Photodynamic Inactivation of Microorganisms Using Semisynthetic Chlorophyll a Derivatives as Photosensitizers
Abstract
:1. Introduction
2. Results and Discussion
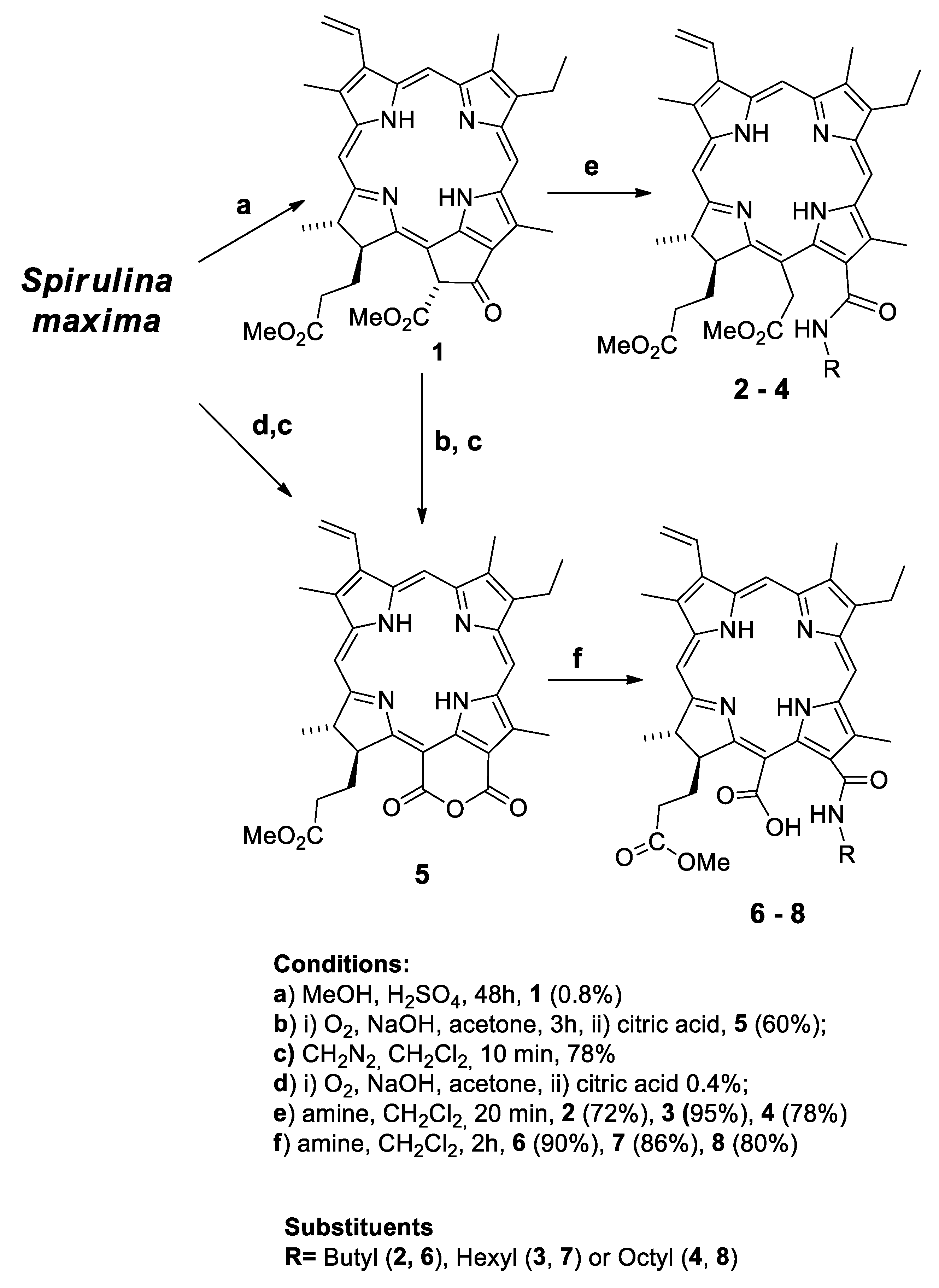
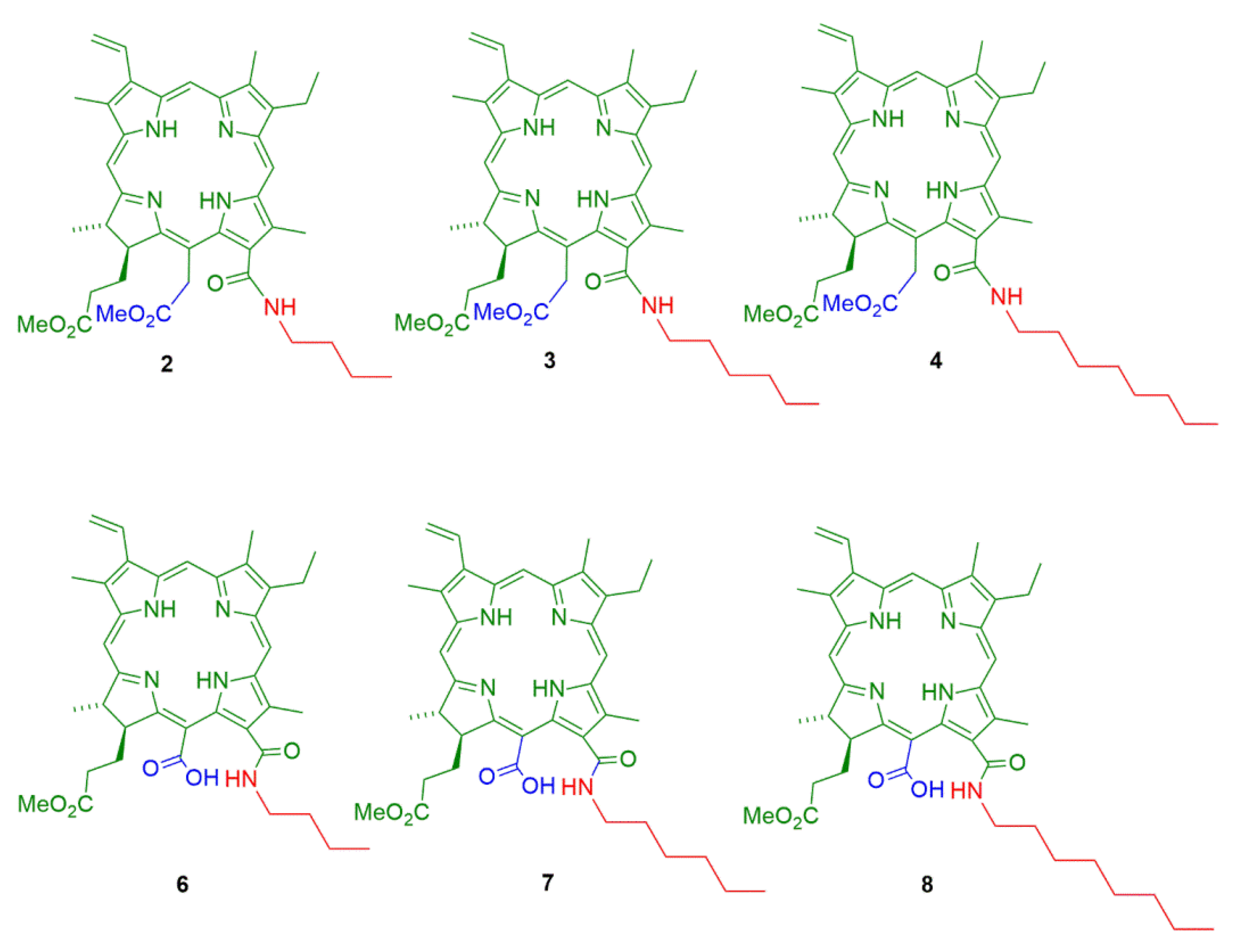
2.1. Semisynthesis of Photosensitizers
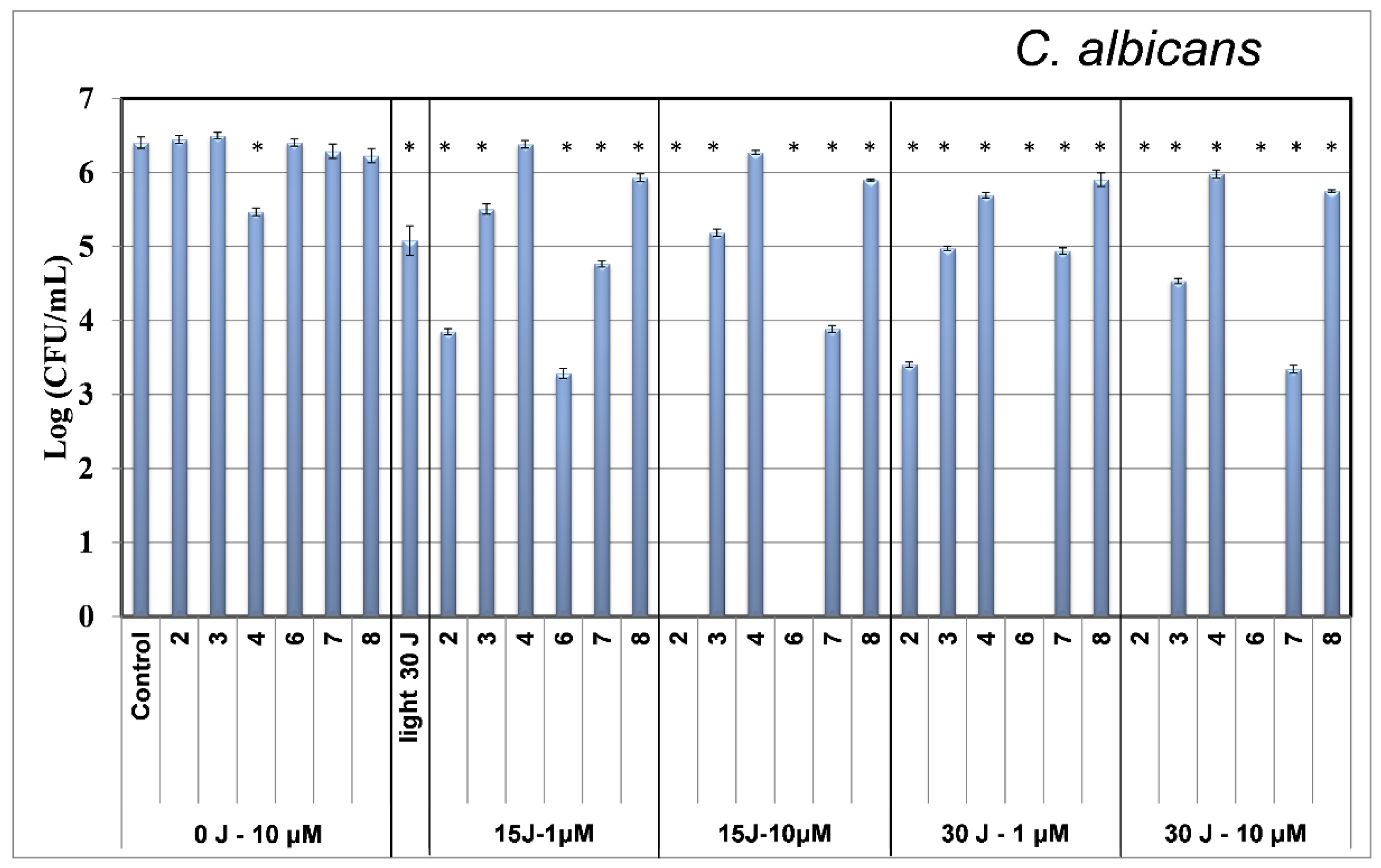
2.2. Photodynamic Inactivation
3. Materials and Methods
3.1. Semisynthesis of Photosensitizers
3.2. Photodynamic Inactivation
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA-A Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Betrouni, N.; Boukris, S.; Benzaghou, F. Vascular targeted photodynamic therapy with TOOKAD (R) Soluble (WST11) in localized prostate cancer: Efficiency of automatic pre-treatment planning. Lasers Med. Sci. 2017, 32, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Mazor, O.; Brandis, A.; Plaks, V.; Neumark, E.; Rosenbach-Belkin, V.; Salomon, Y.; Scherz, A. WST11, a novel water-soluble bacteriochlorophyll derivative; Cellular uptake, pharmacokinetics, biodistribution and vascular-targeted photodynamic activity using melanoma tumors as a model. Photochem. Photobiol. 2005, 81, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of third generation photosensitizers used in anticancer photodynamic therapy: Review. Photodiagn. Photodyn. Ther. 2021, 34, 102091. [Google Scholar] [CrossRef] [PubMed]
- Alzeibak, R.; Mishchenko, T.A.; Shilyagina, N.Y.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Targeting immunogenic cancer cell death by photodynamic therapy: Past, present and future. J. Immunother. Cancer 2021, 9, e001926. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, M.X. A clinical review of phototherapy for psoriasis. Lasers Med. Sci. 2018, 33, 173–180. [Google Scholar] [CrossRef]
- Marotti, J.; Aranha, A.C.C.; Eduardo, C.D.; Ribeiro, M.S. Photodynamic Therapy Can Be Effective as a Treatment for Herpes Simplex Labialis. Photomed. Laser Surg. 2009, 27, 357–363. [Google Scholar] [CrossRef]
- Issa, M.C.A.; Manela-Azulay, M. Photodynamic therapy: A review of the literature and image documentation. An. Bras. Dermatol. 2010, 85, 501–511. [Google Scholar] [CrossRef]
- De Carvalho, G.G.; Sanchez-Puetate, J.C.; Donatoni, M.C.; Huacho, P.M.M.; Rastelli, A.N.D.; de Oliveira, K.T.; Spolidorio, D.M.P.; Zandim-Barcelos, D.L. Photodynamic inactivation using a chlorin-based photosensitizer with blue or red-light irradiation against single-species biofilms related to periodontitis. Photodiagn. Photodyn. Ther. 2020, 31, 101916. [Google Scholar] [CrossRef]
- Seeger, M.G.; Ries, A.S.; Gressler, L.T.; Botton, S.A.; Iglesias, B.A.; Cargnelutti, J.F. In vitro antimicrobial photodynamic therapy using tetra-cationic porphyrins against multidrug-resistant bacteria isolated from canine otitis. Photodiagn. Photodyn. Ther. 2020, 32, 101982. [Google Scholar] [CrossRef]
- Hirose, M.; Yoshida, Y.; Horii, K.; Hasegawa, Y.; Shibuya, Y. Efficacy of antimicrobial photodynamic therapy with Rose Bengal and blue light against cariogenic bacteria. Arch. Oral Biol. 2021, 122, 105024. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.C.M.; Regis, W.F.M.; Rodrigues, L.K.A. Scientific evidence in antimicrobial photodynamic therapy: An alternative approach for reducing cariogenic bacteria. Photodiagn. Photodyn. Ther. 2019, 26, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Geralde, M.C.; Leite, I.S.; Inada, N.M.; Salina, A.C.G.; Medeiros, A.I.; Kuebler, W.M.; Kurachi, C.; Bagnato, V.S. Pneumonia treatment by photodynamic therapy with extracorporeal illumination—An experimental model. Physiol. Rep. 2017, 5, e13190. [Google Scholar] [CrossRef] [PubMed]
- Babu, B.; Sindelo, A.; Mack, J.; Nyokong, T. Thien-2-yl substituted chlorins as photosensitizers for photodynamic therapy and photodynamic antimicrobial chemotherapy. Dyes Pigm. 2021, 185, 108886. [Google Scholar] [CrossRef]
- Kate, C.; Blanco, N.M.I.; Carbinatto, F.M.; Bagnato, V.S. Antimicrobial Efficacy of Curcumin Formulations by Photodynamic Therapy. J. Pharm. Pharmacol. 2017, 5, 506–511. [Google Scholar]
- Amos-Tautua, B.M.; Songca, S.P.; Oluwafemi, O.S. Application of Porphyrins in Antibacterial Photodynamic Therapy. Molecules 2019, 24, 2456. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, L.S.; de Annunzio, S.R.; de Freitas, L.M.; Dantas, L.O.; de Boni, L.; Donatoni, M.C.; de Oliveira, K.T.; Fontana, C.R. Influence of light intensity and irradiation mode on methylene blue, chlorin-e6 and curcumin-mediated photodynamic therapy against Enterococcus faecalis. Photodiagn. Photodyn. Ther. 2020, 31, 101925. [Google Scholar] [CrossRef]
- Mesquita, M.Q.; Dias, C.J.; Neves, M.; Almeida, A.; Faustino, M.A.F. Revisiting Current Photoactive Materials for Antimicrobial Photodynamic Therapy. Molecules 2018, 23, 2424. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.P.; Uliana, M.P.; Guimaraes, F.E.G.; de Oliveira, K.T.; Blanco, K.C.; Bagnato, V.S.; Inada, N.M. Investigation on the in vitro anti-Trichophyton activity of photosensitizers. Photochem. Photobiol. Sci. 2022, 21, 1185–1192. [Google Scholar] [CrossRef]
- Habermeyer, B.; Guilard, R. Some activities of PorphyChem illustrated by the applications of porphyrinoids in PDT, PIT and PDI. Photochem. Photobiol. Sci. 2018, 17, 1675–1690. [Google Scholar] [CrossRef]
- Ziganshyna, S.; Guttenberger, A.; Lippmann, N.; Schulz, S.; Bercker, S.; Kahnt, A.; Rffer, T.; Voigt, A.; Gerlach, K.; Werdehausen, R. Tetrahydroporphyrin-tetratosylate (THPTS)-based photodynamic inactivation of critical multidrug-resistant bacteria in vitro. Int. J. Antimicrob. Agents 2020, 55, 105976. [Google Scholar] [CrossRef]
- De Souza, L.M.; Inada, N.M.; Venturini, F.P.; Carmona-Vargas, C.C.; Pratavieira, S.; de Oliveira, K.T.; Kurachi, C.; Bagnato, V.S. Photolarvicidal effect of curcuminoids from Curcuma longa Linn. against Aedes aegypti larvae. J. Asia-Pac. Entomol. 2019, 22, 151–158. [Google Scholar] [CrossRef]
- Oriel, S.; Nitzan, Y. Photoinactivation of Candida albicans by Its Own Endogenous Porphyrins. Curr. Microbiol. 2010, 60, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Dai, T.; Hamblin, M.R. Antimicrobial photodynamic inactivation and photodynamic therapy for infections. Methods Mol. Biol. 2010, 635, 155–173. [Google Scholar] [CrossRef] [PubMed]
- Gois, M.M.; Kurachi, C.; Santana, E.J.B.; Mima, E.G.O.; Spolidorio, D.M.P.; Pelino, J.E.P.; Bagnato, V.S. Susceptibility of Staphylococcus aureus to porphyrin-mediated photodynamic antimicrobial chemotherapy: An in vitro study. Lasers Med. Sci. 2010, 25, 391–395. [Google Scholar] [CrossRef] [PubMed]
- De Souza, L.M.; Inada, N.M.; Pratavieira, S.; Corbi, J.J.; Kurachi, C.; Bagnato, V.S. Efficacy of Photogem® (Hematoporphyrin Derivative) as a Photoactivatable Larvicide against Aedes aegypti (Diptera: Culicidae) Larvae. J. Life Sci. 2017, 11, 74–81. [Google Scholar] [CrossRef]
- Wiehe, A.; O’Brien, J.M.; Senge, M.O. Trends and targets in antiviral phototherapy. Photochem. Photobiol. Sci. 2019, 18, 2565–2612. [Google Scholar] [CrossRef]
- Conrado, P.C.V.; Sakita, K.M.; Arita, G.S.; Galinari, C.B.; Goncalves, R.S.; Lopes, L.D.G.; Lonardoni, M.V.C.; Teixeira, J.J.V.; Bonfim-Mendonca, P.S.; Kioshima, E.S. A systematic review of photodynamic therapy as an antiviral treatment: Potential guidance for dealing with SARS-CoV-2. Photodiagn. Photodyn. Ther. 2021, 34, 102221. [Google Scholar] [CrossRef]
- Svyatchenko, V.A.; Nikonov, S.D.; Mayorov, A.P.; Gelfond, M.L.; Loktev, V.B. Antiviral photodynamic therapy: Inactivation and inhibition of SARS-CoV-2 in vitro using methylene blue and Radachlorin. Photodiagn. Photodyn Ther. 2021, 33, 102112. [Google Scholar] [CrossRef]
- Sternberg, E.D.; Dolphin, D.; Bruckner, C. Porphyrin-based photosensitizers for use in photodynamic therapy. Tetrahedron 1998, 54, 4151–4202. [Google Scholar] [CrossRef]
- Ben Amor, T.; Jori, G. Sunlight-activated insecticides: Historical background and mechanisms of phototoxic activity. Insect Biochem. Mol. Biol. 2000, 30, 915–925. [Google Scholar] [CrossRef]
- Nesi-Reis, V.; Lera-Nonose, D.; Oyama, J.; Silva-Lalucci, M.P.P.; Demarchi, I.G.; Aristides, S.M.A.; Teixeira, J.J.V.; Silveira, T.G.V.; Lonardoni, M.V.C. Contribution of photodynamic therapy in wound healing: A systematic review. Photodiagn. Photodyn. Ther. 2018, 21, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, S.; Chiodo, F.; Mazzone, G.; Richeter, S.; Sicilia, E. Porphyrins and Metalloporphyrins Combined with N-heterocyclic Carbene (NHC) Gold (I) Complexes for Photodynamic Therapy Application. What Is the Weight of the Heavy Atom Effect? Molecules 2022, 27, 4046. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.L.; Sun, X.; Wang, W.L.; Yang, D.L.; Yang, C.J.; Shen, Q.; Shao, J.J. Diketopyrrolopyrrole-derived organic small molecular dyes for tumor phototheranostics. Chin. Chem. Lett. 2022, 33, 1681–1692. [Google Scholar] [CrossRef]
- Sampaio, F.J.P.; de Oliveira, S.; Crugeira, P.J.L.; Monteiro, J.S.C.; Fagnani, S.; Pepe, I.M.; de Almeida, P.F.; Pinheiro, A.L.B. aPDT using nanoconcentration of 1,9-dimethylmethylene blue associated to red light is efficacious in killing Enterococcus faecalis ATCC 29212 in vitro. J. Photochem. Photobiol. B 2019, 200, 11654. [Google Scholar] [CrossRef]
- Sobotta, L.; Skupin-Mrugalska, P.; Piskorz, J.; Mielcarek, J. Porphyrinoid photosensitizers mediated photodynamic inactivation against bacteria. Eur. J. Med. Chem. 2019, 175, 72–106. [Google Scholar] [CrossRef]
- Yook, K.; Kim, J.; Song, W. In vitro study on the effects of photodynamic inactivation using methyl pheophorbide a, PhotoMed, PhotoCure, and 660 nm diode laser on Candida albicans. Photodiagn. Photodyn. Ther. 2022, 38, 102871. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.M.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef]
- Santezi, C.; Reina, B.D.; Dovigo, L.N. Curcumin-mediated Photodynamic Therapy for the treatment of oral infections—A review. Photodiagn. Photodyn. Ther. 2018, 21, 409–415. [Google Scholar] [CrossRef]
- Pratavieira, S.; Uliana, M.P.; Lopes, N.S.D.; Donatoni, M.C.; Linares, D.R.; Anibal, F.D.; de Oliveira, K.T.; Kurachi, C.; de Souza, C.W.O. Photodynamic therapy with a new bacteriochlorin derivative: Characterization and in vitro studies. Photodiagn. Photodyn. Ther. 2021, 34, 102251. [Google Scholar] [CrossRef]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.Y.; Dai, T.H.; Hamblin, M.R. Photodynamic Therapy for Infections: Clinical Applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Simplicio, F.I.; Maionchi, F.; Hioka, N. Photodynamic therapy: Pharmacological aspects, applications and news from medications development. Quim. Nova 2002, 25, 801–807. [Google Scholar] [CrossRef]
- Kubrak, T.P.; Kołodziej, P.; Sawicki, J.; Mazur, A.; Koziorowska, K.; Aebisher, D. Some Natural Photosensitizers and Their Medicinal Properties for Use in Photodynamic Therapy. Molecules 2022, 27, 1192. [Google Scholar] [CrossRef]
- Uliana, M.P.; Pires, L.; Pratavieira, S.; Brocksom, T.J.; de Oliveira, K.T.; Bagnato, V.S.; Kurachi, C. Photobiological characteristics of chlorophyll a derivatives as microbial PDT agents. Photochem. Photobiol. Sci. 2014, 13, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, K.T.; Silva, A.M.S.; Tomé, A.C.; Neves, M.G.P.M.S.; Neri, C.R.; Garcia, V.S.; Serra, O.A.; Iamamoto, Y.; Cavaleiro, J.A.S. Synthesis of new amphiphilic chlorin derivatives from protoporphyrin-IX dimethyl ester. Tetrahedron 2008, 64, 8709–8715. [Google Scholar] [CrossRef]
- Petrilli, R.; Praça, F.S.G.; Carollo, A.R.H.; Medina, W.S.G.; de Oliveira, K.T.; Fantini, M.C.A.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Serra, O.A.; Yassuko Iamamoto, Y.; et al. Nanoparticles of Lyotropic Liquid Crystals: A Novel Strategy for the Topical Delivery of a Chlorin Derivative for Photodynamic Therapy of Skin Cancer. Curr. Nanosci. 2013, 9, 434–444. [Google Scholar] [CrossRef]
- Dos Santos, F.A.B.; Uchoa, A.F.; Baptista, M.S.; Iamamoto, Y.; Serra, O.A.; Brocksom, T.J.; de Oliveira, K.T. Synthesis of functionalized chlorins sterically-prevented from self-aggregation. Dyes Pigm. 2013, 99, 402–411. [Google Scholar] [CrossRef]
- Uchoa, A.F.; de Oliveira, K.T.; Baptista, M.S.; Bortoluzzi, A.J.; Iamamoto, Y.; Serra, O.A. Chlorin Photosensitizers Sterically Designed To Prevent Self-Aggregation. J. Org. Chem. 2011, 76, 8824–8832. [Google Scholar] [CrossRef]
- Gushchina, O.I.; Larkina, E.A.; Nikolskaya, T.A.; Mironov, A.F. Synthesis of amide derivatives of chlorin e(6) and investigation of their biological activity. J. Photochem. Photobiol. B 2015, 153, 76–81. [Google Scholar] [CrossRef]
- Belykh, D.V.; Pushkareva, E.I. Amidation of the Ester Group of Methylpheoforbide a with Sterically Nonhindered Primary and Secondary Aliphatic Amines. Russ. J. Gen. Chem. 2011, 81, 1216–1221. [Google Scholar] [CrossRef]
- Belykh, D.V.; Kopylov, E.A.; Gruzdev, I.V.; Kuchin, A.V. Opening of the extra ring in pheophorbide a methyl ester by the action of amines as a one-step method for introduction of additional fragments at the periphery of chlorin macroring. Russ. J. Org. Chem. 2010, 46, 577–585. [Google Scholar] [CrossRef]
- Louda, J.W.; Li, J.; Liu, L.; Winfree, M.N.; Baker, E.W. Chlorophyll-a degradation during cellular senescence and death. Org. Geochem. 1998, 29, 1233–1251. [Google Scholar] [CrossRef]
- Drogat, N.; Barriere, M.; Granet, R.; Sol, V.; Krausz, P. High yield preparation of purpurin-18 from Spirulina maxima. Dyes Pigm. 2011, 88, 125–127. [Google Scholar] [CrossRef]
- Lee, S.J.H.; Jagerovic, N.; Smith, K.M. Use of the chlorophyll derivative, purpurin-18, for syntheses of sensitizers for use in photodynamic therapy. J. Chem. Soc.-Perkin Trans. 1 1993, 19, 2369–2377. [Google Scholar] [CrossRef]
- Zheng, G.; Potter, W.R.; Camacho, S.H.; Missert, J.R.; Wang, G.S.; Bellnier, D.A.; Henderson, B.W.; Rodgers, M.A.J.; Dougherty, T.J.; Pandey, R.K. Synthesis, photophysical properties, tumor uptake, and preliminary in vivo photosensitizing efficacy of a homologous series of 3-(1 ’-alkyloxy) ethyl-3-devinaylpurpurin-18-N-alkylimides with variable lipophilicity. J. Med. Chem. 2001, 44, 1540–1559. [Google Scholar] [CrossRef] [PubMed]
- Kozyrev, A.N.; Zheng, G.; Zhu, C.F.; Dougherty, T.J.; Smith, K.M.; Pandey, R.K. Syntheses of stable bacteriochlorophyll-a derivatives as potential photosensitizers for photodynamic therapy. Tetrahedron Lett. 1996, 37, 6431–6434. [Google Scholar] [CrossRef]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef]
- Moreira, X.; Santos, P.; Faustino, M.A.F.; Raposo, M.M.M.; Costa, S.P.G.; Moura, N.M.M.; Gomes, A.; Almeida, A.; Neves, M. An insight into the synthesis of cationic porphyrin-imidazole derivatives and their photodynamic inactivation efficiency against Escherichia coli. Dyes Pigm. 2020, 178, 108330. [Google Scholar] [CrossRef]
- Ladeira, B.M.F.; Dias, C.J.; Gomes, A.T.P.C.; Tomé, A.C.; Neves, M.G.P.M.S.; Moura, N.M.; Almeida, A.; Faustino, M.A.F. Cationic Pyrrolidine/Pyrroline-Substituted Porphyrins as Efficient Photosensitizers against E. coli. Molecules 2021, 26, 464. [Google Scholar] [CrossRef]
- De Oliveira, B.P.; Lins, C.C.d.S.A.; Diniz, F.A.; Melo, L.L.; de Castro, C.M.M.B. In Vitro antimicrobial photoinactivation with methylene blue in different microorganisms. Braz. J. Oral Sci. 2014, 13, 53–57. [Google Scholar] [CrossRef]
- Omar, G.S.; Wilson, M.; Nair, S.P. Lethal photosensitization of wound-associated microbes using indocyanine green and near-infrared light. BMC Microbiol. 2008, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Topaloglu, N.; Gulsoy, M.; Yuksel, S. Antimicrobial Photodynamic Therapy of Resistant Bacterial Strains by Indocyanine Green and 809-nm Diode Laser. Photomed. Laser Surg. 2013, 31, 155–162. [Google Scholar] [CrossRef]
- Hargus, J.A.; Fronczek, F.R.; Graca, M.; Vicente, H.; Smith, K.M. Mono-(L)-aspartylchlorin-e(6). Photochem. Photobiol. 2007, 83, 1006–1015. [Google Scholar] [CrossRef]
- Ma, L.; Dolphin, D. Nucleophilic reaction of 1,8- diazabicyclo[5.4.0]undec-7-ene and 1,5- diazabicyclo[4.3.0]non-5-ene with methyl pheophorbide a. Unexpected products. Tetrahedron 1996, 52, 849–860. [Google Scholar] [CrossRef]
- Kustov, A.V.; Belykh, D.V.; Startseva, O.M.; Kruchin, S.O.; Venediktov, E.A.; Berezin, D.B. New Sensitizers Developed on a Methylpheophorbide a Platform for Photodynamic Therapy: Synthesis, Singlet Oxygen Generation and Modeling of Passive Membrane Transport. Pharm. Anal. Acta 2016, 7, 480. [Google Scholar] [CrossRef]
- Belykh, D.V.; Tarabukinaa, I.S.; Gruzdevb, I.V.; Kodessc, M.I.; Kutchin, A.V. Aminomethylation of chlorophyll a derivatives using bis(N,N-dimethylamino)methane. J. Porphyr. Phthalocyanines 2009, 13, 949–956. [Google Scholar] [CrossRef]
- Redmond, R.W.; Gamlin, J.N. A Compilation of Singlet Oxigen Yields from Biologically Relevant Molecules. Photochem. Photobiol. 1999, 70, 391–475. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uliana, M.P.; da Cruz Rodrigues, A.; Ono, B.A.; Pratavieira, S.; de Oliveira, K.T.; Kurachi, C. Photodynamic Inactivation of Microorganisms Using Semisynthetic Chlorophyll a Derivatives as Photosensitizers. Molecules 2022, 27, 5769. https://doi.org/10.3390/molecules27185769
Uliana MP, da Cruz Rodrigues A, Ono BA, Pratavieira S, de Oliveira KT, Kurachi C. Photodynamic Inactivation of Microorganisms Using Semisynthetic Chlorophyll a Derivatives as Photosensitizers. Molecules. 2022; 27(18):5769. https://doi.org/10.3390/molecules27185769
Chicago/Turabian StyleUliana, Marciana Pierina, Andréia da Cruz Rodrigues, Bruno Andrade Ono, Sebastião Pratavieira, Kleber Thiago de Oliveira, and Cristina Kurachi. 2022. "Photodynamic Inactivation of Microorganisms Using Semisynthetic Chlorophyll a Derivatives as Photosensitizers" Molecules 27, no. 18: 5769. https://doi.org/10.3390/molecules27185769





