Polynaphthylimide–Azomethines Containing Triphenylamine or Carbazole Moieties with Tuned Optoelectronic Properties through Molecular Design
Abstract
:1. Introduction
2. Experimental
2.1. Starting Materials
2.2. Polymers Synthesis
- P1, 1H NMR (DMSO-d6, 400.13 MHz, δ ppm): 8.74–8.72 (m, 6H), 7.96–7.83 (dd, 4H), 7.50–7.47 (t, 2H), 7.33–7.24 (m, 5H), 7.18–7.16 (d, 2H).
- P2, 1H NMR (DMSO-d6, 400.13 MHz, δ ppm): 8.89–8.68 (m, 6H), 8.22–8.20 (dd, 4H), 8.06 (s, 2H), 7.86–7.83 (m, 2H), 4.42–4.39 (m, 2H), 2.04 (s, 1H), 1.33–1.19 (m, 8H), 0.88–0.77 (m, 6H).
- P3, 1H NMR (DMSO-d6, 400.13 MHz, δ ppm): 8.97–8.91 (m, 2H), 8.64–8.49 (m, 4H), 8.13–8.19 (m, 2H), 7.92–7.81 (m, 6H), 7.47–7.45 (d, 2H), 7.29–7.14 (m, 7H).
- P4, 1H NMR (CDCl3, 400.13 MHz, δ ppm): 8.84–8.50 (m, 8H), 8.30–8.22 (m, 2H), 8.11–7.95 (m, 2H), 7.71–7.43 (m, 4H), 4.34–4.21 (m, 2H), 2.10 (s, 1H), 1.40–1.25 (m, 8H), 0.97–0.86 (m, 6H).
2.3. Preparation of Polymer Films (Coatings)
2.4. Measurements
3. Results and Discussions
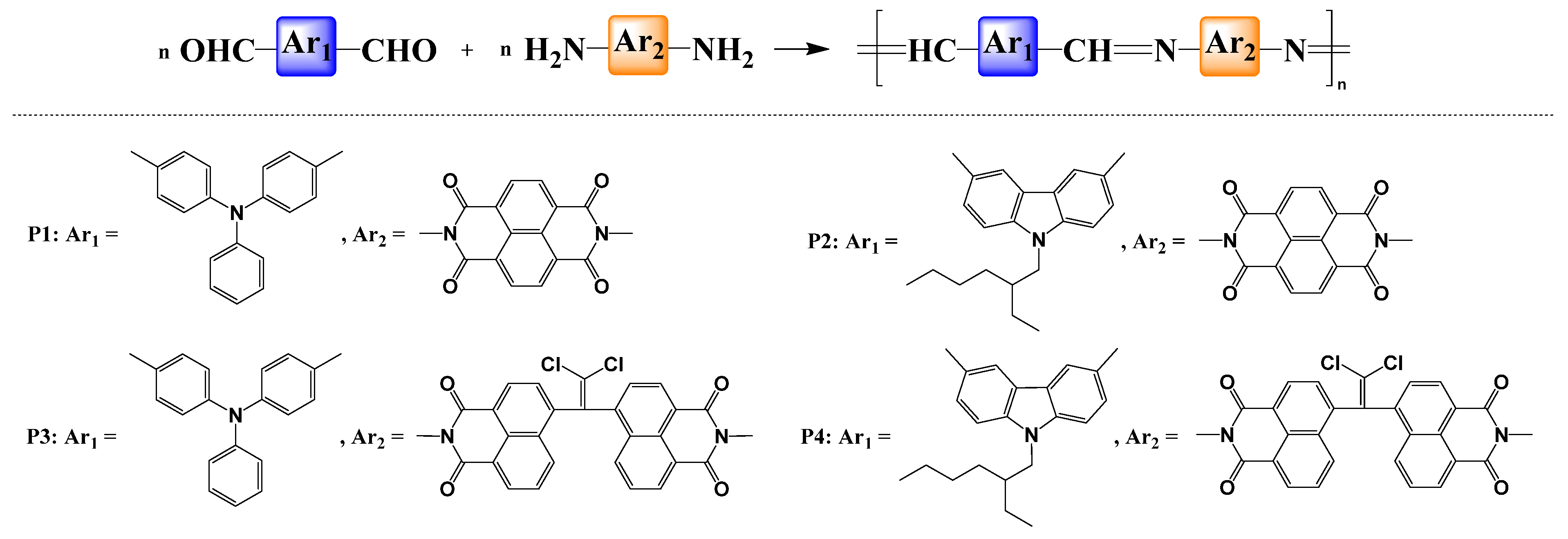
3.1. Synthesis and Structural Characterization
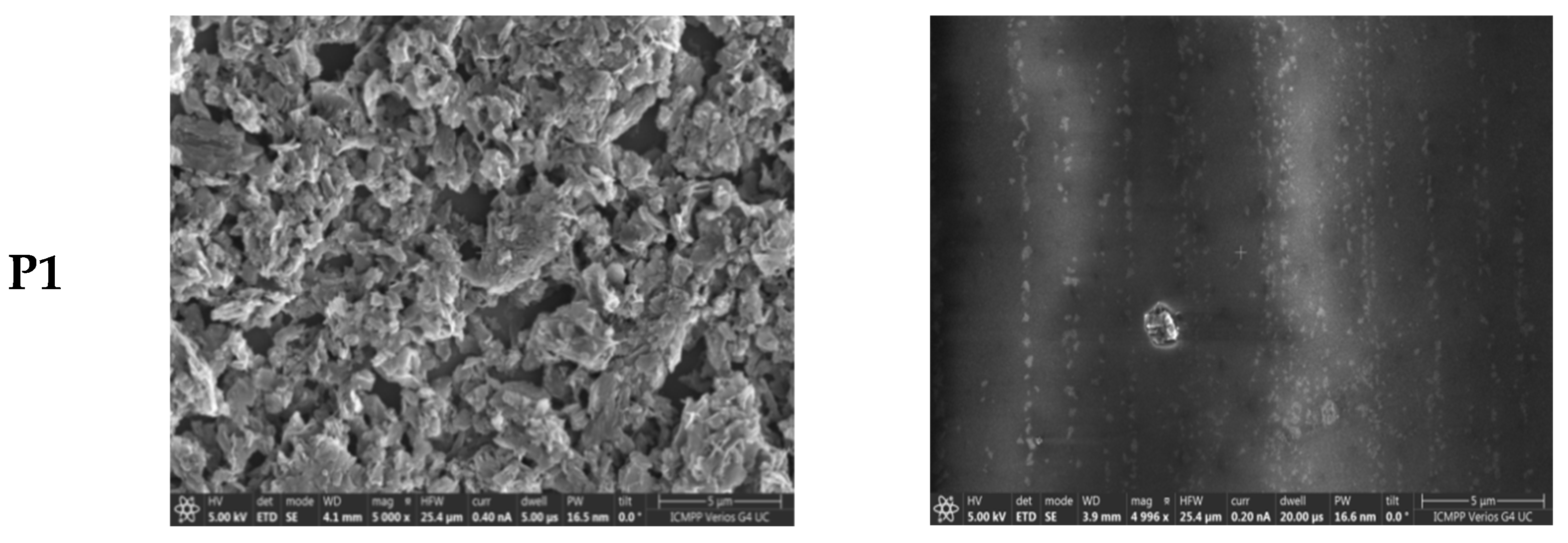
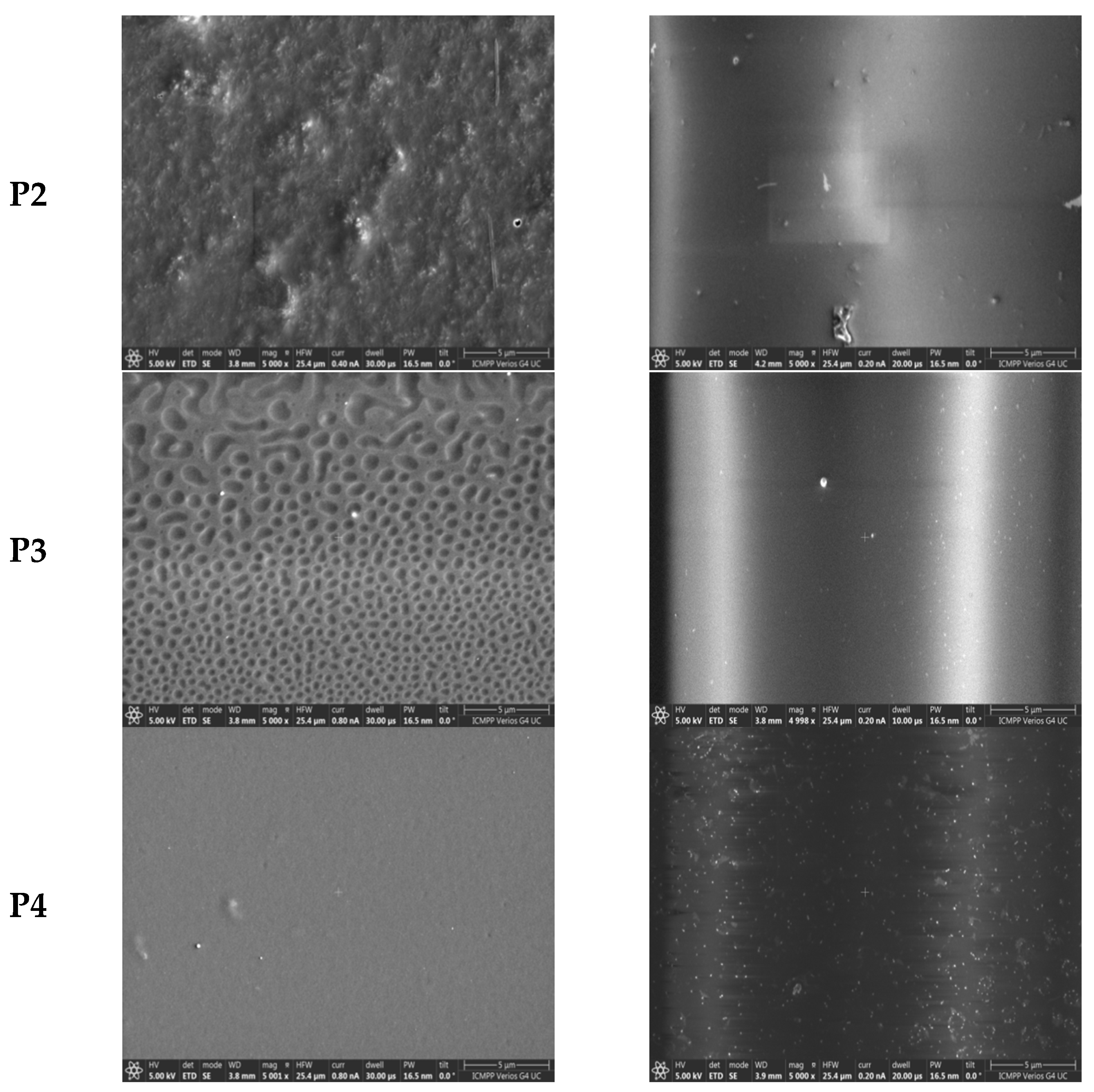
3.2. Films Morphology
3.3. Thermal Stability
3.4. Photophysical Studies
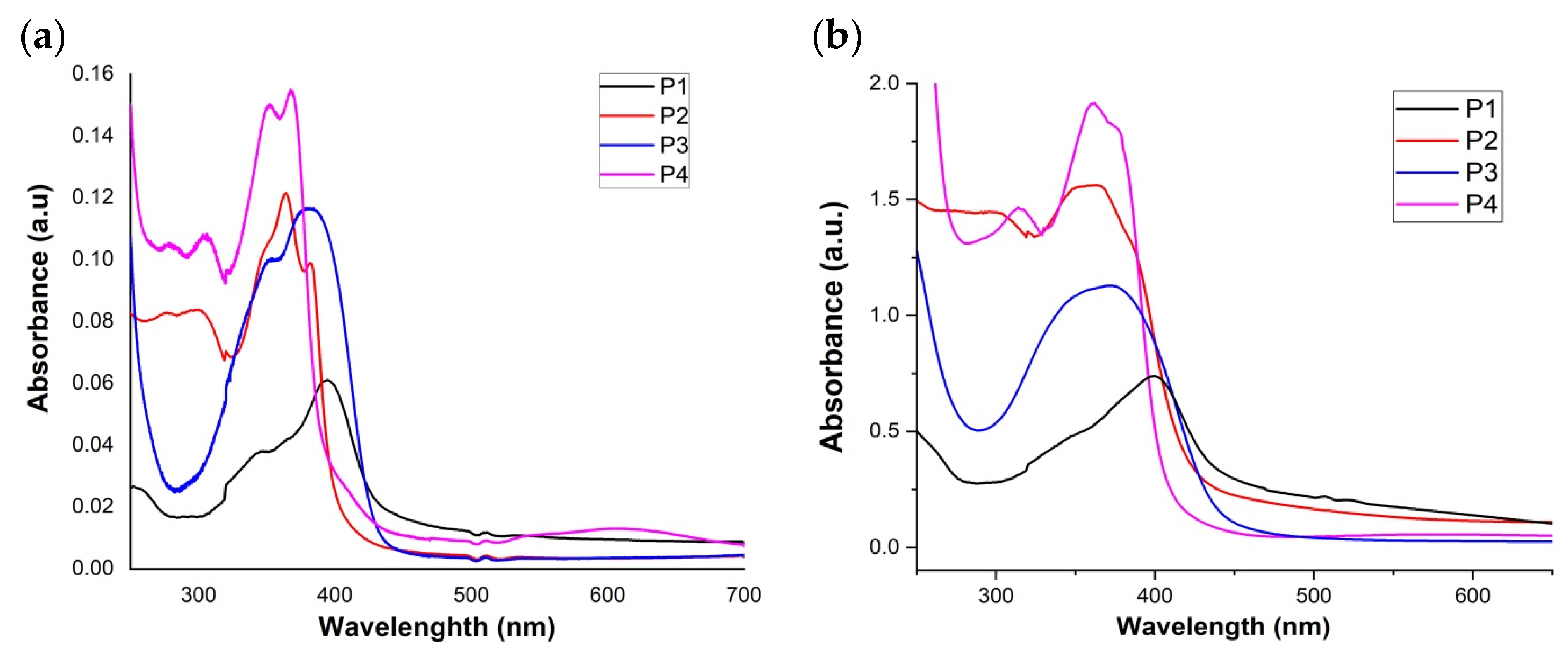
3.4.1. UV-Vis Absorption Characteristics
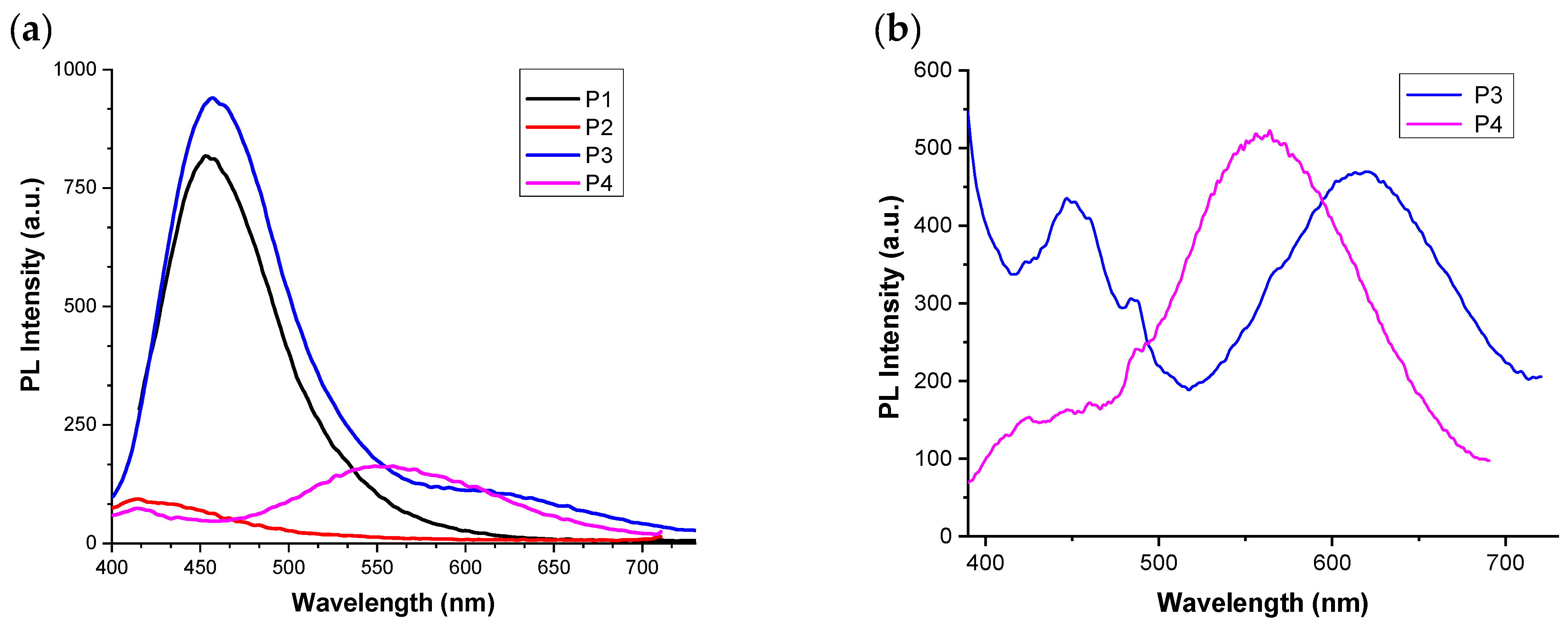
3.4.2. Fluorescence Characteristics
3.4.3. Optical Bandgap Energy
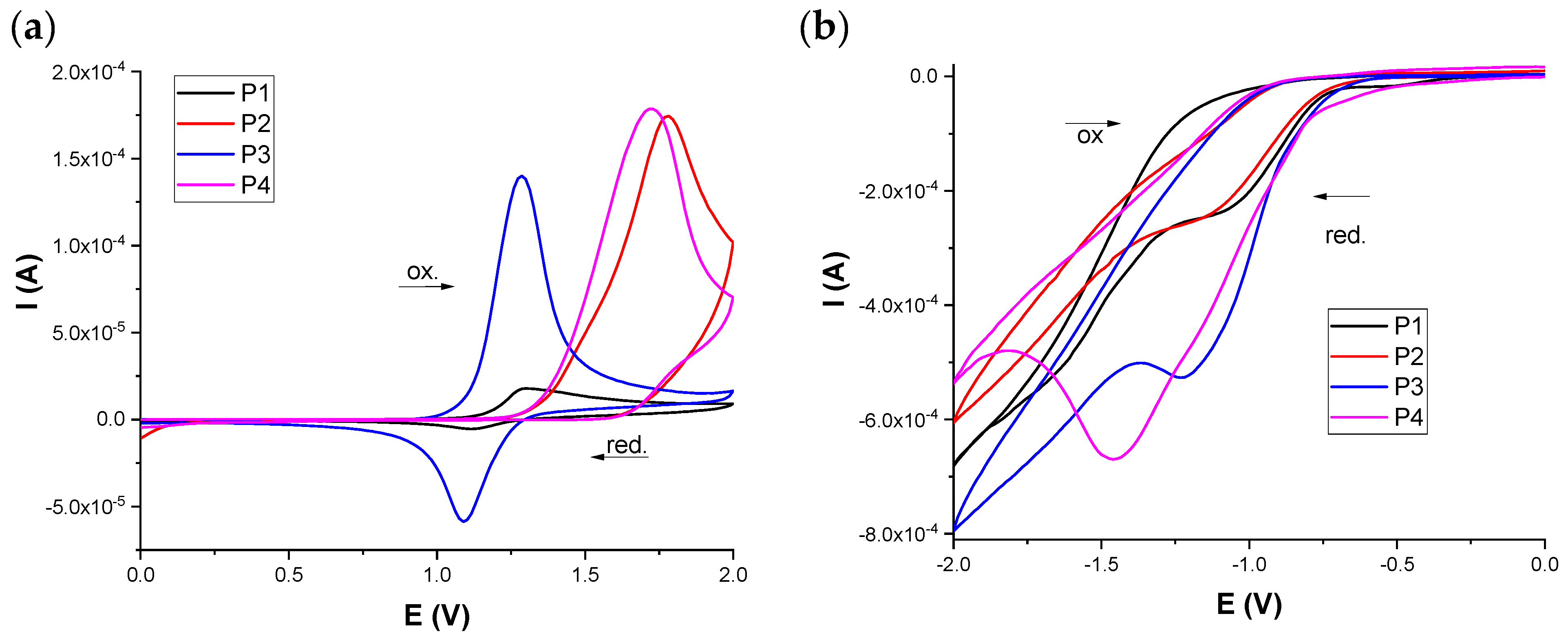
3.5. Polymer’s Electroactivity and Electronic Structure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Y.; Liu, Y.; Emrick, T.; Russell, T.P. Polymer design to promote low work function surfaces in organic electronics. Prog. Polym. Sci. 2020, 103, 101222. [Google Scholar] [CrossRef]
- Guo, X.; Baumgarten, M.; Müllen, K. Designing π-conjugated polymers for organic electronics. Prog. Polym. Sci. 2013, 38, 1832–1908. [Google Scholar] [CrossRef]
- Pron, A.; Leclerc, M. Imide/amide based π-conjugated polymers for organic electronics. Prog. Polym. Sci. 2013, 38, 1815–1831. [Google Scholar] [CrossRef]
- Giraud, L.; Grelier, S.; Grau, E.; Hadziioannou, G.; Brochon, C.; Cramail, H.; Cloutet, E. Upgrading the chemistry of π-conjugated polymers toward more sustainable materials. J. Mater. Chem. C 2020, 8, 9792–9810. [Google Scholar] [CrossRef]
- Coakley, K.M.; McGehee, M.D. Conjugated Polymer Photovoltaic Cells. Chem. Mater. 2004, 16, 4533–4542. [Google Scholar] [CrossRef]
- Bolduc, A.; Mallet, C.; Skene, W.G. Survey of recent advances of in the field of π-conjugated heterocyclic azomethines as materials with tuneable properties. Sci. China Chem. 2012, 56, 3–23. [Google Scholar] [CrossRef]
- AlSalhi, M.S.; Alam, J.; Dass, L.A.; Raja, M. Recent Advances in Conjugated Polymers for Light Emitting Devices. Int. J. Mol. Sci. 2011, 12, 2036–2054. [Google Scholar] [CrossRef]
- Sek, D.; Iwan, A.; Jarzabek, B.; Kaczmarczyk, B.; Kasperczyk, J.; Mazurak, Z.; Domanski, M.; Karon, K.; Lapkowski, M. Hole Transport Triphenylamine−Azomethine Conjugated System: Synthesis and Optical, Photoluminescence, and Electrochemical Properties. Macromolecules 2008, 41, 6653–6663. [Google Scholar] [CrossRef]
- Temizkan, K.; Kaya, İ. Synthesis of soluble poly(azomethine)s containing thiophene and their fluorescence quantum yields. Polym. Bull. 2020, 77, 3287–3303. [Google Scholar] [CrossRef]
- Chen, C.K.; Lin, Y.C.; Ho, J.C.; Yang, W.C.; Chen, W.C. Biomass-Derived Degradable Poly(azomethine)s for Flexible Bistable Photonic Transistor Memories. ACS Sustain. Chem. Eng. 2022, 10, 5268–5277. [Google Scholar] [CrossRef]
- Nitschke, P.; Jarząbek, B.; Vasylieva, M.; Godzierz, M.; Janeczek, H.; Musioł, M.; Domiński, A. The Effect of Alkyl Substitution of Novel Imines on Their Supramolecular Organization, towards Photovoltaic Applications. Polymers 2021, 13, 1043. [Google Scholar] [CrossRef] [PubMed]
- Vercelli, B.; Pasini, M.; Berlin, A.; Casado, J.; López Navarrete, J.T.; Ortiz, R.P.; Zotti, G. Phenyl- and Thienyl-Ended Symmetric Azomethines and Azines as Model Compounds for n-Channel Organic Field-Effect Transistors: An Electrochemical and Computational Study. J. Phys. Chem. C 2014, 118, 3984–3993. [Google Scholar] [CrossRef]
- Gomulya, W.; Derenskyi, V.; Kozma, E.; Pasini, M.; Loi, M.A. Polyazines and Polyazomethines with Didodecylthiophene Units for Selective Dispersion of Semiconducting Single-Walled Carbon Nanotubes. Adv. Funct. Mater. 2015, 25, 5858–5864. [Google Scholar] [CrossRef]
- Korona, K.P.; Korona, T.; Rutkowska-Zbik, D.; Grankowska-Ciechanowicz, S.; Iwan, A.; Kamińska, M. Polyazomethine as a component of solar cells-theoretical and optical study. J. Phys. Chem. Solids 2015, 86, 186–193. [Google Scholar] [CrossRef]
- Iwan, A.; Sek, D. Processible polyazomethines and polyketanils: From aerospace to light-emitting diodes and other advanced applications. Prog. Polym. Sci. 2008, 33, 289–345. [Google Scholar] [CrossRef]
- Hafeez, A.; Akhter, Z.; Gallagher, J.F.; Khan, N.A.; Gul, A.; Shah, F.U. Synthesis, Crystal Structures, and Spectroscopic Characterization of Bis-aldehyde Monomers and Their Electrically Conductive Pristine Polyazomethines. Polymers 2019, 11, 1498. [Google Scholar] [CrossRef] [PubMed]
- Bejan, A.E.; Damaceanu, M.D. New heterocyclic conjugated azomethines containing triphenylamine units with optical and electrochemical responses towards the acid environment. Synth. Met. 2020, 268, 116498. [Google Scholar] [CrossRef]
- Cai, X.M.; Lin, Y.; Li, Y.; Chen, X.; Wang, Z.; Zhao, X.; Huang, S.; Zhao, Z.; Tang, B.Z. BioAIEgens derived from rosin: How does molecular motion affect their photophysical processes in solid state? Nat. Commun. 2021, 12, 1773. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.M.; Tang, Z.; Chen, X.; Lin, Y.; Zhang, X.; Huang, S. Construction of two rosin-based BioAIEgens with distinct fluorescence and mechanochromic properties for rewritable paper. Dyes Pigm. 2022, 204, 110454. [Google Scholar] [CrossRef]
- Yen, H.-J.; Liou, G.-S. Recent advances in triphenylamine-based electrochromic derivatives and polymers. Polym. Chem. 2018, 9, 3001–3018. [Google Scholar] [CrossRef]
- Bejan, A.-E.; Constantin, C.-P.; Damaceanu, M.D. n-Type Polyimides with 1,3,4-Oxadiazole-Substituted Triphenylamine Units—An Innovative Structural Approach. J. Phys. Chem. C 2019, 123, 15908–15923. [Google Scholar] [CrossRef]
- Li, Z.; Ye, T.; Tang, S.; Wang, C.; Ma, D.; Li, Z. Triphenylamine-based π-conjugated dendrimers: Convenient synthesis, easy solution processability, and good hole-transporting properties. J. Mater. Chem. C 2015, 3, 2016–2023. [Google Scholar] [CrossRef]
- Bekkar, F.; Bettahar, F.; Moreno, I.; Meghabar, R.; Hamadouche, M.; Hernáez, E.; Vilas-Vilela, J.L.; Ruiz-Rubio, L. Polycarbazole and Its Derivatives: Synthesis and Applications. A Review of the Last 10 Years. Polymers 2020, 12, 2227. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Li, C.; Zhang, M.; Chen, Y. Acceptor-donor-acceptor type molecules for high performance organic photovoltaics-chemistry and mechanism. Chem. Soc. Rev. 2020, 49, 2828–2842. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, S.V.; Jani, C.H.; Langford, S.J. Chemistry of naphthalene diimides. Chem. Soc. Rev. 2008, 37, 331–342. [Google Scholar] [CrossRef]
- Constantin, C.P.; Damaceanu, M.D.; Bruma, M.; Pinteala, M. Advanced materials based on new structurally designed poly(naphthylimide-amide)s. Polym. Int. 2015, 64, 361–372. [Google Scholar] [CrossRef]
- Christopherson, C.J.; Mayder, D.M.; Poisson, J.; Paisley, N.R.; Tonge, C.M.; Hudson, Z.M. 1,8-Naphthalimide-Based Polymers Exhibiting Deep-Red Thermally Activated Delayed Fluorescence and Their Application in Ratiometric Temperature Sensing. ACS Appl. Mater. Interfaces 2020, 12, 20000–20011. [Google Scholar] [CrossRef] [PubMed]
- Soroceanu, M.; Constantin, C.P.; Damaceanu, M.D. A straightforward synthetic strategy towards conjugated donor-acceptor naphthylimido-azomethines with tunable films morphologies and optoelectronic properties. Prog. Org. Coat. 2022, 166, 106785. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Wu, C.N. Synthesis and Properties of Redox-Active Polyimides with 3,5-Bis(trifluoromethyl)- or 3,5-Dimethyl-Substituted Triphenylamine Groups. Polym. Plast. Technol. Eng. 2017, 56, 1274–1285. [Google Scholar] [CrossRef]
- Jebnouni, A.; Leclerc, N.; Teka, S.; Mansour, D.; Jaballah, N.S. Vinylene-versus azomethine-bridged carbazole-based polymers for light emission and sensor applications. J. Mol. Struct. 2021, 1244, 130994. [Google Scholar] [CrossRef]
- Damaceanu, M.D.; Rusu, R.D.; Bruma, M.; Rusanov, A.L. New thermally stable and organosoluble heterocyclic poly(naphthaleneimide)s. Polym. Adv. Technol. 2011, 22, 420–429. [Google Scholar] [CrossRef]
- Constantin, C.P.; Damaceanu, M.D. In-depth investigation of the optical effects in rationally designed phenoxazine-based polyazomethines with activated quenched fluorescence. J. Phys. Chem. C 2017, 121, 6300–6313. [Google Scholar] [CrossRef]
- Dufresne, S.; Skene, W.G. Optoelectronic property tailoring of conjugated heterocyclic azomethines—the effect of pyrrole, thiophene and furans. J. Phys. Org. Chem. 2012, 25, 211–221. [Google Scholar] [CrossRef]
- Bourque, A.N.; Dufresne, S.; Skene, W.G. Thiophene-phenyl azomethines with varying rotational barriers-model compounds for examining imine fluorescence deactivation. J. Phys. Chem. C 2009, 113, 19677–19685. [Google Scholar] [CrossRef]
- Tauc, J. Optical Properties and Electronic Structure of Amorphous Semiconductors. In Optical Physics and Engineering; Nudelman, S., Mitra, S.S., Eds.; Springer: Boston, MA, USA, 1969. [Google Scholar] [CrossRef]
- Jarzabek, B.; Weszka, J.; Domański, M.; Jurusik, J.; Cisowski, J. Optical studies of aromatic polyazomethine thin films. J. Non. Cryst. Solids 2008, 354, 856–862. [Google Scholar] [CrossRef]
- Jarząbek, B.; Kaczmarczyk, B.; Jurusik, J.; Siwy, M.; Weszka, J. Optical properties of thin films of polyazomethine with flexible side chains. J. Non. Cryst. Solids 2013, 375, 13–18. [Google Scholar] [CrossRef]
- Stranius, K.; Hertzog, M.; Börjesson, K. Selective manipulation of electronically excited states through strong light-matter interactions. Nat. Commun. 2018, 9, 2273. [Google Scholar] [CrossRef]
- Scharber, M.C.; Sariciftci, N.S. Low Band Gap Conjugated Semiconducting Polymers. Adv. Mater. Technol. 2021, 6, 2000857. [Google Scholar] [CrossRef]
- Schab-Balcerzak, E.; Grucela-Zajac, M.; Krompiec, M.; Janeczek, H.; Siwy, M.; Sek, D. New naphthalene diimide-based compounds containing triarylamine units and imine linkages: Thermal, optical and electrochemical properties. Synth. Met. 2011, 161, 2268–2279. [Google Scholar] [CrossRef]
- Rybakiewicz, R.; Djurado, D.; Cybulski, H.; Dobrzynska, E.; Kulszewicz-Bajer, I.; Boudinet, D.; Verilhac, J.M.; Zagorska, M.; Pron, A. Arylene bisimides with triarylamine N-substituents as new solution processable organic semiconductors: Synthesis, spectroscopic, electrochemical and electronic properties. Synth. Met. 2011, 161, 1600–1610. [Google Scholar] [CrossRef]
- Damaceanu, M.D.; Rusu, R.D.; Nicolescu, A.; Bruma, M. Blue fluorescent polyamides containing naphthalene and oxadiazole rings. J. Polym. Sci. Part A: Polym. Chem. 2011, 49, 893–906. [Google Scholar] [CrossRef]
- Sworakowski, J. How accurate are energies of HOMO and LUMO levels in small-molecule organic semiconductors determined from cyclic voltammetry or optical spectroscopy? Synth. Met. 2018, 235, 125–130. [Google Scholar] [CrossRef]

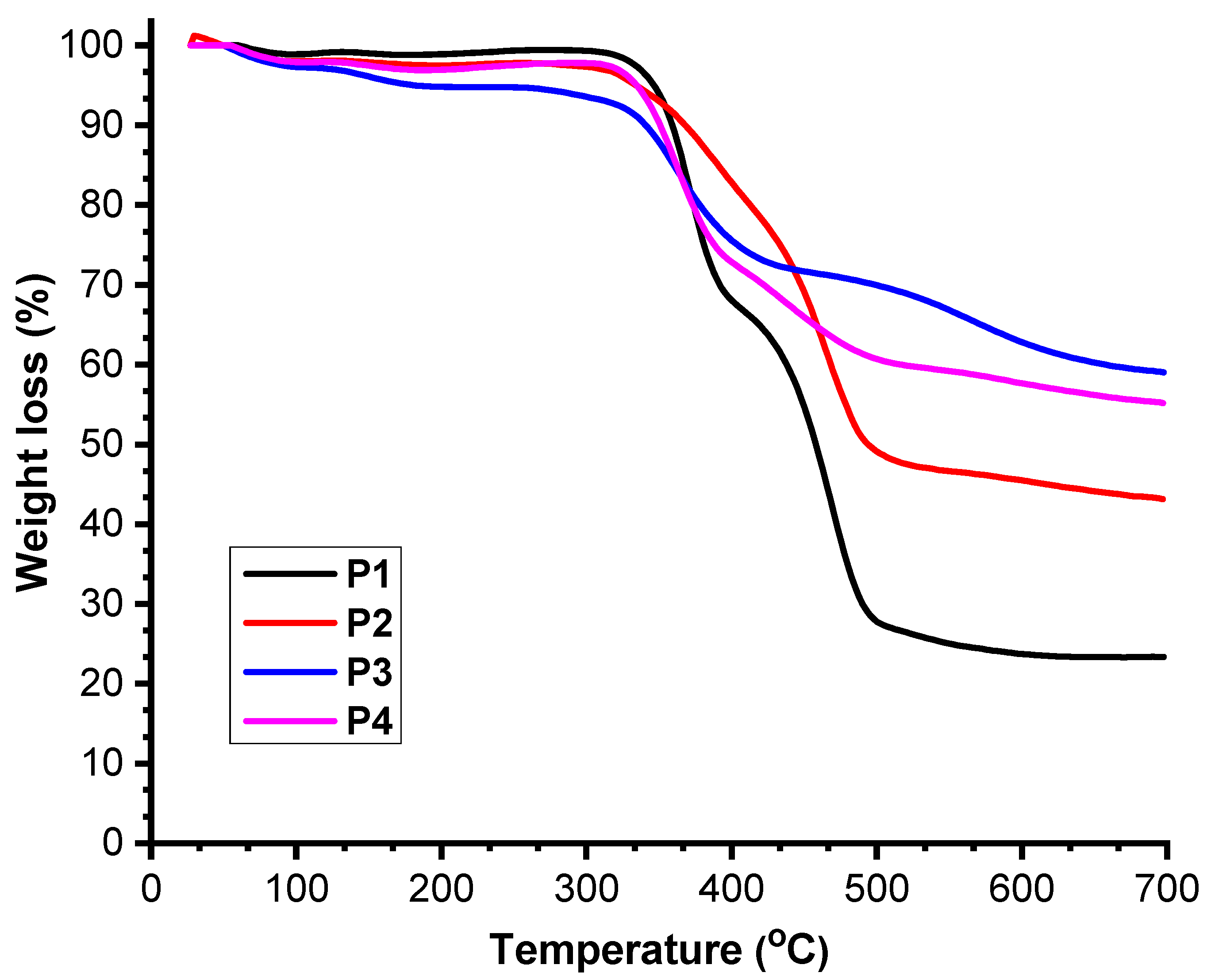

| Polimers | Mn (g/mol) | Mw (g/mol) | Mw/Mn | Tonset (°C) |
|---|---|---|---|---|
| P1 | 1280 | 1540 | 1.19 | 345 |
| P2 | 2150 | 2880 | 1.34 | 318 |
| P3 | 2700 | 5100 | 1.88 | 328 |
| P4 | 3290 | 5660 | 1.72 | 338 |
| Polymer | Eg (eV) | Thickness (µm) |
|---|---|---|
| P1 | 1.8 | 0.512 |
| P2 | 2.7 | 0.351 |
| P3 | 2.6 | 0.638 |
| P4 | 2.9 | 0.357 |
| Polymer Film | Oxidation Potential (V) | Reduction Potential (V) | Energy (eV) | ||||
|---|---|---|---|---|---|---|---|
| Eoxonset | Eox | Eredonset | Ered1 | ELUMO | EHOMO | Eg | |
| P1 | 1.13 | 1.30 | −0.77 | 1.12 −1.07 −1.61 | −3.67 | −5.57 | 1.9 |
| P2 | 1.36 | 1.78 | −0.76 | −1.13 | −3.68 | −5.8 | 2.12 |
| P3 | 1.09 | 1.29 | −0.82 | 1.09 −1.23 | −3.62 | −5.53 | 1.91 |
| P4 | 1.34 | 1.73 | −0.83 | −1.46 | −3.61 | −5.78 | 2.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soroceanu, M.; Constantin, C.-P.; Damaceanu, M.-D. Polynaphthylimide–Azomethines Containing Triphenylamine or Carbazole Moieties with Tuned Optoelectronic Properties through Molecular Design. Molecules 2022, 27, 5761. https://doi.org/10.3390/molecules27185761
Soroceanu M, Constantin C-P, Damaceanu M-D. Polynaphthylimide–Azomethines Containing Triphenylamine or Carbazole Moieties with Tuned Optoelectronic Properties through Molecular Design. Molecules. 2022; 27(18):5761. https://doi.org/10.3390/molecules27185761
Chicago/Turabian StyleSoroceanu, Marius, Catalin-Paul Constantin, and Mariana-Dana Damaceanu. 2022. "Polynaphthylimide–Azomethines Containing Triphenylamine or Carbazole Moieties with Tuned Optoelectronic Properties through Molecular Design" Molecules 27, no. 18: 5761. https://doi.org/10.3390/molecules27185761
APA StyleSoroceanu, M., Constantin, C.-P., & Damaceanu, M.-D. (2022). Polynaphthylimide–Azomethines Containing Triphenylamine or Carbazole Moieties with Tuned Optoelectronic Properties through Molecular Design. Molecules, 27(18), 5761. https://doi.org/10.3390/molecules27185761






