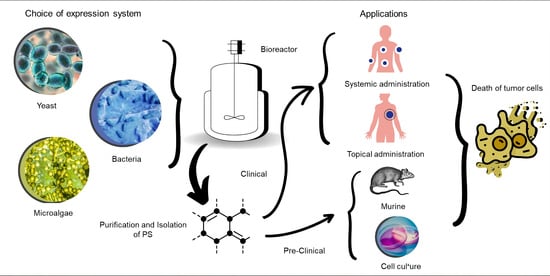
Development of Biotechnological Photosensitizers for Photodynamic Therapy: Cancer Research and Treatment—From Benchtop to Clinical Practice
Abstract
1. Introduction
2. Photosensitizers Compounds
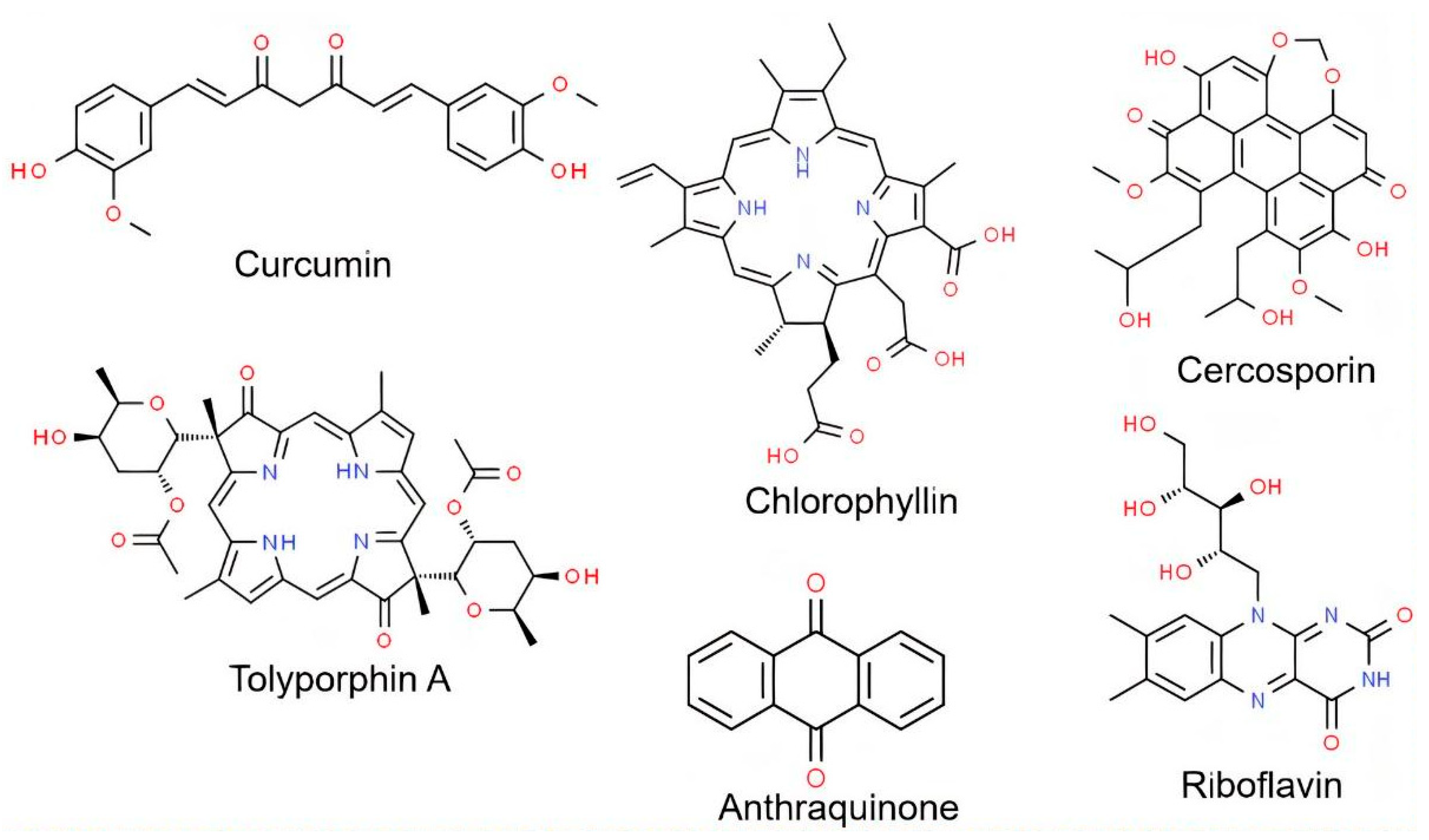
3. Compounds from Microbial Origin—Biotechnological Photosensitizers
4. Application of PDT Photosensitizers in Cancer Treatment
5. Conclusions
6. Challenges and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niculescu, A.G.; Grumezescu, A.M. Photodynamic Therapy—An Up-to-Date Review. Appl. Sci. 2021, 11, 3626. [Google Scholar] [CrossRef]
- Smith, C.B.; Days, L.C.; Alajroush, D.R.; Faye, K.; Khodour, Y.; Beebe, S.J.; Holder, A.A. Photodynamic Therapy of Inorganic Complexes for the Treatment of Cancer. Photochem. Photobiol. 2022, 98, 17–41. [Google Scholar] [CrossRef]
- Sandland, J.; Malatesti, N.; Boyle, R. Porphyrins and Related Macrocycles: Combining Photosensitization with Radio- or Optical-Imaging for next Generation Theranostic Agents. Photodiagnosis Photodyn. Ther. 2018, 23, 281–294. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zhou, F.; Doughty, A.; Hoover, A.R.; Nordquist, R.E.; Chen, W.R. Nanotechnology-Based Photoimmunological Therapies for Cancer. Cancer Lett. 2019, 442, 429–438. [Google Scholar] [CrossRef]
- Silvestre, A.L.P.; di Filippo, L.D.; Besegato, J.F.; de Annunzio, S.R.; Almeida Furquim de Camargo, B.; de Melo, P.B.G.; Rastelli, A.N.de.S.; Fontana, C.R.; Chorilli, M. Current Applications of Drug Delivery Nanosystems Associated with Antimicrobial Photodynamic Therapy for Oral Infections. Int. J. Pharm. 2021, 592, 120078. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic Therapy (PDT): A Short Review on Cellular Mechanisms and Cancer Research Applications for PDT. J. Photochem. Photobiol. B 2009, 96, 1–8. [Google Scholar] [CrossRef]
- Sabir, F.; Barani, M.; Rahdar, A.; Bilal, M.; Zafar, M.N.; Bungau, S.; Kyzas, G.Z. How to Face Skin Cancer with Nanomaterials: A Review. Biointerface Res. Appl. Chem. 2021, 11, 11931–11955. [Google Scholar]
- Sperandio, A.; Vivian de, I.; Elisa Pimenta, V. Nanoemulsões Formuladas Para Uso Tópico: Estudo de Síntese e Toxicidade. Nanoemulsions Formulated for Topical Use: A Study of Synthesis and Toxicity. Revista Fitos 2020, 14, 513–527. [Google Scholar] [CrossRef]
- Lee, N.H.; You, S.; Taghizadeh, A.; Taghizadeh, M.; Kim, H.S. Cell Membrane-Cloaked Nanotherapeutics for Targeted Drug Delivery. Int. J. Mol. Sci. 2022, 23, 2223. [Google Scholar] [CrossRef]
- Nesi-Reis, V.; Lera-Nonose, D.S.S.L.; Oyama, J.; Silva-Lalucci, M.P.P.; Demarchi, I.G.; Aristides, S.M.A.; Teixeira, J.J.V.; Silveira, T.G.V.; Lonardoni, M.V.C. Contribution of Photodynamic Therapy in Wound Healing: A Systematic Review. Photodiagnosis Photodyn. Ther. 2018, 21, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Gorbachev, D.A.; Staroverov, D.B.; Lukyanov, K.A.; Sarkisyan, K.S. Genetically Encoded Red Photosensitizers with Enhanced Phototoxicity. Int. J. Mol. Sci. 2020, 21, 8800. [Google Scholar] [CrossRef]
- Sowa, A.; Voskuhl, J. Host-Guest Complexes—Boosting the Performance of Photosensitizers. Int. J. Pharm. 2020, 586, 119595. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, J.; Zhang, H.; Yin, P.; Li, T.; Li, Q.; Li, Q.; Liu, Y.; Liu, H.-B. A Highly Sensitive Magnetic Nano-Fluorescent Probe for Singlet Oxygen Detection and Screening of Natural Photosensitizers. Sens. Actuators B. Chem. 2022, 369, 132346. [Google Scholar] [CrossRef]
- D’Alessandro, S.; Priefer, R. Non-Porphyrin Dyes Used as Photosensitizers in Photodynamic Therapy. J. Drug Deliv. Sci. Technol. 2020, 60, 101979. [Google Scholar] [CrossRef]
- Gulati, L.K.; Gulati, G.K.; Kumar, S. Photochromic Materials as a Photosensitizer in Reversible Reactive Singlet Oxygen Generation. Dye. Pigment. 2022, 199, 110104. [Google Scholar] [CrossRef]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of Third Generation Photosensitizers Used in Anticancer Photodynamic Therapy: Review. Photodiagnosis Photodyn. Ther. 2021, 34, 102091. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-T.; Sui, S.-Y.; He, Y.-X.; Yu, C.-H.; Peng, Q. Nanomaterials-Based Photosensitizers and Delivery Systems for Photodynamic Cancer Therapy. Biomater. Adv. 2022, 135, 212725. [Google Scholar] [CrossRef] [PubMed]
- Weijer, R.; Broekgaarden, M.; Kos, M.; van Vught, R.; Rauws, E.A.J.; Breukink, E.; van Gulik, T.M.; Storm, G.; Heger, M. Enhancing Photodynamic Therapy of Refractory Solid Cancers: Combining Second-Generation Photosensitizers with Multi-Targeted Liposomal Delivery. J. Photochem. Photobiol. C Photochem. Rev. 2015, 23, 103–131. [Google Scholar] [CrossRef]
- Mussagy, C.; Khan, S.; Kot, A.M. Current Developments on the Application of Microbial Carotenoids as an Alternative to Synthetic Pigments. Crit. Rev. Food Sci. Nutr. 2021, 62, 6932–6946. [Google Scholar] [CrossRef]
- Mussagy, C.; Winterburn, J.; Santos-Ebinuma, V.C.; Pereira, J.F.B. Production and Extraction of Carotenoids Produced by Microorganisms. Appl. Microbiol. Biotechnol. 2019, 103, 1095–1114. [Google Scholar] [CrossRef]
- Lagashetti, A.C.; Dufossé, L.; Singh, S.K.; Singh, P.N. Fungal Pigments and Their Prospects in Different Industries. Microorganisms 2019, 7, 604. [Google Scholar] [CrossRef]
- Dufossé, L. Microbial Pigments From Bacteria, Yeasts, Fungi, and Microalgae for the Food and Feed Industries. In Natural and Artificial Flavoring Agents and Food Dyes; Elsevier: London, UK, 2018; pp. 113–132. [Google Scholar]
- Mussagy, C.U.; Pereira, J.F.B.; Dufossé, L.; Raghavan, V.; Santos-Ebinuma, V.C.; Pessoa, A. Advances and Trends in Biotechnological Production of Natural Astaxanthin by Phaffia rhodozyma Yeast. Crit. Rev. Food Sci. Nutr. 2021, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Velho, S.R.K.; Brum, L.F.W.; Petter, C.O.; dos Santos, J.H.Z.; Šimunić, Š.; Kappa, W.H. Development of Structured Natural Dyes for Use into Plastics. Dye. Pigment. 2017, 136, 248–254. [Google Scholar] [CrossRef]
- Schmidt, I.; Schewe, H.; Gassel, S.; Jin, C.; Buckingham, J.; Hümbelin, M.; Sandmann, G.; Schrader, J. Biotechnological Production of Astaxanthin with Phaffia rhodozyma/Xanthophyllomyces dendrorhous. Appl. Microbiol. Biotechnol. 2011, 89, 555–571. [Google Scholar] [CrossRef]
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic Astaxanthin Is Significantly Inferior to Algal-Based Astaxanthin as an Antioxidant and May Not Be Suitable as a Human Nutraceutical Supplement. Nutrafoods 2013, 12, 145–152. [Google Scholar] [CrossRef]
- Kubrak, T.P.; Kołodziej, P.; Sawicki, J.; Mazur, A.; Koziorowska, K.; Aebisher, D. Some Natural Photosensitizers and Their Medicinal Properties for Use in Photodynamic Therapy. Molecules 2022, 27, 1192. [Google Scholar] [CrossRef]
- Gunaydin, G.; Emre Gedik, M.; Ayan, S. Photodynamic Therapy-Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Zhang, X.; Guo, W.; Li, F.; Yu, M.; Kong, X.; Wu, W.; Hong, Z. Highly Water-Soluble and Tumor-Targeted Photosensitizers for Photodynamic Therapy. Org. Biomol. Chem. 2015, 13, 7681–7694. [Google Scholar] [CrossRef]
- Li, Y.; Xie, J.; Um, W.; You, D.G.; Kwon, S.; Zhang, L.; Zhu, J.; Park, J.H. Sono/Photodynamic Nanomedicine-Elicited Cancer Immunotherapy. Adv. Funct. Mater. 2021, 31, 2008061. [Google Scholar] [CrossRef]
- Kuruppuarachchi, M.; Savoie, H.; Lowry, A.; Alonso, C.; Boyle, R.W. Polyacrylamide Nanoparticles as a Delivery System in Photodynamic Therapy. Mol. Pharm. 2011, 8, 920–931. [Google Scholar] [CrossRef]
- Rossetti, F.C.; Lopes, L.B.; Carollo, A.R.H.; Thomazini, J.A.; Tedesco, A.C.; Bentley, M.V.L.B. A Delivery System to Avoid Self-Aggregation and to Improve in vitro and in vivo Skin Delivery of a Phthalocyanine Derivative Used in the Photodynamic Therapy. J. Control. Release 2011, 155, 400–408. [Google Scholar] [CrossRef]
- Barnhart-Dailey, M.; Zhang, Y.; Zhang, R.; Anthony, S.M.; Aaron, J.S.; Miller, E.S.; Lindsey, J.S.; Timlin, J.A. Cellular Localization of Tolyporphins, Unusual Tetrapyrroles, in a Microbial Photosynthetic Community Determined Using Hyperspectral Confocal Fluorescence Microscopy. Photosynth. Res. 2019, 141, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, I.; Ali, S.E.; El-Tayeb, T.A.; Abdel-kader, M.H. Chlorophyll Derivative Mediated PDT versus Methotrexate: An in vitro Study Using MCF-7 Cells. Photodiagnosis Photodyn. Ther. 2012, 9, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Mussagy, C.U.; Gonzalez-Miquel, M.; Santos-Ebinuma, V.C.; Pereira, J.F.B. Microbial Torularhodin-a Comprehensive Review. Crit. Rev. Biotechnol. 2022, 1–19. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.; Hirai, P.R.; Teixeira, M.F.S.; Pereira, J.F.B.; Santos-Ebinuma, V.C. Talaromyces amestolkiae Cell Disruption and Colorant Extraction Using Imidazolium-Based Ionic Liquids. Sep. Purif. Technol. 2021, 257, 117759. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Kurnia, K.A.; Dias, A.C.R.V.; Raghavan, V.; Santos-Ebinuma, V.C.; Pessoa Jr, A. An Eco-Friendly Approach for the Recovery of Astaxanthin and β-Carotene from Phaffia rhodozyma Biomass Using Bio-Based Solvents. Bioresour. Technol. 2022, 345, 126555. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Ahmad, S.; Ahmad, A. Green Extraction Methods and Environmental Applications of Carotenoids-a Review. RSC Adv. 2015, 5, 62358–62393. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Pereira, J.F.B.; Santos-Ebinuma, V.C.; Pessoa, A.; Raghavan, V. Insights into Using Green and Unconventional Technologies to Recover Natural Astaxanthin from Microbial Biomass. Crit. Rev. Food Sci. Nutr. 2022, 1–15. [Google Scholar] [CrossRef]
- Comini, L.R.; Fernandez, I.M.; Vittar, N.B.R.; Núñez Montoya, S.C.; Cabrera, J.L.; Rivarola, V.A. Photodynamic Activity of Anthraquinones Isolated from Heterophyllaea pustulata Hook f. (Rubiaceae) on MCF-7c3 Breast Cancer Cells. Phytomedicine 2011, 18, 1093–1095. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Lin, J.-N.; Ma, J.-W.; Yang, N.-S.; Ho, C.-T.; Kuo, S.-C.; Way, T.-D. Demethoxycurcumin Induces Autophagic and Apoptotic Responses on Breast Cancer Cells in Photodynamic Therapy. J. Funct. Foods 2015, 12, 439–449. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, R.; Nazari, M.; Bagley, M.C.; Miller, E.S.; Williams, P.G.; Muddiman, D.C.; Lindsey, J.S. Mass Spectrometric Detection of Chlorophyll a and the Tetrapyrrole Secondary Metabolite Tolyporphin A in the Filamentous Cyanobacterium HT-58-2. Approaches to High-Throughput Screening of Intact Cyanobacteria. J. Porphyr. Phthalocyanines 2017, 21, 759–768. [Google Scholar] [CrossRef]
- Singh, V.L.; Chakravarty, S.; Chandra, N.; Mallick, N. Production of Sodium Copper Chlorophyllin from a Green Microalga Chlorella minutissima: A Value-Added Co-Product for Sustainable Microalgal Refinery. Food Bioprod. Process. 2020, 123, 322–334. [Google Scholar] [CrossRef]
- You, J.; Yang, C.; Pan, X.; Hu, M.; Du, Y.; Osire, T.; Yang, T.; Rao, Z. Metabolic Engineering of Bacillus subtilis for Enhancing Riboflavin Production by Alleviating Dissolved Oxygen Limitation. Bioresour. Technol. 2021, 333, 125228. [Google Scholar] [CrossRef]
- Kim, E.J.; Cha, M.N.; Kim, B.-G.; Ahn, J.-H. Production of Curcuminoids in Engineered Escherichia coli. J. Microbiol. Biotechnol. 2017, 27, 975–982. [Google Scholar] [CrossRef]
- Amantino, C.F.; de Baptista-Neto, Á.; Badino, A.C.; Siqueira-Moura, M.P.; Tedesco, A.C.; Primo, F.L. Anthraquinone Encapsulation into Polymeric Nanocapsules as a New Drug from Biotechnological Origin Designed for Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2020, 31, 101815. [Google Scholar] [CrossRef]
- Galanie, S.; Smolke, C.D. Optimization of Yeast-Based Production of Medicinal Protoberberine Alkaloids. Microb. Cell Fact. 2015, 14, 144. [Google Scholar] [CrossRef]
- Zhou, T.; Yu, S.; Hu, Y.; Zhang, Y.; Song, Y.; Chu, J.; Liu, C.; Rao, Y. Enhanced Cercosporin Production by Co-Culturing Cercospora Sp. JNU001 with Leaf-Spot-Disease-Related Endophytic Bacteria. Microb. Cell Fact. 2021, 20, 100. [Google Scholar] [CrossRef]
- dos Santos, A.F.; de Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic Therapy in Cancer Treatment-an Update Review. J. Cancer Metastasis Treatment 2019, 5, 25. [Google Scholar] [CrossRef]
- Cui, S.; Yin, D.; Chen, Y.; Di, Y.; Chen, H.; Ma, Y.; Achilefu, S.; Gu, Y. In vivo Targeted Deep-Tissue Photodynamic Therapy Based on near-Infrared Light Triggered Upconversion Nanoconstruct. ACS Nano. 2013, 7, 676–688. [Google Scholar] [CrossRef]
- Hammerle, F.; Bingger, I.; Pannwitz, A.; Magnutzki, A.; Gstir, R.; Rutz, A.; Wolfender, J.L.; Peintner, U.; Siewert, B. Targeted Isolation of Photoactive Pigments from Mushrooms Yielded a Highly Potent New Photosensitizer: 7,7′-Biphyscion. Sci. Rep. 2022, 12, 1108. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, A.; Balalas, T.; Mitrakas, A.; Vrazas, V.; Katsani, K.R.; Koumbis, A.E.; Koukourakis, M.I.; Litinas, K.E.; Fylaktakidou, K.C. 6-Nitro-Quinazolin−4(3H)−one Exhibits Photodynamic Effects and Photodegrades Human Melanoma Cell Lines. A Study on the Photoreactivity of Simple Quinazolin−4(3H)−ones. Photochem. Photobiol. 2021, 97, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kaur, K.; Karelia, D.N.; Beniwal, V.; Gupta, G.K.; Sharma, A.K.; Gupta, A.K. Synthesis and Biological Evaluation of Some 2-(3,5-Dimethyl-1H-Pyrazol-1- Yl)-1-Arylethanones: Antibacterial, DNA Photocleavage, and Anticancer Activities. Eur. J. Med. Chem. 2014, 81, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; He, C.; Lin, W. Nanoscale Metal-Organic Framework for Highly Effective Photodynamic Therapy of Resistant Head and Neck Cancer. J. Am. Chem. Soc. 2014, 136, 16712–16715. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Cai, Z.; Li, J.; Xiao, H.; Qi, R.; Zheng, M. Light Triggered Release of a Triple Action Porphyrin-Cisplatin Conjugate Evokes Stronger Immunogenic Cell Death for Chemotherapy, Photodynamic Therapy and Cancer Immunotherapy. J. Nanobiotechnol. 2022, 20, 329. [Google Scholar] [CrossRef]
- Tian, J.; Ding, L.; Xu, H.J.; Shen, Z.; Ju, H.; Jia, L.; Bao, L.; Yu, J.S. Cell-Specific and PH-Activatable Rubyrin-Loaded Nanoparticles for Highly Selective near-Infrared Photodynamic Therapy against Cancer. J. Am. Chem. Soc. 2013, 135, 18850–18858. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Zhao, G.; Zhang, Y.; Zhan, F.; Chen, Z.; He, T.; Cao, Y.; Hao, L.; Wang, Z.; et al. Homologous Targeting Nanoparticles for Enhanced PDT against Osteosarcoma HOS Cells and the Related Molecular Mechanisms. J. Nanobiotechnol. 2022, 20, 83. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, R.; Yu, H.; Xu, Z.; Kang, Y.; Cui, H.; Xue, P. MnO2-Capped Silk Fibroin (SF) Nanoparticles with Chlorin E6 (Ce6) Encapsulation for Augmented Photo-Driven Therapy by Modulating the Tumor Microenvironment. J. Mater. Chem. B 2021, 9, 3677–3688. [Google Scholar] [CrossRef]
- Muehlmann, L.A.; Ma, B.C.; Longo, J.P.; Almeida Santos, M.F.; Azevedo, R.B. Aluminum-phthalocyanine chloride associated to poly(methyl vinyl ether-co-maleic anhydride) nanoparticles as a new third-generation photosensitizer for anticancer photodynamic therapy. Int. J. Nanomed. 2014, 9, 1199–1213. [Google Scholar] [CrossRef]
- Zheng, B.Y.; Shen, X.M.; Zhao, D.M.; Cai, Y.B.; Ke, M.R.; Huang, J.D. Silicon (IV) Phthalocyanines Substituted Axially with Different Nucleoside Moieties. Effects of Nucleoside Type on the Photosensitizing Efficiencies and in vitro Photodynamic Activities. J. Photochem. Photobiol. B 2016, 159, 196–204. [Google Scholar] [CrossRef]
- Qi, S.; Guo, L.; Yan, S.; Lee, R.J.; Yu, S.; Chen, S. Hypocrellin A-Based Photodynamic Action Induces Apoptosis in A549 Cells through ROS-Mediated Mitochondrial Signaling Pathway. Acta. Pharm. Sin. B 2019, 9, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Li, C.; Jiang, Z.; Li, W.; Wang, A.; Wei, J. Preclinical Study of Antineoplastic Sinoporphyrin Sodium-PDT via in vitro and In vivo Models. Molecules 2017, 22, 112. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, X.; Liu, Q.; Wingnang Leung, A.; Wang, P.; Xu, C. Sinoporphyrin Sodium Mediated Photodynamic Therapy Inhibits the Migration Associated with Collapse of F-Actin Filaments Cytoskeleton in MDA-MB-231 Cells. Photodiagnosis Photodyn. Ther. 2016, 13, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xiao, Q.; Zhang, N.; Xue, C.; Leung, A.W.; Zhang, H.; Tang, Q.J.; Xu, C. Palmatine Hydrochloride Mediated Photodynamic Inactivation of Breast Cancer MCF-7 Cells: Effectiveness and Mechanism of Action. Photodiagnosis Photodyn. Ther. 2016, 15, 133–138. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, Q.; Zhang, N.; Xue, C.; Leung, A.W.; Zhang, H.; Xu, C.; Tang, Q.J. Photodynamic Action of Palmatine Hydrochloride on Colon Adenocarcinoma HT-29 Cells. Photodiagnosis Photodyn. Ther. 2016, 15, 53–58. [Google Scholar] [CrossRef]
- Gonçalves, J.L.da.S.; Bernal, C.; Imasato, H.; Perussi, J.R. Hypericin Cytotoxicity in Tumor and Non-Tumor Cell Lines: A Chemometric Study. Photodiagnosis Photodyn. Ther. 2017, 20, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Pucelik, B.; Arnaut, L.G.; Stochel, G.; Dabrowski, J.M. Design of Pluronic-Based Formulation for Enhanced Redaporfin-Photodynamic Therapy against Pigmented Melanoma. ACS Appl. Mater. Interfaces 2016, 8, 22039–22055. [Google Scholar] [CrossRef]
- Lucky, S.S.; Idris, N.M.; Huang, K.; Kim, J.; Li, Z.; Thong, P.S.P.; Xu, R.; Soo, K.C.; Zhang, Y. In vivo Biocompatibility, Biodistribution and Therapeutic Efficiency of Titania Coated Upconversion Nanoparticles for Photodynamic Therapy of Solid Oral Cancers. Theranostics 2016, 6, 1844–1865. [Google Scholar] [CrossRef]
- Baskaran, R.; Lee, J.; Yang, S.G. Clinical Development of Photodynamic Agents and Therapeutic Applications. Biomater. Res. 2018, 22, 1–8. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Foresto, E.; Gilardi, P.; Ibarra, L.E.; Cogno, I.S. Light-Activated Green Drugs: How We Can Use Them in Photodynamic Therapy and Mass-Produce Them with Biotechnological Tools. Phytomedicine Plus 2021, 1, 100044. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy for the Treatment and Diagnosis of Cancer–A Review of the Current Clinical Status. Front. Chem. 2021, 9, 608. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Photodynamic Therapy for Cancer: What’s Past Is Prologue. Photochem. Photobiol. 2020, 96, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Nanashima, A.; Abo, T.; Nonaka, T.; Nonaka, Y.; Morisaki, T.; Uehara, R.; Ohnita, K.; Fukuda, D.; Murakami, G.; Tou, K.; et al. Photodynamic Therapy Using Talaporfin Sodium (Laserphyrin ®) for Bile Duct Carcinoma: A Preliminary Clinical Trial. Anticancer Res. 2012, 32, 4931–4938. [Google Scholar] [CrossRef]
- Nanashima, A.; Hiyoshi, M.; Imamura, N.; Hamada, T.; Nishida, T.; Kawakami, H.; Ban, T.; Kubota, Y.; Nakashima, K.; Yano, K.; et al. Two Cases of Bile Duct Carcinoma Patients Who Underwent the Photodynamic Therapy Using Talaporfin Sodium (Laserphyrin ®). Clin. J. Gastroenterol 2020, 13, 102–109. [Google Scholar] [CrossRef]
- Sultan, A.A.; Jerjes, W.; Berg, K.; Høgset, A.; Mosse, C.A.; Hamoudi, R.; Hamdoon, Z.; Simeon, C.; Carnell, D.; Forster, M.; et al. Disulfonated Tetraphenyl Chlorin (TPCS2a)-Induced Photochemical Internalisation of Bleomycin in Patients with Solid Malignancies: A Phase 1, Dose-Escalation, First-in-Man Trial. Lancet. Oncol. 2016, 17, 1217–1229. [Google Scholar] [CrossRef]
- Ji, W.; Yoo, J.W.; Bae, E.K.; Lee, J.H.; Choi, C.M. The Effect of Radachlorin ® PDT in Advanced NSCLC: A Pilot Study. Photodiagnosis Photodyn. Ther. 2013, 10, 120–126. [Google Scholar] [CrossRef]
- Shafirstein, G.; Rigual, N.R.; Arshad, H.; Cooper, M.T.; Bellnier, D.A.; Wilding, G.; Tan, W.; Merzianu, M.; Henderson, B.W. Photodynamic Therapy with 3-(1 0-Hexyloxyethyl) Pyropheophorbide-a for Early-Stage Cancer of the Larynx: Phase Ib Study. Head Neck 2015. [Google Scholar] [CrossRef]
- Santos, L.L.; Oliveira, J.; Monteiro, E.; Santos, J.; Sarmento, C. Treatment of Head and Neck Cancer with Photodynamic Therapy with Redaporfin: A Clinical Case Report. Case Rep. Oncol. 2018, 11, 769. [Google Scholar] [CrossRef]
- Baron, E.D.; Malbasa, C.L.; Santo-Domingo, D.; Fu, P.; Miller, J.D.; Hanneman, K.K.; Hsia, A.H.; Oleinick, N.L.; Colussi, V.C.; Cooper, K.D. Silicon Phthalocyanine (Pc 4) Photodynamic Therapy Is a Safe Modality for Cutaneous Neoplasms: Results of a Phase 1 Clinical Trial. Lasers Surg. Med. 2010, 42, 888–895. [Google Scholar] [CrossRef]
- Chen, C.; Wu, C.; Yu, J.; Zhu, X.; Wu, Y.; Liu, J.; Zhang, Y. Photodynamic-Based Combinatorial Cancer Therapy Strategies: Tuning the Properties of Nanoplatform According to Oncotherapy Needs. Coord. Chem. Rev. 2022, 461, 214495. [Google Scholar] [CrossRef]
- Sharma, H.; Bagaria, A.; Poddar, N.K. Photodynamic Therapy-A Novel and Promising Treatment for Cancer. Proc. Mater. Today: Proc. 2021, 43, 2861–2863. [Google Scholar] [CrossRef]
- Verger, A.; Brandhonneur, N.; Molard, Y.; Cordier, S.; Kowouvi, K.; Amela-Cortes, M.; Dollo, G. From Molecules to Nanovectors: Current State of the Art and Applications of Photosensitizers in Photodynamic Therapy. Int. J. Pharm. 2021, 604, 120763. [Google Scholar] [CrossRef] [PubMed]
- Escudero, A.; Carrillo-Carrión, C.; Castillejos, M.C.; Romero-Ben, E.; Rosales-Barrios, C.; Khiar, N. Photodynamic Therapy: Photosensitizers and Nanostructures. Mater. Chem. Front. 2021, 5, 3788–3812. [Google Scholar] [CrossRef]
- Cogno, I.S.; Gilardi, P.; Comini, L.; Núñez-Montoya, S.C.; Cabrera, J.L.; Rivarola, V.A. Natural Photosensitizers in Photodynamic Therapy: In vitro Activity against Monolayers and Spheroids of Human Colorectal Adenocarcinoma SW480 Cells. Photodiagnosis Photodyn. Ther. 2020, 31, 101852. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, L.; Zhang, Z.; Liu, Z. Advances in Photosensitizer-Related Design for Photodynamic Therapy. Asian J. Pharm. Sci. 2021, 16, 668–686. [Google Scholar] [CrossRef]


| Photosensitizer (s) | PS Class | Cancer Type (s) | Application | PS-PDT Effective Concentrations | Ref. |
|---|---|---|---|---|---|
| Upconversion nanoconstruct—targeted with folate-modified amphiphilic chitosan loaded with zinc(II) phthalocyanine (FASOC-UCNP-ZnPc) | Phthalocyanines Derivatives | Hepatocellular carcinoma and sarcoma | In vivo | <150 mg.kg−1 triggered by 660 and 980 nm light (0.2 W.cm−2, 30 min) | [51] |
| Dimeric anthraquinone (–)-7,7′-biphyscion | Anthraquinones Derivatives | lung cancer, cervical cancer, stomach cancer and urinary bladder carcinoma | In vitro | 0.064 µmol.L−1 combined with 9.3 J.cm−2 light dose (λexc = 468 nm) | [52] |
| 6-Nitro-Quinazolin-4(3H)-one | Quinazoline Derivatives | glioblastoma and melanoma | In vitro | 50 μmol.L−1 and then irradiated at 365 nm using a UVA lamp for 1 and 2 h. | [53] |
| 2-(3,5-dimethyl-1Hpyrazol-1-yl)-1-arylethanones | Pyrazole Derivatives | colon cancer, prostate cancer, ovarian cancer, and lung cancer. | In vitro | Not reported. | [54] |
| Hf–porphyrin nanoscale metal–organic framework, (DBP–UiO) | Porphyrin derivative | head and neck cancer | In vitro/in vivo | 5–100 μmol.L−1 and 3.5 mg.kg−1(in vivo) combined with 90–180 J.cm−2 light dose (100 mW.cm−2, 15 and 30 min). | [55] |
| Cationic porphyrin-cisplatin conjugate (Pt-1)- polymeric nanoparticles (NP@Pt-1) | Porphyrin derivative | colon carcinoma | In vitro/ in vivo | 0.025–20 μmol.L−1 and 3.5 mg.kg−1(in vivo) combined with 6.95 J.cm−2 light dose (100 mW.cm−2, 15 and 30 min). | [56] |
| Selenium-rubyrin (NMe2Se4N2)-loaded nanoparticles functionalized with folate (FA) | Transition metal complex | cervical carcinoma | In vitro/ in vivo | 35 μg.mL−1 and 0.5 mg.kg−1(in vivo) combined with 30 J.cm−2 light dose (635 nm and 808 nm laser, 100 mW.cm−2, 30 min). | [57] |
| Constructed homologous targeting-based nanoplatform (MH-PLGA-IR780 NPs) | NIR-absorbing PSs | osteosarcoma | In vitro/in vivo | 5 μg.mL−1, 808 nm laser, 1 and 1.5 W.cm−2. | [58] |
| MnO2-capped silk fibroin (SF) nanoparticles with chlorin e6 (Ce6) encapsulated | Chlorin | breast cancer | In vitro/ in vivo | 40 μg.mL−1 and 1 mg.kg−1 (in vivo), 808/660 nm laser, 1.5/1.0 W.cm−2. | [59] |
| Aluminum-phthalocyanine chloride associated with poly(methyl vinyl ether-co-maleic anhydride) nanoparticles | Phthalocyanines Derivatives | breast cancer | In vitro | 0.25 μmol.L−1 combined with 3.82 J.cm−2 light dose | [60] |
| Silicon (IV) phthalocyanine (SiPC) | Phthalocyanines Derivatives | hepatocarcinoma and gastric cancer | In vitro | 9 nmol.L−1 to 33 nM combined with 27 J.cm−2 light dose (λ > 610 nm, 15 mW.cm-2, 30 min). | [61] |
| Hypocrellin A (HA) | Natural hypocrellins | lung adenocarcinoma | In vitro | 0.08 μmol.L−1, 470 nm LED light irradiation. | [62] |
| Sinoporphyrin Sodium | Photofrin | eleven human cancer cell line | In vitro/ in vivo | 0.1–0.8 μg.mL−1, 630 nm laser at fluence rate of 30 mW.cm−2 total illumination power 5.4 J.well−1. 0.5–2 mg.kg−1, fluence rate of 127.7 mW.cm−2 total illumination power 60 J/animal. | [63] |
| Sinoporphyrin Sodium | Photofrin | breast cancer | In vitro | 2–4 μ.mol.L− at fluence rate of 23.85 mW.cm−2 combined with 5.72 J.cm−2 light dose. | [64] |
| Palmatine hydrochloride (PaH) | Quinolone-based alkaloids | breast cancer | In vitro | 0.087 μ.mol.L−1, 470 nm LED light irradiation, combined with 10.8 J.cm−2 light dose. | [65] |
| Palmatine hydrochloride (PaH) | Quinolone-based alkaloids | colon adenocarcinoma | In vitro | 5 μ.mol.L−1, 470 nm LED light irradiation, combined with 10.8 J.cm−2 light dose. | [66] |
| Hypericin | Perylenequinone/Natural PS | larynx carcinoma | In vitro | 11 and 25 nmol.L−1, combined with 6 and 12 J.cm−2 light dose. | [67] |
| Redaporfin-P123 micelles | bacteriochlorin derivative | melanoma | In vitro/ in vivo | 5 μ.mol.L−1, 735 nm LED light irradiation, combined with 0.97 J.cm−2 light dose. 1.5 mg.Kg−1, 750 nm LED light irradiation, combined with 74 J.cm−2. | [68] |
| Photosensitizer (s) | PS Class | Cancer Type (s) | PS-PDT Effective Concentrations | Ref. |
|---|---|---|---|---|
| Talaporfin, mono-L-aspartyl chlorin e6, NPe6, LS11 (Laserphyrin) | chlorin(e6) derivative | bile duct carcinoma | 40 mg.m−2 combined with 100 J.cm−2 light dose (664 nm, 4–6 h). | [75] |
| Talaporfin, mono-L-aspartyl chlorin e6, NPe6, LS11 (Laserphyrin) | chlorin(e6) derivative | bile duct carcinoma | 40 mg.m−2 combined with 100 J.cm−2 light dose (664 nm, 6 h). | [76] |
| Disulfonated tetraphenyl chlorin (TPCS2a) | chlorin | Solid tumor | 0.25 mg.kg−1, fluence rate of 100 mW.cm−2, 60 J.cm−2 light dose (652 nm, 4–6 h). | [77] |
| Radachlorin | chlorin derivative | obstructive advanced non-small-cell lung cancer | 1 mg.kg−1 combined with 200 J.cm−2 light dose (662 nm, 11 min 6 s). | [78] |
| 2-(1-Hexyloxyethyl)-2-devinyl pyropheophorbide-a (HPPH) | chlorin derivative | laryngeal cancer | 4 mg.kg−1 at fluence rate of 100 mW.cm−2 combined with below 100 J.cm−2 light dose (665 nm). | [79] |
| Redaporfin, LUZ11 or F-2BMet, 5,10,15,20-tetrakis(2,6-difluoro-3-N-methylsulfamoylphenyl)-bacteriochlorin | bacteriochlorin derivative | head and neck squamous carcinom | 0.75 mg.kg−1combined with 50 J.cm−2 light dose (749 nm). | [80] |
| Silicon (IV) phthalocyanine (SiPC) | Phthalocyanines Derivatives | non-melanoma skin cancer | 0.1 mg.mL−1 combined with 100–150 mJ.cm−1 light dose. | [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aires-Fernandes, M.; Botelho Costa, R.; Rochetti do Amaral, S.; Mussagy, C.U.; Santos-Ebinuma, V.C.; Primo, F.L. Development of Biotechnological Photosensitizers for Photodynamic Therapy: Cancer Research and Treatment—From Benchtop to Clinical Practice. Molecules 2022, 27, 6848. https://doi.org/10.3390/molecules27206848
Aires-Fernandes M, Botelho Costa R, Rochetti do Amaral S, Mussagy CU, Santos-Ebinuma VC, Primo FL. Development of Biotechnological Photosensitizers for Photodynamic Therapy: Cancer Research and Treatment—From Benchtop to Clinical Practice. Molecules. 2022; 27(20):6848. https://doi.org/10.3390/molecules27206848
Chicago/Turabian StyleAires-Fernandes, Mariza, Ramon Botelho Costa, Stéphanie Rochetti do Amaral, Cassamo Ussemane Mussagy, Valéria C. Santos-Ebinuma, and Fernando Lucas Primo. 2022. "Development of Biotechnological Photosensitizers for Photodynamic Therapy: Cancer Research and Treatment—From Benchtop to Clinical Practice" Molecules 27, no. 20: 6848. https://doi.org/10.3390/molecules27206848
APA StyleAires-Fernandes, M., Botelho Costa, R., Rochetti do Amaral, S., Mussagy, C. U., Santos-Ebinuma, V. C., & Primo, F. L. (2022). Development of Biotechnological Photosensitizers for Photodynamic Therapy: Cancer Research and Treatment—From Benchtop to Clinical Practice. Molecules, 27(20), 6848. https://doi.org/10.3390/molecules27206848