Robust Superhydrophobic Brass Mesh with Electrodeposited Hydroxyapatite Coating for Versatile Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Fabrication of the Superhydrophobic Brass Mesh
2.2. Characterization
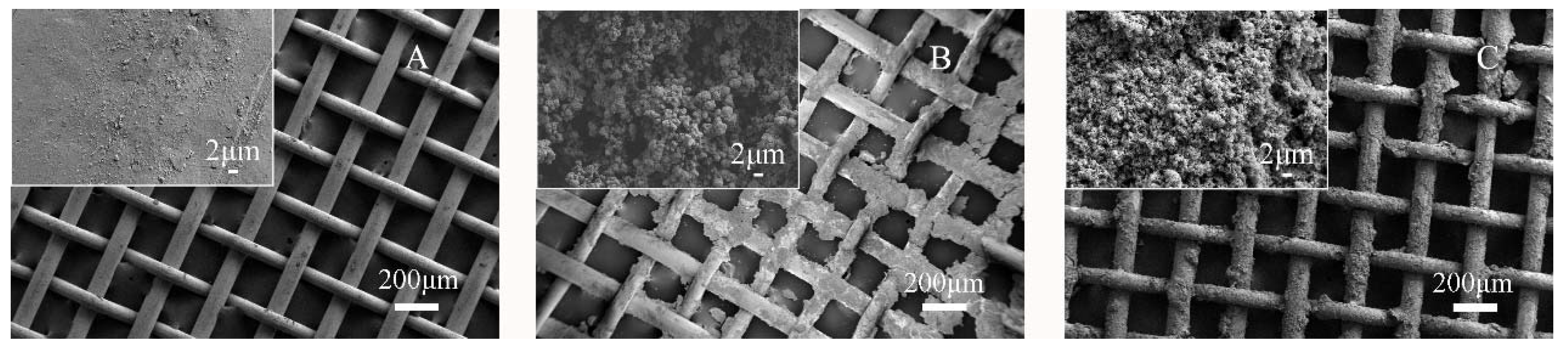
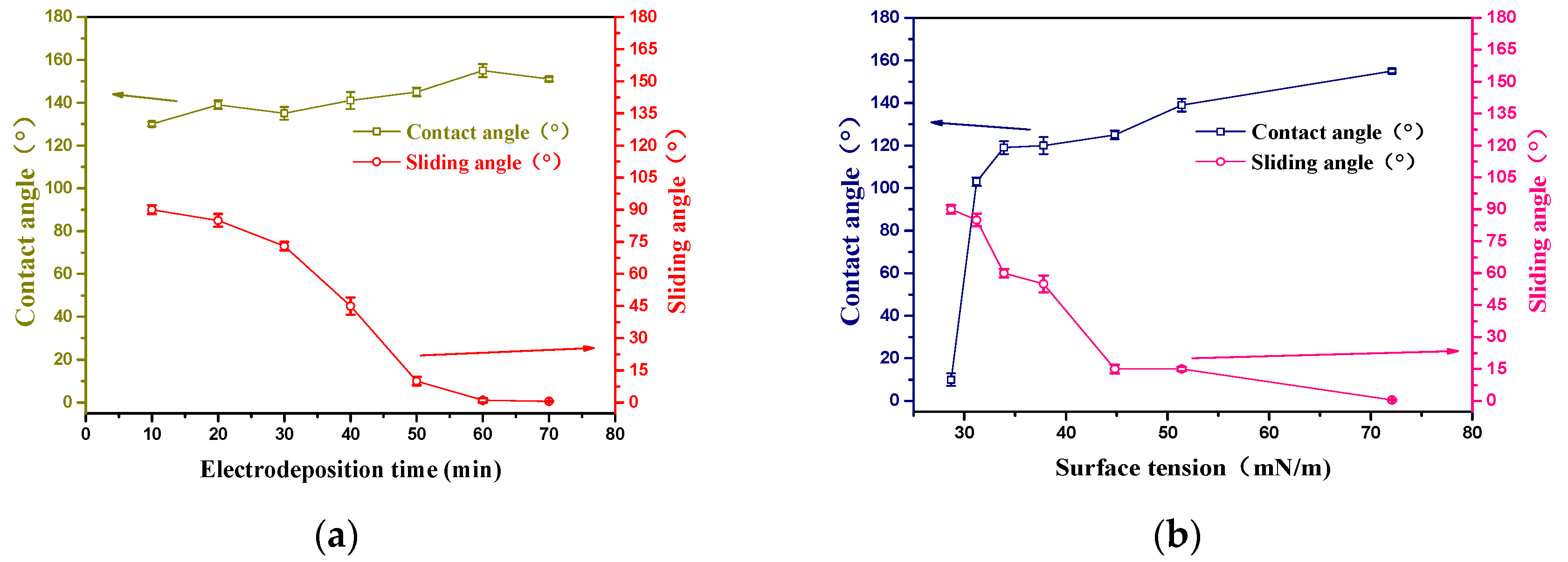
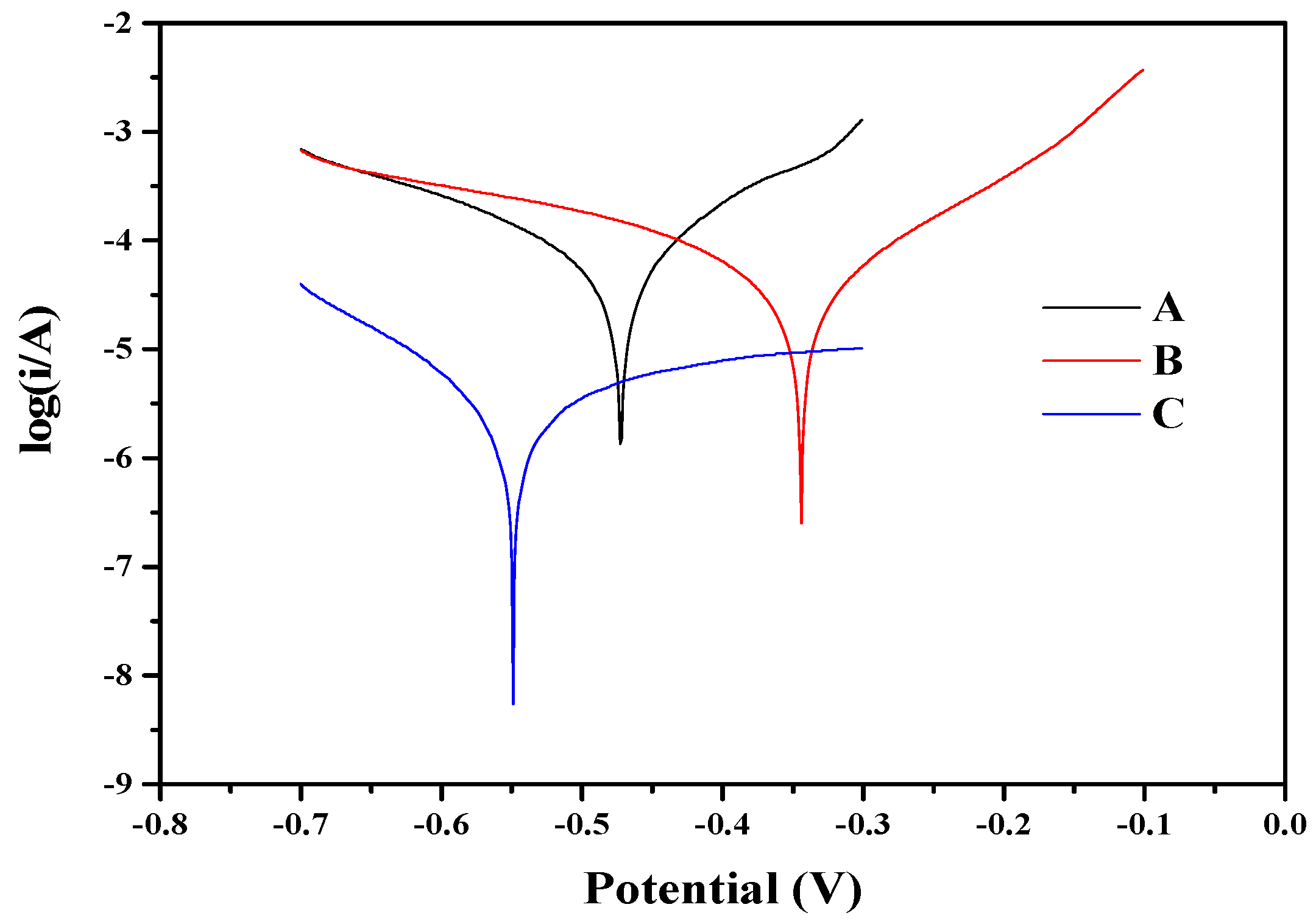
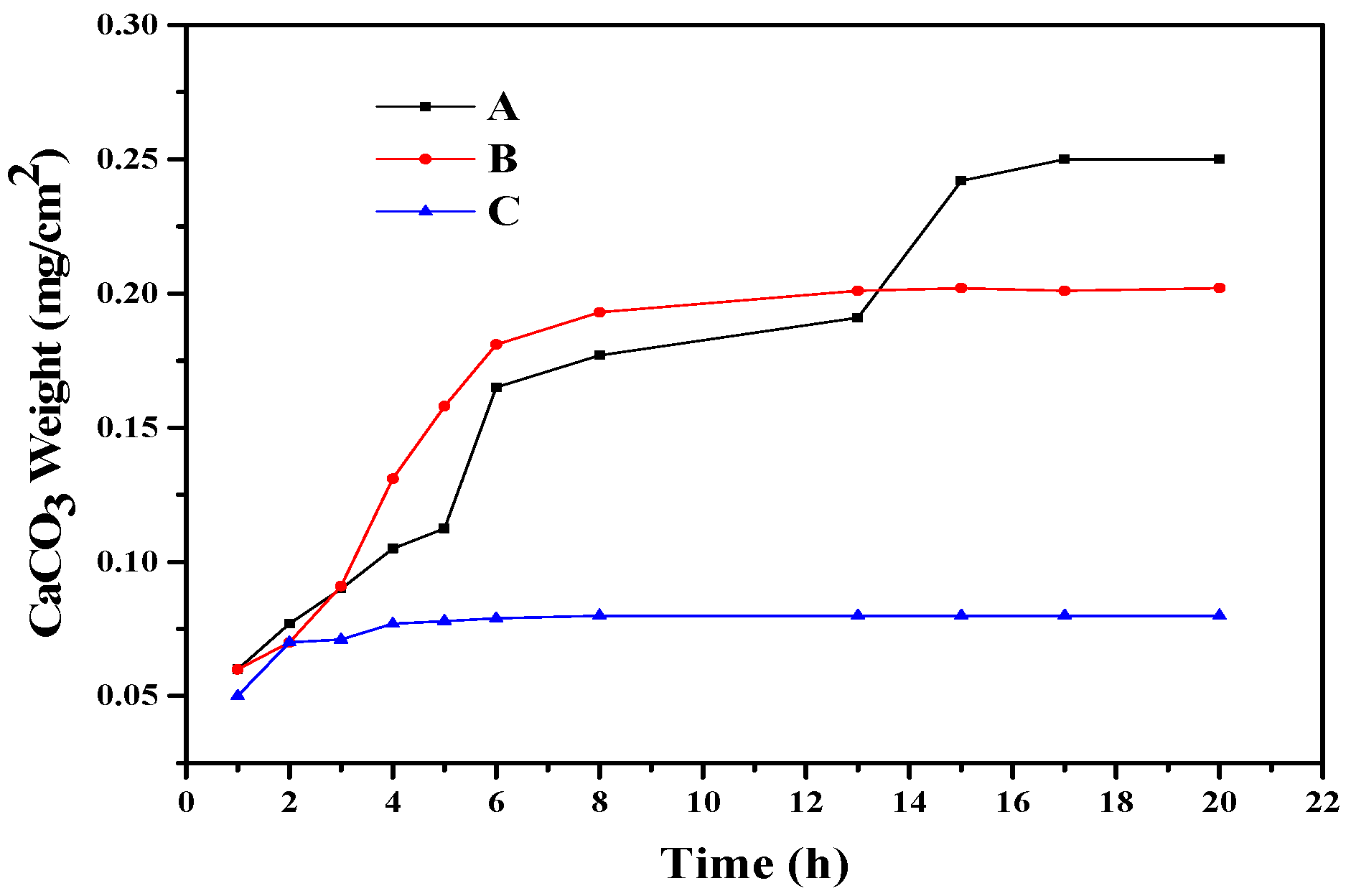
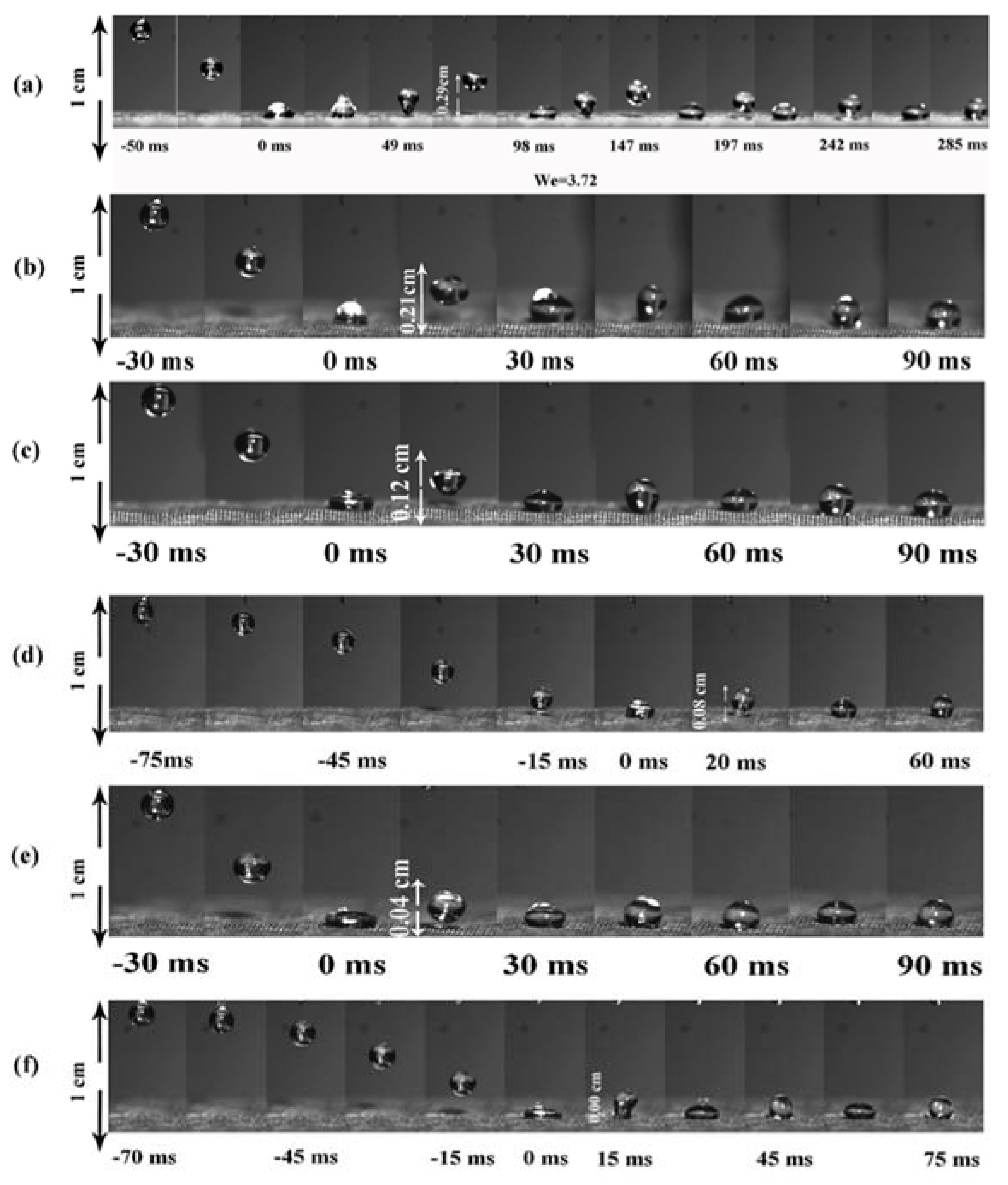
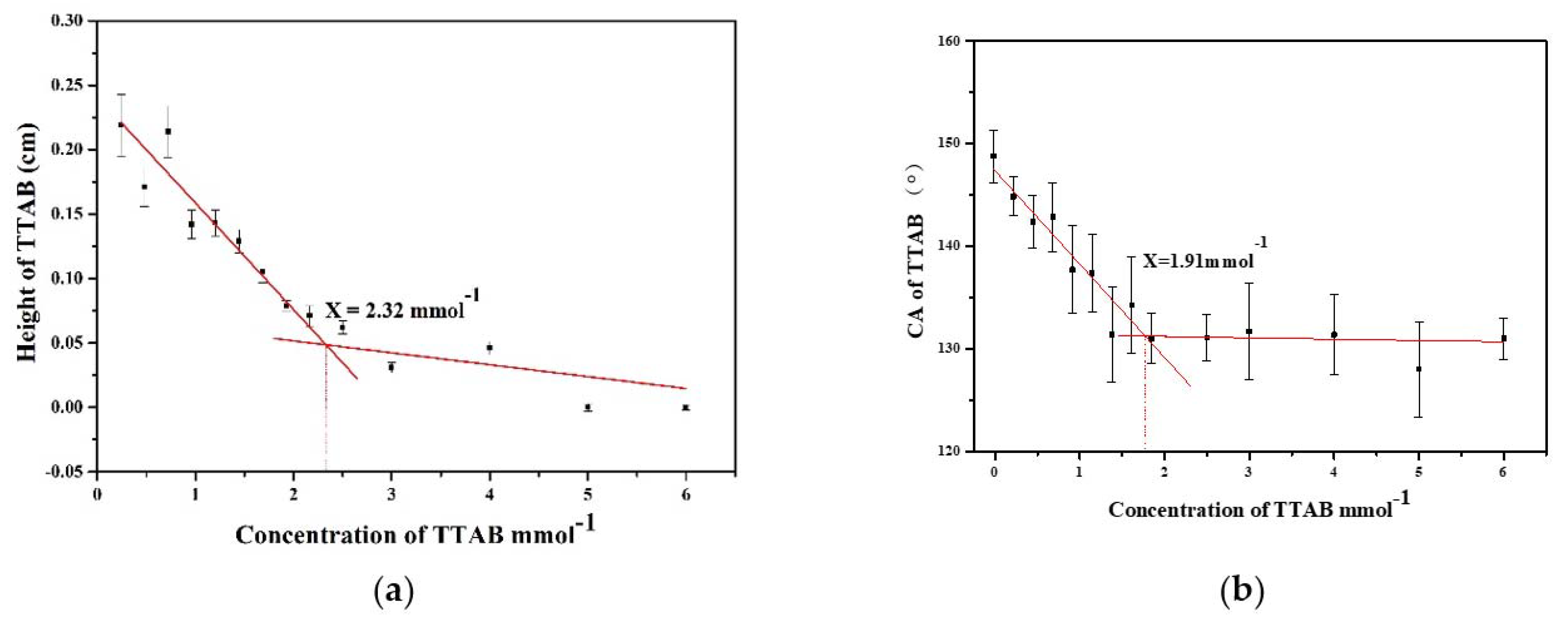
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, L.; McCarthy, T.J. The “Lotus Effect” Explained: Two Reasons Why Two Length Scales of Topography Are Important. Langmuir 2006, 22, 2966–2967. [Google Scholar] [CrossRef]
- Bhushan, B.; Her, E.K. Fabrication of Superhydrophobic Surfaces with High and Low Adhesion Inspired from Rose Petal. Langmuir 2010, 26, 8207–8217. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Ju, J.; Qiu, Y.; He, Y.; Wang, X.; Dou, S.; Liu, K.; Jiang, L. Peanut leaf inspired multifunctional surfaces. Small 2014, 10, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Gao, X.; Jiang, L. Directional Adhesion of Superhydrophobic Butterfly Wings. Soft Mat. 2007, 3, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, K.; Yao, X.; Jiang, L. Bioinspired Surfaces with Superwettability: New Insight on Theory, Design, and Applications. Chem. Rev. 2015, 115, 8230–8293. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, S.; Wei, Z.; Song, Y.; Jiang, L. Bioinspired Design of a Superoleophobic and Low Adhesive Water/Solid Interface. Adv. Mater. 2009, 21, 665–669. [Google Scholar] [CrossRef]
- Guo, J.; Qian, H.; Liu, P.; Ma, J. Fabrication of Durability Superhydrophobic LDH Coating on Zinc Sheet Surface via NH4F-assisted in-situ Growth and Post-modification for Enhancing anti-Corrosion and anti-Icing. Appl. Clay Sci. 2019, 180, 105182. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.; Liu, S.; Zhang, Y.; Du, Z.; Qu, L. Bio-inspired Fabrication of Fire-retarding, Magnetic-responsive, Superhydrophobic Sponges for Oil and Organics Collection. Appl. Clay Sci. 2019, 172, 19–27. [Google Scholar] [CrossRef]
- Mokabber, T.; Lu, L.Q.; van Rijn, P.; Vakis, A.I.; Pei, Y.T. Crystal Growth Mechanism of Calcium Phosphate Coatings on Titanium by Electrochemical Deposition. Surf. Coatings Technol. 2018, 334, 526–535. [Google Scholar] [CrossRef]
- Sun, L.; Berndt, C.C.; Gross, K.A.; Kucuk, A. Material Fundamentals and Clinical Performance of PlasmaSprayed Hydroxyapatite Coatings: A Review. J. Biomed. Mater. Res. 2001, 58, 570–592. [Google Scholar] [CrossRef]
- Müller, V.; Pagnier, T.; Tadier, S.; Gremillard, L.; Jobbagy, M.; Djurado, E. Design of Advanced One-step Hydroxyapatite Coatings for Biomedical Applications using the Electrostatic Spray Deposition. Appl. Surf. Sci. 2021, 541, 148462. [Google Scholar] [CrossRef]
- Luo, D.-F.; Ning, P.; Zhang, F.; Zhou, Y.; Zhang, H.-M.; Fu, T. Hydrothermal Calcification Surface Modification of Biomedical Tantalum. Rare Metals 2020, 40, 928–933. [Google Scholar] [CrossRef]
- Molaei, A.; Yari, M.; Afshar, M.R. Investigation of Halloysite Nanotube Content on Electrophoretic Deposition (EPD) of Chitosan-bioglass-hydroxyapatite-halloysite Nanotube Nanocomposites Films in Surface Engineering. Appl. Clay Sci. 2017, 135, 75–81. [Google Scholar] [CrossRef]
- Domínguez-Trujillo, C.; Peón, E.; Chicardi, E.; Pérez, H.; Rodríguez-Ortiz, J.A.; Pavón, J.J.; García-Couce, J.; Galván, J.C.; García-Moreno, F.; Torres, Y. Sol-gel Deposition of Hydroxyapatite Coatings on Porous Titanium for Biomedical Applications. Surf. Coat. Technol. 2018, 333, 158–162. [Google Scholar] [CrossRef]
- Ma, Q.; Liao, J.; Tian, T.; Zhang, Q.; Cai, X. A Potential Flower-like Coating Consisting of Calcium-phosphate Nanosheets on Titanium Surface. Chin. Chem. Let. 2017, 28, 1893–1896. [Google Scholar] [CrossRef]
- Fu, C.; Song, B.; Wan, C.; Savino, K.; Wang, Y.; Zhang, X.; Yates, M.Z. Electrochemical Growth of Composite Hydroxyapatite Coatings for Controlled Release. Surf. Coat. Technol. 2015, 276, 618–625. [Google Scholar] [CrossRef]
- Montalbert-Smith, R.; Palma, C.A.; Arias, J.D.; Montero, M.L. Formation of Hydroxyapatite Nanosized and other Apatites by Electrolysis Process. Key Eng. Mater. 2008, 396–398, 579–582. [Google Scholar] [CrossRef]
- Yin, X.; Wang, Z.; Shen, Y.; Mu, P.; Zhu, G.; Li, J. Facile fabrication of Superhydrophobic Copper Hydroxide Ccoated Mesh for Effective Separation of Water-in-oil Emulsions. Sep. Purif. Technol. 2020, 230, 115856. [Google Scholar] [CrossRef]
- Yan, L.; Xiang, Y.; Yu, J.; Wang, Y.; Cui, W. Fabrication of Antibacterial and Antiwear Hydroxyapatite Coatings via In Situ Chitosan-Mediated Pulse Electrochemical Deposition. ACS Appl. Mater. Inter. 2017, 9, 5023–5030. [Google Scholar] [CrossRef]
- Schutzius, T.M.; Jung, S.; Maitra, T.; Graeber, G.; Köhme, M.; Poulikakos, D. Spontaneous Droplet Trampolining on Rigid Superhydrophobic Surfaces. Nature 2015, 527, 82–85. [Google Scholar] [CrossRef]
- Liu, Y.; Moevius, L.; Xu, X.; Qian, T.; Yeomans, J.M.; Wang, Z. Pancake Bouncing on Superhydrophobic Surfaces. Nat. Phys. 2014, 10, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Alkawareek, M.Y.; Akkelah, B.M.; Mansour, S.M.; Amro, H.M.; Abulateefeh, S.R.; Alkilany, A.M. Simple Experiment to Determine Surfactant Critical Micelle Concentrations Using Contact-Angle Measurements. J. Chem. Educ. 2018, 95, 2227–2232. [Google Scholar] [CrossRef]
- Zhu, F.; Cao, C.; Cao, L.; Li, F.; Du, F.; Huang, Q. Wetting Behavior and Maximum Retention of Aqueous Surfactant Solutions on Tea Leaves. Molecules 2019, 24, 2094. [Google Scholar] [CrossRef]
- Goronja, J.; Pejić, N.; Janošević Ležaić, A.; Stanisavljev, D.; Malenović, A. Using a Combination of Experimental and Mathematical Method To Explore Critical Micelle Concentration of a Cationic Surfactant. J. Chem. Educ. 2016, 93, 1277–1281. [Google Scholar] [CrossRef]
- Hu, B.-B.; Yuan, Y.; Zhou, X.-P.; Li, S.-M. Synthesis and Properties of a Novel Bolaamphiphile Surfactant Derived from Proline. Chin. Chem. Let. 2016, 27, 447–450. [Google Scholar] [CrossRef]
- Zhiqing, Z.; Fang, W.; Chao, R. Experimental Comparison of Critical Micelle Concentration of Surfactant by Conductivity Method and Max Bubble Pressure Method. Experi. Technol. Manage. 2013, 30, 44–46. [Google Scholar]
- More, U.; Vaid, Z.; Bhamoria, P.; Kumar, A.; Malek, N.I. Effect of [C n mim][Br] Based Ionic Liquids on the Aggregation Behavior of Tetradecyltrimethylammonium Bromide in Aqueous Medium. J. Sol. Chem. 2015, 44, 850–874. [Google Scholar] [CrossRef]
- Ruso, J.M.; Sarmiento, F. The interaction between n -alkyl trimethylammonium bromides with poly(l -aspartate): A thermodynamics study. Colloid Polym. Sci. 2000, 278, 800–804. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-P.; Zhang, S.-M.; Liu, P.-F.; Chen, D.-L.; Chen, Y.; Chen, M.-J.; Zhao, C.-H. Robust Superhydrophobic Brass Mesh with Electrodeposited Hydroxyapatite Coating for Versatile Applications. Molecules 2022, 27, 5624. https://doi.org/10.3390/molecules27175624
Zhang Y-P, Zhang S-M, Liu P-F, Chen D-L, Chen Y, Chen M-J, Zhao C-H. Robust Superhydrophobic Brass Mesh with Electrodeposited Hydroxyapatite Coating for Versatile Applications. Molecules. 2022; 27(17):5624. https://doi.org/10.3390/molecules27175624
Chicago/Turabian StyleZhang, Yu-Ping, Shi-Ming Zhang, Peng-Fei Liu, De-Liang Chen, Yuan Chen, Meng-Jun Chen, and Chang-Hua Zhao. 2022. "Robust Superhydrophobic Brass Mesh with Electrodeposited Hydroxyapatite Coating for Versatile Applications" Molecules 27, no. 17: 5624. https://doi.org/10.3390/molecules27175624
APA StyleZhang, Y.-P., Zhang, S.-M., Liu, P.-F., Chen, D.-L., Chen, Y., Chen, M.-J., & Zhao, C.-H. (2022). Robust Superhydrophobic Brass Mesh with Electrodeposited Hydroxyapatite Coating for Versatile Applications. Molecules, 27(17), 5624. https://doi.org/10.3390/molecules27175624




