Effect of ZnO Nanofiller on Structural and Electrochemical Performance Improvement of Solid Polymer Electrolytes Based on Polyvinyl Alcohol–Cellulose Acetate–Potassium Carbonate Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PVA–CA–K2CO3/ZnO-NPs Composite
2.3. Physicochemical Characterization
2.4. Electrochemical Characterization
3. Results
3.1. Physicochemical Characterization
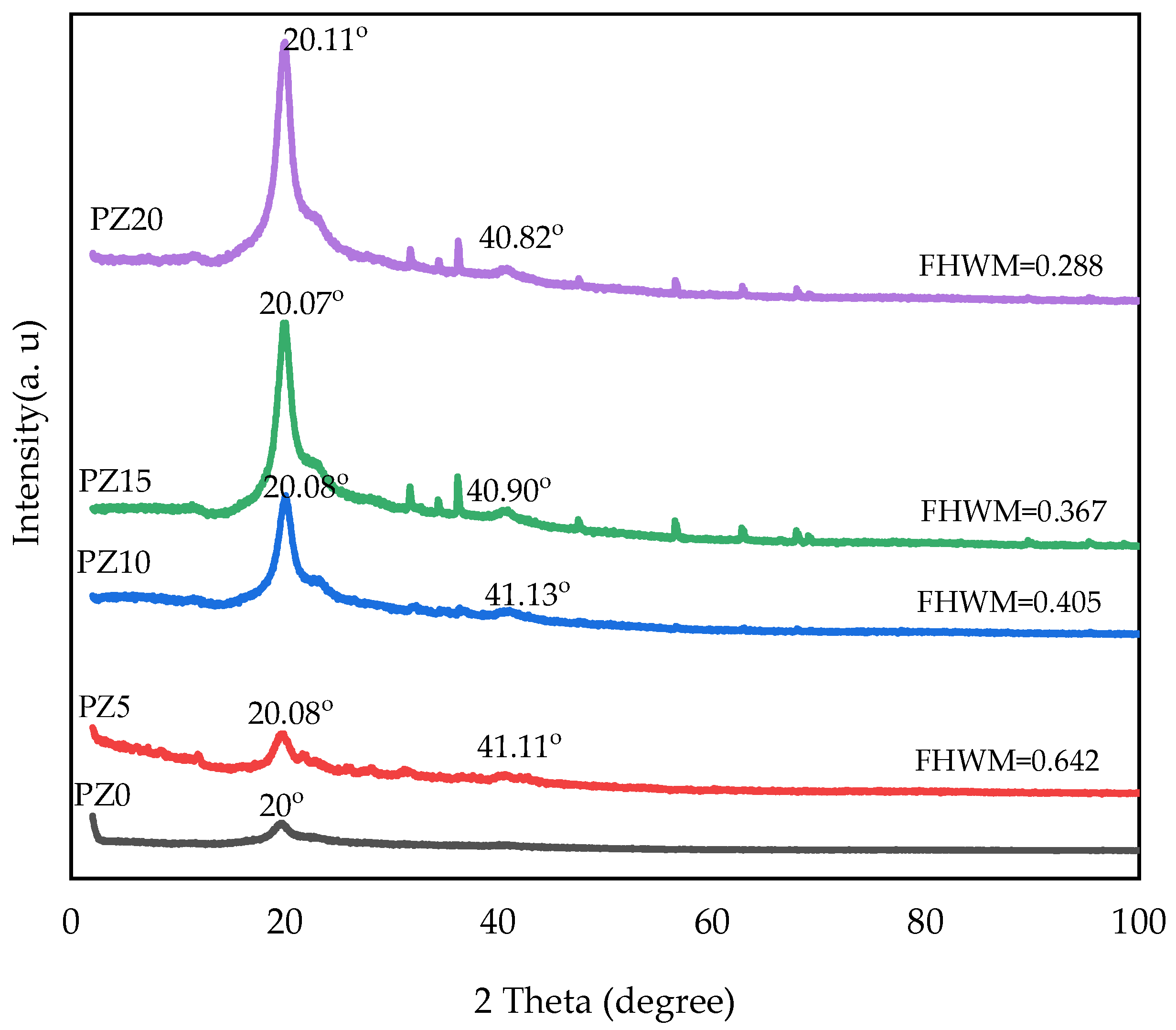
3.1.1. Crystal Phase for PVA–CA–K2CO3/ZnO-NPs
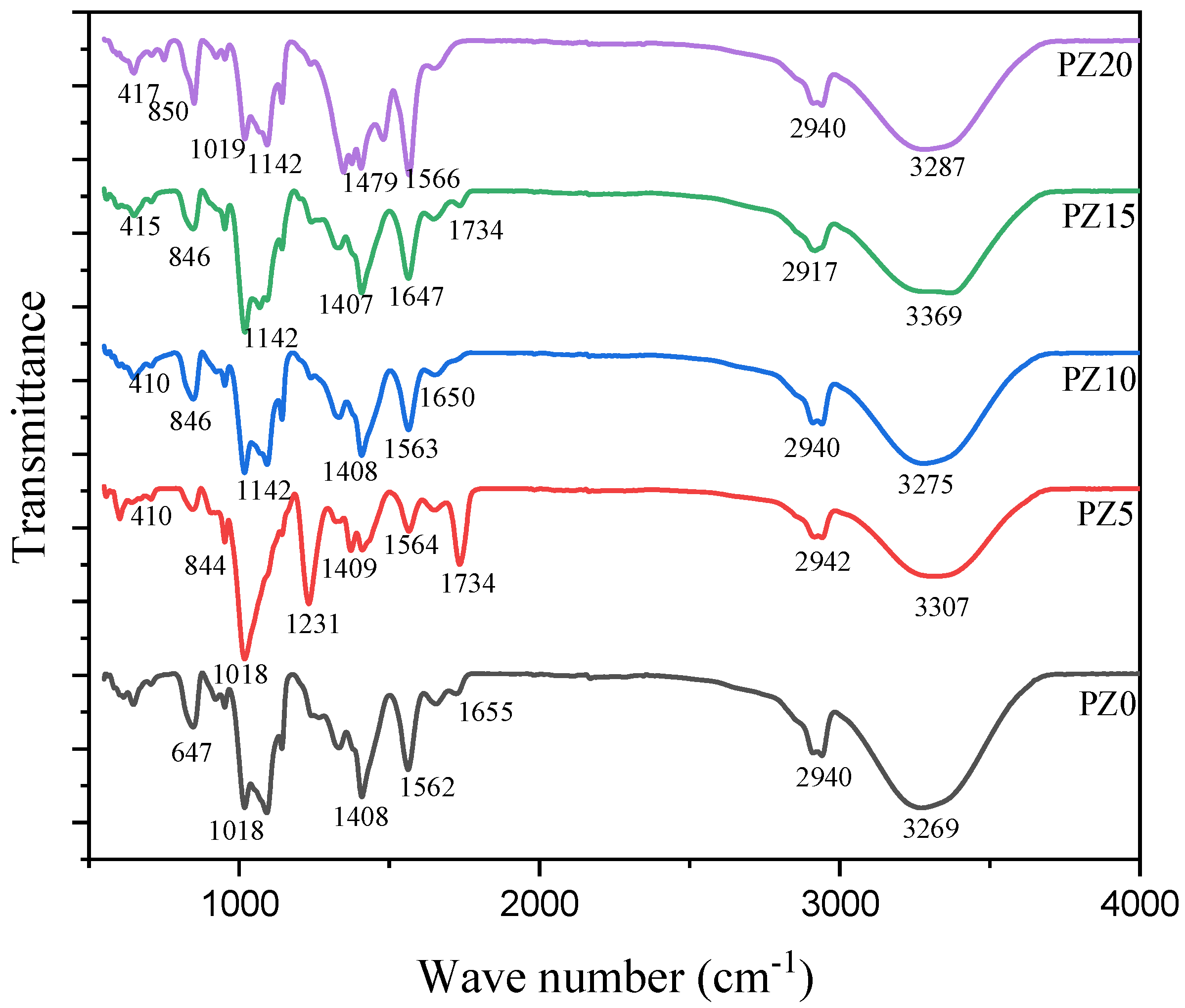
3.1.2. FTIR Analysis of PVA–CA–K2CO3/ZnO-NPs
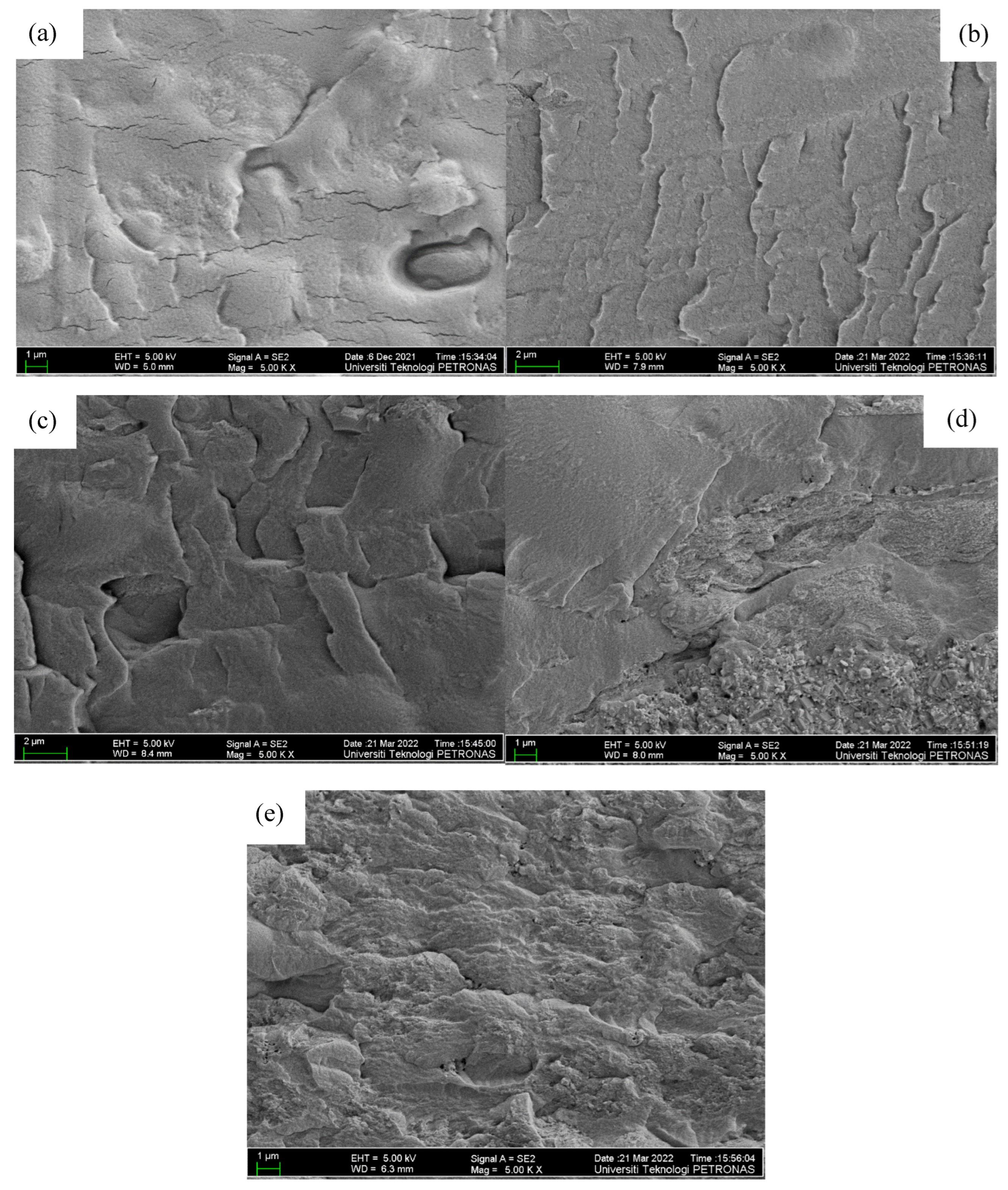
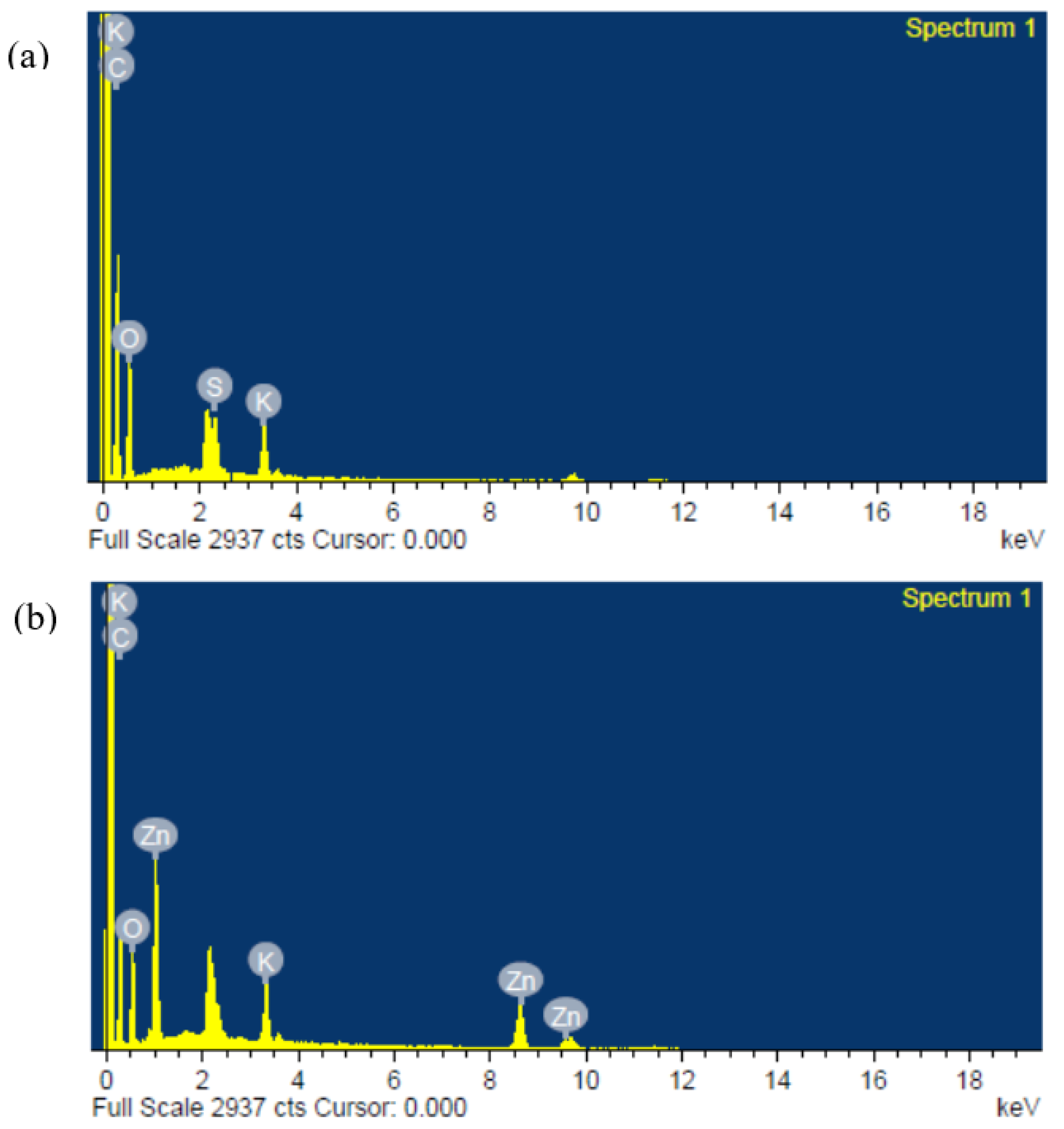
3.1.3. Morphological and Structural Analysis of PVA–CA–K2CO3/ZnO-NPs
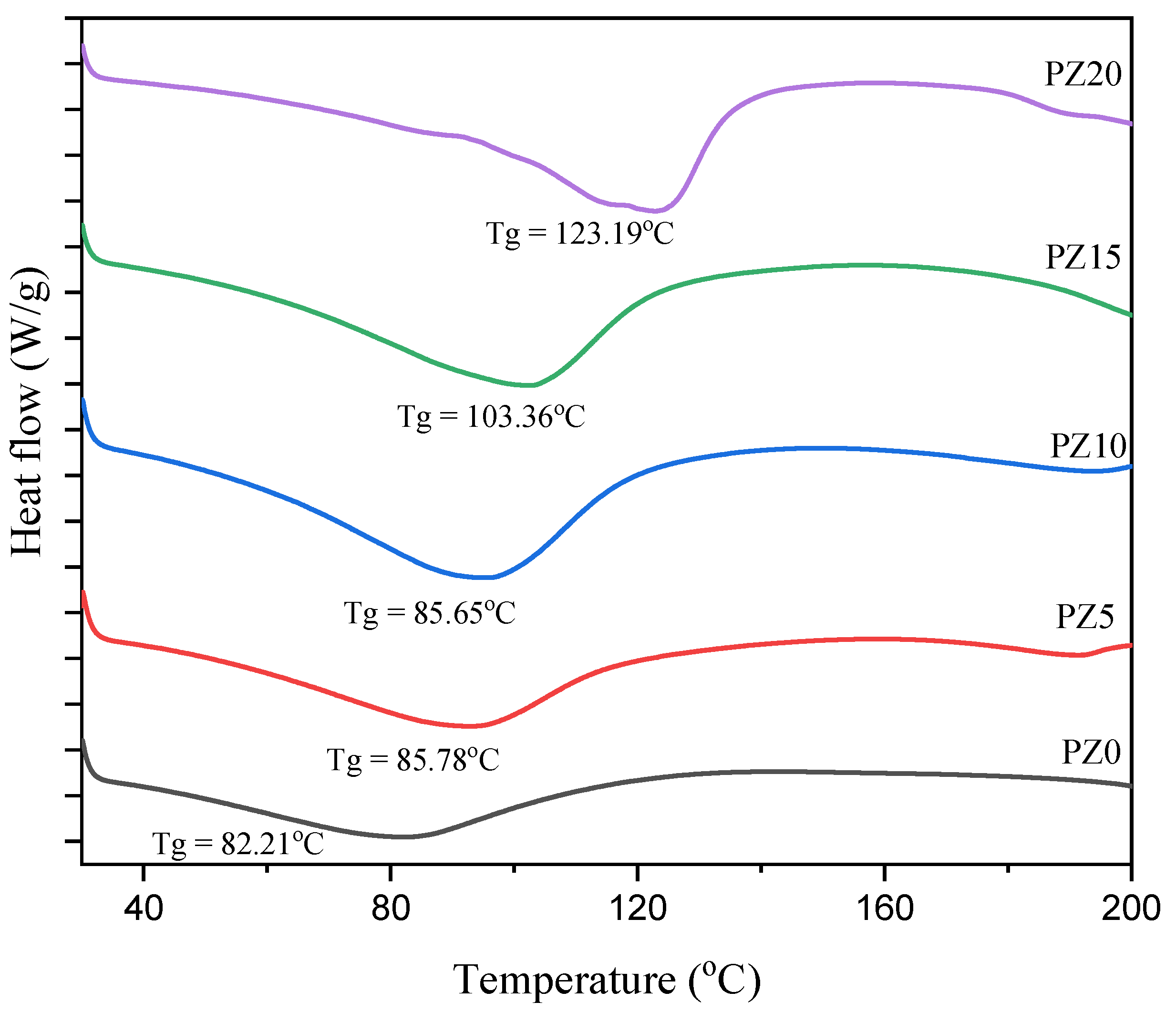
3.1.4. Glass Transition Temperature of PVA–CA–K2CO3/ZnO-NPs
3.2. Electrochemical Characterization
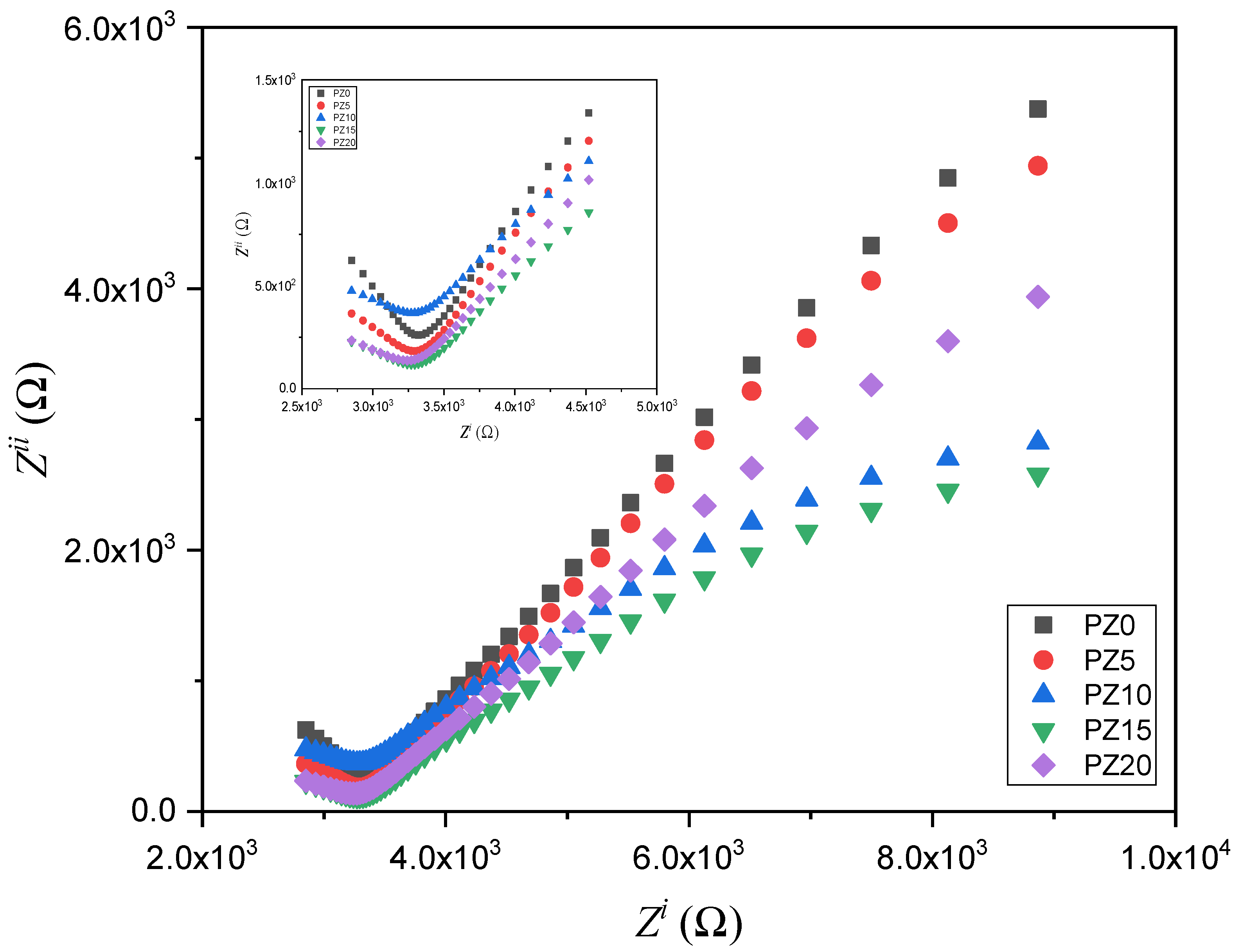
3.2.1. SPE Resistance
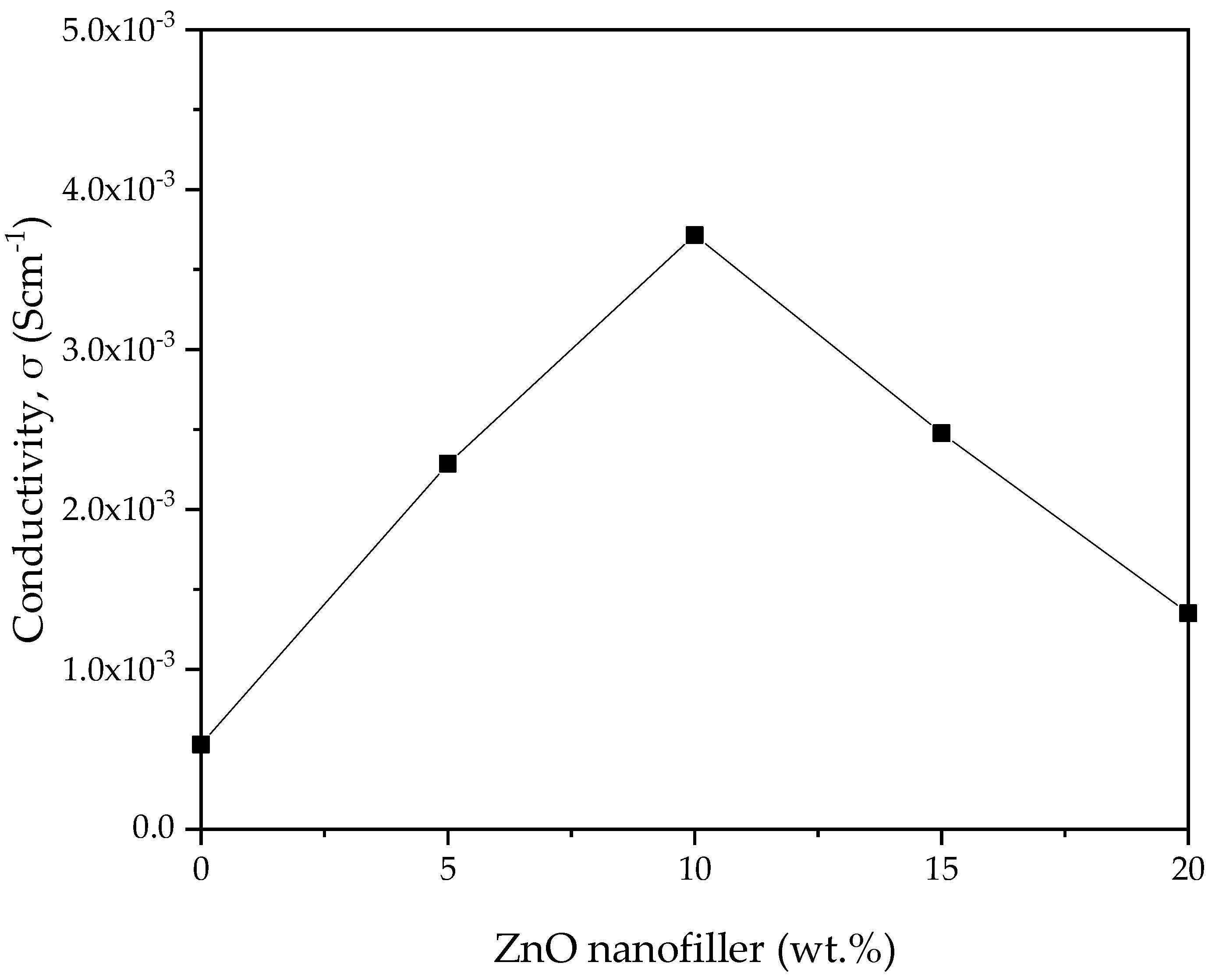
3.2.2. Ionic Conductivity
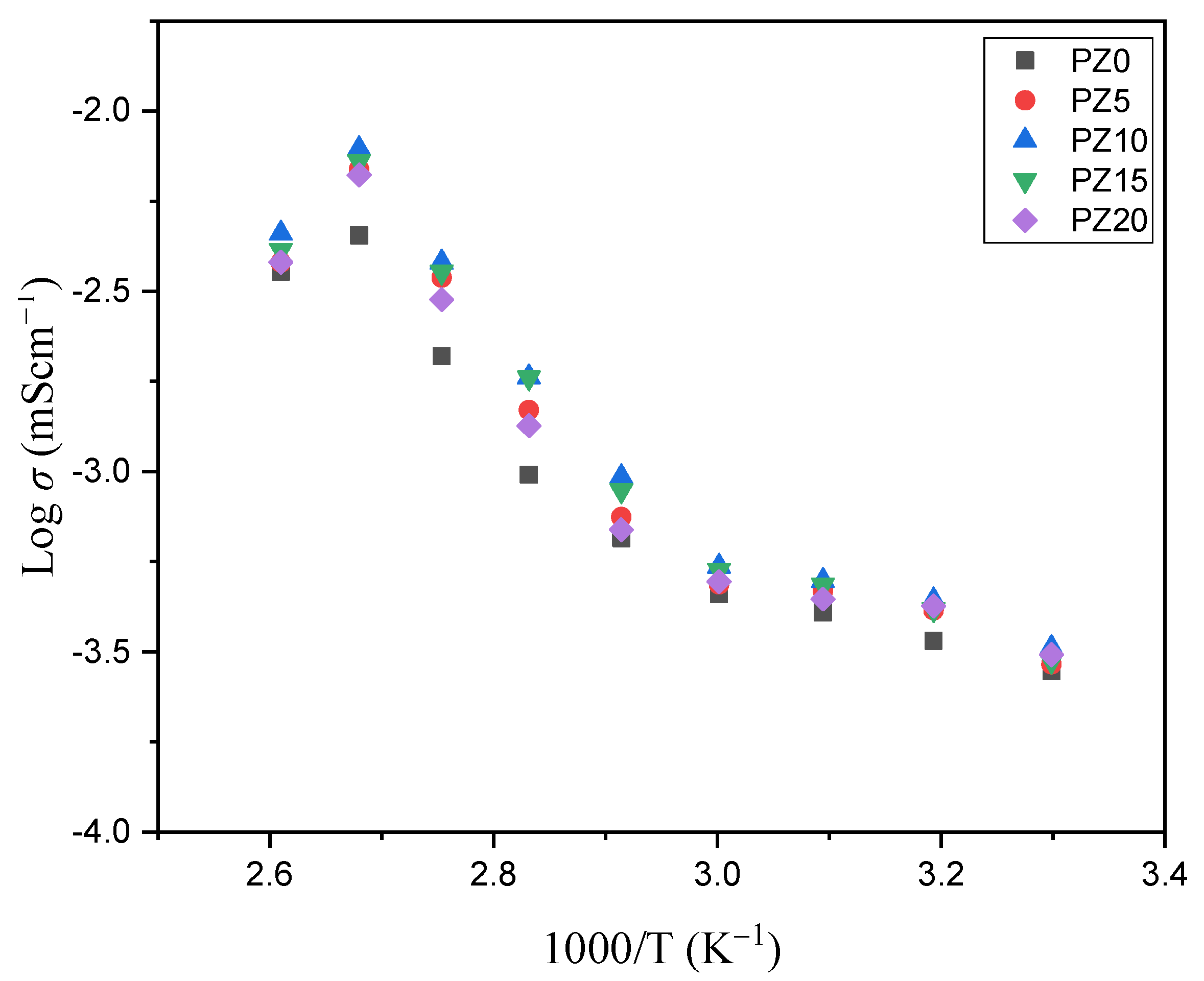
3.2.3. Temperature versus Conductivity Relationship
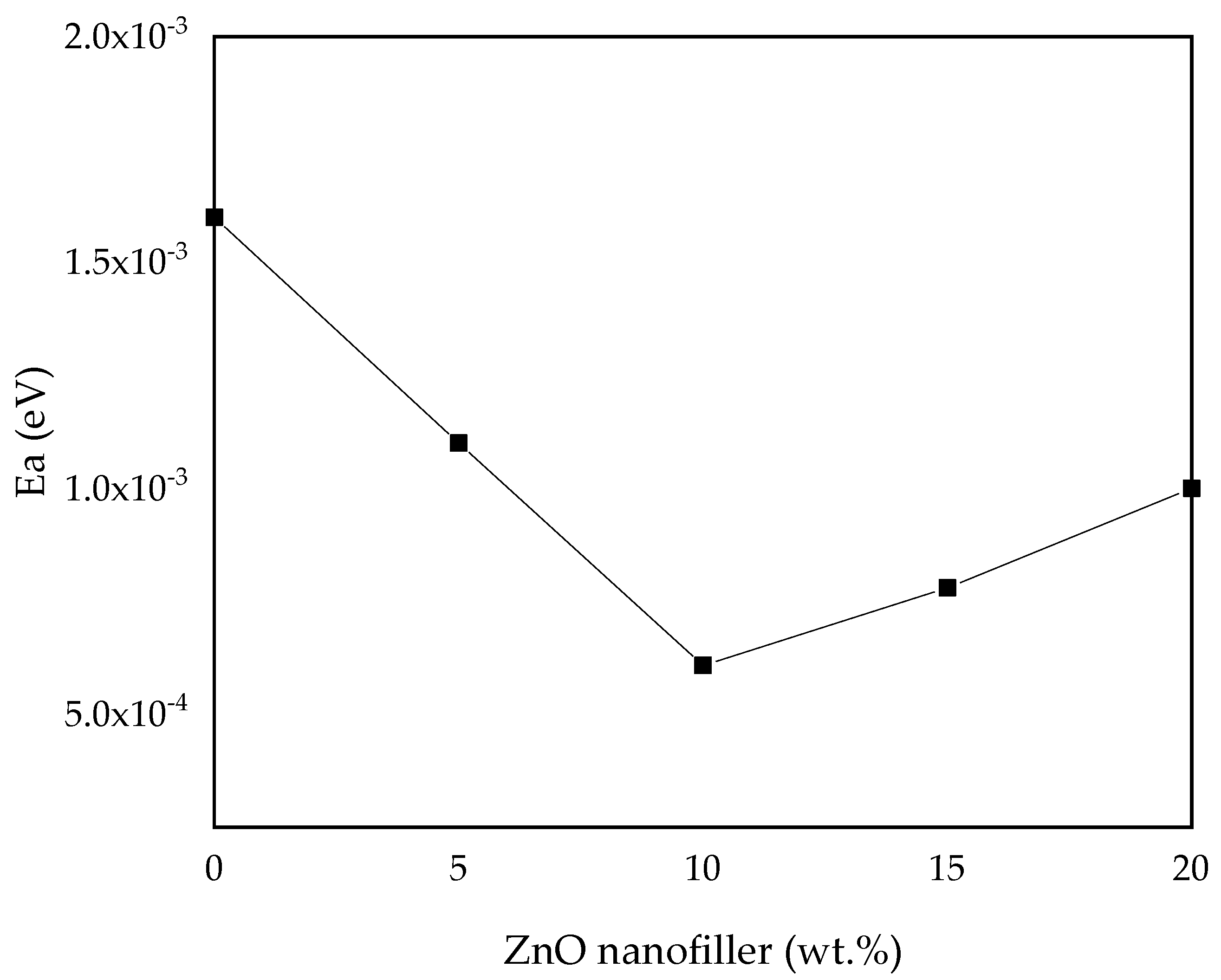
3.2.4. Activation Energy (Ea)
3.2.5. Electrochemical Stability Window
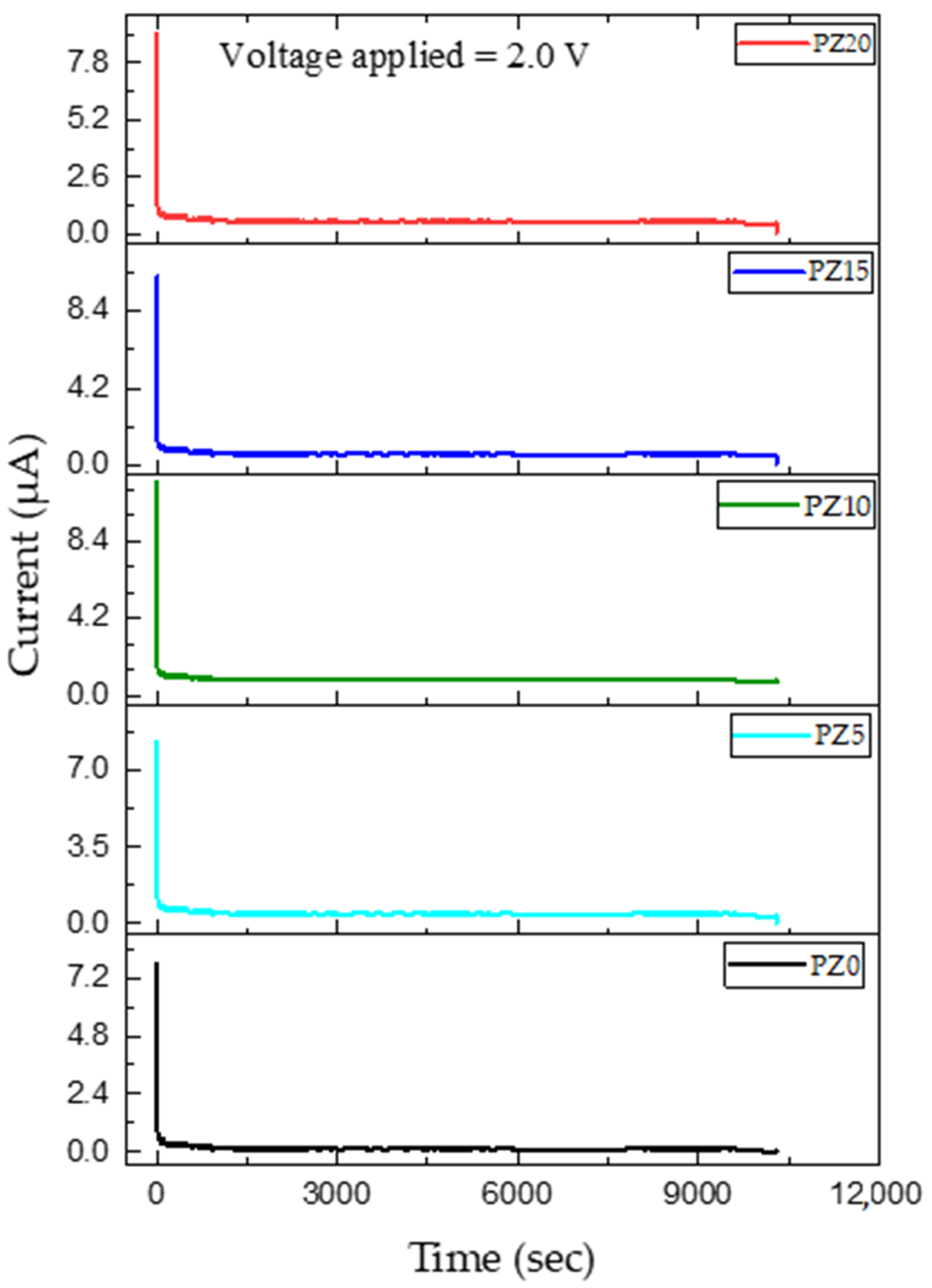
3.3. Transference Number (TNM) Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lingjuan, D.; Jianfang, W.; Gang, Z.; Liping, K.; Zhengping, H.; Zhibin, L.; Zupei, Y.; Zong-Huai, L. RuO2/graphene hybrid material for high performance electrochemical capacitor. J. Power Sources 2014, 248, 407–415. [Google Scholar]
- Arof, A.K.; Kufian, M.Z.; Shukur, M.F.; Aziz, A.; Abdelrahman, A.-E.; Majid, S.-R. Electrical double layer capacitor using poly(methyl methacrylate)–C4BO8 Li gel polymer electrolyte and carbonaceous material from shells of mata kucing (Dimocarpus longan) fruit. Electrochim. Acta 2012, 74, 39–45. [Google Scholar] [CrossRef]
- Na, R.; Lu, N.; Zhang, S.; Huo, G.; Yang, Y.; Zhang, C.; Mu, Y.; Luo, Y.; Wang, G. Facile synthesis of a high-performance, fire-retardant organic gel polymer electrolyte for flexible solid-state supercapacitors. Electrochim. Acta 2018, 290, 262–272. [Google Scholar] [CrossRef]
- Gao, H.; Lian, K. Proton-Conducting Polymer Electrolytes and Their Applications in Solid Supercapacitors: A Review. RSC Adv. 2014, 4, 33091–33113. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Naoi, K.; Ishimoto, S.; Miyamoto, J.-I.; Naoi, W. Second generation ‘nanohybrid supercapacitor’: Evolution of capacitive energy storage devices. Energy Environ. Sci. 2012, 5, 9363–9373. [Google Scholar] [CrossRef]
- Polu, A.R.; Kumar, R. Preparation and characterization of PVA based solid polymer electrolytes for electrochemical cell applications. Chin. J. Polym. Sci. 2013, 31, 641–648. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Xu, X.; Wang, Z.; Liu, Z.; Shen, J.; Liu, J.; Zhu, M. Interface engineering for composite cathodes in sulfide-based all-solid-state lithium batteries. J. Energy Chem. 2021, 60, 32–60. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, D.; Xu, X.; Liu, Z.; Liu, J. Challenges and Development of Composite Solid Electrolytes for All-solid-state Lithium Batteries. Chem. Res. Chin. Univ. 2021, 37, 210–231. [Google Scholar] [CrossRef]
- Chen, D.; Lou, Z.; Jiang, K.; Shen, G. Device Configurations and Future Prospects of Flexible/Stretchable Lithium-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1805596. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, X.; Qin, Y.; Ji, S.; Huo, Y.; Wang, Z.; Liu, Z.; Shen, J.; Liu, J. Recent Progress of Organic-Inorganic Composite Solid Electrolytes for All-Solid-State Lithium Batteries. Chem. A Eur. J. 2020, 26, 1720–1736. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.-B.; Abdullah, O.-G. Effect of PVA Blending on Structural and Ion Transport Properties of CS:AgNt-Based Polymer. Polymers 2017, 9, 622. [Google Scholar] [CrossRef]
- Omeh, G.-A.; Ahmed, H.-T.; Tahir, D.-A.; Jamal, G.-M.; Mohamad, A.-H. Influence of PEG plasticizer content on the proton-conducting PEO:MC-NH4I blend polymer electrolytes based films. Results Phys. 2021, 23, 104073. [Google Scholar]
- Nadirah, B.-N.; Ong, C.-C.; Saheed, M.-S.-M.; Yusof, Y.M. Structural and conductivity studies of polyacrylonitrile/methylcellulose blend based electrolytes embedded with lithium iodide. Int. J. hydrog. Energy 2020, 45, 19590–19600. [Google Scholar] [CrossRef]
- Dodda, J.M.; Bělský, P.; Chmelař, J.; Remiš, T.; Smolná, K.; Tomáš, M.; Kullová, L.; Kadlec, J. Comparative study of PVA/SiO2 and PVA/SiO2/glutaraldehyde (GA) nanocomposite membranes prepared by single-step solution casting method. J. Mater. Sci. 2015, 50, 6477–6490. [Google Scholar] [CrossRef]
- Bashir, A.-A.; John, O.-D.; Al-Hadeethi, Y.; Shukur, M.F.; Mkawi, E.M.; Nuha, A.; Ibnaouf, K.H.; Aldaghri, O.; Fahad, U.; Abdullahi, A.A. Optimization of the Electrochemical Performance of a Composite Polymer Electrolyte Based on PVA-K2CO3-SiO2 Composite. Polymers 2021, 13, 92. [Google Scholar]
- Abdullahi, A.A.; Hassan, S.; Muhammad, F.S.; John, O.D.; Yarima, M.H.; Bashir, A.A.; Jemilat, Y.Y.; Omar, S.S.A.; Shahira, S.S.; Saba, A.; et al. Novel composite polymer electrolytes based on methylcellulose-pectin blend complexed with potassium phosphate and ethylene carbonate. Biomass Convers. Biorefinery 2022, 1–18. [Google Scholar] [CrossRef]
- Choo, K.; Ching, Y.C.; Chuah, C.H.; Julai, S.; Liou, N.-S. Preparation and Characterization of Polyvinyl Alcohol-Chitosan Composite Films Reinforced with Cellulose Nanofiber. Materials 2016, 9, 644. [Google Scholar] [CrossRef]
- Wu, W.; Wang, S.; Wu, W.; Chen, K.; Hong, S.; Lai, Y. A critical review of battery thermal performance and liquid based battery thermal management. Energy Convers. Manag. 2019, 182, 262–281. [Google Scholar] [CrossRef]
- Fan, L.; Wang, M.; Zhang, Z.; Qin, G.; Hu, X.; Chen, Q. Preparation and Characterization of PVA Alkaline Solid Polymer Electrolyte with Addition of Bamboo Charcoal. Materials 2018, 11, 679. [Google Scholar] [CrossRef]
- Kadir, M.-F.Z.; Majid, S.-R.; Arof, A.K. Plasticized chitosan–PVA blend polymer electrolyte-based proton battery. Electrochim. Acta 2010, 55, 1475–1482. [Google Scholar] [CrossRef]
- Rehman, M.; Tuoqeer, M.; Rasheed, T. Lithium Salt Doped Poly(Vinylidene Fluoride)/Cellulose Acetate Composite Gel Electrolyte Membrane for Lithium Ion Battery. Mater. Sci. Eng. 2019, 654, 012017. [Google Scholar]
- Abdulkadir, B.A.; Dennis, J.O.; Shukur, M.F.; Nasef, M.M.E.; Usman, F. Preparation and characterization of gel polymer electrolyte based on PVA-K2CO3. Polym. Technol. Mater. 2020, 59, 1679–1697. [Google Scholar] [CrossRef]
- Abdullahi, A.A.; Hassan, S.; Shukur, M.F.; John, O.D.; Bashir, A.A.; Hassan, Y.M.; Jemilat, Y.Y.; Shamsuri, N.A.B. A new approach to understanding the interaction effect of salt and plasticizer on solid polymer electrolytes using statistical model and artificial intelligence algorithm. J. Non. Cryst. Solids 2022, 587, 121597. [Google Scholar]
- Hu, X.; Chen, Y.; Hu, Z.; Li, Y.; Ling, Z. All-Solid-State Supercapacitors Based on a Carbon-Filled Porous/Dense/Porous Layered Ceramic Electrolyte. J. Electrochem. Soc. 2018, 165, 1269–1274. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.H.; Lee, Y.G.; Park, J.K.; Seung, D.Y. Effect of silica on the electrochemical characteristics of the plasticized polymer electrolytes based on the P(AN-co-MMA) copolymer. Solid State Ion. 2000, 133, 257–263. [Google Scholar] [CrossRef]
- Dennis, J.O.; Adam, A.A.; Ali, M.K.M.; Soleimani, H.; Shukur, M.F.B.A.; Ibnaouf, K.H.; Aldaghri, O.; Eisa, M.H.; Ibrahem, M.A.; Bashir Abdulkadir, A.; et al. Substantial Proton Ion Conduction in Methylcellulose/Pectin/Ammonium Chloride Based Solid Nanocomposite Polymer Electrolytes: Effect of ZnO Nanofiller. Membranes 2022, 12, 706. [Google Scholar] [CrossRef]
- Xiong, H.; Zhao, X.; Chen, J. New Polymer-Inorganic Nanocomposites: PEO-ZnO and PEO-ZnO-LiClO4 Films. J. Phys. Chem. B 2001, 105, 10169–10174. [Google Scholar] [CrossRef]
- Zebardastan, N.; Ramesh, M.H.S.; Ramesh, K. Performance enhancement of poly(vinylidene fl uoride-co-hexa fl uoro propylene)/polyethylene oxide based nanocomposite polymer electrolyte with ZnO nano filler for dye-sensitized solar cell. Org. Electron. 2017, 49, 292–299. [Google Scholar] [CrossRef]
- Omed, G.A.; Yahya, A.K.S.; Dana, A.T.; Gelas, M.J.; Hawzhin, T.A.; Azhin, H.M.; Auday, K.A. Effect of ZnO Nanoparticle Content on the Structural and Ionic Transport Parameters of Polyvinyl Alcohol Based Proton-Conducting Polymer Electrolyte Membranes. Membranes 2021, 11, 163. [Google Scholar]
- Bashir, A.A.; John, O.D.; Abbas, A.A.; Yerima, M.H.; Nurrul, A.S.; Shukur, M.F. Preparation and characterization of solid biopolymer electrolytes based on polyvinyl alcohol/cellulose acetate blend doped with potassium carbonate (K2CO3) salt. J. Electroanal. Chem. 2022, 919, 116539. [Google Scholar]
- Raghavan, P.; Choi, J.; Ahn, J.; Cheruvally, G.; Chauhan, G.S.; Ahn, H.; Nah, C. Novel electrospun poly(vinylidene fluoride-co-hexafluoropropylene)–in situ SiO2 composite membrane-based polymer electrolyte for lithium batteries. J. Power Sources 2008, 184, 437–443. [Google Scholar] [CrossRef]
- Mahalakshmi, M.; Selvanayagam, S.; Selvasekarapandian, S.; Chandra, M.V.L.; Sangeetha, P. Magnesium ion-conducting solid polymer electrolyte based on cellulose acetate with magnesium nitrate (Mg(NO3)2·6H2O) for electrochemical studies. Ionics 2020, 3, 4553–4565. [Google Scholar] [CrossRef]
- Arya, A.; Sharma, A.L. Dielectric Relaxations and Transport Properties Parameter Analysis of Novel Blended Solid Polymer Electroly. J. Mater. Sci. 2019, 54, 7131–7155. [Google Scholar]
- Abdulwahid, R.T.; Woo, H.J. Increase of metallic silver nanoparticles in Chitosan: AgNt based polymer electrolytes incorporated with alumina filler. Results Phys. 2019, 13, 102326. [Google Scholar]
- He, T.; Jia, R.; Lang, X.; Wu, X.; Wang, Y. Preparation and Electrochemical Performance of PVdF Ultrafine Porous Fiber Separator-Cum-Electrolyte for Supercapacitor. J. Electrochem. Soc. 2017, 164, 379–384. [Google Scholar] [CrossRef]
- Liew, C.; Ramesh, S.; Arof, A.K. Characterization of ionic liquid added poly(vinyl alcohol)-based proton conducting polymer electrolytes and electrochemical studies on the supercapacitors. Int. J. hydrog. Energy 2014, 40, 852–862. [Google Scholar] [CrossRef]
- Monisha, S.; Mathavan, T.; Selvasekarapandian, S.; Benial, A.M.F.; Aristatil, G.; Mani, N.; Premalatha, M.; Vinoth, D.-P. Investigation of bio polymer electrolyte based on cellulose acetate-ammonium nitrate for potential use in electrochemical devices. Carbohydr. Polym. 2017, 157, 38–47. [Google Scholar] [CrossRef]
- Liew, C.; Ramesh, S.; Arof, A.K. A novel approach on ionic liquid-based poly(vinyl alcohol) proton conductive polymer electrolytes for fuel cell applications. Int. J. hydrog. Energy 2013, 39, 2917–2928. [Google Scholar] [CrossRef]
- Gan, W.; Zhou, D.; Zhou, L.; Zhang, Z.; Zhao, J. Zinc electrode with anion conducting polyvinyl alcohol/poly(diallyldimethylammonium chloride) film coated ZnO for secondary zinc air batteries. Electrochim. Acta 2015, 182, 430–436. [Google Scholar] [CrossRef]
- Mansour, A.F.; Mansour, S.F.; Abdo, M.A. Improvement Structural and Optical Properties of ZnO/PVA Nanocomposites. IOSR J. Appl. Phys. 2015, 7, 60–69. [Google Scholar]
- Padmaraj, O.; Venkateswarlu, M.; Satyanarayana, N. Effect of ZnO filler concentration on the conductivity, structure and morphology of PVdF-HFP nanocomposite solid polymer electrolyte for lithium battery application. Ionics 2013, 10, 1835–1842. [Google Scholar] [CrossRef]
- Chang, J.; Lai, H.; Samarahan, K. Polyvinyl alcohol/silica/clay composites: Effect of clay on surface morphology and thermo-mechanical properties. J. Teknol. Sci. Eng. 2016, 78, 45–53. [Google Scholar] [CrossRef]
- Aziz, S.B.; Faraj, M.G.; Abdullah, O.G. Impedance Spectroscopy as a Novel Approach to Probe the Phase Transition and Microstructures Existing in CS:PEO Based Blend Electrolytes. Sci. Rep. 2018, 8, 1–14. [Google Scholar]
- Rafique, S.; Bashir, S.; Akram, R.; Kiyani, F.B.; Raza, S.; Hussain, M.; Fatima, S.K. Variation in the Performance of MWCNT/ZnO Hybrid Material with pH for Efficient Antibacterial Agent. Biomed. Res. Int. 2022, 57, 1–11. [Google Scholar] [CrossRef]
- Abdulkadir, B.A.; Dennis, J.O.; Abdullahi, A.A.; Adel, A.; Shukur, M.F. Novel electrospun separator-electrolyte based on in flexible energy storage devices. J. Appl. Polym. Sci. 2022, 139, 52308. [Google Scholar] [CrossRef]
- Yup, J.; Kang, D.A.; Un, N.; Min, J.; Hak, J. Bicontinuously crosslinked polymer electrolyte membranes with high ion conductivity and mechanical strength. J. Memb. Sci. 2019, 589, 117250. [Google Scholar]
- Çavuş, S.; Durgun, E. Poly(vinyl alcohol) Based Polymer Gel Electrolytes: Investigation on Their Conductivity and Characterization. Acta Phys. Pol. A 2016, 129, 621–624. [Google Scholar] [CrossRef]
- Kadir, M.F.Z.; Hamsan, M.H. Green electrolytes based on dextran-chitosan blend and the effect of NH4SCN as proton provider on the electrical response studies. Ionics 2017, 11, 2379–2398. [Google Scholar] [CrossRef]
- Liew, C.; Ramesh, S. Comparing Triflate and Hexafluorophosphate Anions of Ionic Liquids in Polymer Electrolytes for Supercapacitor Applications. Materials 2014, 75, 4019–4033. [Google Scholar] [CrossRef]
- Mishra, K.; Garg, A.; Sharma, R.; Gautam, R.; Pundir, S.S. Effect of blending of PMMA on PVdF-HFP + NaCF3SO3-EC-PC gel polymer electrolyte. Mater. Today Proc. 2019, 12, 621–627. [Google Scholar] [CrossRef]
- Yusof, Y.M.; Illias, H.A.; Shukur, M.F.; Kadir, M.F.Z. Characterization of starch-chitosan blend-based electrolyte doped with ammonium iodide for application in proton batteries. Ionics 2017, 23, 681–697. [Google Scholar] [CrossRef]
- Aziz, S.B.; Woo, T.J.; Kadir, M.F.Z.; Ahmed, H.M. A conceptual review on polymer electrolytes and ion transport models. J. Sci. Adv. Mater. Devices 2018, 3, 1–17. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Z.; Song, S.; Ma, Q.; Liu, R. High Performance Poly(vinyl alcohol)-Based Li-Ion Conducting Gel Polymer Electrolyte Films for Electric Double-Layer Capacitors. Polymers 2018, 10, 1179. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fu, J.; Lu, Q.; Shi, L.; Li, M.; Dong, L.; Xu, Y.; Jia, R. Cross-linked polymeric ionic liquids ion gel electrolytes by in situ radical polymerization. Chem. Eng. J. 2019, 378, 122245. [Google Scholar] [CrossRef]
- Hyeon, S.; Yong, J.; Wook, J.; Kim, W.; Chung, C. Faradaic reaction of dual-redox additive in zwitterionic gel electrolyte boosts the performance of flexible supercapacitors. Electrochim. Acta 2019, 319, 672–681. [Google Scholar] [CrossRef]
- Shukur, M.F.; Ithnin, R.; Illias, H.A.; Kadir, M.F.Z. Proton conducting polymer electrolyte based on plasticized chitosan-PEO blend and application in electrochemical devices. Opt. Mater. 2013, 35, 1834–1841. [Google Scholar] [CrossRef]
- Morsi, M.A.; Oraby, A.H.; Elshahawy, A.G.; El-hady, R.M.A. Preparation, structural analysis, morphological investigation and electrical properties of gold nanoparticles filled polyvinyl alcohol/carboxymethyl cellulose blend. J. Mater. Res. Technol. 2019, 8, 5996–6010. [Google Scholar] [CrossRef]
- Yusof, Y.M.; Majid, N.A.; Kasmani, R.M.; Illias, H.A.; Kadir, M.F.Z. The Effect of Plasticization on Conductivity and Other Properties of Starch/Chitosan Blend Biopolymer Electrolyte Incorporated with Ammonium Iodide. Mol. Cryst. Liq. Cryst. 2014, 603, 73–88. [Google Scholar] [CrossRef]
- Shukur, M.F.; Azmi, M.S.; Zawawi, S.M.M.; Majid, N.A.; Illias, H.A.; Kadir, M.F.Z. Conductivity studies of biopolymer electrolytes based on chitosan incorporated with NH4Br. Phys. Scr. 2013, 2013, 014049. [Google Scholar] [CrossRef]
- Patil, S.U.; Yawale, S.S.; Yawale, S.P. Conductivity study of PEO–LiClO4 polymer electrolyte doped with ZnO nanocomposite ceramic filler. Bull. Mater. Sci. 2014, 37, 1403–1409. [Google Scholar] [CrossRef]
- Wang, X.; Hao, X.; Xia, Y.; Liang, Y.; Xia, X.; Tu, J. A polyacrylonitrile (PAN)-based double-layer multifunctional gel polymer electrolyte for lithium-sulfur batteries. J. Memb. Sci. 2019, 582, 37–47. [Google Scholar] [CrossRef]
- Agrawal, R.C.; Mahipal, Y.K.; Ashrafi, R. Materials and ion transport property studies on hot-press casted solid polymer electrolyte membranes: [(1 − x) PEO: X KIO3]. Solid State Ion. 2011, 192, 6–8. [Google Scholar] [CrossRef]
- Devi, G.N.; Chitra, S.; Selvasekarapandian, S.; Premalatha, M.; Monisha, S. Synthesis and characterization of dextrin-based polymer electrolytes for potential applications in energy storage devices. Ionics 2017, 23, 3377–3388. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.G.; Al-zangana, S. Solid Polymer Electrolytes Based on Chitosan: NH4Tf Modified by Various Amounts of TiO2 Filler and its Electrical and Dielectric Characteristics. Int. J. Electrochem. Sci. 2019, 14, 1909–1925. [Google Scholar] [CrossRef]
- Tripathi, M.; Kumar, A. Zinc oxide nanofiller-based composite polymer gel electrolyte for application in EDLCs. Electrochim. Acta 2018, 24, 3155–3165. [Google Scholar] [CrossRef]
- Zhuang, Z.; Tang, Y.; Ju, B.; Tu, F. In situ synthesis of graphitic C3N4–poly(1,3-dioxolane) composite interlayers for stable lithium metal anodes. Sustain. Energy Fuels 2021, 5, 2433. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Abdullah, R.M.; Kadir, M.F.Z. A Promising Polymer Blend Electrolytes Based on Chitosan: Methyl Cellulose for EDLC Application with High Specific Capacitance and Energy Density. Molecules 2019, 24, 2503. [Google Scholar] [CrossRef]
- Shukur, M.F.; Kadir, M.F.Z. Hydrogen ion conducting starch-chitosan blend-based electrolyte for application in electrochemical devices. Electrochim. Acta 2015, 158, 152–165. [Google Scholar] [CrossRef]
- Shamsudin, I.J.; Ahmad, A.; Hassan, N.H.; Kaddami, H. Bifunctional ionic liquid in conductive biopolymer based on chitosan for electrochemical devices application. Solid State Ion. 2015, 278, 11–19. [Google Scholar] [CrossRef]
- Shuhaimi, N.E.A.; Majid, S.R.; Arof, A.K. On complexation between methyl cellulose and ammonium nitrate on complexation between methyl cellulose and ammonium nitrate. Mater. Res. Innov. 2013, 13, 239–242. [Google Scholar] [CrossRef]
- Selvasekarapandian, S.; Baskaran, R.; Hema, M. Complex AC impedance, transference number and vibrational spectroscopy studies of proton conducting PVAc–NH4SCN polymer electrolytes. Phys. B 2005, 357, 412–419. [Google Scholar] [CrossRef]
- Eric-Koh, R.W.; Sun, C.C.; Yap, Y.L.; Cheang, P.L.; You, A.H. Measurement of Lithium Transference Number in PMMA Solid Polymer Electrolytes Doped with Micron-sized Fillers. J. Mech. Eng. Technol. 2021, 13, 30–42. [Google Scholar]
- Anil, A.; Nilesh, G.S.; Sharma, A.L. Impact of shape (nanofiller vs. nanorod) of TiO2 nanoparticle on free-standing solid polymeric separator for energy storage/conversion devices. J. Appl. Polym. Sci. 2018, 136, 47361. [Google Scholar]


| Description | PVA–CA Polymer Blend Ratio | K2CO3 Content (wt %) | ZnO-NPs Content (wt %) |
|---|---|---|---|
| PZ0 | 80:20 | 20 | 0 |
| PZ5 | 80:20 | 20 | 5 |
| PZ10 | 80:20 | 20 | 10 |
| PZ15 | 80:20 | 20 | 15 |
| PZ20 | 80:20 | 20 | 20 |
| Samples | Tg (°C) | FWHM |
|---|---|---|
| PZ0 | 82.21 | 0.642 |
| PZ5 | 85.78 | 0.470 |
| PZ10 | 85.65 | 0.405 |
| PZ15 | 103.36 | 0.367 |
| PZ20 | 123.19 | 0.288 |
| Polymers | Salt | Filler/Plasticizer | Conductivity (S/cm) | Ref. |
|---|---|---|---|---|
| PVA–CA | K2CO3 | ZnO-NPs | 3.70 × 10–3 | This study |
| Starch–chitosan | NH4I | Glycerol | 1.28 × 10–3 | [58] |
| Chitosan–PEO | NH4NO3 | Ethylene carbonate (EC) | 2.06 × 10–3 | [56] |
| Chitosan-based solid biopolymer | NH4Br | Glycerol | 1.51 × 10–3 | [59] |
| Pectin–methylcellulose | K3PO4 | Glycerol | 3.00 × 10–4 | [24] |
| PEO–MC | NH4I | Polyethylene glycol | 3.37 × 10−3 | [10] |
| PVA | NH4NO3 | ZnO-NPs | 4.71 × 10–4 | [30] |
| PEO | LiClO4 | ZnO | 1⋅28 × 10–5 | [60] |
| S/N | Samples | Activation (Ea) (eV) |
|---|---|---|
| 1 | PZ0 | 1.60 × 10–3 |
| 2 | PZ5 | 1.00 × 10–3 |
| 3 | PZ10 | 6.08 × 10–4 |
| 4 | PZ15 | 7.80 × 10–4 |
| 5 | PZ20 | 1.10 × 10–3 |
| Samples | Electron Transference Number (tel) | Transference Number (tion) |
|---|---|---|
| PZ0 | 0.090 | 0.909 |
| PZ5 | 0.089 | 0.911 |
| PZ10 | 0.034 | 0.965 |
| PZ15 | 0.078 | 0.922 |
| PZ20 | 0.082 | 0.918 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojur Dennis, J.; Ali, M.K.M.; Ibnaouf, K.H.; Aldaghri, O.; Abdel All, N.F.M.; Adam, A.A.; Usman, F.; Hassan, Y.M.; Abdulkadir, B.A. Effect of ZnO Nanofiller on Structural and Electrochemical Performance Improvement of Solid Polymer Electrolytes Based on Polyvinyl Alcohol–Cellulose Acetate–Potassium Carbonate Composites. Molecules 2022, 27, 5528. https://doi.org/10.3390/molecules27175528
Ojur Dennis J, Ali MKM, Ibnaouf KH, Aldaghri O, Abdel All NFM, Adam AA, Usman F, Hassan YM, Abdulkadir BA. Effect of ZnO Nanofiller on Structural and Electrochemical Performance Improvement of Solid Polymer Electrolytes Based on Polyvinyl Alcohol–Cellulose Acetate–Potassium Carbonate Composites. Molecules. 2022; 27(17):5528. https://doi.org/10.3390/molecules27175528
Chicago/Turabian StyleOjur Dennis, John, Mohammed Khalil Mohammed Ali, Khalid Hassan Ibnaouf, Osama Aldaghri, Naglaa F. M. Abdel All, Abdullahi Abbas Adam, Fahad Usman, Yarima Mudassir Hassan, and Bashir Abubakar Abdulkadir. 2022. "Effect of ZnO Nanofiller on Structural and Electrochemical Performance Improvement of Solid Polymer Electrolytes Based on Polyvinyl Alcohol–Cellulose Acetate–Potassium Carbonate Composites" Molecules 27, no. 17: 5528. https://doi.org/10.3390/molecules27175528
APA StyleOjur Dennis, J., Ali, M. K. M., Ibnaouf, K. H., Aldaghri, O., Abdel All, N. F. M., Adam, A. A., Usman, F., Hassan, Y. M., & Abdulkadir, B. A. (2022). Effect of ZnO Nanofiller on Structural and Electrochemical Performance Improvement of Solid Polymer Electrolytes Based on Polyvinyl Alcohol–Cellulose Acetate–Potassium Carbonate Composites. Molecules, 27(17), 5528. https://doi.org/10.3390/molecules27175528










