How Computations Can Assist the Rational Design of Drugs for Photodynamic Therapy: Photosensitizing Activity Assessment of a Ru(II)-BODIPY Assembly
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photophysical Properties of BDP, Ru and Ru-BDP Conjugate
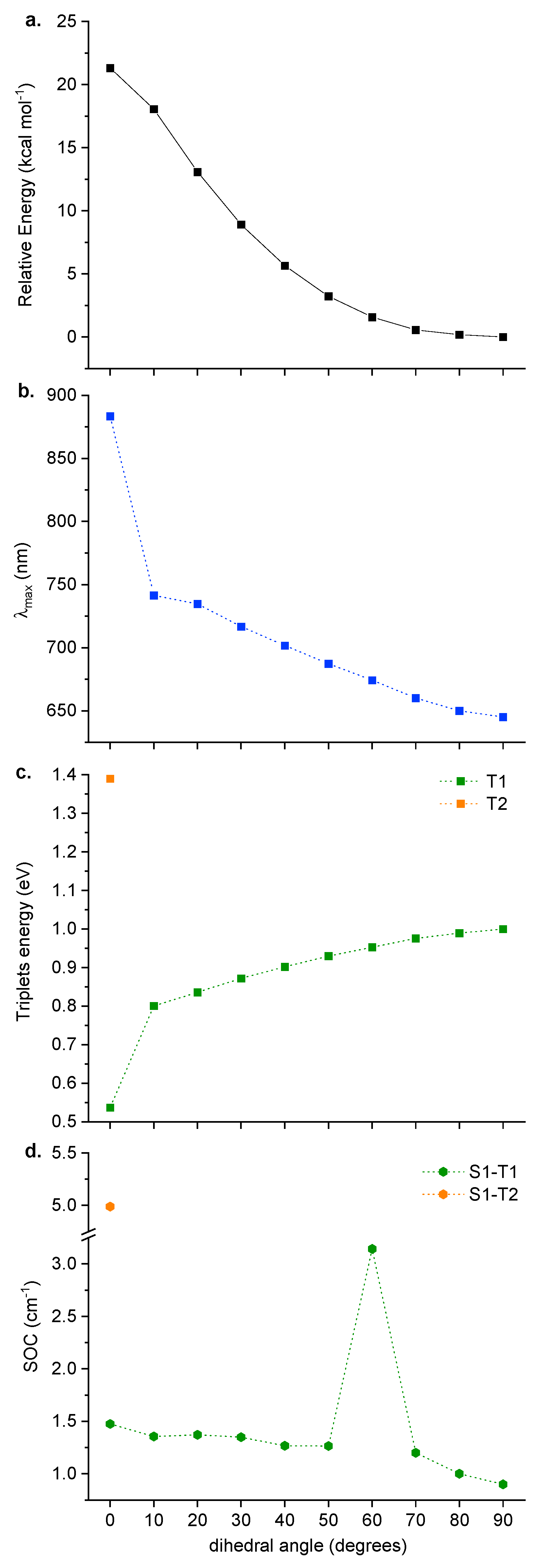
2.2. Structural Features of the Conjugate
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and Photodynamic Therapy: Mechanisms, Monitoring and Optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [PubMed]
- Monro, S.; Colón, K.L.; Yin, H.; Roque, J.; Konda, P.; Gujar, S.; Thummel, R.P.; Lilge, L.; Cameron, C.G.; McFarland, S.A. Transition Metal Complexes and Photodynamic Therapy from a Tumor-Centered Approach: Challenges, Opportunities, and Highlights from the Development of TLD1433. Chem. Rev. 2019, 119, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, J. Reactive Oxygen Species in Photodynamic Therapy: Mechanisms of Their Generation and Potentiation. In Advances in Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; Volume 70, pp. 343–394. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy—Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Lilge, L.; Portnoy, M.; Wilson, B.C. Apoptosis Induced in Vivo by Photodynamic Therapy in Normal Brain and Intracranial Tumour Tissue. Br. J. Cancer 2000, 83, 1110–1117. [Google Scholar] [CrossRef]
- Dąbrowski, J.M.; Arnaut, L.G. Photodynamic Therapy (PDT) of Cancer: From Local to Systemic Treatment. Photochem. Photobiol. Sci. 2015, 14, 1765–1780. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, G.; Nogueira, J.J. Stacking Effects on Anthraquinone/DNA Charge-Transfer Electronically Excited States. Molecules 2020, 25, 5927. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Zhang, L.; Chen, Z.; Zheng, B.-Y.; Ke, M.; Li, X.; Huang, J.-D. Nanostructured Phthalocyanine Assemblies with Efficient Synergistic Effect of Type I Photoreaction and Photothermal Action to Overcome Tumor Hypoxia in Photodynamic Therapy. J. Am. Chem. Soc. 2021, 143, 13980–13989. [Google Scholar] [CrossRef]
- Nash, G.T.; Luo, T.; Lan, G.; Ni, K.; Kaufmann, M.; Lin, W. Nanoscale Metal–Organic Layer Isolates Phthalocyanines for Efficient Mitochondria-Targeted Photodynamic Therapy. J. Am. Chem. Soc. 2021, 143, 2194–2199. [Google Scholar] [CrossRef]
- Ponte, F.; Mazzone, G.; Russo, N.; Sicilia, E. BODIPY for Photodynamic Therapy Applications: Computational Study of the Effect of Bromine Substitution on 1O2 Photosensitization. J. Mol. Model. 2018, 24, 183. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Yang, X. Singlet Oxygen Generation and Triplet Excited-State Spectra of Brominated BODIPY. J. Phys. Chem. B 2013, 117, 5533–5539. [Google Scholar] [CrossRef] [PubMed]
- Masood, M.A.; Wu, Y.; Chen, Y.; Yuan, H.; Sher, N.; Faiz, F.; Yao, S.; Qi, F.; Khan, M.I.; Ahmed, M.; et al. Optimizing the Photodynamic Therapeutic Effect of BODIPY-Based Photosensitizers against Cancer and Bacterial Cells. Dye. Pigment. 2022, 202, 110255. [Google Scholar] [CrossRef]
- Nguyen, V.N.; Ha, J.; Cho, M.; Li, H.; Swamy, K.M.K.; Yoon, J. Recent Developments of BODIPY-Based Colorimetric and Fluorescent Probes for the Detection of Reactive Oxygen/Nitrogen Species and Cancer Diagnosis. Coord. Chem. Rev. 2021, 439, 213936. [Google Scholar] [CrossRef]
- Simone, B.C.D.; Mazzone, G.; Pirillo, J.; Russo, N.; Sicilia, E. Halogen Atom Effect on the Photophysical Properties of Substituted Aza-BODIPY Derivatives. Phys. Chem. Chem. Phys. 2017, 19, 2530–2536. [Google Scholar] [CrossRef]
- Sun, T.; Guan, X.; Zheng, M.; Jing, X.; Xie, Z. Mitochondria-Localized Fluorescent BODIPY-Platinum Conjugate. ACS Med. Chem. Lett. 2015, 6, 430–433. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Chen, L.; Xie, Z. Near Infrared BODIPY-Platinum Conjugates for Imaging, Photodynamic Therapy and Chemotherapy. Dye. Pigment. 2017, 141, 5–12. [Google Scholar] [CrossRef]
- Imberti, C.; Zhang, P.; Huang, H.; Sadler, P.J. New Designs for Phototherapeutic Transition Metal Complexes. Angew. Chem. Int. Ed. 2020, 59, 61–73. [Google Scholar] [CrossRef]
- Karges, J. Clinical Development of Metal Complexes as Photosensitizers for Photodynamic Therapy of Cancer. Angew. Chem. Int. Ed. 2022, 61, e202112236. [Google Scholar] [CrossRef]
- Lazic, S.; Kaspler, P.; Shi, G.; Monro, S.; Sainuddin, T.; Forward, S.; Kasimova, K.; Hennigar, R.; Mandel, A.; McFarland, S.; et al. Novel Osmium-Based Coordination Complexes as Photosensitizers for Panchromatic Photodynamic Therapy. Photochem. Photobiol. 2017, 93, 1248–1258. [Google Scholar] [CrossRef]
- Zamora Martínez, A.; Vigueras Bautista, G.; Rodriguez, V.; Santana, M.; Ruiz, J. Cyclometalated Iridium(III) Luminescent Complexes in Therapy and Phototherapy. Coord. Chem. Rev. 2018, 360, 34–76. [Google Scholar] [CrossRef]
- Roque, J.A.; Barrett, P.C.; Cole, H.D.; Lifshits, L.M.; Shi, G.; Monro, S.; von Dohlen, D.; Kim, S.; Russo, N.; Deep, G.; et al. Breaking the Barrier: An Osmium Photosensitizer with Unprecedented Hypoxic Phototoxicity for Real World Photodynamic Therapy. Chem. Sci. 2020, 11, 9784–9806. [Google Scholar] [CrossRef] [PubMed]
- Ponte, F.; Alberto, M.E.; De Simone, B.C.; Russo, N.; Sicilia, E. Photophysical Exploration of Dual-Approach PtII–BODIPY Conjugates: Theoretical Insights. Inorg. Chem. 2019, 58, 9882–9889. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Yuan, H.; Chen, Y.; Guo, Y.; Zhang, S.; Liu, Z.; He, W.; Guo, Z. BODIPY-Based Monofunctional Pt (II) Complexes for Specific Photocytotoxicity against Cancer Cells. J. Inorg. Biochem. 2021, 218, 111394. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Gunawan, Y.F.; Liu, G.; Tse, M.-K.; Zhu, G. Optimization of Axial Ligands to Promote the Photoactivation of BODIPY-Conjugated Platinum(IV) Anticancer Prodrugs. Dalton Trans. 2021, 50, 13737–13747. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Gautam, S.; Raza, M.K.; Kondaiah, P.; Chakravarty, A.R. Oxoplatin-B, a Cisplatin-Based Platinum(IV) Complex with Photoactive BODIPY for Mitochondria Specific “Chemo-PDT” Activity. J. Inorg. Biochem. 2021, 223, 111526. [Google Scholar] [CrossRef]
- Karges, J.; Tharaud, M.; Gasser, G. Polymeric Encapsulation of a Ru(II)-Based Photosensitizer for Folate-Targeted Photodynamic Therapy of Drug Resistant Cancers. J. Med. Chem. 2021, 64, 4612–4622. [Google Scholar] [CrossRef]
- Karges, J.; Heinemann, F.; Jakubaszek, M.; Maschietto, F.; Subecz, C.; Dotou, M.; Vinck, R.; Blacque, O.; Tharaud, M.; Goud, B.; et al. Rationally Designed Long-Wavelength Absorbing Ru(II) Polypyridyl Complexes as Photosensitizers for Photodynamic Therapy. J. Am. Chem. Soc. 2020, 142, 6578–6587. [Google Scholar] [CrossRef]
- Padilla, R.; Maza, W.A.; Dominijanni, A.J.; Winkel, B.S.J.; Morris, A.J.; Brewer, K.J. Pushing the Limits of Structurally-Diverse Light-Harvesting Ru(II) Metal-Organic Chromophores for Photodynamic Therapy. J. Photochem. Photobiol. A Chem. 2016, 322–323, 67–75. [Google Scholar] [CrossRef]
- Higgins, S.L.H.; Brewer, K.J. Designing Red-Light-Activated Multifunctional Agents for the Photodynamic Therapy. Angew. Chem. Int. Ed. 2012, 51, 11420–11422. [Google Scholar] [CrossRef]
- Campagna, S.; Puntoriero, F.; Nastasi, F.; Bergamini, G.; Balzani, V. Photochemistry and Photophysics of Coordination Compounds: Ruthenium. In Photochemistry and Photophysics of Coordination Compounds I; Balzani, V., Campagna, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 280, pp. 117–214. ISBN 3540733469. [Google Scholar]
- Paul, S.; Kundu, P.; Kondaiah, P.; Chakravarty, A.R. BODIPY-Ruthenium(II) Bis-Terpyridine Complexes for Cellular Imaging and Type-I/-II Photodynamic Therapy. Inorg. Chem. 2021, 60, 16178–16193. [Google Scholar] [CrossRef]
- Qiao, L.; Liu, J.; Han, Y.; Wei, F.; Liao, X.; Zhang, C.; Xie, L.; Ji, L.; Chao, H. Rational Design of a Lysosome-Targeting and near-Infrared Absorbing Ru(II)–BODIPY Conjugate for Photodynamic Therapy. Chem. Commun. 2021, 57, 1790–1793. [Google Scholar] [CrossRef] [PubMed]
- Adamo, C.; Jacquemin, D. The Calculations of Excited-State Properties with Time-Dependent Density Functional Theory. Chem. Soc. Rev. 2013, 42, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, G.; Quartarolo, A.D.; Russo, N. PDT-Correlated Photophysical Properties of Thienopyrrole BODIPY Derivatives. Theoretical Insights. Dye. Pigment. 2016, 130, 9–15. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, K.; Yang, W.; Wang, Z.; Zhong, F. The Triplet Excited State of Bodipy: Formation, Modulation and Application. Chem. Soc. Rev. 2015, 44, 8904–8939. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, X.-F.; Lu, X.; Lan, S.; Tian, D.; Li, T.; Wang, L.; Zhao, S.; Feng, M.; Zhang, J. Attaching Electron Donating Groups on the Meso-Phenyl and Meso-Naphthyl Make Aryl Substituted BODIPYs Act as Good Photosensitizer for Singlet Oxygen Formation. J. Lumin. 2018, 194, 185–192. [Google Scholar] [CrossRef]
- Prieto-Montero, R.; Prieto-Castañeda, A.; Katsumiti, A.; Sola-Llano, R.; Agarrabeitia, A.R.; Cajaraville, M.P.; Ortiz, M.J.; Martinez-Martinez, V. Red HaloBODIPYs as Theragnostic Agents: The Role of the Substitution at Meso Position. Dye. Pigment. 2022, 198, 110015. [Google Scholar] [CrossRef]
- Mazzone, G.; Alberto, M.E.; De Simone, B.C.; Marino, T.; Russo, N. Can Expanded Bacteriochlorins Act as Photosensitizers in Photodynamic Therapy? Good News from Density Functional Theory Computations. Molecules 2016, 21, 288. [Google Scholar] [CrossRef]
- El-Sayed, M.A. Triplet State. Its Radiative and Nonradiative Properties. Acc. Chem. Res. 1968, 1, 8–16. [Google Scholar] [CrossRef]
- Marian, C.M. Spin–Orbit Coupling and Intersystem Crossing in Molecules. WIREs Comput. Mol. Sci. 2012, 2, 187–203. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; GaussView, 5.0; Gaussian, Inc.; European University Association (EUA): Wallingford, CT, USA, 2016. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B Condens. Matter. 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuß, H. Energy-Adjustedab Initio Pseudopotentials for the Second and Third Row Transition Elements. Theoret. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, Molecules, Solids, and Surfaces: Applications of the Generalized Gradient Approximation for Exchange and Correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Wang, Y. Accurate and Simple Analytic Representation of the Electron-Gas Correlation Energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A New Hybrid Exchange–Correlation Functional Using the Coulomb-Attenuating Method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-Range Corrected Hybrid Density Functionals with Damped Atom–Atom Dispersion Corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [Green Version]
- Tao, J.; Perdew, J.P.; Staroverov, V.N.; Scuseria, G.E. Climbing the Density Functional Ladder: Nonempirical Meta--Generalized Gradient Approximation Designed for Molecules and Solids. Phys. Rev. Lett. 2003, 91, 146401. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Ernzerhof, M.; Scuseria, G.E. Assessment of the Perdew–Burke–Ernzerhof Exchange-Correlation Functional. J. Chem. Phys. 1999, 110, 5029–5036. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Peverati, R.; Truhlar, D.G. Improving the Accuracy of Hybrid Meta-GGA Density Functionals by Range Separation. J. Phys. Chem. Lett. 2011, 2, 2810–2817. [Google Scholar] [CrossRef]
- Peverati, R.; Truhlar, D.G. An Improved and Broadly Accurate Local Approximation to the Exchange–Correlation Density Functional: The MN12-L Functional for Electronic Structure Calculations in Chemistry and Physics. Phys. Chem. Chem. Phys. 2012, 14, 13171–13174. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.S.; He, X.; Li, S.L.; Truhlar, D.G. MN15: A Kohn–Sham Global-Hybrid Exchange–Correlation Density Functional with Broad Accuracy for Multi-Reference and Single-Reference Systems and Noncovalent Interactions. Chem. Sci. 2016, 7, 5032–5051. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.S.; He, X.; Truhlar, D.G. MN15-L: A New Local Exchange-Correlation Functional for Kohn-Sham Density Functional Theory with Broad Accuracy for Atoms, Molecules, and Solids. J. Chem. Theory Comput. 2016, 12, 1280–1293. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System, Version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Neese, F. The ORCA Program System. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
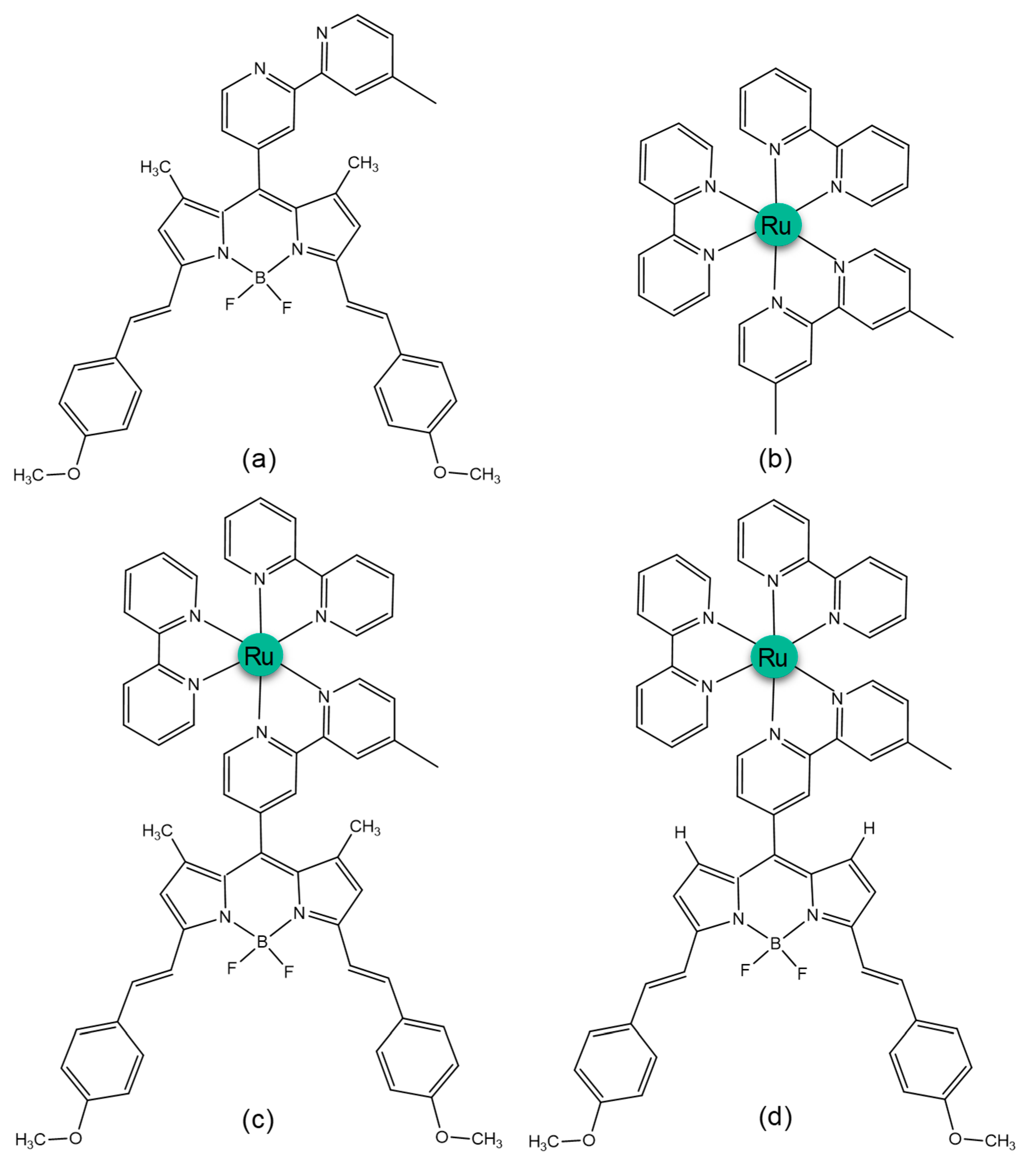
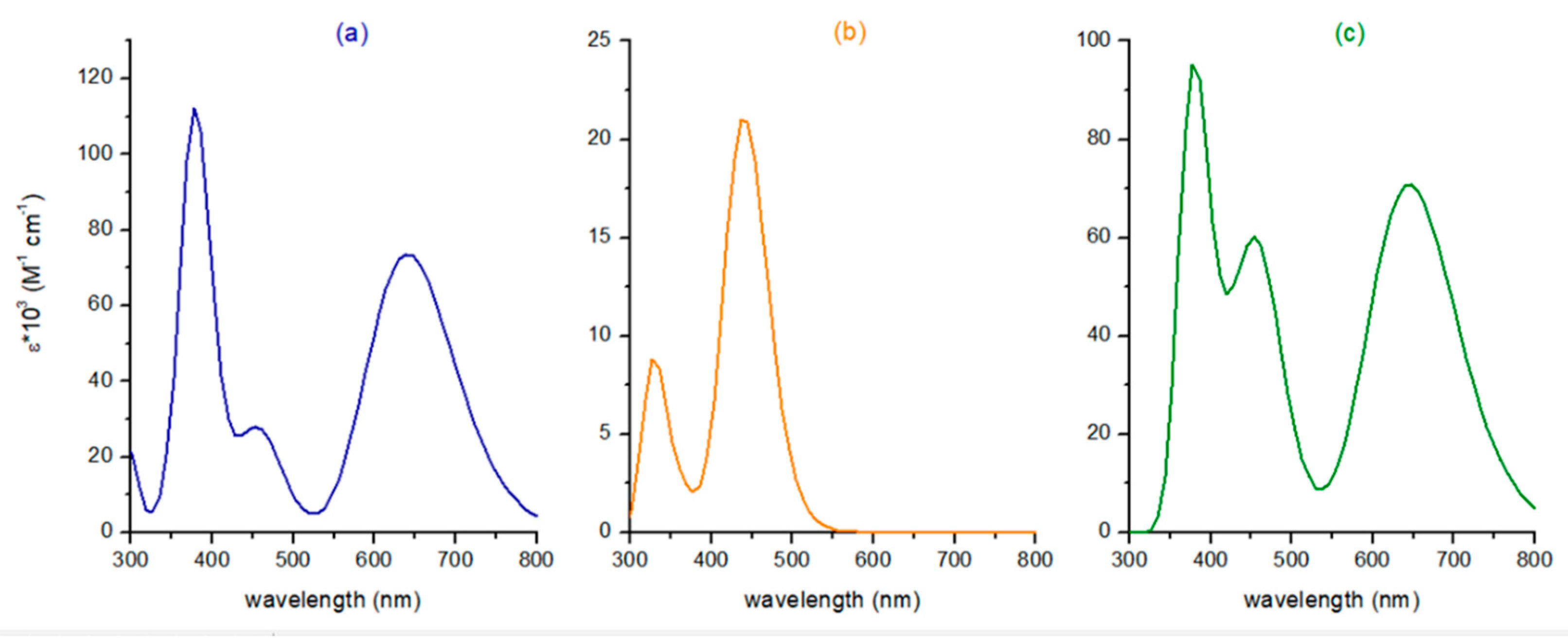
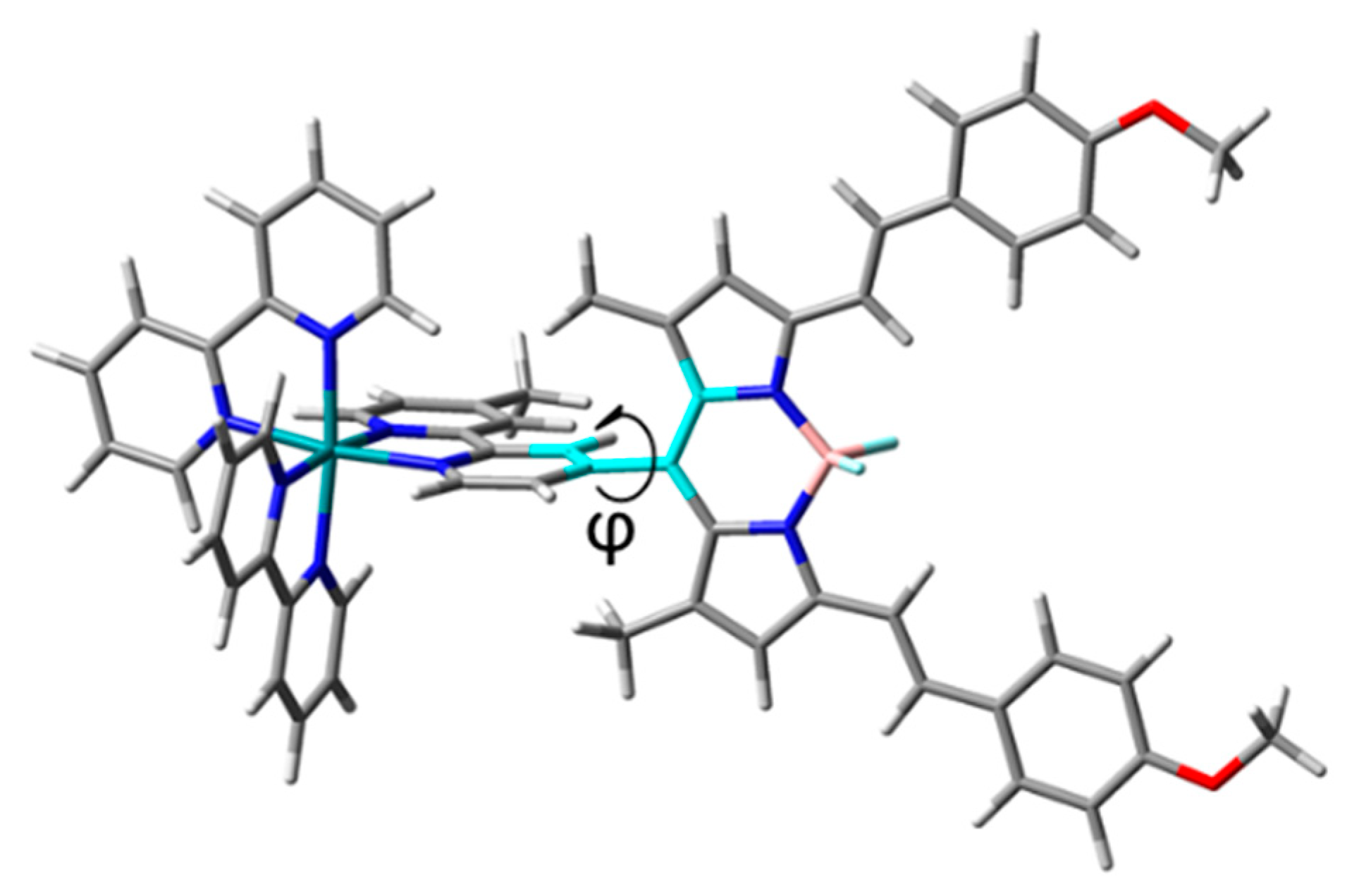
| Compound | Tr a | Band | ΔE | λ | f | MO Contribution b | Theoretical Assignment |
|---|---|---|---|---|---|---|---|
| BDP | 1 | I | 1.93 | 641 | 1.04 | H→L 100% | π-π* |
| 2 | II | 2.71 | 457 | 0.38 | H-1→L 87% | π-π* | |
| 3 | III | 3.02 | 410 | 0.08 | H→L+1 89% | π-π* | |
| 4 | 3.27 | 379 | 1.36 | H→L+2 72% | |||
| 5 | 3.28 | 378 | 0.17 | H-2→L 49%, H-3→L 18% | |||
| 6 | 3.53 | 351 | 0.04 | H→L+4 59%, H→L+3 34% | |||
| 7 | 3.56 | 348 | 0.05 | H-5→L 99% | |||
| 8 | 3.64 | 341 | 0.02 | H→L+3 41%, H→L+4 36% | |||
| 9 | IV | 4.16 | 298 | 0.03 | H→L+6 85% | π-π* | |
| 10 | 4.20 | 295 | 0.24 | H-9→L 77% | |||
| 11 | 4.21 | 294 | 0.06 | H→L+7 71% | |||
| Ru | 1 | I | 2.47 | 502 | 0.00 | H→L 92% | MLCT/ILCT |
| 2 | 2.67 | 464 | 0.01 | H-1→L+1 52%, H-2→L 28% | |||
| 3 | 2.74 | 451 | 0.06 | H-2→L 62%, H-2→L+2 22% | |||
| 4 | 2.83 | 438 | 0.08 | H-2→L+2 67%, H-1→L+1 25% | |||
| 5 | 2.85 | 436 | 0.14 | H-2→L+1 46%, H-1→L+2 43% | MLCT | ||
| 6 | II | 3.31 | 374 | 0.01 | H→L+3 98% | MLCT/ILCT | |
| 7 | 3.49 | 355 | 0.01 | H-1→L+3 98% | |||
| 8 | 3.52 | 352 | 0.01 | H-2→L+3 99% | |||
| 9 | 3.66 | 338 | 0.01 | H→L+5 97% | |||
| 10 | 3.80 | 326 | 0.06 | H-1→L+6 79% | |||
| Ru-BDP | 1 | I | 1.92 | 645 | 1.00 | H→L 100% | ILCT |
| 2 | 2.33 | 532 | 0.01 | H→L+1 84% | LMCT/ILCT | ||
| 3 | II | 2.68 | 463 | 0.49 | H-4→L 47%, H-2→L+1 44% | ILCT | |
| 4 | 2.69 | 462 | 0.07 | H-3→L+1 45%, H-2→L+1 21% | LMCT/ILCT | ||
| 5 | 2.70 | 458 | 0.04 | H-2→L+2 42%, H-2→L+1 18% | MLCT | ||
| 6 | 2.71 | 457 | 0.04 | H-3→L+1 40%, H-3→L+3 25% | MLCT | ||
| 7 | 2.83 | 438 | 0.12 | H-3→L+3 40%, H-2→L+2 26% | MLCT/ILCT | ||
| 8 | 2.85 | 436 | 0.13 | H-2→L+3 36%, H-3→L+2 35% | MLCT | ||
| 9 | III | 3.04 | 408 | 0.19 | H→L+4 85% | LLCT/ILCT | |
| 10 | 3.28 | 378 | 1.27 | H→L+5 76% | LLCT/ILCT |
| Compound | State | ΔE | MO Contribution | Theoretical Assignment |
|---|---|---|---|---|
| BDP | T1 | 1.01 | H→L 99% | π-π* |
| Ru | T1 | 2.33 | H→L+1 88% | MLCT/ILCT |
| T2 | 2.34 | H→L 63%, H→L+2 26% | MLCT/ILCT | |
| T3 | 2.40 | H-2→L+1 62%, H-1→L+2 25% | MLCT/ILCT | |
| T4 | 2.41 | H→L+2 61%, H→L 27% | MLCT | |
| T5 | 2.48 | H-1→L+1 40%, H-2→L 39% | MLCT/ILCT | |
| T6 | 2.53 | H-1→L+2 68%, H-2→L+1 25% | MLCT | |
| T7 | 2.58 | H-1→L 85% | MLCT/ILCT | |
| T8 | 2.60 | H-1→L+1 46%, H-2→L 44% | MLCT/ILCT | |
| T9 | 2.70 | H-2→L+2 74% | MLCT/LLCT | |
| Ru-BDP | T1 | 1.00 | H→L 99% | ILCT |
| Tm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sn | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| BDP | 1 | 0.02 (0.92) | ||||||||
| Ru | 1 | 64.31 (0.14) | 17.43 (0.13) | 174.79 (0.07) | 3.92 (0.06) | |||||
| 2 | 35.10 (0.17) | 59.63 (0.16) | 326.53 (0.10) | 8.99 (0.09) | 277.95 (0.02) | |||||
| 3 | 24.47 (0.25) | 26.99 (0.24) | 143.18 (0.18) | 61.56 (0.16) | 74.62 (0.09) | 316.15 (0.04) | ||||
| 4 | 323.15 (0.34) | 220.03 (0.33) | 269.23 (0.27) | 7.92 (0.26) | 4.39 (0.19) | 62.45 (0.14) | 72.67 (0.09) | 41.38 (0.07) | ||
| 5 | 144.13 (0.34) | 339.81 (0.33) | 9.60 (0.28) | 138.15 (0.26) | 267.93 (0.19) | 28.83 (0.14) | 17.97 (0.09) | 131.45 (0.07) | ||
| 6 | 51.57 (0.42) | 65.83 (0.41) | 59.51 (0.35) | 378.67 (0.34) | 16.05 (0.27) | 143.54 (0.22) | 239.22 (0.17) | 11.13 (0.14) | 27.29 (0.05) | |
| 7 | 222.18 (0.51) | 313.33 (0.49) | 6.87 (0.44) | 97.27 (0.42) | 37.20 (0.35) | 270.91 (0.30) | 146.0 (0.25) | 38.06 (0.23) | 27.92 (0.14) | |
| 8 | 256.01 (0.52) | 35.11 (0.51) | 3.48 (0.45) | 294.79 (0.43) | 155.84 (0.37) | 31.32 (0.31) | 28.09 (0.27) | 179.42 (0.24) | 161.21 (0.15) | |
| Ru-BDP | 1 | 0.92 (0.92) | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponte, F.; Scopelliti, D.M.; Sanna, N.; Sicilia, E.; Mazzone, G. How Computations Can Assist the Rational Design of Drugs for Photodynamic Therapy: Photosensitizing Activity Assessment of a Ru(II)-BODIPY Assembly. Molecules 2022, 27, 5635. https://doi.org/10.3390/molecules27175635
Ponte F, Scopelliti DM, Sanna N, Sicilia E, Mazzone G. How Computations Can Assist the Rational Design of Drugs for Photodynamic Therapy: Photosensitizing Activity Assessment of a Ru(II)-BODIPY Assembly. Molecules. 2022; 27(17):5635. https://doi.org/10.3390/molecules27175635
Chicago/Turabian StylePonte, Fortuna, Davide Maria Scopelliti, Nico Sanna, Emilia Sicilia, and Gloria Mazzone. 2022. "How Computations Can Assist the Rational Design of Drugs for Photodynamic Therapy: Photosensitizing Activity Assessment of a Ru(II)-BODIPY Assembly" Molecules 27, no. 17: 5635. https://doi.org/10.3390/molecules27175635
APA StylePonte, F., Scopelliti, D. M., Sanna, N., Sicilia, E., & Mazzone, G. (2022). How Computations Can Assist the Rational Design of Drugs for Photodynamic Therapy: Photosensitizing Activity Assessment of a Ru(II)-BODIPY Assembly. Molecules, 27(17), 5635. https://doi.org/10.3390/molecules27175635









