Developing a Chromatographic 99mTc Generator Based on Mesoporous Alumina for Industrial Radiotracer Applications: A Potential New Generation Sorbent for Using Low-Specific-Activity 99Mo
Abstract
1. Introduction
2. Results and Discussion
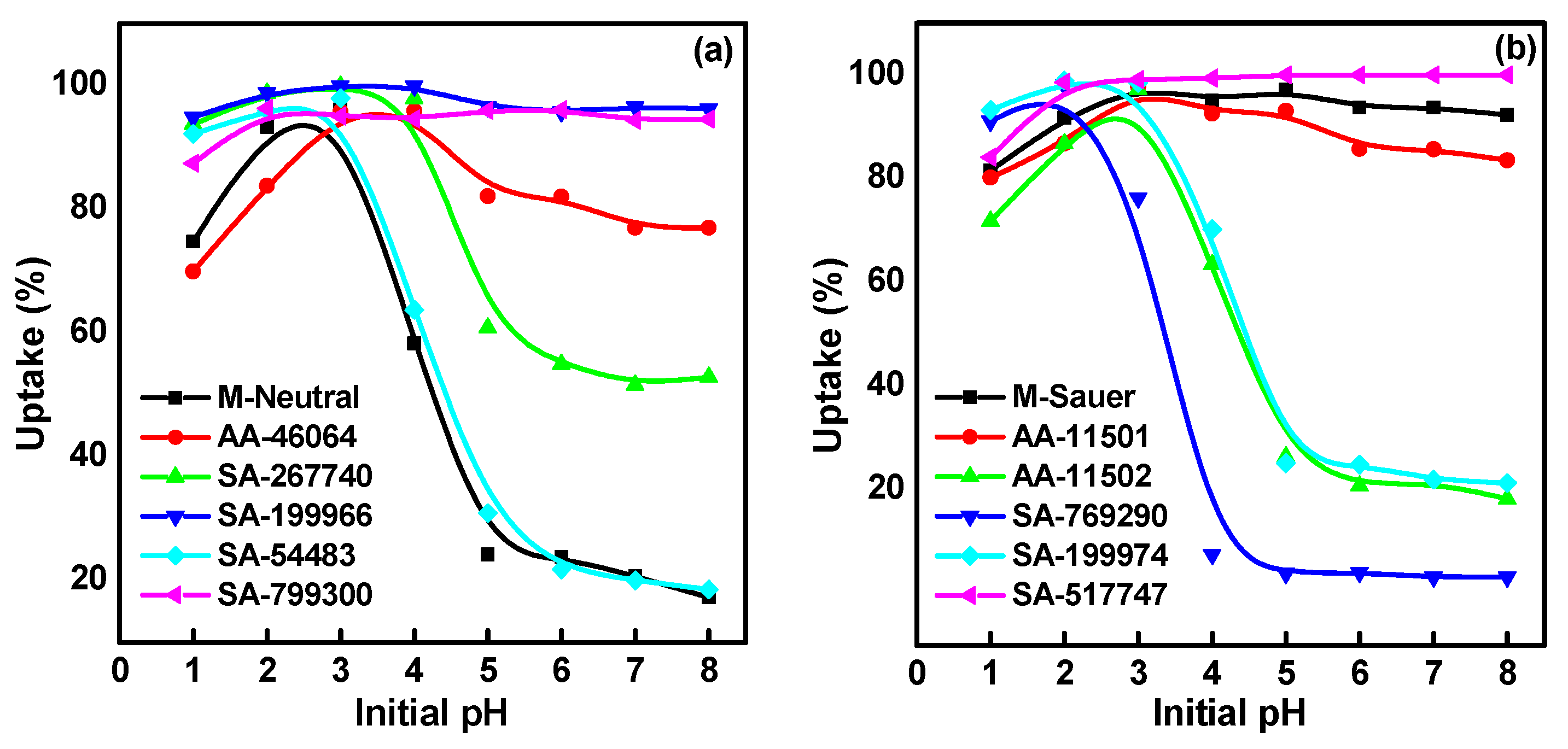
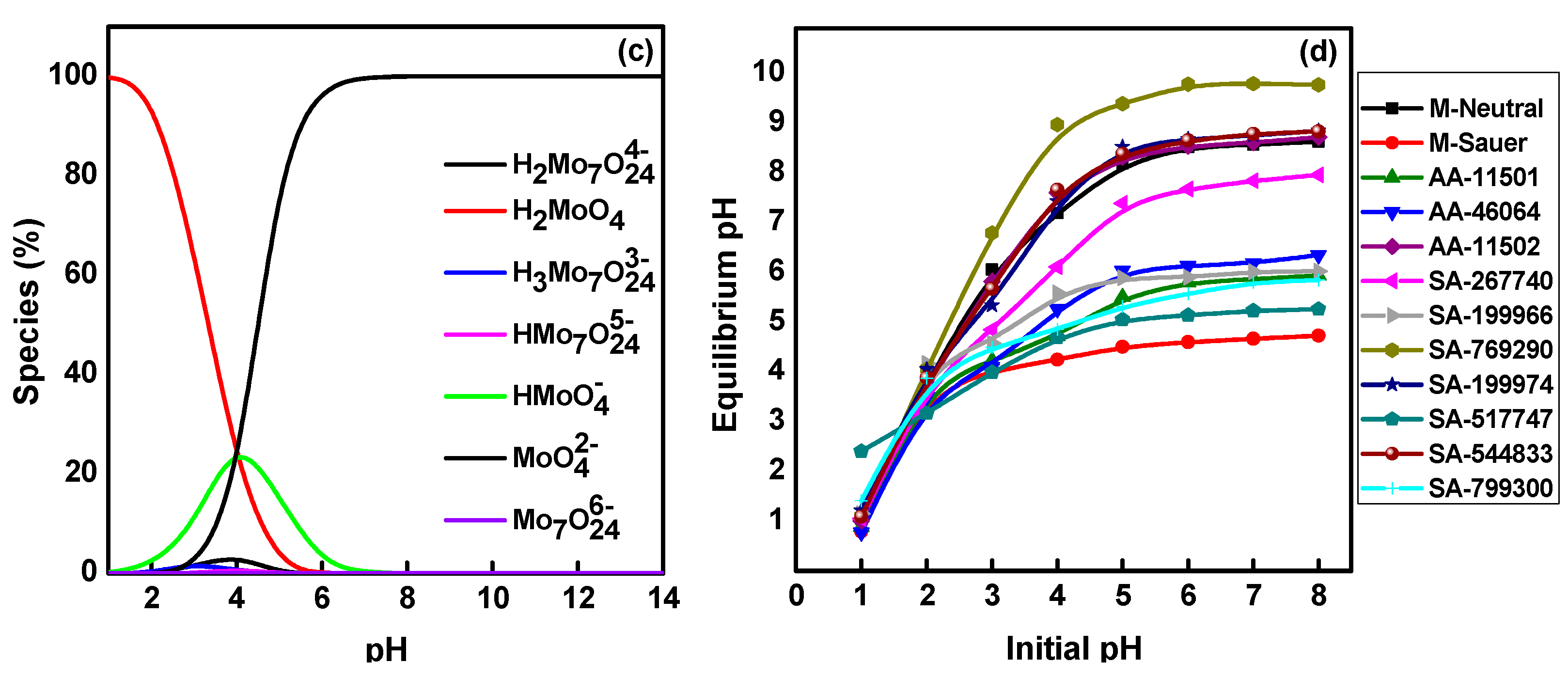
2.1. Effect of Solution pH
2.2. Thermodynamic Studies
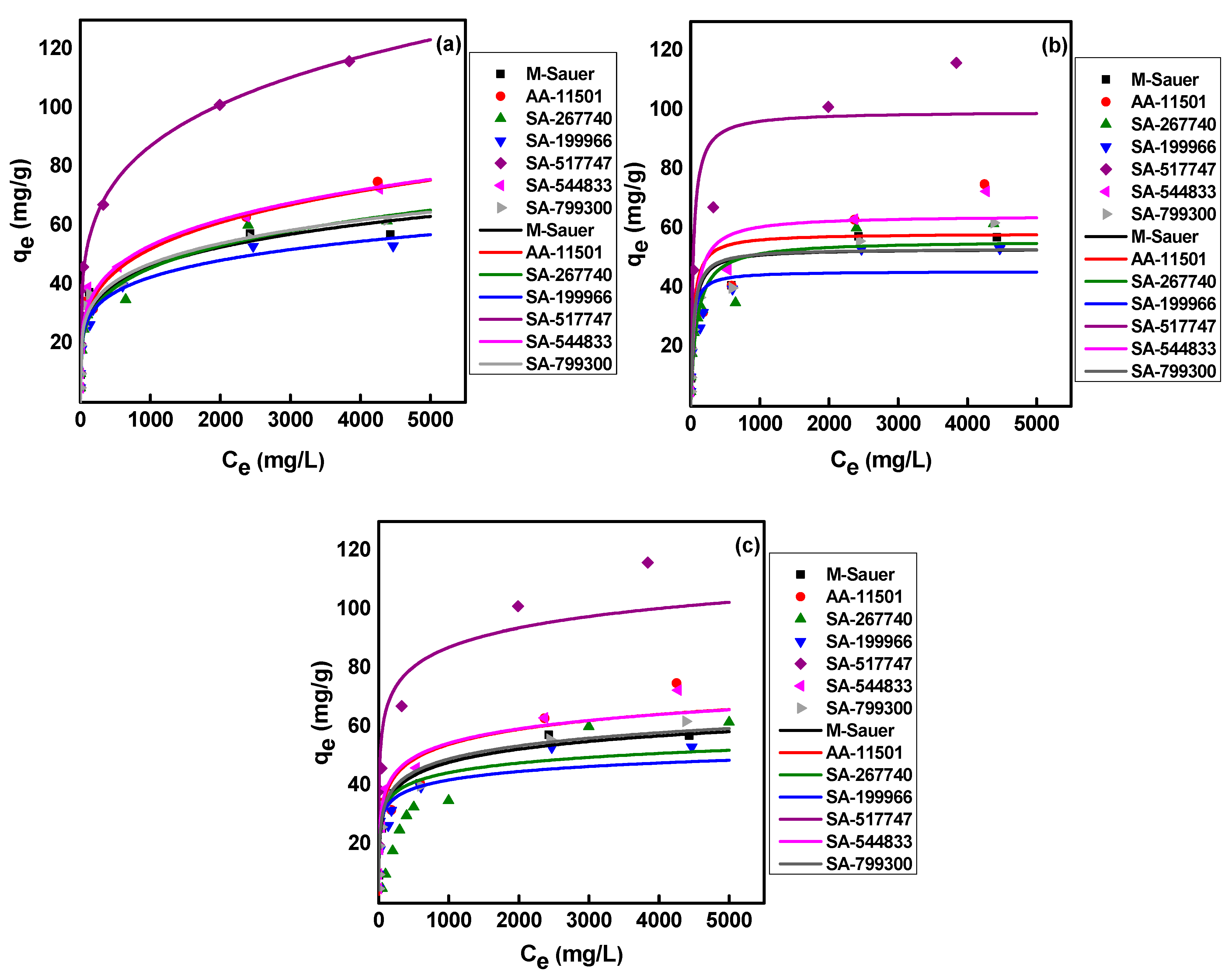
2.3. Adsorption Isotherms
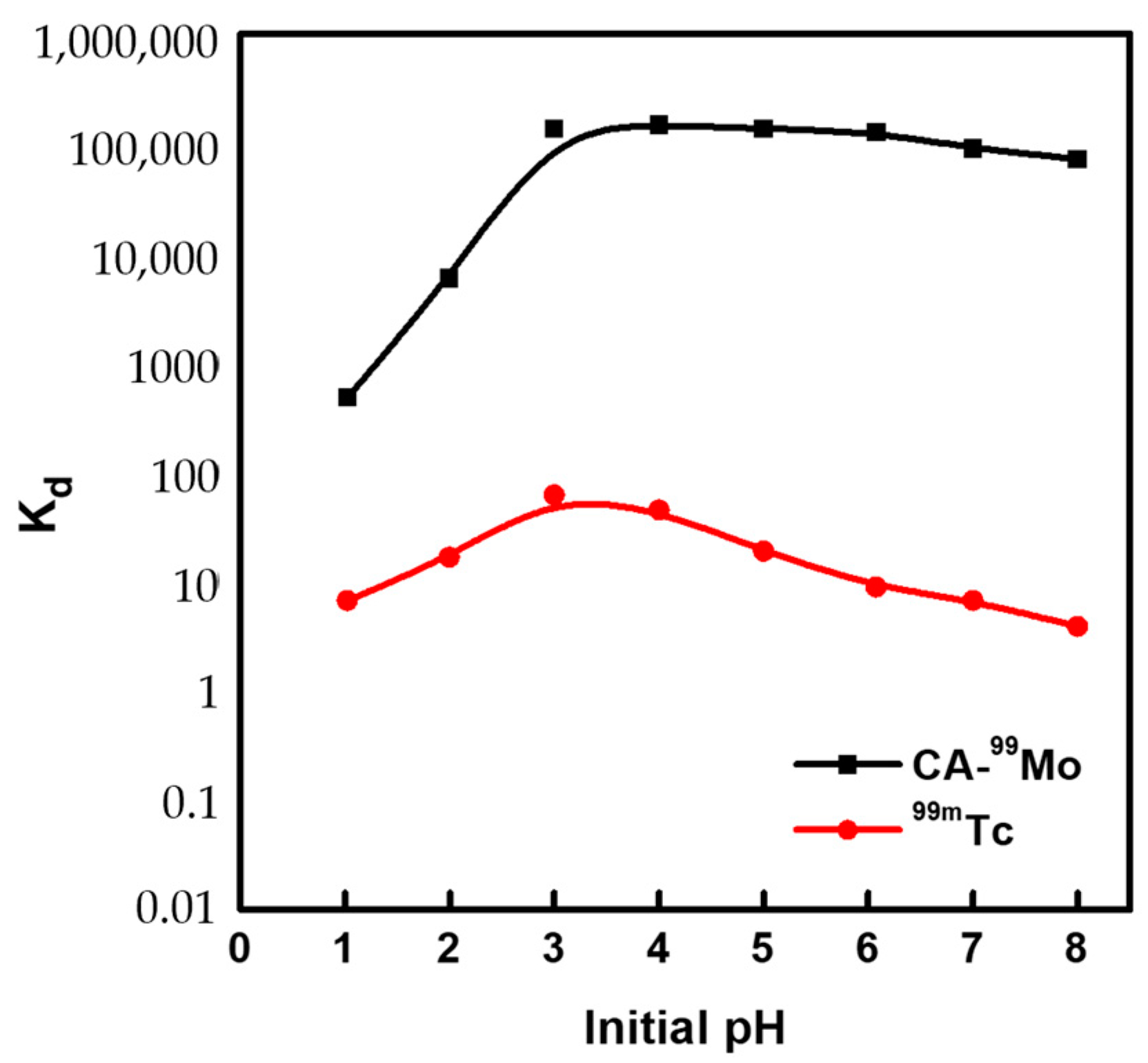
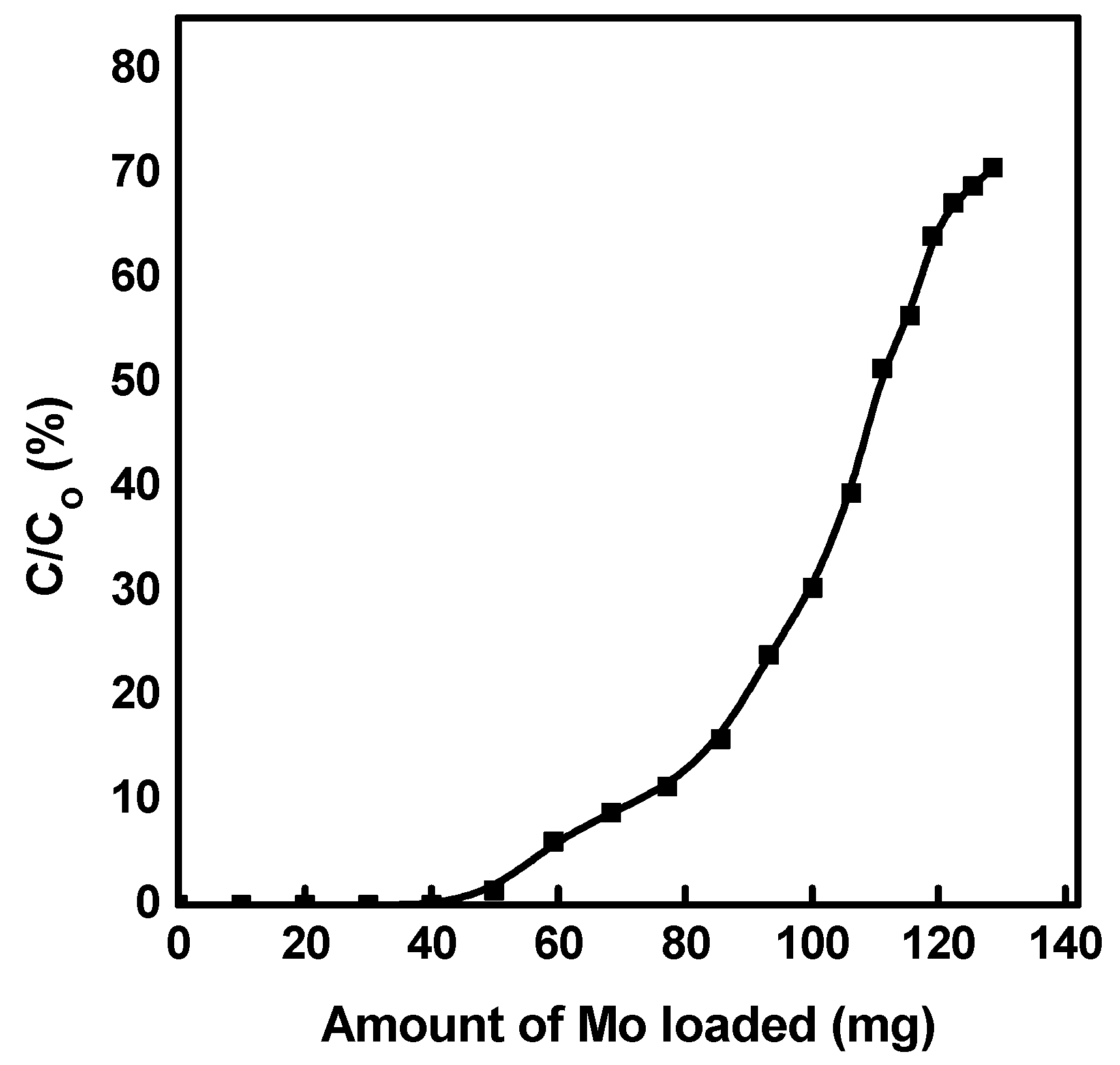
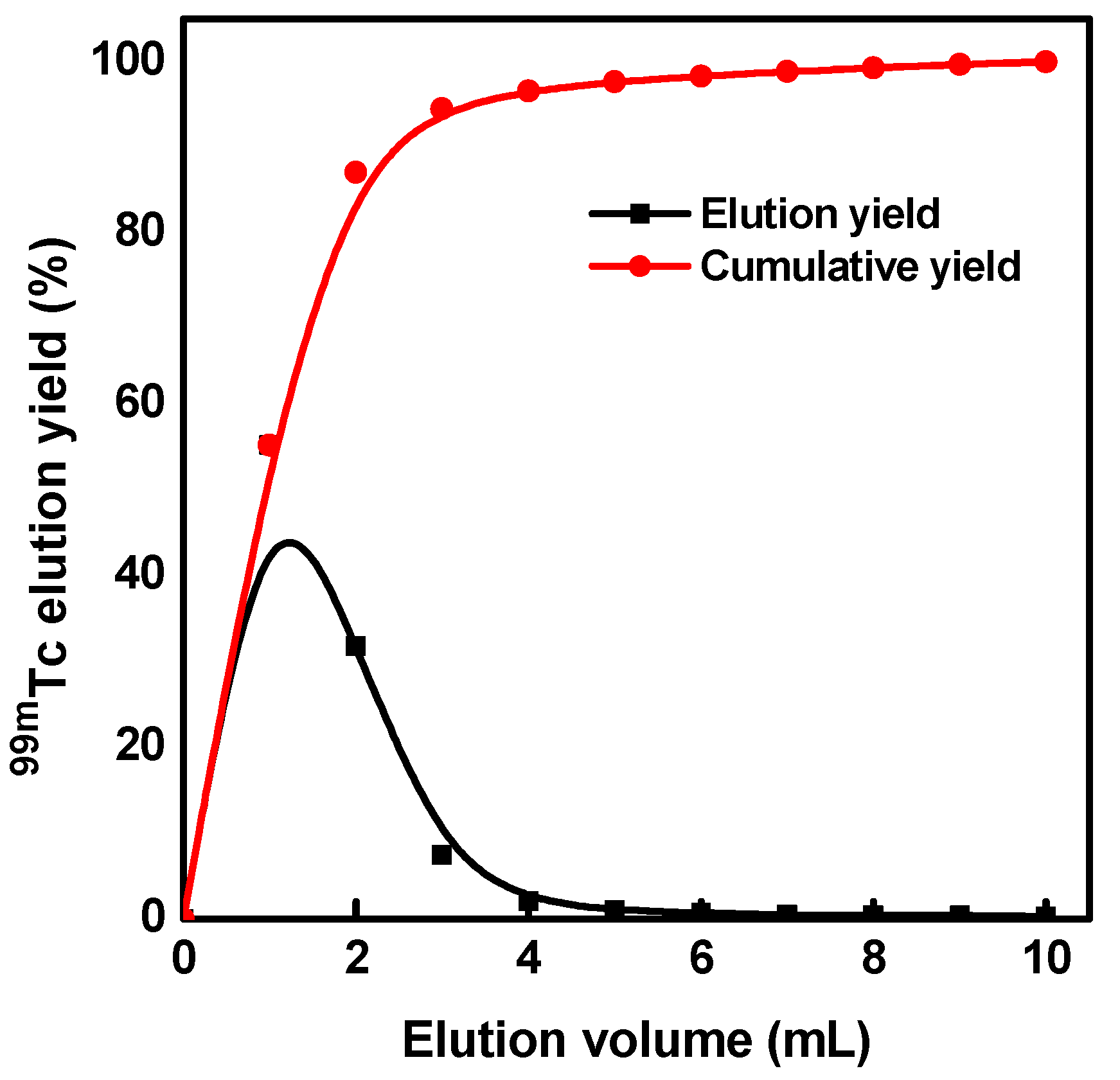
2.4. Preparation of 99Mo/99mTc Generator
3. Materials and Methods
3.1. Materials
3.2. Instrumentation
3.3. Static Equilibrium Studies
Data Presentation
3.4. Application of Mesoporous Alumina in Preparing a 99Mo/99mTc Generator
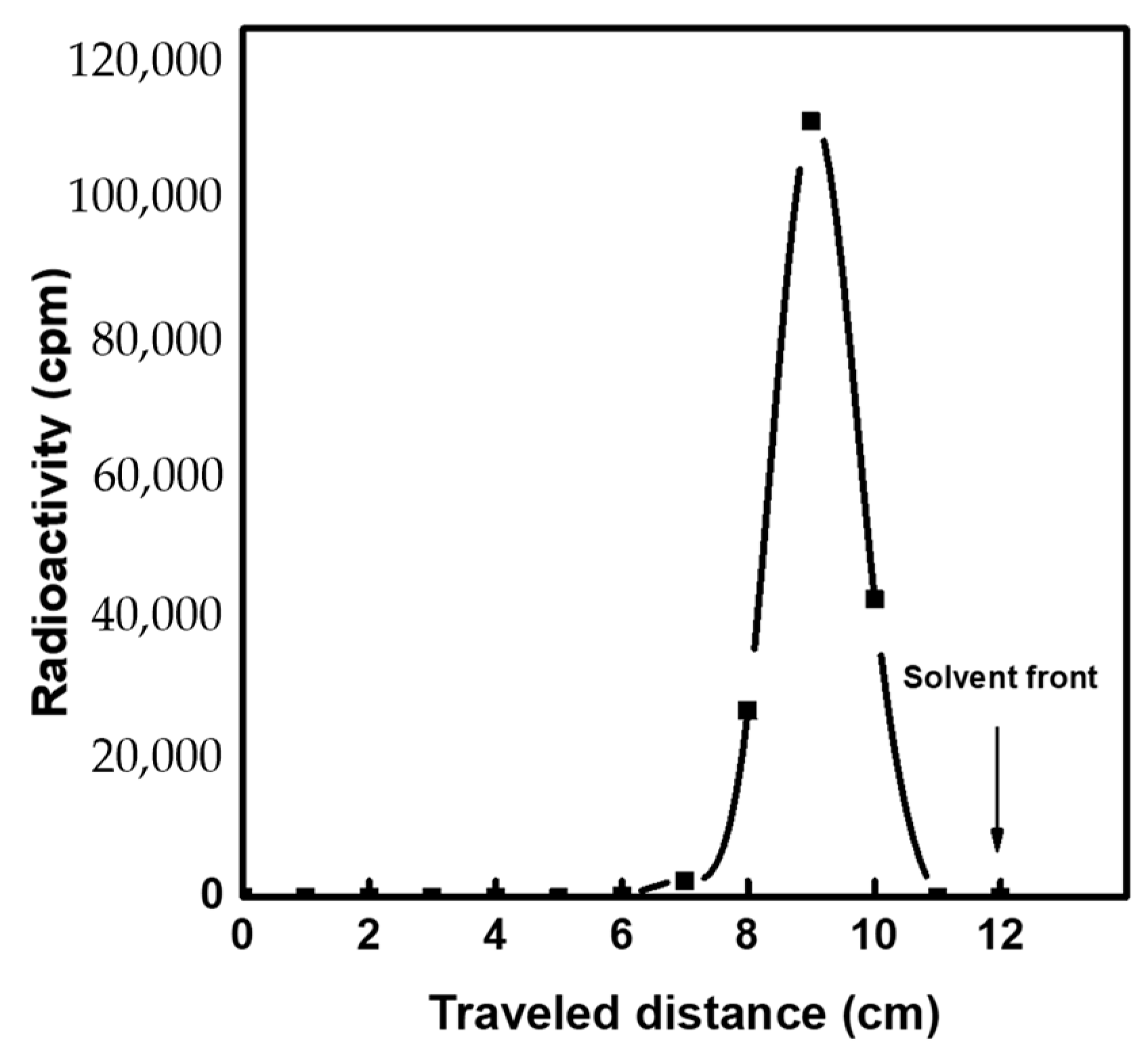
3.5. Elution Performance of 99mTc Eluate
3.6. Recovery of 99Mo from the Spent Generator
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| AA | Alfa Aesar |
| M | Merk |
| N.A | Not Available |
| SA | Sigma-Aldrich |
References
- International Atomic Energy Agency. Radiotracer Generators for Industrial Applications; International Atomic Energy Agency: Vienna, Austria, 2013. [Google Scholar]
- Wetchagarun, S.A.; Petchrak, A.; Tippayakul, C. Preliminary study of the use of radiotracers for leak detection in industrial applications. J. Phys. Conf. Ser. 2015, 611, 012018. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Radiotracer Applications in Industry-A Guidebook; Technical Reports Series 2004; International Atomic Energy Authority: Vienna, Austria, 2004. [Google Scholar]
- International Atomic Energy Agency. Radiotracer Residence Time Distribution Method for Industrial and Environmental Applications; Training Course Series 2008; International Atomic Energy Authority: Vienna, Austria, 2008. [Google Scholar]
- Othman, N.; Kamarudin, S.K. Radiotracer technology in mixing processes for industrial applications. Sci. World J. 2014, 30, 768604. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, J.V.; Sabino, C.S.; Aun, P.E.; Mendes, V.L.; Agudo, E.G. Development of a technique for using 99mTc as an absorbable tracer for hydrodynamic studies of fine sediments in suspension. Int. J. Appl. Radiat. Isot. 2002, 57, 85–92. [Google Scholar] [CrossRef]
- Berne, P.P.; Thereska, J. Simulation of a radiotracer experiment by flow and detection-chain modelling: A first step towards better interpretation. Int. J. Appl. Radiat. Isot. 2004, 60, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Borroto, J.I.; Dominguez, J.; Griffith, J.; Fick, M.; Leclerc, J.P. Technetium-99m as a tracer for the liquid RTD measurement in opaque anaerobic digester: Application in a sugar wastewater treatment plant. Chem. Eng. Process. 2003, 42, 857–865. [Google Scholar] [CrossRef]
- Fauquex, P.F.; Flaschel, E.; Renken, A.; Do, H.P.; Friedli, C.; Lerch, P. 24Na and 99mTc tracers applied to the characterization of liquid–solid fluidized bed and hydraulic transport reactors. Int. J. Appl. Radiat. Isot. 1983, 34, 1465–1468. Available online: https://core.ac.uk/download/pdf/147916299.pdf (accessed on 5 August 2022). [CrossRef]
- Hughes, C.E.; Airey, P.L.; Duran, E.B.; Miller, B.M.; Sombrito, E. Using radiotracer techniques for coastal hydrodynamic model evaluation. J. Environ. Radioact. 2004, 76, 195–206. [Google Scholar] [CrossRef]
- Mohd Yunos, M.A.S.; Hussain, S.A.; Sipaun, S.M. Industrial radiotracer application in flow rate measurement and flowmeter calibration using 99mTc and 198Au nanoparticles radioisotope. Appl. Rad. Isot. 2019, 143, 24–28. [Google Scholar] [CrossRef]
- Pavlović, M.M.; Pavlović, M.R.P.; Bartl, P.; Stevanović, J.; Radak, B. Optimization of injected radiotracer volume for flow rate measurement in closed conduits. Hemijska Industrija 2020, 74, 305–312. [Google Scholar] [CrossRef]
- Thýn, J.; Žitný, R. Radiotracer applications for the analysis of complex flow structure in industrial apparatuses. Nucl. Instrum. Methods Phys. Res. B. 2004, 213, 339–347. [Google Scholar] [CrossRef]
- Sakr, T.M.; Nawar, M.F.; Fasih, T.; El-Bayoumy, S.; Abd El-Rehim, H.A. Nano-technology contributions towards the development of high performance radioisotope generators: The future promise to meet the continuing clinical demand. Appl. Radiat. Isot. 2017, 129, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Mausolf, E.J.; Johnstone, E.V.; Mayordomo, N.; Williams, D.L.; Guan, E.Y.Z.; Gary, C.K. Fusion-Based Neutron Generator Production of Tc-99m and Tc-101: A Prospective Avenue to Technetium Theranostics. Pharmaceuticals 2021, 14, 875. [Google Scholar] [CrossRef] [PubMed]
- Gumiela, M. Cyclotron production of 99mTc: Comparison of known separation technologies for isolation of 99mTc from molybdenum targets. Nucl. Med. Biol. 2018, 58, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Nuclear Energy Agency. Medical Isotope Supply Review: 99Mo/99mTc Market Demand and Production Capacity Projection 2017–2022; Nuclear Development NEA/SEN/HLGMR, Nuclear Energy Agency: Paris, France, 2017. [Google Scholar]
- Paterson, A.; Druce, M.; Killen, E. Six problems with the 6-day Curie and a solution. J. Radioanal. Nucl. Chem. 2015, 305, 13–22. [Google Scholar] [CrossRef]
- Nawar, M.F.; Türler, A. Development of New Generation of 99Mo/99mTc Radioisotope Generators to Meet the Continuing Clinical Demands. In Proceedings of the 2nd International Conference on Radioanalytical and Nuclear Chemistry (RANC 2019), Budapest, Hungary, 5–10 May 2019. [Google Scholar]
- National Academies Press. Opportunities and Approaches for Supplying Molybdenum-99 and Associated Medical Isotopes to Global Markets: Proceedings of a Symposium; National Academies Press: Washington, DC, USA, 2018. [Google Scholar]
- Nuclear Energy Agency. The Supply of Medical Radioisotopes: 2019 Medical Isotope Demand and Capacity Projection for the 2019–2024 Period; Nuclear Development NEA/SEN/HLGMR; Nuclear Energy Agency: Paris, France, 2019; Available online: https://www.oecd-nea.org/med-radio/docs/sen-hlgmr2019-1.pdf (accessed on 5 August 2022).
- Nawar, M.F.; Türler, A. New strategies for a sustainable 99mTc supply to meet increasing medical demands: Promising solutions for current problems. Front. Chem. 2022, 10, 926258. [Google Scholar] [CrossRef]
- Chang, S.H. Types of bulk liquid membrane and its membrane resistance in heavy metal removal and recovery from wastewater. Desalin. Water Treat. 2016, 57, 19785–19793. [Google Scholar] [CrossRef]
- Molinski, V.J. A review of 99mTc generator technology. Int. J. Appl. Rad. Isot. 1982, 33, 811–819. [Google Scholar] [CrossRef]
- Knapp, F., Jr.; Baum, R. Radionuclide generators-a new renaissance in the development of technologies to provide diagnostic and therapeutic radioisotopes for clinical applications. Curr. Radiopharm. 2012, 5, 175–177. [Google Scholar] [CrossRef]
- Osso, J.A., Jr.; Catanoso, M.F.; Barrio, G.; Brambilla, T.P.; Teodoro, R.; Dias, C.R.B.R.; Suzuki, K.N. Technetium-99m: New Production and Processing Strategies to Provide Adequate Levels for SPECT Imaging. Curr. Radiopharm. 2012, 5, 178–186. [Google Scholar] [CrossRef]
- Maoliang, L. Production of gel-type Tc-99m generator for nuclear medicine. In Proceedings of the 12th KAIF/KNS Annual Conference, Seoul, Korea, 3–4 April 1997. [Google Scholar]
- Kadarisman, K.; Sriyono, S.; Abidin, A.; Lestari, E.; Marlina, M.; Saptiama, I.; Setiawan, H. Synthesis of Nano-α-Al2O3 for 99Mo Adsorbent. At. Indones. 2018, 44, 17–21. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, N.; Ray, S.S. Recent advances in carbon nanomaterial-based adsorbents for water purification. Coord. Chem. Rev. 2020, 405, 213111. [Google Scholar] [CrossRef]
- Mousavi, D.V.; Ahmadipouya, S.; Shokrgozar, A.; Molavi, H.; Rezakazemi, M.; Ahmadijokani, F.; Arjmand, M. Adsorption performance of UiO-66 towards organic dyes: Effect of activation conditions. J. Mol. Liq. 2021, 321, 114487. [Google Scholar] [CrossRef]
- Gulicovski, J.J.; Čerović, L.S.; Milonjić, S.K. Point of Zero Charge and Isoelectric Point of Alumina. Mater. Manuf. Process 2008, 23, 615–619. [Google Scholar] [CrossRef]
- Wang, Y.; Persson, P.; Michel, F.M.; Brown, G.E. Comparison of isoelectric points of single-crystal and polycrystalline α-Al2O3 and α-Fe2O3 surfaces. Am. Mineral. 2016, 101, 2248–2259. [Google Scholar] [CrossRef]
- Nawar, M.F.; El-Daoushy, A.F.; Madkour, M.; Türler, A. Sorption Profile of Low Specific Activity 99Mo on Nanoceria-Based Sorbents for the Development of 99mTc Generators: Kinetics, Equilibrium, and Thermodynamic Studies. Nanomaterials 2022, 12, 1587. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, R.J.; Ren, B.Z.; Hou, B.; Hursthouse, A. Preparation of a novel Fe3O4/HCO composite adsorbent and the mechanism for the removal of antimony (III) from aqueous solution. Sci. Rep. 2019, 9, 13021. [Google Scholar] [CrossRef]
- ALOthman, Z.A.; Naushad, M.; Ali, R. Kinetic, equilibrium isotherm and thermodynamic studies of Cr(VI) adsorption onto low-cost adsorbent developed from peanut shell activated with phosphoric acid. Environ. Sci. Pollut. Res. Int. 2013, 20, 3351–3365. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Y.; Zheng, Y.; Zhang, H.; Liang, S.; Long, M. Equilibrium, kinetic and thermodynamic studies on the sorption of 4-hydroxyphenol on Cr-bentonite. Chem. Eng. J. 2008, 143, 117–123. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Naffrechoux, E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon. Part I. Two-parameter models and equations allowing determination of thermodynamic parameters. J. Hazard Mater. 2007, 147, 381–394. [Google Scholar] [CrossRef]
- Nawar, M.F.; El-Daoushy, A.F.; Ashry, A.; Soliman, M.A.; Türler, A. Evaluating the Sorption Affinity of Low Specific Activity 99Mo on Different Metal Oxide Nanoparticles. Inorganics, 2022; submitted. [Google Scholar]
- Ashry, A. Adsorption and Time Dependent Fixation of Uranium (VI) in Synthetic and Natural Matrices. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2017. [Google Scholar]
- Ashry, A.; Bailey, E.H.; Chenery, S.R.N.; Young, S.D. Kinetic study of time-dependent fixation of U(VI) on biochar. J. Hazard Mater. 2016, 320, 55–66. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Temkin, M.; Pyzhev, V. Recent modifications to Langmuir isotherms. Acta Physiochim. URSS 1940, 12, 217–225. [Google Scholar]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard Mater. 2020, 5, 122383. [Google Scholar] [CrossRef]
- Akbas, Y.A.; Yusan, S.; Sert, S.; Aytas, S. Sorption of Ce(III) on magnetic/olive pomace nanocomposite: Isotherm, kinetic and thermodynamic studies. Environ. Sci. Pollut. Res. 2021, 28, 56782–56794. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Quality Control in the Production of Radiopharmaceuticals; IAEA TECDOC SERIES: IAEA-TECDOC-1856; International Atomic Energy Agency: Vienna, Austria, 2018; Available online: https://www-pub.iaea.org/MTCD/Publications/PDF/TE-1856web.pdf (accessed on 5 August 2022).
- Lever, S.Z.; Lever, J.R. Technetium-99m Pharmaceuticals: Preparation and Quality Control in Nuclear Medicine. J. Nucl. Med. 2009, 50, 831. [Google Scholar] [CrossRef][Green Version]
- British Pharmacopoeia Commission. British Pharmacopoeia; The Stationery Office: Norwich, UK, 2008. [Google Scholar]
- United States Pharmacopeia Convention. Sodium Pertechnetate Tc-99m Injection. In Official Monographs: USP 28; United States Pharmacopeia (USP) 28-National Formulary (NF) 23; United States Pharmacopeia: Rockville, MD, USA, 2005. [Google Scholar]
- Uzunov, N.; Yordanova, G.; Salim, S.; Stancheva, N.; Mineva, V.; Meléndez-Alafort, L.; Rosato, A. Quality assurance of 99Mo/99mTc radionuclide generators. Acta Sci. Nat. 2018, 5, 40–47. [Google Scholar]
- Saha, G.B. Quality Control of Radiopharmaceuticals. In Fundamentals of Nuclear Pharmacy; Springer: New York, NY, USA, 1992. [Google Scholar]


| Adsorbent | Temperature (K) | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (kJ/mol∙K) |
|---|---|---|---|---|
| M-Sauer | 298 | −10.508 | −8.934 | 0.005 |
| 313 | −10.588 | |||
| 323 | −10.640 | |||
| 333 | −10.693 | |||
| AA-11501 | 298 | −10.602 | −7.225 | 0.011 |
| 313 | −10.772 | |||
| 323 | −10.885 | |||
| 333 | −10.998 | |||
| SA-267740 | 298 | −9.563 | 5.877 | 0.052 |
| 313 | −10.340 | |||
| 323 | −10.859 | |||
| 333 | −11.377 | |||
| SA-199966 | 298 | −9.503 | 9.208 | 0.063 |
| 313 | −10.444 | |||
| 323 | −11.072 | |||
| 333 | −11.700 | |||
| SA-517747 | 298 | −10.405 | 22.132 | 0.109 |
| 313 | −12.043 | |||
| 323 | −13.135 | |||
| 333 | −14.227 | |||
| SA-544833 | 298 | −10.975 | 4.089 | 0.051 |
| 313 | −11.733 | |||
| 323 | −12.238 | |||
| 333 | −12.744 | |||
| SA-799300 | 298 | −9.787 | 7.756 | 0.059 |
| 313 | −10.670 | |||
| 323 | −11.258 | |||
| 333 | −11.847 |
| Isotherm Model | Parameter | M-Sauer | AA-11501 | SA-267740 | SA-199966 | SA-517747 | SA-544833 | SA-799300 |
|---|---|---|---|---|---|---|---|---|
| Freundlich | nf | 5.046 | 4.332 | 4.484 | 5.485 | 4.637 | 4.561 | 5.066 |
| Kf (mg1−nLn/g) | 11,698.05 | 10,581.47 | 9779.30 | 12,073.05 | 19,643.67 | 11,720.94 | 12,051.36 | |
| R2 | 0.93 | 0.96 | 0.97 | 0.96 | 0.99 | 0.98 | 0.95 | |
| Langmuir | nL (mg/g) | 53,211.56 | 58,393.90 | 55,956.60 | 45,611.11 | 99,583.12 | 64,505.84 | 53,278.67 |
| KL (L/mg) | 0.0234 | 0.0253 | 0.0115 | 0.0350 | 0.0290 | 0.0152 | 0.0258 | |
| R2 | 0.94 | 0.8 | 0.89 | 0.83 | 0.9 | 0.94 | 0.89 | |
| Temkin | AT (L/g) | 1.5688 | 1.48864 | 11.7524 | 22.3752 | 9.66192 | 2.26332 | 1.983 |
| bT (KJ/mol) | 0.00428 | 0.00376 | 0.00588 | 0.00665 | 0.00293 | 0.00395 | 0.00431 | |
| R2 | 0.97 | 0.92 | 0.81 | 0.88 | 0.91 | 0.95 | 0.98 |
| Elution No. | 99mTc Growth Period, h | Quality Control of the Eluted 99mTc | |||
|---|---|---|---|---|---|
| 99mTc Elution Yield, % | R.C. Purity, (99mTcO4−, %) | Chemical Purity | |||
| Al Content, µg/mL | pH Value | ||||
| 1 | 24 | 84.6 | >99 | <1 | 6 |
| 2 | 24 | 84.5 | 6 | ||
| 3 | 24 | 85.0 | 6 | ||
| 4 | 24 | 84.6 | 6 | ||
| 5 | 24 | 83.7 | 6 | ||
| 6 | 24 | 85.0 | 6 | ||
| 7 | 24 | 83.8 | 6 | ||
| 8 | 24 | 83.5 | 6 | ||
| 9 | 24 | 83.4 | 6.5 | ||
| 10 | 48 | 84.0 | 6.5 | ||
| 12 | 24 | 82.9 | 6.5 | ||
| No. | Name | Supplier | Description | Particle Size | Surface Area | pH |
|---|---|---|---|---|---|---|
| 1 | M-Neutral | Merck | Activity stage I, neutral | 63–200 µm | 120 m2/g | 6.8–7.8 |
| 2 | M-Sauer | Merck | Activity stage I, acidic | 63–200 µm | 120 m2/g | 3.5–4.5 |
| 3 | AA-11501 | Alfa Aesar | Activated, acidic | 60 mesh | 150 m2/g | 4.5 ± 0.5 |
| 4 | AA-46064 | Alfa Aesar | Activated, acidic | 50–200 µm | N.A | N.A |
| 5 | AA-11502 | Alfa Aesar | Activated, neutral | 60 mesh | 150 m2/g | N.A |
| 6 | SA-267740 | Sigma-Aldrich | Weakly acidic | 150 mesh | 155 m2/g | 6.0 |
| 7 | SA-199966 | Sigma-Aldrich | Activated, acidic | 50–300 mesh | 155 m2/g | 4.5 ± 0.5 |
| 8 | SA-769290 | Sigma-Aldrich | Ultra-dry | 63 µm | 120–190 m2/g | N.A |
| 9 | SA-199974 | Sigma-Aldrich | Activated, neutral | 40–160 µm | 205 m2/g | 7.0 ± 0.5 |
| 10 | SA-517747 | Sigma-Aldrich | Nano mesoporous (Pore size = 3.8 nm) | N.A | N.A | N.A |
| 11 | SA-544833 | Sigma-Aldrich | Nanopowder | <50 nm | >40 m2/g | N.A |
| 12 | SA-799300 | Sigma-Aldrich | Activated, acidic | 50–300 mesh | 155 m2/g | 4.5 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawar, M.F.; El-Daoushy, A.F.; Ashry, A.; Türler, A. Developing a Chromatographic 99mTc Generator Based on Mesoporous Alumina for Industrial Radiotracer Applications: A Potential New Generation Sorbent for Using Low-Specific-Activity 99Mo. Molecules 2022, 27, 5667. https://doi.org/10.3390/molecules27175667
Nawar MF, El-Daoushy AF, Ashry A, Türler A. Developing a Chromatographic 99mTc Generator Based on Mesoporous Alumina for Industrial Radiotracer Applications: A Potential New Generation Sorbent for Using Low-Specific-Activity 99Mo. Molecules. 2022; 27(17):5667. https://doi.org/10.3390/molecules27175667
Chicago/Turabian StyleNawar, Mohamed F., Alaa F. El-Daoushy, Ahmed Ashry, and Andreas Türler. 2022. "Developing a Chromatographic 99mTc Generator Based on Mesoporous Alumina for Industrial Radiotracer Applications: A Potential New Generation Sorbent for Using Low-Specific-Activity 99Mo" Molecules 27, no. 17: 5667. https://doi.org/10.3390/molecules27175667
APA StyleNawar, M. F., El-Daoushy, A. F., Ashry, A., & Türler, A. (2022). Developing a Chromatographic 99mTc Generator Based on Mesoporous Alumina for Industrial Radiotracer Applications: A Potential New Generation Sorbent for Using Low-Specific-Activity 99Mo. Molecules, 27(17), 5667. https://doi.org/10.3390/molecules27175667







