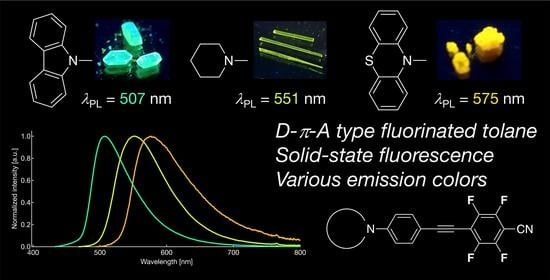
Development of Yellow-to-Orange Photoluminescence Molecules Based on Alterations in the Donor Units of Fluorinated Tolanes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Photophysical Behavior in Solution State
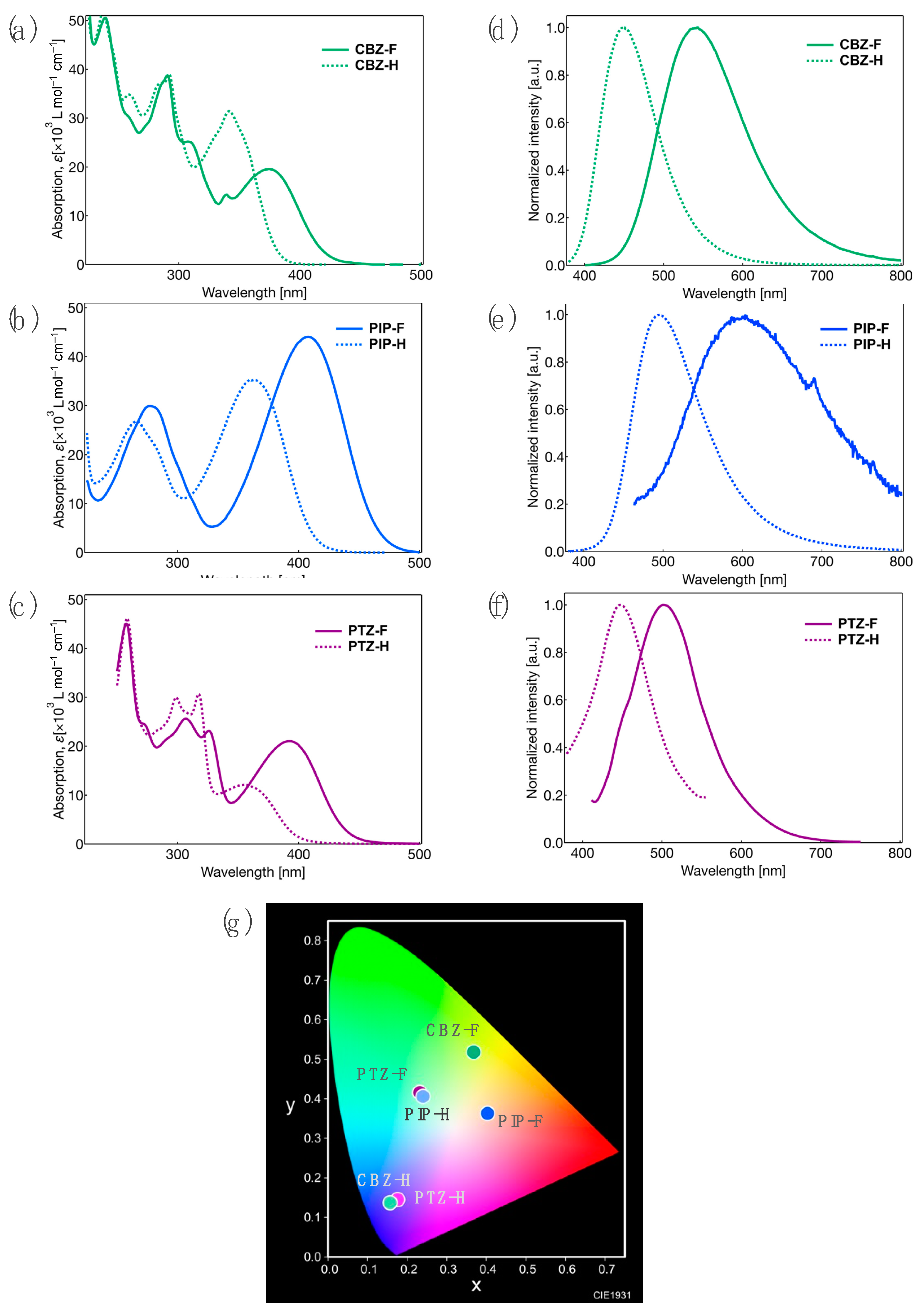
2.3. Photophysical Properties in Crystalline State
- (i)
- The ΦPL of CBZ-H in the crystalline state is lower than that in the solution state because of the promotion of non-radiative deactivation through intermolecular energy transfer via tight π/π stacking.
- (ii)
- The increase in the ΦPL of PIP-F and PTZ-F in the crystalline state compared to that in the solution state is due to the effective suppression of non-radiative deactivation and the formation of rigid molecular aggregated structures. This is supported by the significant reduction in the nonradiative rate constant (knr) in the crystalline state, rather than in the solution state.
- (iii)
- Comparing PL decay among the three fluorinated tolanes, viz., CBZ-F, PIP-F, and PTZ-F, however, irregular PL decay profiles were observed depending on the donor moiety. This can be attributed to the significant alteration of molecular aggregated structures in the crystalline state. Thus, the retardation of PL in case of CBZ-F and PTZ-F crystals is due to the dimer-like molecular packing induced by strong CT interactions between the electron-donating aromatic ring-substituted amine-donor moieties and the electron-withdrawing fluorinated aromatic rings. In contrast, PIP-F crystals demonstrated rapid PL decay due to structural relaxation of the donor moiety, which is similar to solution-state PL decay.
- (iv)
- The red-shift of the PL band of PTZ-F in the crystalline state compared to that in the solution state is due to the formation of dimer-like molecular packing structures through tight π/π stacking caused by the orthogonal molecular structure characteristic of PTZ-F [15]. This is supported by the significantly delayed τ in PTZ-F compared with that in CBZ-F and PIP-F (Figure S19).
2.4. Aggregation-Induced Emission Behavior
3. Materials and Methods
3.1. General Method
3.2. Synthesis
3.2.1. Typical Procedure for the Nucleophilic Aromatic Substitution Reaction of Lithium 4-Carbazol-9-ylphenylacetylide with Pentafluorobenzonitrile
4-(2-(4-Carbazol-9-ylphenyl)ethynyl)-2,3,5,6-Tetrafluorobenzonitrile (CBZ-F)
4-(2-(4-Piperidylphenyl)ethynyl)-2,3,5,6-Tetrafluorobenzonitrile (PIP-F)
4-(2-(4-Phenothiazin-10-ylphenyl)ethynyl)-2,3,5,6-Tetrafluorobenzonitrile (PTZ-F)
3.2.2. Typical Procedure for the Pd(0)-Catalyzed Sonogashira Cross-Couplilng Reaction of 4-Carbazol-9-ylphenylacetylene with 4-Bromobenzonitrile
4-(2-(4-Carbazol-9-ylphenyl)ethynyl)benzonitrile (CBZ-H)
4-(2-(4-Piperidylphenyl)ethynyl)-2,3,5,6-Tetrafluorobenzonitrile (PIP-H)
4-(2-(4-Phenothiazin-10-ylphenyl)ethynyl)benzonitrile (PTZ-H)
3.3. Single Crystal X-ray Diffraction
3.4. Photophysical Properties
3.5. Theoretical Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gao, R.; Kodaimati, M.S.; Yan, D. Recent advances in persistent luminescence based on molecular hybrid materials. Chem. Soc. Rev. 2021, 50, 5564–5589. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.; Fradgley, J.D.; Wong, K. The design of responsive luminescent lanthanide probes and sensors. Chem. Soc. Rev. 2021, 50, 8193–8231. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Leung, N.L.C.; Zhang, J.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. AIE-based luminescence probes for metal ion detection. Coord. Chem. Rev. 2021, 429, 213693. [Google Scholar] [CrossRef]
- Jackson, C.T.; Jeong, S.; Dorlhiac, G.F.; Landry, M.P. Advances in engineering near-infrared luminescent materials. iScience 2021, 24, 102156. [Google Scholar] [CrossRef] [PubMed]
- Jenekhe, S.A.; Osaheni, J.A. Excimers and exciplexes of conjugated polymers. Science 1994, 265, 765–768. [Google Scholar] [CrossRef]
- Thomas, S.W.; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescent conjugated polymer. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef]
- Birks, J.B. (Ed.) Photophysics of Aromatic Molecules; Wiley: London, UK, 1970. [Google Scholar]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Xhan, X.; Liu, Y.; Xhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef]
- Suzuki, S.; Sasaki, S.; Sairi, A.S.; Iwai, R.; Tang, B.Z.; Konishi, G. Principles of aggregation-induced emission: Design of deactivation pathways for advanced AIEgens and applications. Angew. Chem. Int. Ed. 2020, 59, 9856–9867. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Yan, D.; Wu, Q.; Song, N.; Zhang, H.; Wang, D. Aggregation-induced emission: A rising star in chemistry and materials science. Chin. J. Chem. 2021, 39, 677–689. [Google Scholar] [CrossRef]
- Dong, Y.; Lam, J.W.Y.; Qin, A.; Li, Z.; Sun, J.; Sung, H.H.Y.; Williams, I.D.; Tang, B.Z. Switching the light emission of (4-biphenylyl)phenyldibenzofulvene by morphological modulation: Crystallization-induced emission enhancement. Chem. Commun. 2007, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, S.K.; Reddy, V.S.; Hariharan, M. Crystallization induced green-yellow-orange emitters based on benzoylpyrenes. CrystEngComm 2016, 18, 5089–5094. [Google Scholar] [CrossRef]
- Naito, H.; Nishino, K.; Morisaki, Y.; Tanaka, K.; Chujo, Y. Solid-state emission of the anthracene-o-carborane dyad from the twisted-intermolecular charge transfer in the crystalline state. Angew. Chem. Int. Ed. 2017, 56, 254–259. [Google Scholar] [CrossRef]
- Morita, M.; Yamada, S.; Konno, T. Fluorine-induced emission enhancement of tolanes via formation of tight molecualr aggregates. New J. Chem. 2020, 44, 6704–6708. [Google Scholar] [CrossRef]
- Morita, M.; Yamada, S.; Konno, T. Systematic studies on the effect of fluorine atoms in fluorinated tolanes on their photophysical properties. Molecules 2021, 26, 2274. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Yamada, S.; Konno, T. Halogen atom effect of fluorinated tolanes on their luminescence characteristics. New J. Chem. 2022, 46, 4562–4569. [Google Scholar] [CrossRef]
- Yamada, S.; Kobayashi, K.; Morita, M.; Konno, T. D-p-A-type fluorinated tolanes with adiphenylamino group: Crystal polymorphism formation and photophysical behavior. CrystEngComm 2022, 24, 936–941. [Google Scholar] [CrossRef]
- Lee, Y.; Chiang, C.; Chen, C. Solid-state highly fluorescent diphenyaminospirobifluorenylfumaronitrile red emitters for non-doped organic light-emitting diodes. Chem. Commun. 2008, 217–219. [Google Scholar] [CrossRef]
- Sudyoadsuk, T.; Chasing, P.; Chaiwai, C.; Chawanpunyawat, T.; Kaewpuang, T.; Manyum, T.; Namuangruk, S.; Promarak, V. Highly fluorescent solid-state benzothiadiazole derivatives as saturated red emitters for efficient solution-processed non-doped electroluminescent devices. J. Mater. Chem. C 2020, 8, 10464–10473. [Google Scholar] [CrossRef]
- Roy, I.; David, A.H.G.; Das, P.J.; Pe, D.J.; Stoddart, J.F. Fluorescent cyclophanes and their applications. Chem. Soc. Rev. 2022, 51, 5557–5605. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, H.; Fu, W.; Stoddart, J.F. Color-tunable supramolecular luminescent materials. Adv. Mater. 2022, 34, 2105405. [Google Scholar] [CrossRef]
- Wu, T.; Huang, J.; Yan, Y. Self-assembly of aggregation-induced-emission molecules. Chem. Asian J. 2019, 14, 730–750. [Google Scholar] [CrossRef]
- Wang, H.; Li, Q.; Zhang, J.; Zhang, H.; Shu, Y.; Zhao, Z.; Jiang, W.; Du, L.; Phillips, D.L.; Lam, J.W.Y.; et al. Visualization and manipulation of solid-state molecular motions in cocrystallization processes. J. Am. Chem. Soc. 2021, 143, 9468–9477. [Google Scholar] [CrossRef]
- Bera, M.K.; Pal, P.; Malik, S. Solid-state emissive organic chromophores: Design, strategy and building blocks. J. Mater. Chem. C 2020, 8, 788–802. [Google Scholar] [CrossRef]
- Duan, C.; Zhou, Y.; Shan, G.G.; Chen, Y.; Zhao, W.; Yuan, D.; Zeng, L.; Huang, X.; Niu, G. Bright solid-state red-emissive BODIPYs: Facile synthesis and their high-contrast mechanochromic properties. J. Mater. Chem. C 2019, 7, 3471–3478. [Google Scholar] [CrossRef]
- Lv, C.; Liu, W.; Luo, Q.; Yi, H.; Yu, H.; Yang, Z.; Zou, B.; Zhang, Y. A highly emissive AIE-active luminophore exhibiting deep-red to near-infrared piezochromism and high-quality lasing. Chem. Sci. 2020, 11, 4007–4015. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Takeda, Y.; Higashi, M.; Hiyama, T. 1,4-Bis(alkenyl)-2,5-dipiperidinobenzenes: Minimal fluorophores exhibiting highly efficient emission in the solid state. Angew. Chem. Int. Ed. 2009, 121, 3707–3710. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Hohenstein, E.G.; Chill, S.T.; Sherrill, C.D. Assessment of the performance of the M05-2X and M06-2X exchange-correlation functionals for noncovalent interactions in biomolecules. J. Chem. Theory Comput. 2008, 4, 1996–2000. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jensen, J.H. Improving the efficiency and convergence of geometry optimization with the polarizable continuum model: New energy gradients and molecular surface tesselation. J. Comput. Chem. 2004, 25, 1449–1462. [Google Scholar] [CrossRef]
- Mataga, N.; Kaifu, Y.; Koizumi, M. Solvent effect on fluorescence spectrum, change of solute-solvent interaction during the lifetime of excited solute molecule. Bull. Chem. Soc. Jpn. 1955, 28, 690–691. [Google Scholar] [CrossRef]
- Mataga, N.; Kaifu, Y.; Koizumi, M. Solvent effects on fluorescence spectra and dipole moments of excited molecules. Bull. Chem. Soc. Jpn. 1956, 29, 465–470. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Chi, W.; Qiao, Q.; Tan, D.; Xu, Z.; Liu, X. Twisted intramolecular charge transfer (TICT) and twisted beyond TICT: From mechanisms to rational designs of bright and sensitive fluorophores. Chem. Soc. Rev. 2021, 50, 12656–12678. [Google Scholar] [CrossRef]
- Zgierski, M.Z.; Lim, E.C. Nature of the ‘dark’ state in diphenylacetylene and related molecules: State switch from the linear pp* state to the bent ps*state. Chem. Phys. Lett. 2004, 387, 352–355. [Google Scholar] [CrossRef]
- Saltiel, J.; Kumar, V.K.R. Photophysics of diphenylacetylene: Light from the “dark state”. J. Phys. Chem. A 2012, 116, 10548–10558. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space group and crystal structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement, and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]






| λabs [nm] 1 | HOMO 2 | LUMO 2 | ΔEH−L | λPL [nm] 1,3 | τAVE | τ1 | τ2 | kr5 | knr6 | |
|---|---|---|---|---|---|---|---|---|---|---|
| (ε × 10−3 L mol−1 cm−1) | [eV] | [eV] | [eV] | (ΦPL) 4 | [ns] | [ns] | [ns] | [107 s−1] | [107 s−1] | |
| CBZ-F | 238 (52), 291 (39), 375 (20) | –7.03 | –2.09 | 4.94 | 542 (0.65) | 5.38 | – | – | 12.1 | 6.5 |
| CBZ-H | 238 (52), 292 (39), 342 (31) | –6.97 | –1.58 | 5.39 | 447 (1.0) | 3.51 | – | – | 28.5 | 0.0 |
| PIP-F | 277 (30), 408 (44) | –6.94 | –1.93 | 5.01 | 603 (0.02) | 1.18 | 1.02 | 4.40 | 1.7 | 83.1 |
| PIP-H | 266 (27), 363 (35) | –6.60 | –1.34 | 5.26 | 496 (0.26) | 2.50 | – | – | 10.4 | 29.6 |
| PTZ-F | 258 (45), 307 (26), 326 (23), 393 (21) | –6.75 | –2.12 | 4.63 | 501 (0.01) | 2.38 | <1.0 | 3.39 | 0.42 | 41.6 |
| PTZ-H | 258 (49), 299 (32), 317 (33), 356 (13) | –6.72 | –1.60 | 5.12 | 447 (0.02) | 1.03 | <1.0 | 5.14 | 1.9 | 95.2 |
| Molecule | Theoretical Transition | Theoretical Transition Energy [eV] | Theoretical λabs [nm] | Oscillator Strength |
|---|---|---|---|---|
| CBZ-F | HOMO → LUMO | 3.4871 | 355.55 | 1.3880 |
| HOMO–1 → LUMO+1 | 4.7119 | 263.13 | 0.4074 | |
| CBZ-H | HOMO → LUMO | 3.7348 | 331.97 | 1.6078 |
| HOMO−1 → LUMO+1 | 4.7075 | 263.37 | 0.5154 | |
| PIP-F | HOMO → LUMO | 3.3143 | 374.09 | 1.6677 |
| HOMO → LUMO+2 | 5.1695 | 239.84 | 0.2601 | |
| PIP-H | HOMO → LUMO | 3.5015 | 354.09 | 1.7485 |
| HOMO → LUMO+3 | 4.4592 | 278.04 | 0.0599 | |
| PTZ-F | HOMO → LUMO | 3.4666 | 357.65 | 0.0000 |
| HOMO−2 → LUMO | 3.9363 | 314.98 | 1.7773 | |
| PTZ-H | HOMO → LUMO | 3.7800 | 328.00 | 0.0003 |
| HOMO−1 → LUMO | 4.0564 | 305.65 | 1.8205 |
| λPL [nm] 1 | τAVE | τ1 | τ2 | kr3 | knr 4 | |
|---|---|---|---|---|---|---|
| (ΦPL) 2 | [ns] | [ns] | [ns] | [107 s−1] | [107 s−1] | |
| CBZ-F | 507 (0.42) | 52.8 | 19.4 | 62.1 | 0.8 | 1.1 |
| CBZ-H | 442 (0.48) | 5.32 | 2.67 | 5.27 | 9.0 | 9.8 |
| PIP-F | 551 (0.18) | 8.82 | 7.41 | 13.1 | 2.0 | 9.3 |
| PIP-H | 485 (0.30) | 3.18 | 1.95 | 5.75 | 9.4 | 22.0 |
| PTZ-F | 575 (0.16) | 93.1 | 32.0 | 276 | 0.2 | 0.9 |
| PTZ-H | 478 (0.06) | 2.31 | – | – | 2.6 | 40.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, S.; Kobayashi, K.; Konno, T. Development of Yellow-to-Orange Photoluminescence Molecules Based on Alterations in the Donor Units of Fluorinated Tolanes. Molecules 2022, 27, 5782. https://doi.org/10.3390/molecules27185782
Yamada S, Kobayashi K, Konno T. Development of Yellow-to-Orange Photoluminescence Molecules Based on Alterations in the Donor Units of Fluorinated Tolanes. Molecules. 2022; 27(18):5782. https://doi.org/10.3390/molecules27185782
Chicago/Turabian StyleYamada, Shigeyuki, Kazuki Kobayashi, and Tsutomu Konno. 2022. "Development of Yellow-to-Orange Photoluminescence Molecules Based on Alterations in the Donor Units of Fluorinated Tolanes" Molecules 27, no. 18: 5782. https://doi.org/10.3390/molecules27185782
APA StyleYamada, S., Kobayashi, K., & Konno, T. (2022). Development of Yellow-to-Orange Photoluminescence Molecules Based on Alterations in the Donor Units of Fluorinated Tolanes. Molecules, 27(18), 5782. https://doi.org/10.3390/molecules27185782