexia | 21 | Early-stage lung cancer (LC, n = 7) Chronic hemodialysis (HD, n = 7) Healthy volunteers (CT, n = 7) | Muscle biopsies (LC: latissimus dorsi; HD and CT: vastus lateralis) |
| Costa et al. (2019) [24] | Cachexia | 45 | Cachectic (n = 25) Weight stable (n = 20) | Plasma |
| Narasimhan et al. (2020) [25] | Cachexia | 29 | Weight loss (n = 23) Weight stable (n = 6) | Serum |
| Neto et al. (2018) [26] | Cachexia | 16 | Cachectic (n = 9) Weight stable (n = 7) | Peritumoral adipose tissue |
| Muqaku et al. (2017) [27] | Cachexia | 9 | Melanoma (n = 6) Healthy (n = 3) | Serum |
| Skipworth et al. (2010) [28] | Cachexia | 16 | Weight-stable (n = 8) Weight-loss (n = 8) | Urine |
| Arner et al. (2015) [29] | Cachexia | 59 | Weight-stable (n = 27) Weight-loss (n = 32) | Plasma |
| Ebhardt et al. (2017) [21] | Sarcopenia | 18 | Sarcopenic (n = 8) Non-sarcopenic (n = 10) | Quadriceps muscle biopsy |
| Gueugneau et al. (2021) [30] | Sarcopenia (metabolic syndrome)/aging | 39 | Healthy young (n = 15) Healthy old (n = 15) Old with metabolic syndrome (n = 9) | Vastus lateralis muscle biopsy |
| Bergen et al. (2015) [31] | Sarcopenia | 240 | Young (20–40y, n = 80) Old with normal rASM (>65y, n = 80) Old with low rASM (>65y, n = 80) | Serum |
| L’hôte et al. (2021) [32] | Sarcopenia | 20 | Control and pre-sarcopenia (n = 10) Sarcopenia and severe sarcopenia (n = 10) | Serum |
| Lin et al. (2017) [33] | Frailty/ Aging | 12 | Frail (n = 6) Non-frail (n = 6) | Serum |
| Brocca et al. (2017) [34] | Aging | 20 | Elderly (70.9y, n = 10) Young control (23.0y, n = 10) | Vastus lateralis muscle biopsies |
| Ubaida-Mohien et al. (2019) [35] | Aging | 58 | 20–34 years (n = 13) 35–49 years (n = 11) 50–64 years (n = 12) 65–79 years (n = 12) 80+ years (n = 10) | Vastus lateralis muscle biopsy |
| Lourenço Dos Santos et al. (2015) [36] | Aging | 22 | Young healthy (0–12y, n = 11) Old healthy (52–76y, n = 11) | Rectus abdominis muscle biopsies |
| Gueugneau et al. (2014) [37] | Aging | 24 | Mature women (48–61y, n = 11) Older women (76–82y, n = 13) | Vastus lateralis muscle |
| Théron et al. (2014) [38] | Aging | 10 | Mature healthy (53.0y, n = 6) Old healthy (77.6y, n = 4) | Vastus lateralis muscle |
| Staunton et al. (2012) [39] | Aging | 8 | Middle aged (47–62y, n = 4) Older (76–82y, n = 4) | Vastus lateralis muscle biopsies |
| Rittweger et al. (2018) [40] | Muscle wasting | 2 | Crew members of the ISS assessed pre and post 6 month stay in space | Skeletal (soleus) muscle |
| Capri et al. (2019) [41] | Muscle wasting | 2 | Crew members of the ISS assessed pre and post 6 month stay in space | Muscle biopsy |
| Husi et al. (2018) [42] | Muscle wasting | 49 | Low strength (22/49) Low power (23/42) Low strength and power (n = 13) | Urine |
| Lakhdar et al. (2017) [43] | Muscle wasting | 27 | COPD with low FFMI, (n = 10) COPD with normal FFMI (n = 8) Matched healthy controls (n = 9) | Vastus lateralis muscle of COPD patients |
| Husi et al. (2018) [44] | Muscle wasting | 55 | Myosteatotic (n = 31) Non-myosteatotic (n = 24) | Urine |
| Cachexia | Sarcopenia | Muscle Wasting | Aging | |||||
|---|---|---|---|---|---|---|---|---|
| Tissue * | Biofluid † | Tissue * | Biofluid † | Tissue * | Biofluid † | Tissue * | Biofluid † | |
| Number of studies | 4 | 5 | 2 | 2 | 3 | 2 | 6 | 1 |
| Total number of proteins | 391 | 97 | 41 | 4 | 20 | 26 | 113 | 37 |
| Number of proteins overlapping across studies | 21 | 1 | 3 | 0 | 0 | 1 | 34 | NA |
| Number of overlapping proteins with same directionality | 17 | 0 | 3 | 0 | 0 | 1 | 15 | NA |
| Atrophy Condition | Proteins in Tissue Samples * | Proteins in Biofluid Samples * |
|---|---|---|
| Cachexia | S100A8, ENO3, PKM, MYH6, ATP2A1, TNNC2, GAPDH, ACTA2, TPM1, PGK1, ANXA6, PFKM, AK1, MYBPC2, TPD52L2, ACTBL2, SERBP1, CDS2, STT3B, GMPR, FBLN5 (21/391) | SPTAN1 (1/97) |
| Sarcopenia | HSPB1, GOT1, MYL2 (3/41) | – (0/4) |
| Muscle wasting | – (0/20) | GFAP (1/26) |
| Aging | CKM, PYGM, CA3, ACTA1, HSPB6, TNNT3, ANXA5, TNNT1, MYL1, ANKRD2, GAPDH, PKM, ENO3, MYL2, ACTC1, ALDOA, PRDX2, MYH1, LDHB, TPI1, PGM1, PARK7, GPD1, MYOZ1, ATP5B, DLDH, CRYAB, COX5A, PRDX3, ALDH2, FH, TTN, FABP4, TF (34/113) | – (0/37) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stalmach, A.; Boehm, I.; Fernandes, M.; Rutter, A.; Skipworth, R.J.E.; Husi, H. Gene Ontology (GO)-Driven Inference of Candidate Proteomic Markers Associated with Muscle Atrophy Conditions. Molecules 2022, 27, 5514. https://doi.org/10.3390/molecules27175514
Stalmach A, Boehm I, Fernandes M, Rutter A, Skipworth RJE, Husi H. Gene Ontology (GO)-Driven Inference of Candidate Proteomic Markers Associated with Muscle Atrophy Conditions. Molecules. 2022; 27(17):5514. https://doi.org/10.3390/molecules27175514
Chicago/Turabian StyleStalmach, Angelique, Ines Boehm, Marco Fernandes, Alison Rutter, Richard J. E. Skipworth, and Holger Husi. 2022. "Gene Ontology (GO)-Driven Inference of Candidate Proteomic Markers Associated with Muscle Atrophy Conditions" Molecules 27, no. 17: 5514. https://doi.org/10.3390/molecules27175514
APA StyleStalmach, A., Boehm, I., Fernandes, M., Rutter, A., Skipworth, R. J. E., & Husi, H. (2022). Gene Ontology (GO)-Driven Inference of Candidate Proteomic Markers Associated with Muscle Atrophy Conditions. Molecules, 27(17), 5514. https://doi.org/10.3390/molecules27175514









