Ni-Coated Diamond-like Carbon-Modified TiO2 Nanotube Composite Electrode for Electrocatalytic Glucose Oxidation
Abstract
:1. Introduction
2. Results and Discussion
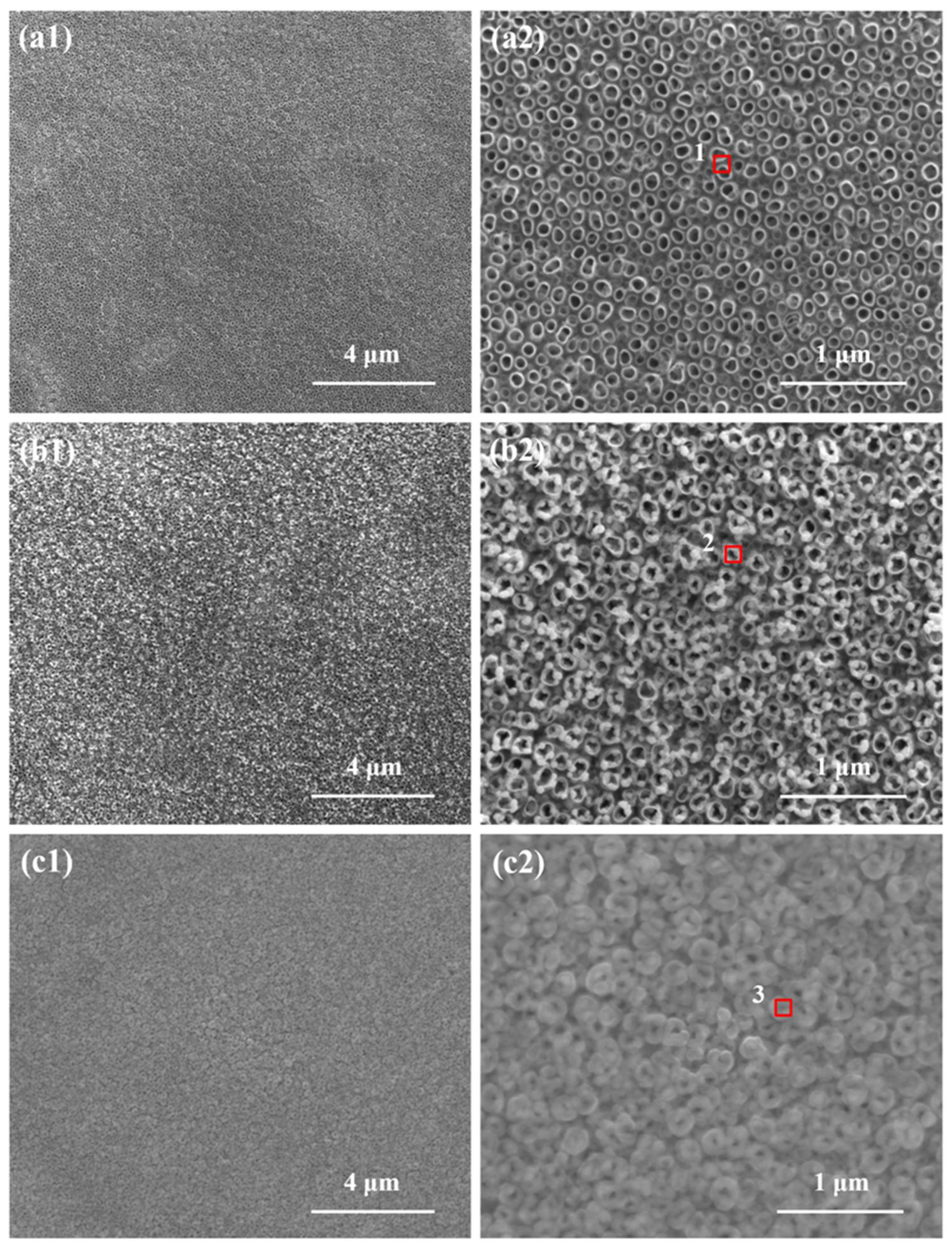
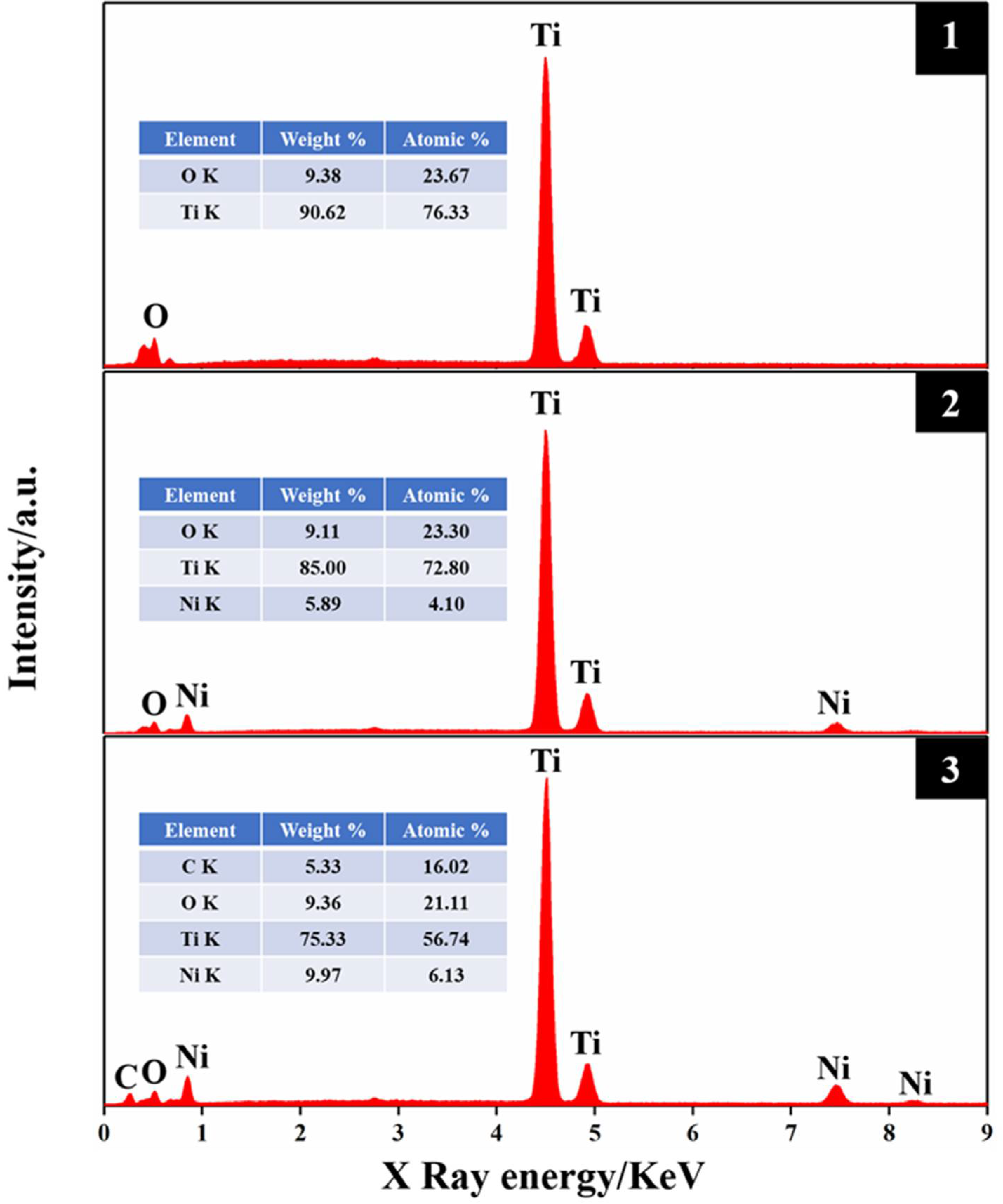
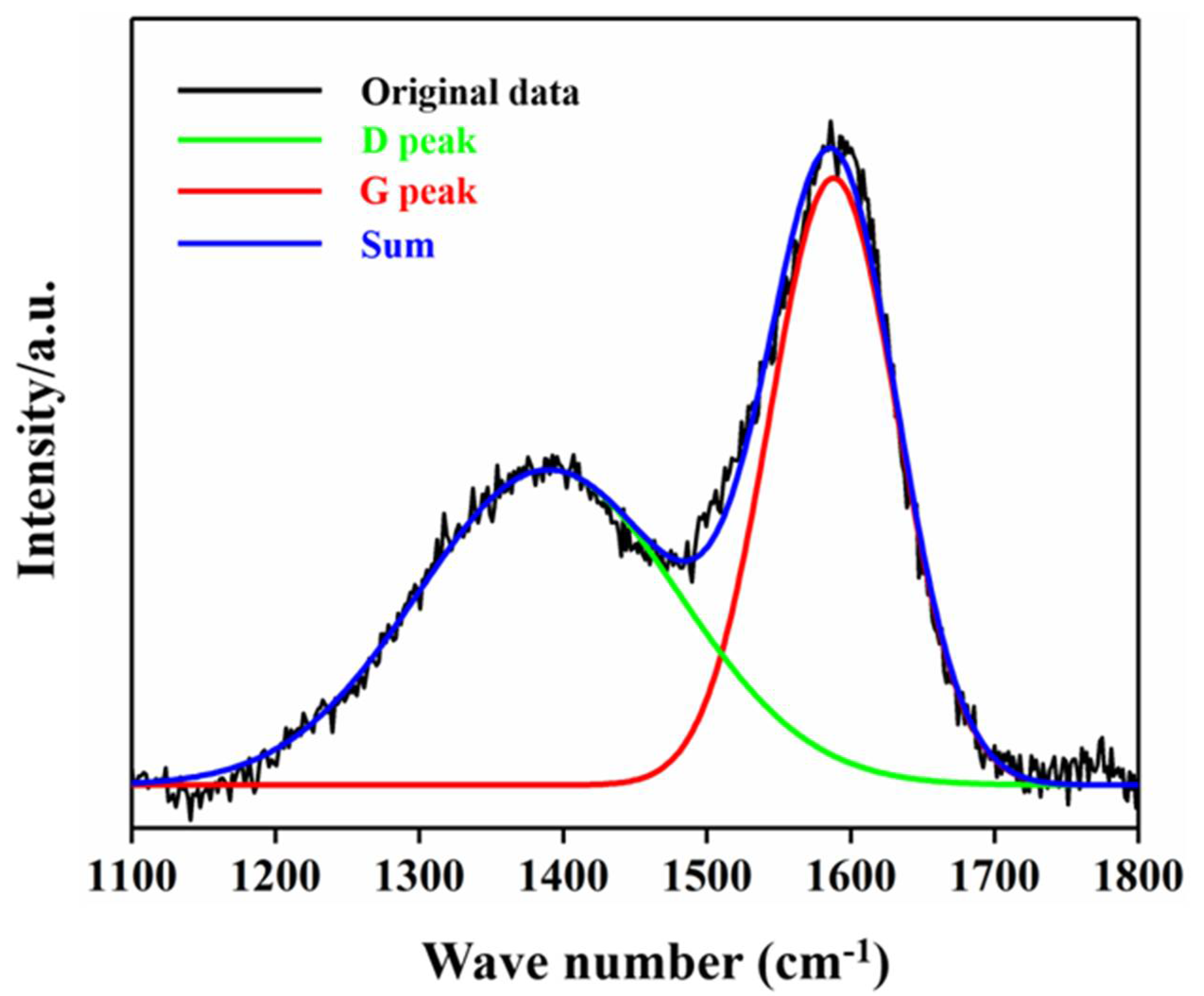
2.1. Electrode Characteristics
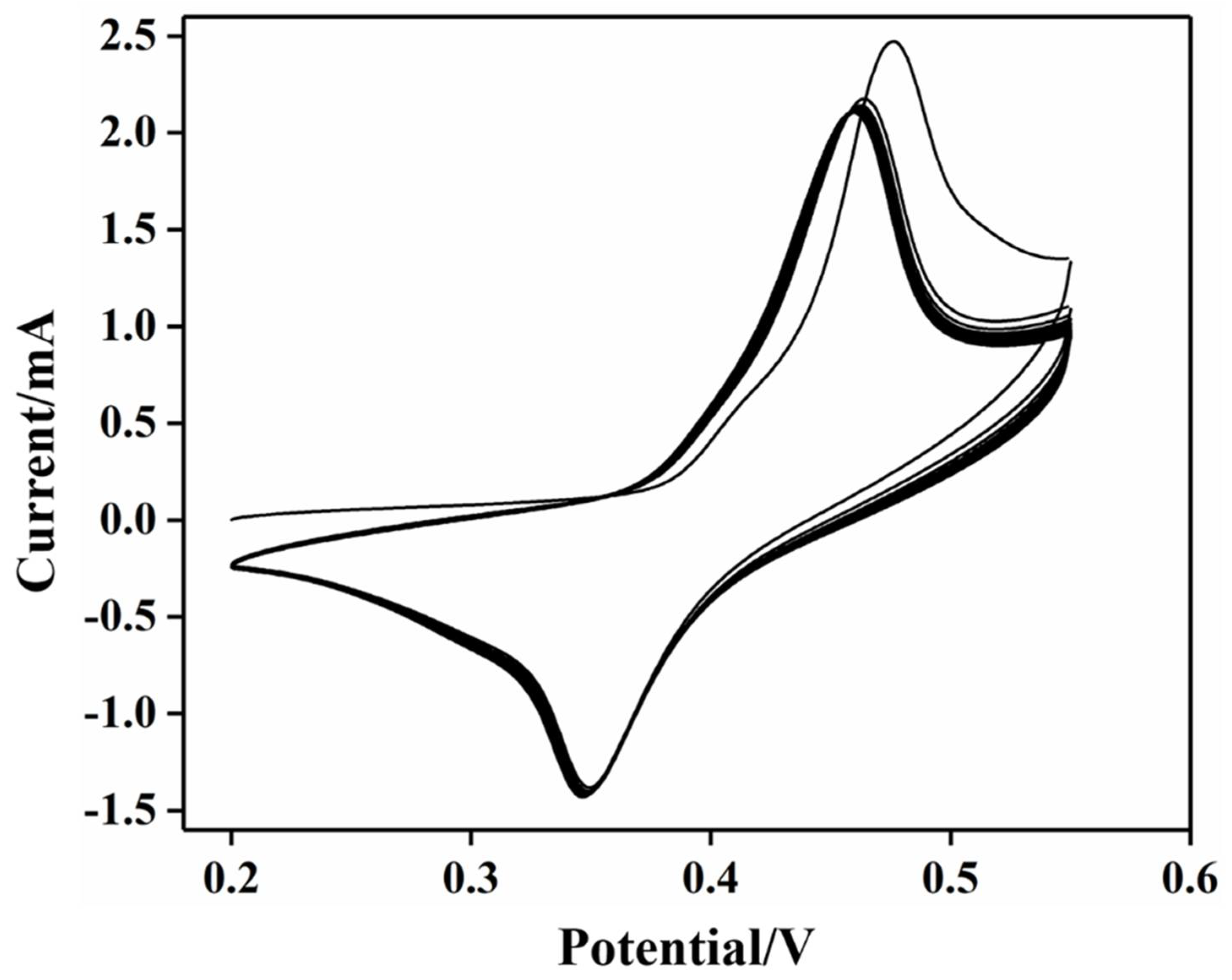
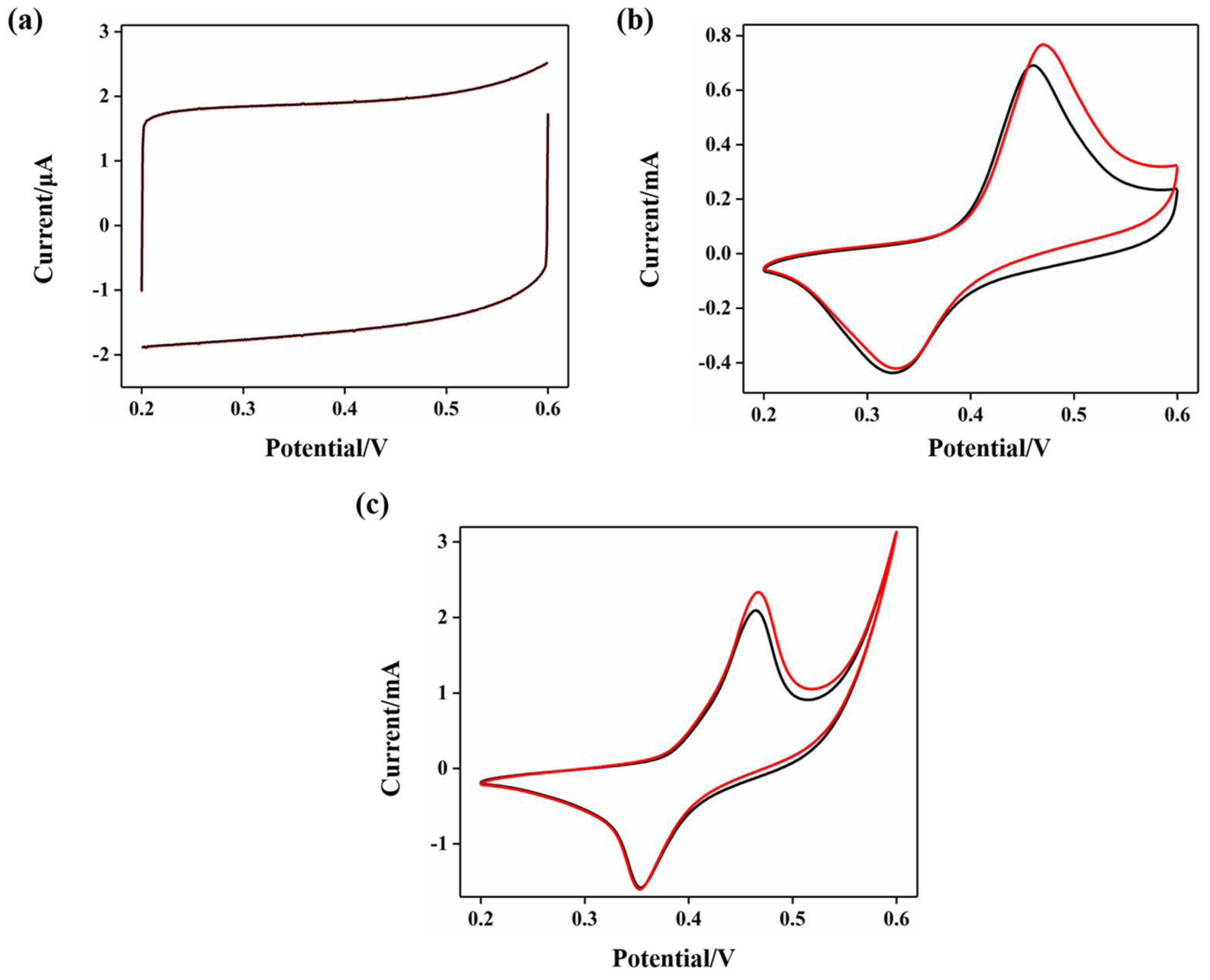
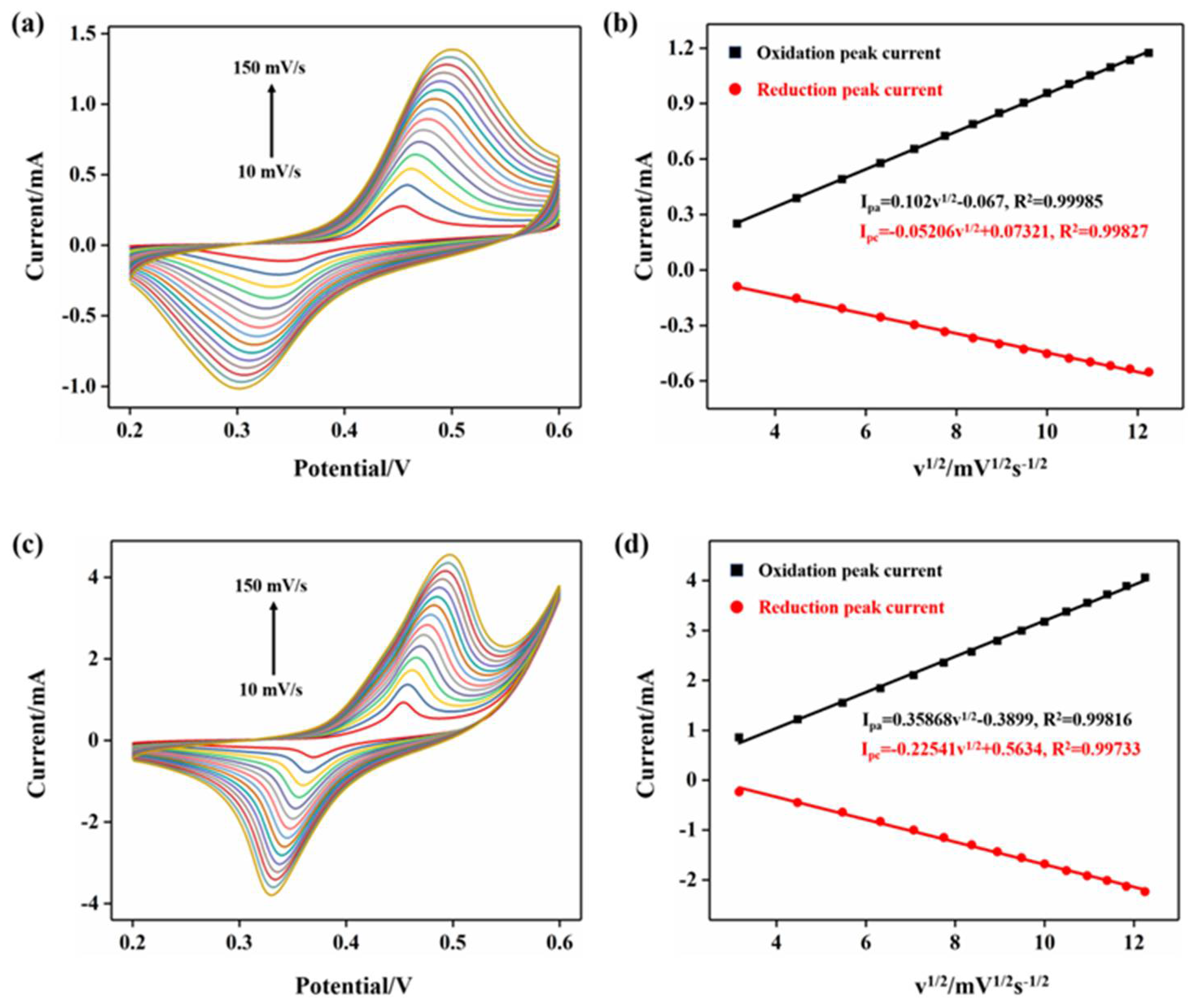
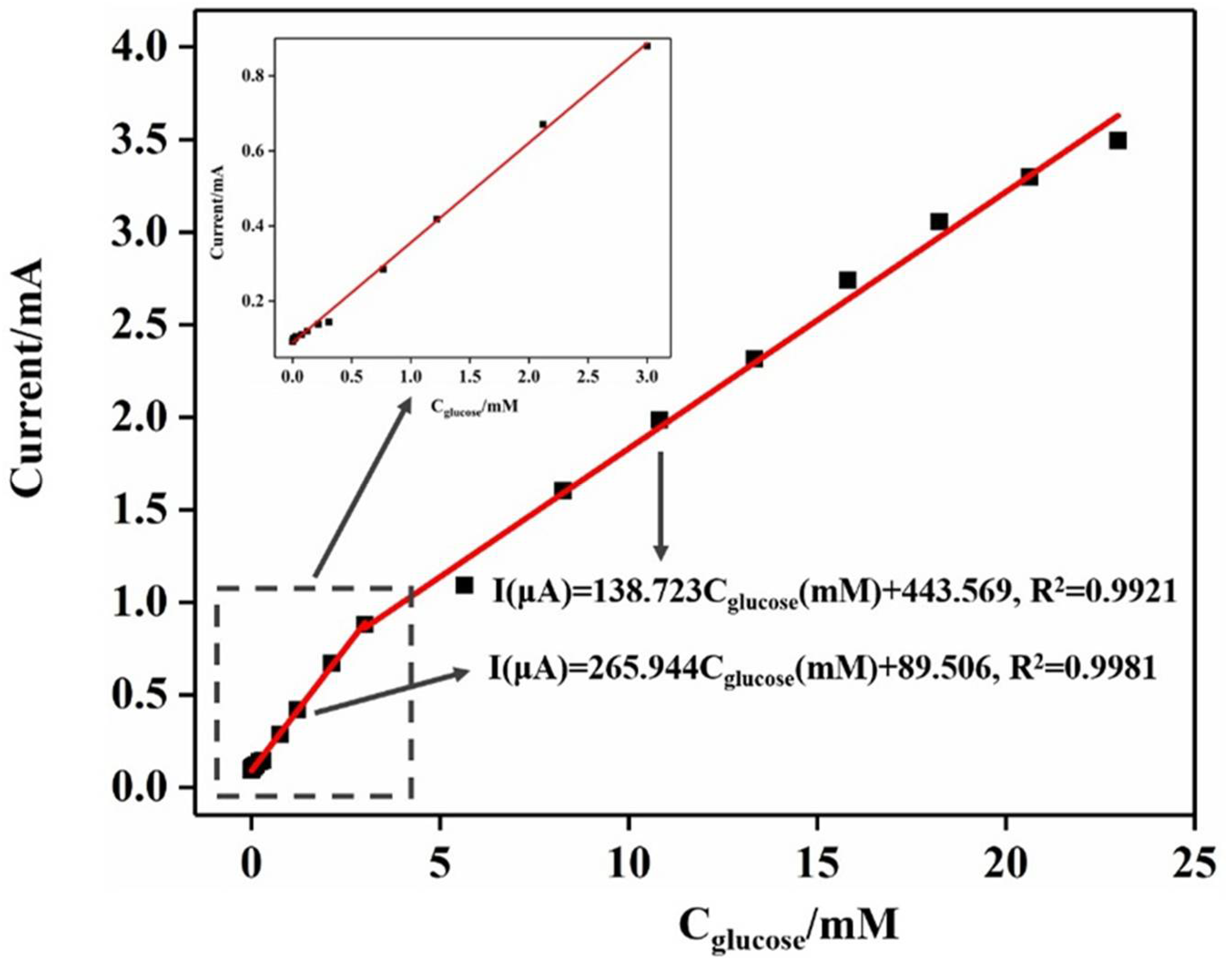
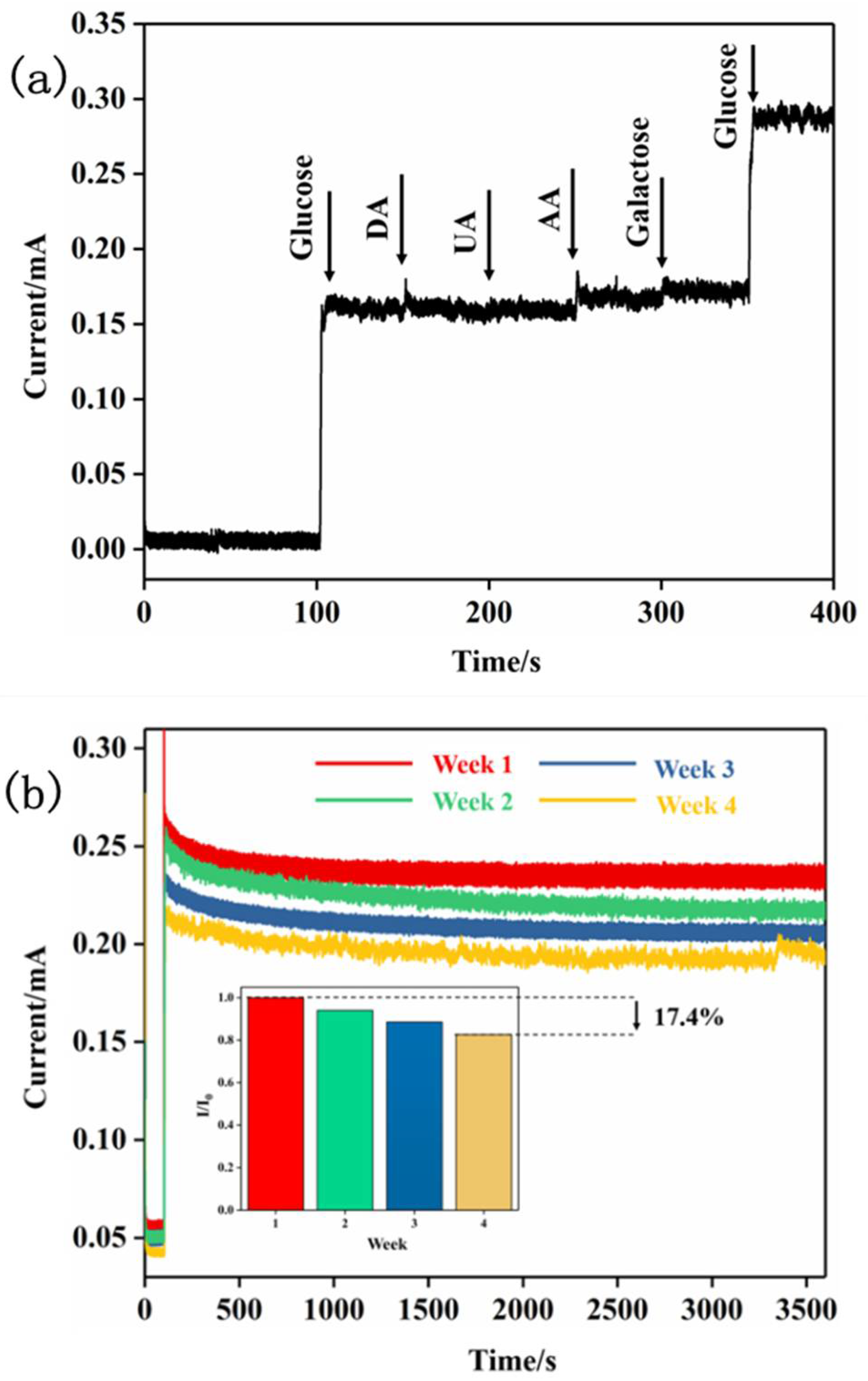
2.2. Performance of Electrode Electrocatalytic Oxidation of Glucose
3. Materials and Methods
3.1. Reagents
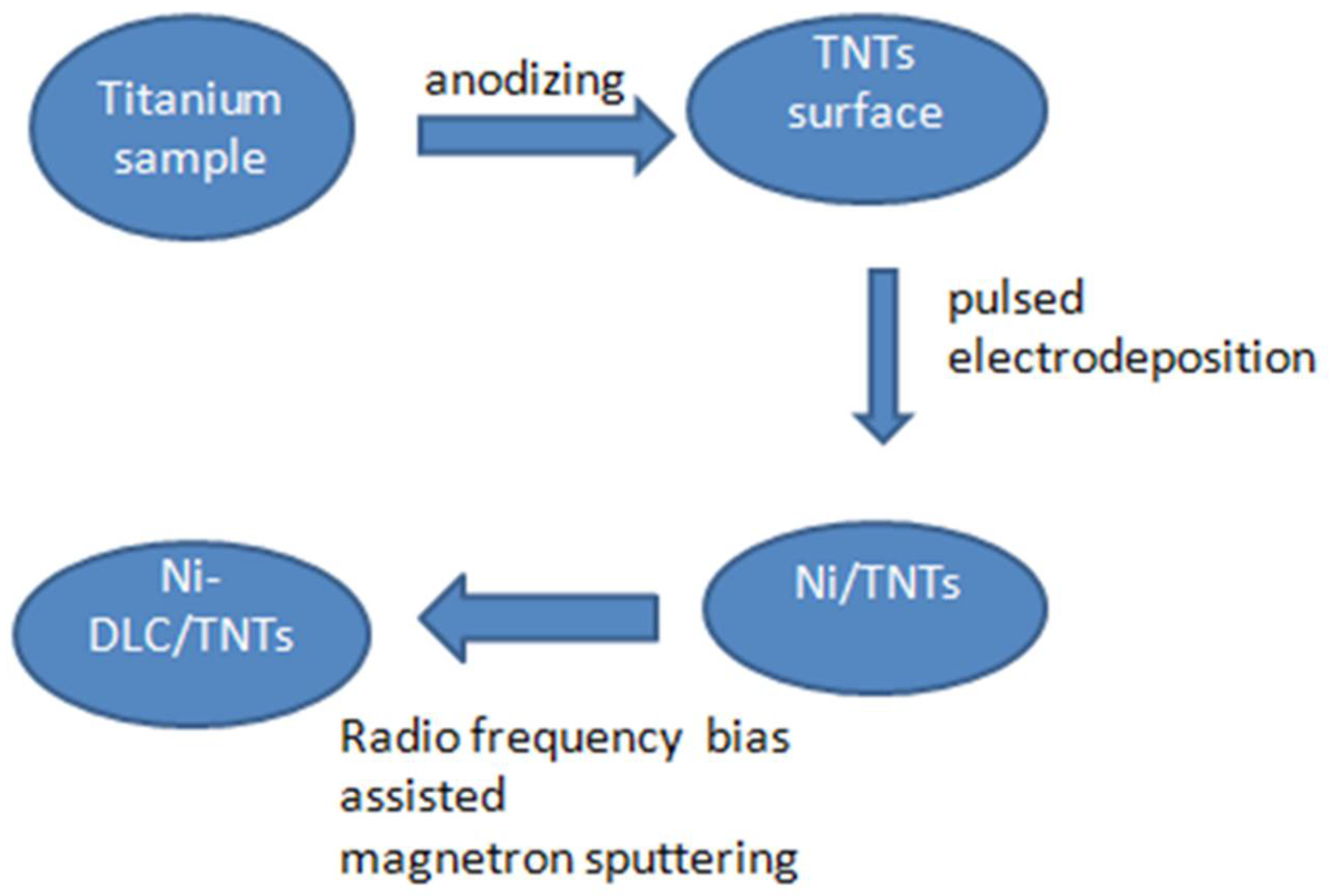
3.2. Sample Preparation
3.3. Sample Characterization and Electrochemical Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; He, X.; Wang, L.; Gu, A.; Huang, Y.; Fang, B.; Geng, B.; Zhang, X. Non-enzymatic electrochemical sensing of glucose. Mikrochim. Acta 2012, 180, 161–186. [Google Scholar] [CrossRef]
- Khalil, M.W.; Rahim, M.A.; Zimmer, A.; Hassan, H.B.; Hameed, R.M. Nickel impregnated silicalite-1 as an electro-catalyst for methanol oxidation. J. Electroanal. Chem. Interfacial Electrochem. 1971, 31, 39–49. [Google Scholar] [CrossRef]
- Liu, X.; Yan, R.; Zhu, J.; Zhang, J.; Liu, X. Growing TiO2 Nanotubes on Graphene Nanoplatelets and Applying Thenanona-nocomposite as Scaffold of Electrochemical Tyrosinasebiosensor. Sens. Actuators B Chem. 2015, 209, 328–335. [Google Scholar] [CrossRef]
- Wang, J.W.; Xu, G.Q.; Zhang, X.; Lv, J.; Zhang, X.; Zheng, Z.; Wu, Y. Electrochemical Performance and Biosensor Application of TiO2 Nanotube Arrays with Mesoporous Structures Constructed by chemical Etching. Dalton Trans. 2015, 44, 7662–7672. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Ye, W. Ni(OH)2/MoSx nanocomposite electrodeposited on a flexible CNT/PI membrane as an electrochemical glucose sensor: The synergistic effect of Ni(OH)2 and MoSx. J. Solid State Electrochem. 2016, 20, 133–142. [Google Scholar] [CrossRef]
- Liu, Y.; Teng, H.; Hou, H.; You, T. Nonenzymatic glucose sensor based on renewable electrospun Ni nanoparticle-loaded carbon nanofiber paste electrode. Biosens. Bioelectron. 2009, 24, 3329–3334. [Google Scholar] [CrossRef]
- You, T.; Niwa, O.; Chen, Z.; Haya, K.; Tomita, M.; Hiro, S. An amperometric detector formed of highly dispersed Ni nanoparticles embedded in a graphite-like carbon film electrode for sugar determination. Anal. Chem. 2003, 75, 5191–5196. [Google Scholar] [CrossRef]
- Li, X.; Yao, J.; Liu, F.; He, H.; Ming, Z.; Nan, M.; Peng, X.; Zhang, Y. Nickel/Copper nanoparticles modified TiO2 nanotubes for non-enzymatic glucose biosensors. Sens. Actuators B Chem. 2013, 181, 501–508. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, X.; Sun, Y.; Shi, Y.; Dai, H.C.; Ni, P.J.; Hu, J.T.; Li, Z.; Song, Y.H.; Wang, L. Electrochemical deposition of nickel nanoparticles on reduced graphene oxide film for nonenzymatic glucose sensing. Electroanalysis 2013, 25, 959. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Medishetty, R.; Fischer, R.A. Guest molecules as a design element for metal-organic frameworks. MRS Bull. 2016, 41, 865–869. [Google Scholar] [CrossRef]
- Bo, W.; Li, S.; Liu, J.; Wang, B. Preparation of nickel nanoparticle/graphene composites for non-enzymatic electrochemical glucose biosensor applications. Mater. Res. Bull. 2014, 49, 521–524. [Google Scholar]
- Zeng, A.; Jin, C.; Cho, S.-J.; Seo, H.-O.; Kim, Y.-D.; Lim, D.-C.; Kim, D.-H.; Hong, B.-Y.; Boo, J.-H. Nickel nano-particle modified nitrogen-doped amorphous hydrogenated diamond-like carbon film for glucose sensing. Mater. Res. Bull. 2012, 47, 2713–2716. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, Y.; Yang, W.; Zhou, M.; Hu, X. Facile One-Step Microwave-Assisted Route towards Ni Nanospheres/Reduced Graphene Oxide Hybrids for Non-Enzymatic Glucose Sensing. Sensors 2012, 12, 4860–4869. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Ren, Q.; Xu, T.; Ming, Y.; Tang, W. Morphology-controllable gold nanostructures on phosphorus doped diamond-like carbon surfaces and their electrocatalysis for glucose oxidation. Sens. Actuators B Chem. 2012, 162, 135–142. [Google Scholar] [CrossRef]
- Liu, A.; Liu, E.; Yang, G.; Tang, W. Electrochemical behavior of gold nanoparticles modified nitrogen incorporated diamond-like carbon electrode and its application in glucose sensing. In Proceedings of the 2010 3rd International Nanoelectronics Conference (INEC), Hong Kong, China, 3–8 January 2010; pp. 336–337. [Google Scholar]
- Wang, C.X.; Yin, L.W.; Zhang, L.Y.; Gao, R. Ti/TiO2 Nanotube Array/Ni Composite Electrodes for Nonenzymatic Amperometric Glucose Sensing. J. Phys. Chem. C 2010, 114, 4408–4413. [Google Scholar] [CrossRef]
- Xin, Y.; Zhen, C.; Shuf, C.; Chen, S.F.; Zhu, B. Synthesis of mesoporous CuO microspheres with core-in-hollow-shell structure and its ap-plication for non-enzymatic sensing of glucose. RSC Adv. 2015, 45, 131–138. [Google Scholar]
- Liu, A.; Liu, E.; Yang, G.; Khun, N.W.; Ma, W. Non-enzymatic glucose detection using nitrogen-doped diamond-like carbon electrodes modified with gold nanoclusters. Pure Appl. Chem. 2010, 82, 2217–2229. [Google Scholar] [CrossRef]
- Naragino, H.; Yoshinaga, K.; Tatsuta, S.; Honda, K. Improvement of conductivity by incorporation of boron atoms in hydrogenated amorphous carbon films fabricated by plasma CVD methods and its electrochemical properties. ECS Trans. 2012, 41, 59–68. [Google Scholar] [CrossRef]
- Jin, C.; Zeng, A.; Cho, S.-J.; Boo, J.-H. Investigation of copper and silver nanoparticles deposited on a nitrogen-doped diamond-like carbon (N-DLC) film electrode for bio-sensing. J. Korean Phys. Soc. 2012, 60, 912–915. [Google Scholar] [CrossRef]
- Choi, T.; Kim, S.H.; Lee, C.W.; Kim, H.G.; Cho, S.-K.; Kim, S.-H.; Kim, E.Y.; Park, J.; Kim, H.J. Synthesis of carbon nanotube–nickel nanocomposites using atomic layer deposition for high-performance non-enzymatic glucose sensing. Biosens. Bioelectron. 2015, 63, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.C.; Astruc, D. Structure and dynamics of poly ethylene glycol coated Au nanoparticles. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, G.J. Catalysis by gold. Catal. Today 2005, 100, 55–61. [Google Scholar] [CrossRef]
- Hvolbæk, B.; Janssens TV, W.; Clausen, B.S.; Hanne, F.; Claus, H.C.; Jens, K.N. Catalytic activity of Au nanopart. Nano Today 2007, 2, 14–18. [Google Scholar] [CrossRef]
- Mena, M.L.; Yanez-Sedeno, P.; Pingarron, J.M. A comparison of different strategies for the construction of amperometric en-zyme biosensors using gold nanoparticle-modified electrodes. Anal. Biochem. 2005, 336, 20–27. [Google Scholar] [CrossRef]
- Hrapovic, S.; Liu, Y.L.; Male, K.B.; Luong, J.H.T. Electrochemical Biosensing Platforms Using Platinum Nanoparticles and Carbon Nanotubes. Anal. Chem. 2004, 76, 1083–1088. [Google Scholar] [CrossRef]
- Male, K.B.; Hrapovic, S.; Liu, Y.L.; Wang, D.; Luong, J.H.T. Electrochemical detection of carbohydrates using copper nanoparticles and carbon nanotubes. Anal. Chim. Acta 2004, 516, 35–41. [Google Scholar] [CrossRef]
- Zhu, H.; Ma, L.; Liu, N.; Wei, Q.; Wu, D.; Wang, Y.; Long, H.; Yu, Z. Improvement in anti-corrosion property of hydrogenated diamond-like carbon film by modifying CrC interlayer. Diam. Relat. Mater. 2017, 72, 99–107. [Google Scholar] [CrossRef]
- Ibl, N. Some theoretical aspects of pulse electrolysis. Surf. Technol. 1980, 10, 81–104. [Google Scholar] [CrossRef]
- Ferrari, A.; Kleinsorge, B.; Adamopoulos, G.; Robertson, J.; Milne, W.I.; Stolojan, V.; Brown, L.M.; Libassi, A.; Tanner, B.K. Determination of bonding in amorphous carbons by electron energy loss spectroscopy, Raman scattering and X-ray reflectivity. J. Non-Cryst. Solids 2000, 266, 765–768. [Google Scholar] [CrossRef]
- Yang, G.; Liu, E.; Khun, N.W.; Jiang, S.P. Direct electrochemical response of glucose at nickel-doped diamond like carbon thin film electrodes. J. Electroanal. Chem. 2009, 627, 51–57. [Google Scholar] [CrossRef]
- Kiang, C.-H.; Goddard Iii, W.A.; Beyers, R.; Goddard, W.A. Carbon nanotubes with single-layer walls. Carbon 1995, 33, 903–914. [Google Scholar] [CrossRef]
- Grill, A. Electrical and optical properties of diamond-like carbon. Thin. Solid Film. 1999, 355, 189–193. [Google Scholar] [CrossRef]
- Li, G.; Huo, H.; Xu, C. Ni0.31Co0.69S2 nanoparticles uniformly anchored on a porous reduced graphene oxide framework for a high-performance non-enzymatic glucose sensor. J. Mater. Chem. A 2015, 3, 4922–4930. [Google Scholar] [CrossRef]
- Dai, W.; Li, M.; Gao, S.; Xu, S.; Wu, X.G.; Yang, B.H. Fabrication of Nickel/nanodiamond/boron-doped diamond electrode for non-enzymatic glucose biosensor. Electrochim. Acta 2016, 187, 413–421. [Google Scholar] [CrossRef]
- Yuan, B.; Xu, C.; Deng, D.; Xing, Y.; Liu, L.; Pang, H.; Zhang, D. Graphene oxide/nickel oxide modified glassy carbon electrode for supercapacitor and non-enzymatic glucose sensor. Electrochim. Acta 2013, 88, 708–712. [Google Scholar] [CrossRef]
- Li, M.; Bo, X.; Mu, Z.; Zhang, Y.; Guo, L. Chemical, Electrodeposition of nickel oxide and platinum nanoparticles on electrochemically re-duced graphene oxide film as a nonenzymatic glucose sensor. Sens. Actuators B Chem. 2014, 192, 261–268. [Google Scholar] [CrossRef]
- Sun, A.; Zheng, J.; Sheng, Q. A highly sensitive non-enzymatic glucose sensor based on nickel and multi-walled carbon nanotubes nanohybrid films fabricated by one-step co-electrodeposition in ionic liquids. Electrochim. Acta 2012, 65, 64–69. [Google Scholar] [CrossRef]
- Qiao, N.; Zheng, J. Nonenzymatic glucose sensor based on glassy carbon electrode modified with a nanocomposite composed of nickel hydroxide and graphene. Electrochim. Acta 2012, 177, 103–109. [Google Scholar] [CrossRef]
- Arvinte, A.; Sesay, A.-M.; Virtanen, V.J.T. Carbohydrates electrocatalytic oxidation using CNT–NiCo-oxide modified electrodes. Talanta 2011, 84, 180–186. [Google Scholar] [CrossRef]
- Toghill, K.E.; Xiao, L.; Phillips, M.A.; Compton, R.G. The non-enzymatic determination of glucose using an electrolytically fabricated nickel microparticle modified boron-doped diamond electrode or nickel foil electrode. Sens. Actuators B Chem. 2010, 147, 642–652. [Google Scholar] [CrossRef]
- Amiripour, F.; Ghasemi, S.; Naser Azizi, S. A novel non-enzymatic glucose sensor based on gold-nickel bimetallic nanoparticles doped aluminosilicate framework prepared from agro-waste material. Appl. Surf. Sci. 2021, 1, 537. [Google Scholar] [CrossRef]
- Emir, G.; Dilgin, Y.; Ramanaviciene, A. Amperometric nonenzymatic glucose biosensor based on graphite rod electrode modified by Ni-nanoparticle/polypyrrole composite. Microchem. J. 2021, 2, 161. [Google Scholar] [CrossRef]
- Wei, H.; Xue, Q.; Li, A.; Wan, T.; Huang, Y.; Cui, D.; Pan, D.; Dong, B.; Wei, R.; Naik, N.; et al. Dendritic core-shell copper-nickel alloy@metal oxide for efficient non-enzymatic glucose detection. Sens. Actuator B Chem. 2021, 7, 337. [Google Scholar]
- Kannan, P.; Maiyalagan, T.; Marsili, E.; Ghosh, S.; Niedziolka-Jönsson, J.; Jönsson-Niedziolka, M. Hierarchical 3-dimensional nickel–iron nanosheet arrays on carbon fiber paper as a novel electrode for non-enzymatic glucose sensing. Nanoscale 2016, 8, 843–855. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Xiao, P.; Zhang, X.; Lu, L.; Li, L. Preparation of Ni nanoparticle-TiO2 nanotube composite by pulse electrodeposition. Mater. Lett. 2009, 63, 2429–2431. [Google Scholar] [CrossRef]

| Electrode Material | Linear Range | Detection Limit (μM) | Sensitivity (μA·mM−1·cm−2) | Ref. |
|---|---|---|---|---|
| Ni-NDa/BDD | 0.2–12 μM; 31.3 μM–1.06 mM | 0.05 | 120; 35.6 | [36] |
| NiNPs/GNc | 5 μM–0.55 mM | 1.85 | 865 | [12] |
| NiONPs/GOd/GCE | 3.13 μM–3.05 mM | 1 | 1087 | [37] |
| NiO/Pt/ErGO/GCE | 50 μM–5.66 mM | 0.2 | 668.2 | [38] |
| Ni-MWCNTe/GCE | 3.2 μM–17.5 mM | 0.89 | 67.19 | [39] |
| Ni(OH)2graphene/GCE | 1–10 μM; 10 μM–10 mM | 0.6 | 494; 328 | [40] |
| NiCoO/CNTf | 0.01–0.93 mM; 0.93–12.12 mM | 5 | 66.15; 15.43 | [41] |
| NiNPs/BDD | 10 μM–10 mM | 2.7 | 1040 | [42] |
| NiNPs/CNFg | 2 μM–2.5 mM | 1 | 420.4 | [7] |
| Ni-Rgo/GCE | 1–110 μM | - | 813 | [18] |
| Au/DLC:P | 0.5–25 mM | 300 | 37 | [19] |
| Au-DLC:N | 0.5–25 mM | 120 | - | [20] |
| CuO | 0.005–7.95 mM | 1 | 622.2 | [22] |
| AuNi/NX/MWCNT-21 | 1–60 mM | 0.063 | 662.93 | [43] |
| 60–1900 mM | 0.285 | 147.22 | ||
| Ni-NPs/GRE | 2–800 mM | 0.4 | 1387 | [44] |
| Cu-Ni/NF | 1−600 mM | 2 | 11,340.25 | [45] |
| Ni-DLC/TiO2 | 0.99–22.97 mM | 0.53 | 1063.78 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.; Ren, X.; Li, Y.; Yu, Z. Ni-Coated Diamond-like Carbon-Modified TiO2 Nanotube Composite Electrode for Electrocatalytic Glucose Oxidation. Molecules 2022, 27, 5815. https://doi.org/10.3390/molecules27185815
Kang Y, Ren X, Li Y, Yu Z. Ni-Coated Diamond-like Carbon-Modified TiO2 Nanotube Composite Electrode for Electrocatalytic Glucose Oxidation. Molecules. 2022; 27(18):5815. https://doi.org/10.3390/molecules27185815
Chicago/Turabian StyleKang, Yi, Xuelei Ren, Yejun Li, and Zhiming Yu. 2022. "Ni-Coated Diamond-like Carbon-Modified TiO2 Nanotube Composite Electrode for Electrocatalytic Glucose Oxidation" Molecules 27, no. 18: 5815. https://doi.org/10.3390/molecules27185815




