Development and Validation of a Highly Sensitive and Rapid LC-MS3 Strategy to Determine Oxcarbazepine and Its Active Metabolite in the Serum of Patients with Epilepsy and Its Application in Therapeutic Drug Monitoring
Abstract
1. Introduction
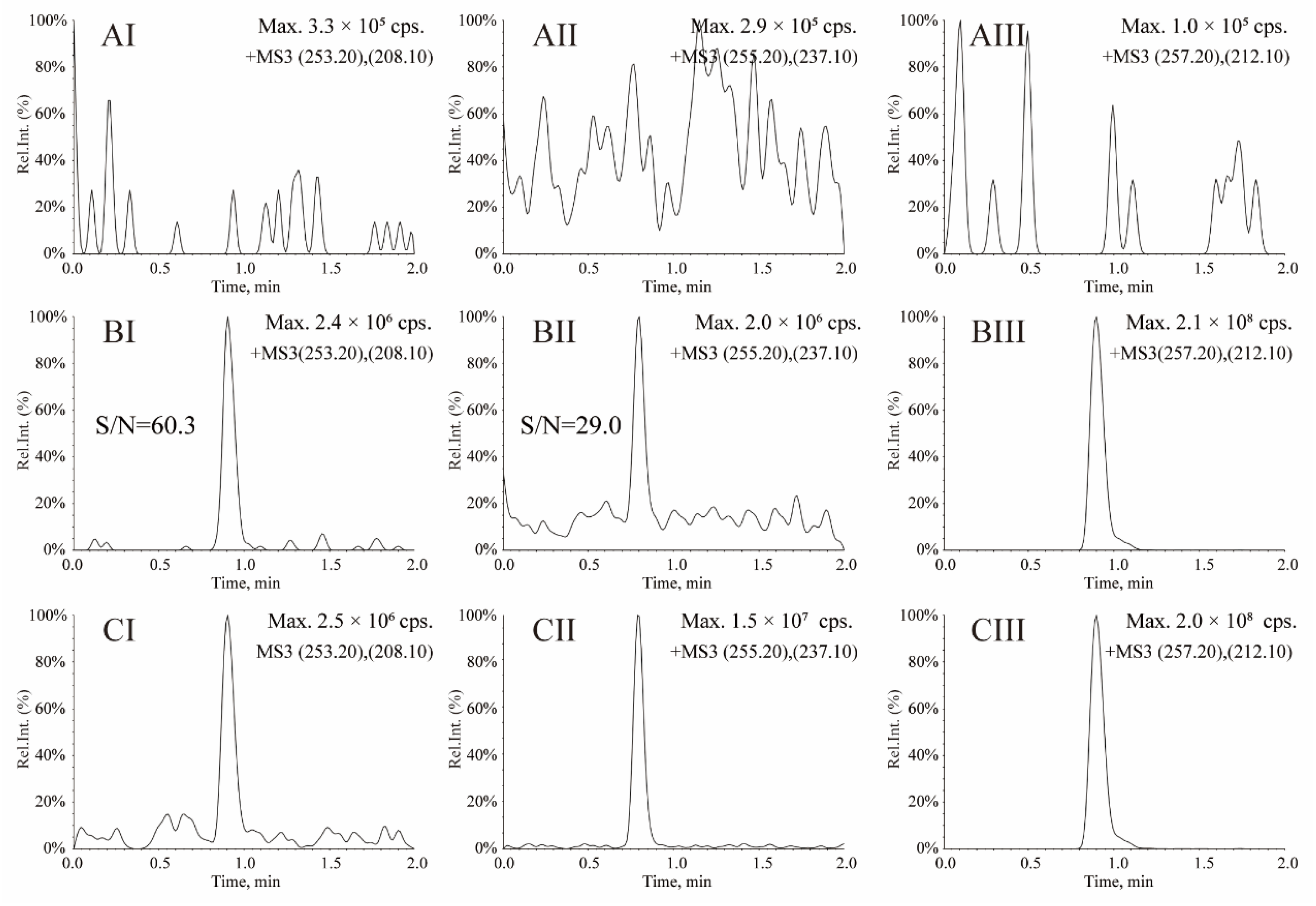
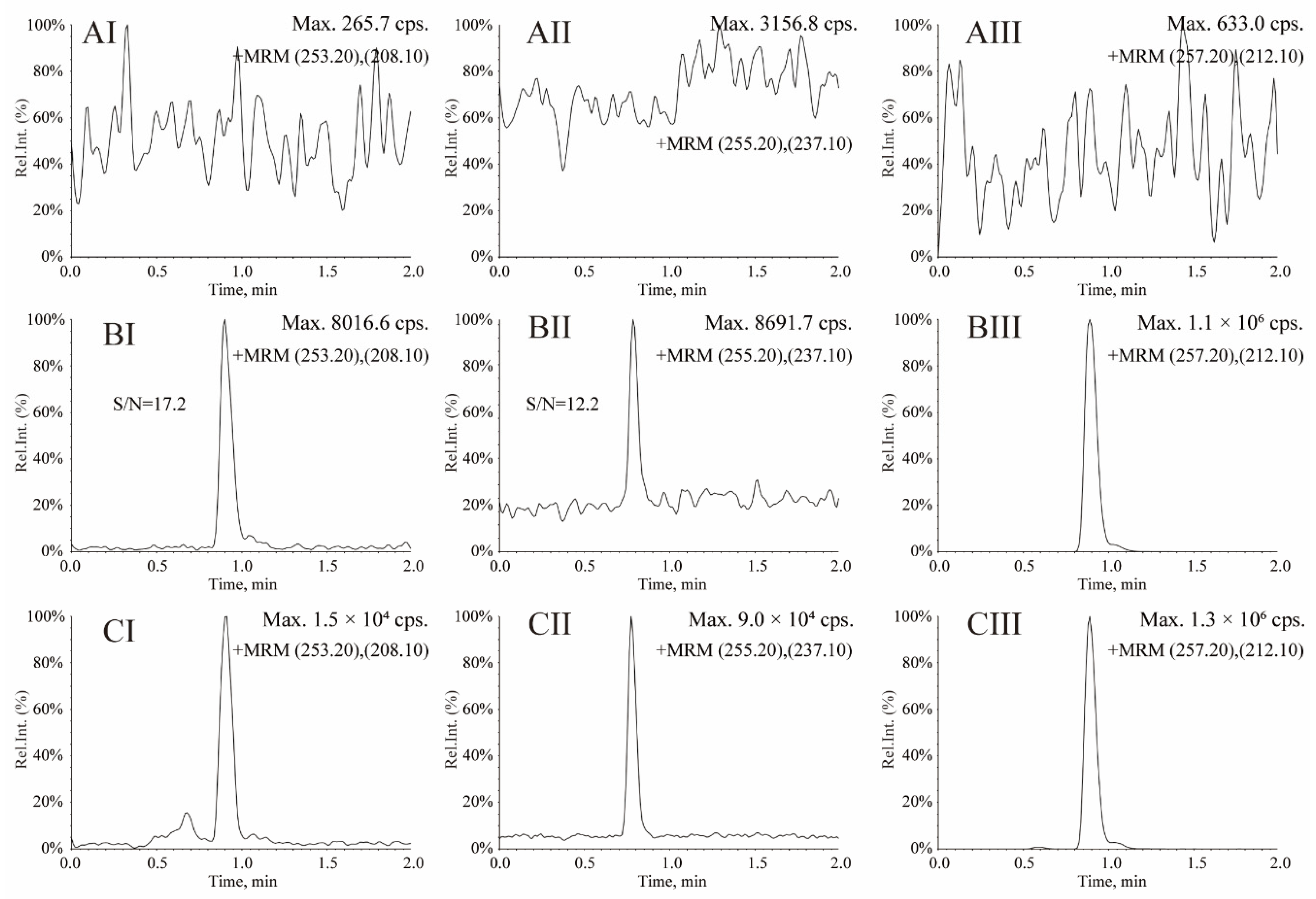
2. Results
Method Validation
3. Discussion
3.1. Optimization of MS Conditions
3.2. Optimization of LC Conditions
3.3. Optimization of Sample Preparation
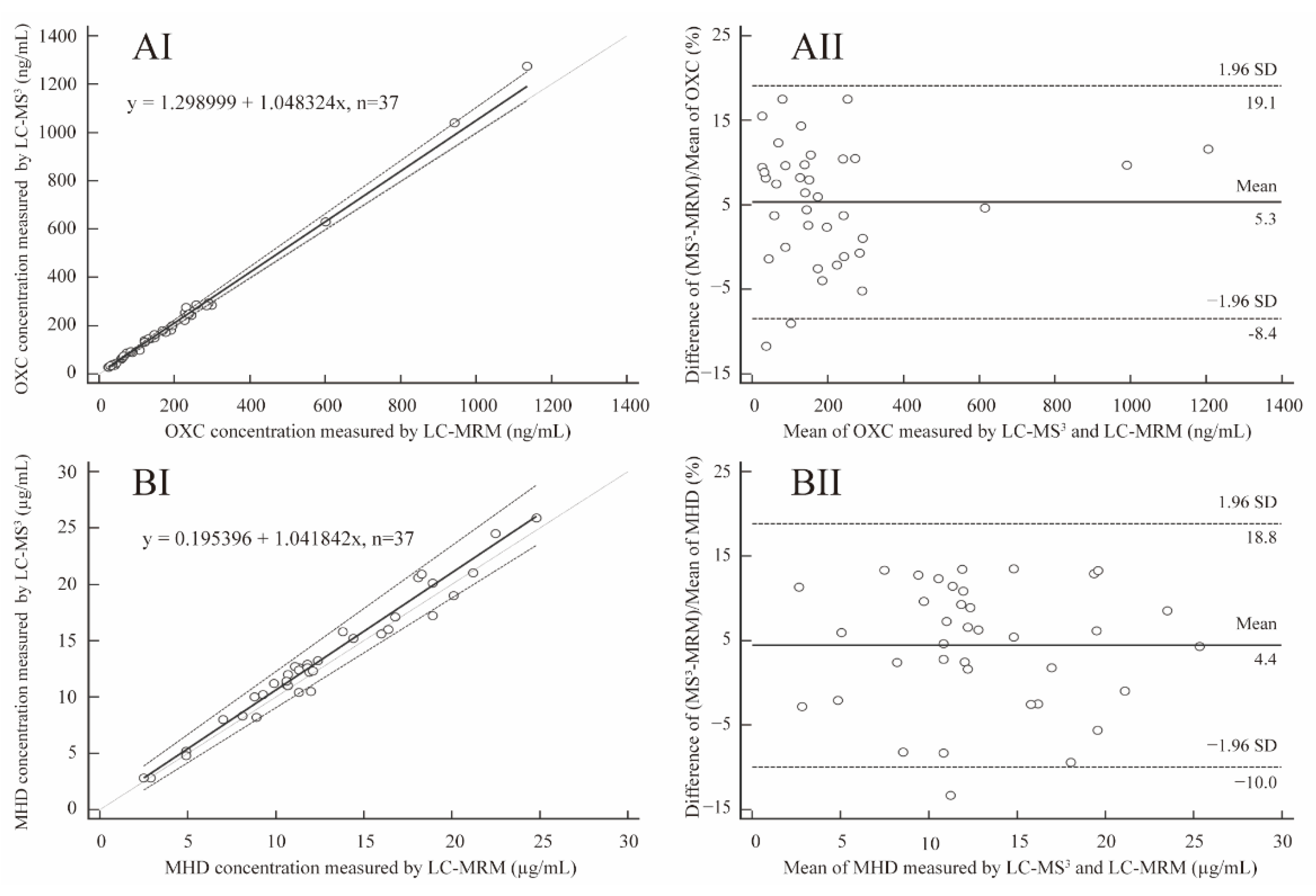
3.4. Method Comparison and Clinical Application
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Chromatographic and Mass Spectrometric Conditions
4.3. Preparation of Stock Solutions, Calibration Standards and Quality Control (QC) Samples
4.4. Serum Sample Pretreatment
4.5. Method Validation for the Quantitation of OXC and MHD in Human Serum
4.6. Clinical Application and Ethics
4.7. Data Acquisition and Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Paglia, G.; D’Apolito, O.; Garofalo, D.; Scarano, C.; Corso, G. Development and validation of a LC/MS/MS method for simultaneous quantification of oxcarbazepine and its main metabolites in human serum. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 860, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Flesch, G.; Czendlik, C.; Renard, D.; Lloyd, P. Pharmacokinetics of the monohydroxy derivative of oxcarbazepine and its enantiomers after a single intravenous dose given as racemate compared with a single oral dose of oxcarbazepine. Drug Metab. Dispos. 2011, 39, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Wellington, K.; Goa, K.L. Oxcarbazepine: An update of its efficacy in the management of epilepsy. CNS Drugs 2001, 15, 137–163. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Kim, K.J. New antiepileptic drugs in pediatric epilepsy. Brain Dev. 2008, 30, 549–555. [Google Scholar] [CrossRef]
- White, H.S. Comparative anticonvulsant and mechanistic profile of the established and newer antiepileptic drugs. Epilepsia 1999, 40 (Suppl. S5), S2–S10. [Google Scholar] [CrossRef] [PubMed]
- Kalis, M.M.; Huff, N.A. Oxcarbazepine, an antiepileptic agent. Clin Ther. 2001, 23, 680–700. [Google Scholar] [CrossRef]
- Flesch, G. Overview of the clinical pharmacokinetics of oxcarbazepine. Clin. Drug Investig. 2004, 24, 185–203. [Google Scholar] [CrossRef]
- Lanckmans, K.; Clinckers, R.; Van Eeckhaut, A.; Sarre, S.; Smolders, I.; Michotte, Y. Use of microbore LC-MS/MS for the quantification of oxcarbazepine and its active metabolite in rat brain microdialysis samples. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006, 831, 205–212. [Google Scholar] [CrossRef]
- Bhatt, M.; Shah, S.; Shivprakash. Rapid ultraperformance liquid chromatography-tandem mass spectrometry method for quantification of oxcarbazepine and its metabolite in human plasma. Biomed. Chromatogr. 2011, 25, 751–759. [Google Scholar] [CrossRef]
- Abou-Khalil, B.W. Update on Antiepileptic Drugs_2019. Continuum 2019, 25, 508–536. [Google Scholar]
- Patsalos, P.N.; Berry, D.J.; Bourgeois, B.F.D.; Cloyd, J.C.; Glauser, T.A.; Johannessen, S.I.; Leppik, I.E.; Tomson, T.; Perucca, E. Antiepileptic drugsbest practice guidelines for therapeutic drug monitoring: A position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia 2008, 49, 1239–1276. [Google Scholar] [CrossRef] [PubMed]
- Sattler, A.; Schaefer, M.; May, T.W. Relationship between mono-hydroxy-carbazepine serum concentrations and adverse effects in patients on oxcarbazepine monotherapy. Seizure 2015, 31, 149–154. [Google Scholar] [CrossRef][Green Version]
- Jang, Y.; Yoon, S.; Kim, T.J.; Lee, S.; Yu, K.S.; Jang, I.J.; Chu, K.; Lee, S.K. Population pharmacokinetic model development and its relationship with adverse events of oxcarbazepine in adult patients with epilepsy. Sci. Rep. 2021, 11, 6370. [Google Scholar] [CrossRef] [PubMed]
- Levert, H.; Odou, P.; Robert, H. LC determination of oxcarbazepine and its active metabolite in human serum. J. Pharm. Biomed. Anal. 2002, 28, 517–525. [Google Scholar] [CrossRef]
- Heideloff, C.; Bunch, D.R.; Wang, S. A novel HPLC method for quantification of 10 antiepileptic drugs or metabolites in serumplasma using a monolithic column. Ther. Drug Monit. 2010, 32, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.V.; Tang, P.H.; Ryan, M.A.; Grim, S.A.; Fakhoury, T.A.; Strawsburg, R.H.; DeGrauw, T.J.; Baumann, R.J. Feasibility and limitations of oxcarbazepine monitoring using salivary monohydroxycarbamazepine (MHD). Ther. Drug Monit. 2004, 26, 300–304. [Google Scholar] [CrossRef]
- Mandrioli, R.; Ghedini, N.; Albani, F.; Kenndler, E.; Raggi, M.A. Liquid chromatographic determination of oxcarbazepine and its metabolites in plasma of epileptic patients after solid-phase extraction. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 783, 253–263. [Google Scholar] [CrossRef]
- Hunek, C.; Burgyan, M.; Wang, S. Measurement of 10,11-dihydro-10-hydroxy-carbamazepine in serum and plasma by high-performance liquid chromatography. Clin. Chem. Lab. Med. 2008, 46, 1429–1433. [Google Scholar] [CrossRef]
- Rani, S.; Malik, A.K. A novel microextraction by packed sorbent-gas chromatography procedure for the simultaneous analysis of antiepileptic drugs in human plasma and urine. J. Sep. Sci. 2012, 35, 2970–2977. [Google Scholar] [CrossRef]
- Erarpat, S.; Bodur, S.; Ayyıldız, M.F.; Günkara, Ö.T.; Erulaş, F.; Chormey, D.S.; Turak, F.; Budak, T.B.; Bakırdere, S. Accurate and simple determination of oxcarbazepine in human plasma and urine samples using switchable-hydrophilicity solvent in GC–MS. Biomed. Chromatogr. 2020, 34, e4915. [Google Scholar] [CrossRef]
- Antunes, N.J.; Wichert-Ana, L.; Coelho, E.B.; Della Pasqua, O.; Alexandre Junior, V.; Takayanagui, O.M.; Marques, M.P.; Lanchote, V.L. Analysis of unbound plasma concentration of oxcarbazepine and the 10-hydroxycarbazepine enantiomers by liquid chromatography with tandem mass spectrometry in healthy volunteers. J. Pharm. Biomed. Anal. 2018, 149, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Karinen, R.; Vindenes, V.; Hasvold, I.; Olsen, K.M.; Christophersen, A.S.; Øiestad, E. Determination of a selection of anti-epileptic drugs and two active metabolites in whole blood by reversed phase UPLC-MS/MS and some examples of application of the method in forensic toxicology cases. Drug Test. Anal. 2015, 7, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Bridgewater, B.; Strickland, E.C.; McIntire, G. A Rapid LC–MS-MS Method for the Quantitation of Antiepileptic Drugs in Urine. J. Anal. Toxicol. 2020, 44, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wang, T.; Shi, M.; Zhang, Y.; Zhao, X.; Yang, Y.; Gu, J. Simultaneous determination of ten antiepileptic drugs in human plasma by liquid chromatography and tandem mass spectrometry with positive/negative ion-switching electrospray ionization and its application in therapeutic drug monitoring. J. Sep. Sci. 2016, 39, 964–972. [Google Scholar] [CrossRef]
- Farouk, F.; ElKady, E.F.; Azzazy, H.M.E. Simultaneous UPLC-MS/MS determination of antiepileptic agents for dose adjustment. Biomed. Chromatogr. 2017, 31. [Google Scholar] [CrossRef]
- Shi, M.; Jiang, Q.; Lyu, Q.; Yuan, Z.; Deng, L.; Yin, L. A LC-MS(3) strategy to determine lamotrigine by Q-Q-trap tandem mass spectrometry coupled with triple stage fragmentation to enhance sensitivity and selectivity. Anal. Methods 2021, 13, 4478–4484. [Google Scholar] [CrossRef]
- Richards, K.H.; Monk, R.; Renko, K.; Rathmann, D.; Rijntjes, E.; Köhrle, J. A combined LC-MS/MS and LC-MS3 multi-method for the quantification of iodothyronines in human blood serum. Anal. Bioanal. Chem. 2019, 411, 5605–5616. [Google Scholar] [CrossRef]
- Ma, D.; Ji, Z.; Cao, H.; Huang, J.; Zeng, L.; Yin, L. LC-MS(3) Strategy for Quantification of Carbamazepine in Human Plasma and Its Application in Therapeutic Drug Monitoring. Molecules 2022, 27, 1224. [Google Scholar] [CrossRef]
- Ji, Z.; Yin, L.; Li, Y.; Yang, X.; Lin, L.; Liu, L.; Jiang, Y.; Huang, J. Development and validation of a liquid chromatography tandem mass spectrometry method with triple stage fragmentation to determine levetiracetam in epileptic patient serum and its application in therapeutic drug monitoring. J. Sep. Sci. 2021, 4255–4263. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidance for Industry: Boianalytical Method Validation; Center for Drug Evaluation and Research; US Department of Health and Human Services: Washington, DC, USA, 2018.
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People Republic of China (Volume Four); Chinese Medical Science and Technology Press: Beijing, China, 2015; pp. 387–393.


| Compound | Spiked Concentration (µg/mL) | Precision (RSD, %) | Accuracy (RE, %) | ||
|---|---|---|---|---|---|
| Intra-day (n = 6) | Inter-day (3 days, n = 18) | Intra-day (n = 6) | Inter-day (3 days, n = 18) | ||
| OXC | 0.05 | 8.3 | 7.0 | 8.6 | 4.9 |
| 0.2 | 7.0 | 6.1 | −6.8 | −2.8 | |
| 0.8 | 1.4 | 0.8 | 1.2 | 0.8 | |
| MHD | 1 | 10.5 | 10.2 | 8.5 | 0.5 |
| 4 | 8.5 | 9.2 | 7.9 | −0.5 | |
| 16 | 9.5 | 9.3 | 7.3 | 0.7 | |
| Compound | Spiked Concentration (µg/mL) | Matrix Effect (%) Mean ± SD (n = 6) | Recovery (%) Mean ± SD (n = 6) |
|---|---|---|---|
| OXC | 0.05 | 92.7 ± 6.4 | 92.1 ± 14.9 |
| 0.2 | 99.8 ± 10.4 | 112.1 ± 11.5 | |
| 0.8 | 101.8 ± 6.8 | 104.4 ± 3.6 | |
| MHD | 1 | 99.1 ± 10.0 | 91.6 ± 4.8 |
| 4 | 96.1 ± 1.0 | 93.0 ± 4.2 | |
| 16 | 103.1 ± 3.9 | 105.0 ± 8.2 |
| Compound | Nominal Concentration (µg/mL) | Long Term −80 °C | Short Term | Freeze–Thaw | Post-Preparative |
|---|---|---|---|---|---|
| OXC | 0.05 | 90.5 ± 6.9 | 97.5 ± 7.4 | 92.7 ± 8.2 | 95.0 ± 5.7 |
| 0.2 | 93.8 ± 5.3 | 91.3 ± 5.1 | 89.3 ± 1.0 | 90.5 ± 2.7 | |
| 0.8 | 99.6 ± 1.8 | 92.4 ± 2.0 | 98.9 ± 1.7 | 97.5 ± 0.9 | |
| MHD | 1 | 95.6 ± 9.3 | 98.6 ± 12.1 | 96.9 ± 4.7 | 97.4 ± 7.1 |
| 4 | 92.5 ± 4.8 | 98.9 ± 0.95 | 100.0 ± 7.5 | 10.9 ± 2.4 | |
| 16 | 104.4 ± 2.5 | 97.7 ± 6.6 | 96.0 ± 9.9 | 96.0 ± 9.9 |
| Parameters | MS3 | MRM | ||||
|---|---|---|---|---|---|---|
| OXC | MHD | OXC-d4 (IS) | OXC | MHD | OXC-d4 (IS) | |
| MS3 transitions/MRM transitions (m/z) | 253.2→208.1→180.2 | 255.2→237.1→194.1 | 257.2→212.1→184.2 | 253.2→236.1 | 255.2→237.1 | 257.2→240.1 |
| Declustering potential (V) | 100 | 60 | 100 | 100 | 60 | 100 |
| Collision energy (eV) | 23.5 | 16.1 | 26.3 | 23.5 | 16.1 | 26.3 |
| AF2 (V) | 0.10 | 0.10 | 0.10 | / | / | / |
| Scan rate (Da/s) | 20000 | 20000 | 20000 | / | / | / |
| LIT fill time (ms) | 20 | 20 | 20 | / | / | / |
| Excitation time (ms) | 25 | 25 | 25 | / | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Z.; Li, T.; Zhao, X.; Ma, W.; Li, Y.; Huang, J. Development and Validation of a Highly Sensitive and Rapid LC-MS3 Strategy to Determine Oxcarbazepine and Its Active Metabolite in the Serum of Patients with Epilepsy and Its Application in Therapeutic Drug Monitoring. Molecules 2022, 27, 5670. https://doi.org/10.3390/molecules27175670
Ji Z, Li T, Zhao X, Ma W, Li Y, Huang J. Development and Validation of a Highly Sensitive and Rapid LC-MS3 Strategy to Determine Oxcarbazepine and Its Active Metabolite in the Serum of Patients with Epilepsy and Its Application in Therapeutic Drug Monitoring. Molecules. 2022; 27(17):5670. https://doi.org/10.3390/molecules27175670
Chicago/Turabian StyleJi, Zhengchao, Tingting Li, Xin Zhao, Wei Ma, Yanyan Li, and Jing Huang. 2022. "Development and Validation of a Highly Sensitive and Rapid LC-MS3 Strategy to Determine Oxcarbazepine and Its Active Metabolite in the Serum of Patients with Epilepsy and Its Application in Therapeutic Drug Monitoring" Molecules 27, no. 17: 5670. https://doi.org/10.3390/molecules27175670
APA StyleJi, Z., Li, T., Zhao, X., Ma, W., Li, Y., & Huang, J. (2022). Development and Validation of a Highly Sensitive and Rapid LC-MS3 Strategy to Determine Oxcarbazepine and Its Active Metabolite in the Serum of Patients with Epilepsy and Its Application in Therapeutic Drug Monitoring. Molecules, 27(17), 5670. https://doi.org/10.3390/molecules27175670




