Toxic Effect of Metal Doping on Diatoms as Probed by Broadband Terahertz Time-Domain Spectroscopy
Abstract
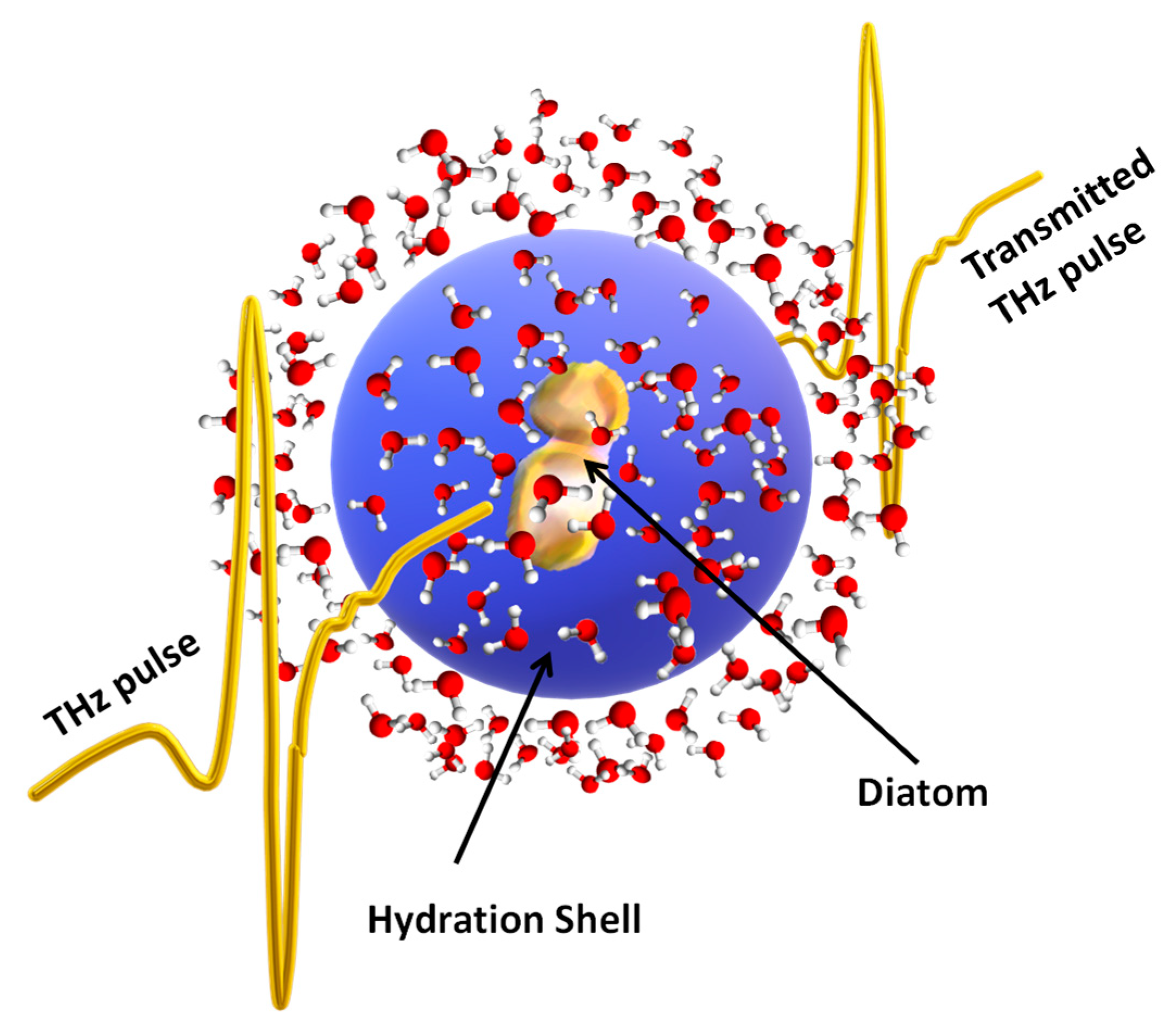
:1. Introduction
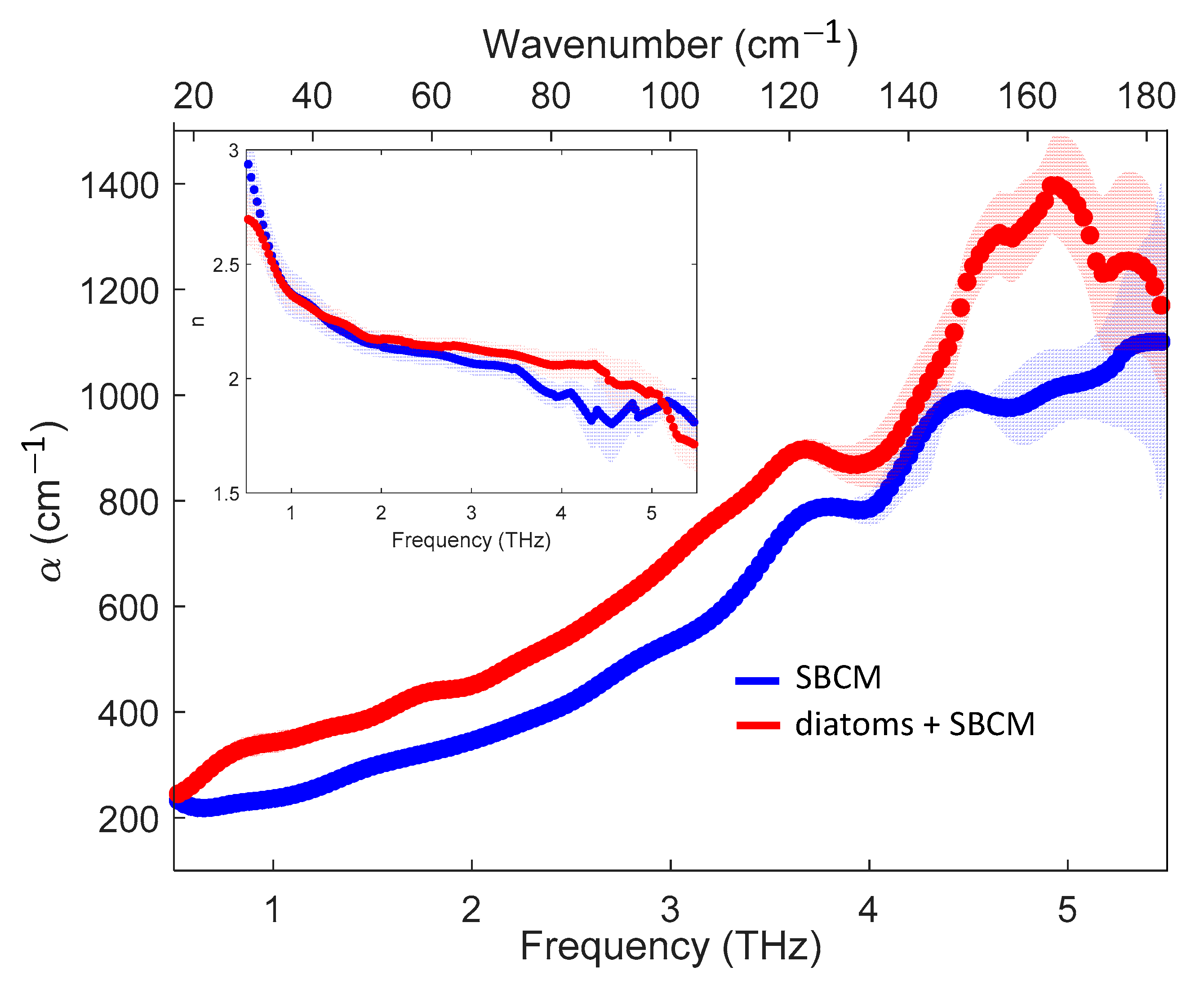
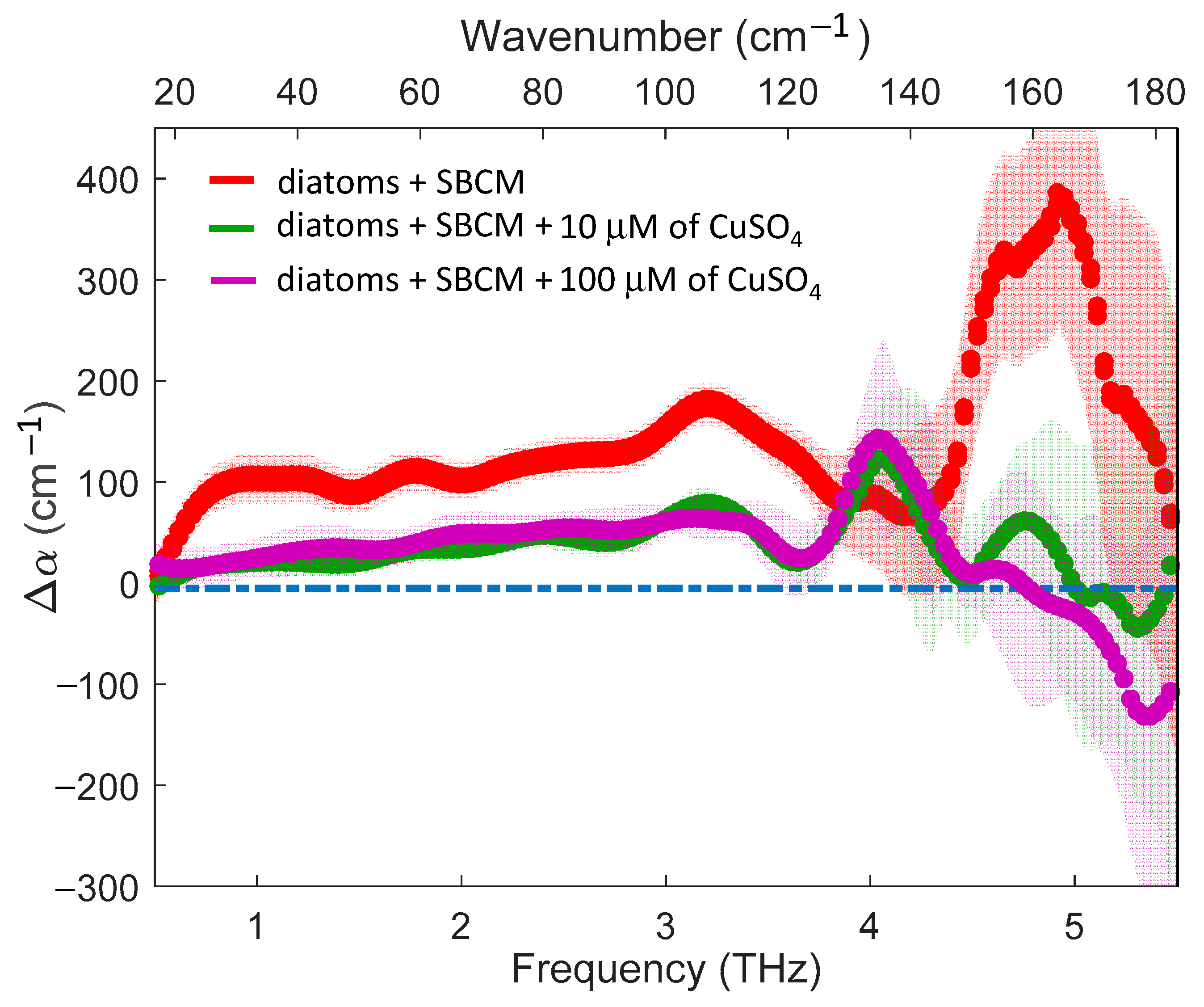
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
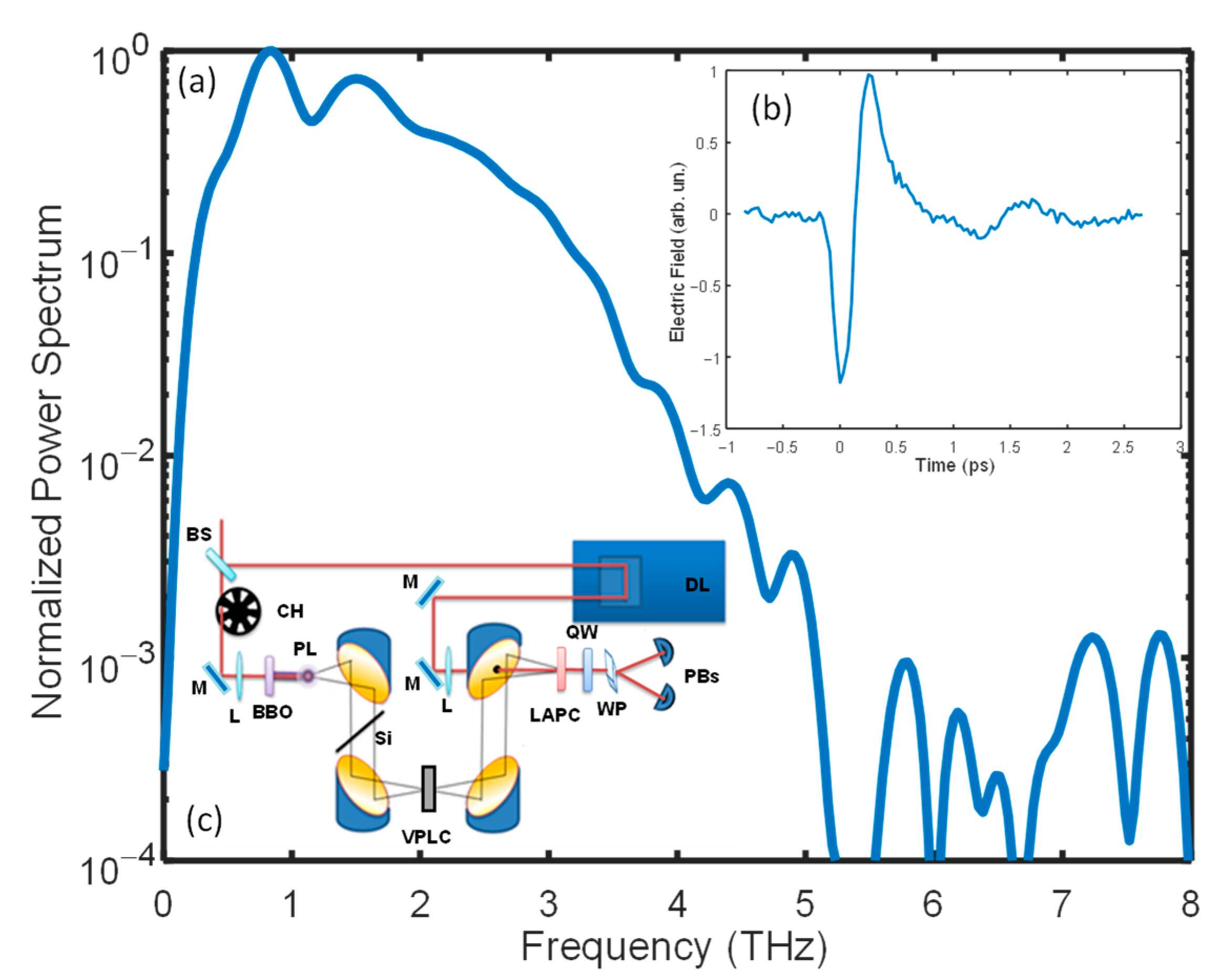
4.2. TDS-THz Experiment
4.3. Absorption Coefficient and Refractive Index Extraction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersson, B.; Godhe, A.; Filipsson, H.L.; Zetterholm, L.; Edler, L.; Berglund, O.; Rengefors, K. Intraspecific variation in metal tolerance modulate competition between two marine diatoms. ISME J. 2022, 16, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exper. Suppl. 2012, 101, 133–164. [Google Scholar]
- He, Z.L.L.; Yang, X.E.; Stoffella, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, P. Manganese metabolism in humans. Front. Biosci. 2018, 23, 1655–1679. [Google Scholar] [CrossRef]
- Schmidt, S.B.; Husted, S. The Biochemical Properties of Manganese in Plants. Plants 2019, 8, 381. [Google Scholar] [CrossRef]
- Gheorghe, S.; Stoica, C.; Vasile, G.G.; Nita-Lazar, M.; Stanescu, E.; Lucaciu, I.E. Metal Toxic Effects in Aquatic Ecosystems: Modulators of Water Quality. InTech 2017. [Google Scholar] [CrossRef]
- Zhou, B.; Ma, J.; Chen, F.; Zou, Y.; Wei, Y.; Zhong, H.; Pan, K. Mechanisms underlying silicon-dependent metal tolerance in the marine diatom Phaeodactylum tricornutum. Environ. Pollut. 2020, 262, 114331. [Google Scholar] [CrossRef]
- Narula, P.; Mahajan, A.; Gurnani, C.; Kumar, V.; Mukhija, S. Microalgae as an Indispensable Tool against Heavy Metals Toxicity to Plants: A Review. Int. J. Pharm. Sci. Rev. Res. 2015, 31, 86–93. [Google Scholar]
- Rocha, G.S.; Parrish, C.C.; Espindola, E.L.G. Effects of copper on photosynthetic and physiological parameters of a freshwater microalga (Chlorophyceae). Algal Res. 2021, 54, 102223. [Google Scholar] [CrossRef]
- Gerringa, L.J.A.; Rijstenbil, J.W.; Poortvliet, T.C.W.; van Drie, J.; Schot, M.C. Speciation of copper and responses of the marine diatom Ditylum brightwellii upon increasing copper concentrations. Aquat. Toxicol. 1995, 31, 77–90. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef] [PubMed]
- Manimaran, K.; Karthikeyan, P.; Ashokkumar, S.; Ashok Prabu, V.; Sampathkumar, P. Effect of Copper on Growth and Enzyme Activities of Marine Diatom, Odontella mobiliensis. Bull. Environ. Contam. Toxicol. 2012, 88, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Melegari, S.P.; Perreault, F.; Costa, R.H.R.; Popovic, R.; Matias, W.G. Evaluation of toxicity and oxidative stress induced by copper oxide nanoparticles in the green alga Chlamydomonas reinhardtii. Aquat. Toxicol. 2013, 142, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Purbonegoro, T.; Suratno; Puspitasari, R.; Husna, N.A. Toxicity of copper on the growth of marine microalgae Pavlova sp. and its chlorophyll-a. IOP Conf. Ser. Earth Environ. Sci. 2018, 118, 012060. [Google Scholar] [CrossRef]
- Chen, Z.; Song, S.; Wen, Y.; Zou, Y.; Liu, H. Toxicity of Cu(II) to the green alga Chlorella vulgaris: A perspective of photosynthesis and oxidant stress. Environ. Sci. Pollut. Res. Int. 2016, 18, 17910-8. [Google Scholar] [CrossRef]
- De Mora, S.; Fowler, S.W.; Wyse, E.; Azemard, S. Distribution of heavy metals in marine bivalves, fish and coastal sediments in the Gulf of Oman. Mar. Pollut. Bull. 2004, 49, 410–424. [Google Scholar] [CrossRef]
- Ndiaye, B.; Ndiaye, M.; Cid, B.P.; Diop, A.; Diagne, I.; Cissé, D.; Dione, C.T.; Hanne, M. Trace Metals in Mussels Mytilus galloprovincialis from Dakar Coast (Senegal). Am. J. Anal. Chem. 2020, 11, 137–145. [Google Scholar] [CrossRef]
- Stauber, J.L.; Davies, C.M. Use and limitations of microbial bioassays for assessing copper bioavailability in the aquatic environment. Environ. Rev. 2000, 8, 255–301. [Google Scholar] [CrossRef]
- Adams, M.S.; Dillon, C.T.; Vogt, S.; Lai, B.; Stauber, J.; Jolley, D.F. Copper Uptake, Intracellular Localization, and Speciation in Marine Microalgae Measured by Synchrotron Radiation X-ray Fluorescence and Absorption Microspectroscopy. Environ. Sci. Technol. 2016, 20, 1–25. [Google Scholar] [CrossRef]
- Squires, L.E.; Rushforth, S.R.; Brotherson, J.D. Algal response to a thermal effluent: Study of a power station on the provo river, Utah, USA. Hydrobiologia 1979, 63, 17–32. [Google Scholar] [CrossRef]
- Vilbaste, S.; Truu, J. Distribution of benthic diatoms in relation to environmental variables in lowland streams. Hydrobiologia 2003, 493, 81–93. [Google Scholar] [CrossRef]
- Nassiri, Y.; Mansot, J.L.; Wery, J.; Ginsburger-Vogel, T.; Amiard, J.C. Ultrastructural and electron energy loss spectroscopy studies of sequestration mechanisms of Cd and Cu in the marine diatom Skeletonema costatum. Arch. Environ. Contam. Toxicol. 1997, 33, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Qin, L.G.; Jin, M.; Zhang, L.L.; Geng, W.W.; Xiao, X.F. Extracellular adsorption, intracellular accumulation and tolerance mechanisms of Cyclotella sp. to Cr(VI) stress. Chemosphere 2021, 270, 128662. [Google Scholar] [CrossRef] [PubMed]
- Pandey, L.K.; Bergey, E.A.; Lyu, J.; Park, J.; Choi, S.; Lee, H.; Depuydt, S.; Oh, Y.-T.; Lee, S.-M.; Han, T. The use of diatoms in ecotoxicology and bioassessment: Insights, advances and challenges. Water Res. 2017, 118, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.; Mortágua, A.; Almeida, S.F.; Serra, S.; Feio, M.J. Diatom size plasticity at regional and global scales. Limnetica 2020, 39, 387–403. [Google Scholar] [CrossRef]
- Heraud, P.; Wood, B.R.; Beardall, J.; McNaughton, D. Probing the Influence of the Environment on Microalgae Using Infrared and Raman Spectroscopy. In New Approaches in Biomedical Spectroscopy; Kneipp, K., Aroca, R., Kneipp, H., Wentrup-Byrne, E., Eds.; ACS Symposium Series; American Chemical Society: Honolulu, HI, USA, 2005; Volume 963, pp. 85–106. [Google Scholar]
- Coat, R.; Montalescot, V.; León, E.S.; Kucma, D.; Perrier, C.; Jubeau, S.; Thouand, G.; Legrand, J.; Pruvost, J.; Gonçalves, O. Unravelling the matrix effect of fresh sampled cells for in vivo unbiased FTIR determination of the absolute concentration of total lipid content of microalgae. Brioprocess Biosyst. Eng. 2014, 37, 2175–2187. [Google Scholar] [CrossRef]
- Rubano, A.; Mou, S.; Marrucci, L.; Paparo, D. Terahertz Hyper-Raman Time-Domain Spectroscopy. ACS Photonics 2019, 6, 1515–1523. [Google Scholar] [CrossRef]
- Mou, S.; Rubano, A.; Paparo, D. Broadband Terahertz Spectroscopy of Imidazolium-Based Ionic Liquids. J. Phys. Chem. B 2018, 122, 3133–3140. [Google Scholar] [CrossRef]
- Novelli, F.; Guchhait, B.; Havenith, M. Towards Intense THz Spectroscopy on Water: Characterization of Optical Rectification by GaP, OH1, and DSTMS at OPA Wavelengths. Materials 2020, 13, 1311–1326. [Google Scholar] [CrossRef]
- Ulbricht, R.; Hendry, E.; Shan, J.; Heinz, T.F.; Bonn, M. Carrier dynamics in semiconductors studied with time-resolved Terahertz spectroscopy. Rev. Mod. Phys. 2011, 83, 543. [Google Scholar] [CrossRef]
- George, D.K.; Markelz, A.G. Terahertz Spectroscopy of Liquids and Biomolecules. In Terahertz Spectroscopy and Imaging; Peiponen, K.-E., Zeitler, J.A., Kuwata-Gonokami, M., Eds.; Springer: Berlin-Heidelberg, Germany, 2013; pp. 229–250. [Google Scholar]
- Jung, S.W.; Yun, S.M.; Lee, S.D.; Kim, Y.-O.; Lee, J.H. Morphological Characteristics of Four Species in the Genus Skeletonema in Coastal Waters of South Korea. Algae 2009, 24, 195–203. [Google Scholar] [CrossRef]
- Cook, D.J.; Hochstrasser, R.M. Intense terahertz pulses by four-wave rectification in air. Opt. Lett. 2000, 25, 1210–1212. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S. Principles of Terahertz Science and Technology; Springer: New York, NY, USA, 2009. [Google Scholar]
- Pupeza, I.; Wilk, R.; Koch, M. Highly accurate optical material parameter determination with THz time-domain spectroscopy. Opt. Express 2007, 15, 4335–4350. [Google Scholar] [CrossRef] [PubMed]
- Heugen, U.; Schwaab, G.; Bruendermann, E.; Havenith, M. Solute-induced retardation of water dynamics probed directly by terahertz spectroscopy. Proc. Natl. Acad. Sci. USA 2006, 103, 12301–12306. [Google Scholar] [CrossRef]
- Ebbinghaus, S.; Kim, S.J.; Heyden, M.; Yu, X.; Heugen, U.; Gruebele, M.; Leitner, D.M.; Havenith, M. An extended dynamical hydration shell around proteins. Proc. Natl. Acad. Sci. USA 2007, 26, 20749–20752. [Google Scholar] [CrossRef]
- Tielrooij, K.J.; Paparo, D.; Piatkowski, L.; Bakker, H.J.; Bonn, M. Dielectric Relaxation Dynamics of Water in Model Membranes Probed by Terahertz Spectroscopy. Biophys. J. 2009, 97, 2484–2492. [Google Scholar] [CrossRef]
- Ball, P. Water as an Active Constituent in Cell Biology. Chem. Rev. 2008, 108, 74–108. [Google Scholar] [CrossRef]
- Ball, P. Water is an active matrix of life for cell and molecular biology. Proc. Natl. Acad. Sci. USA 2017, 114, 13327–13335. [Google Scholar] [CrossRef]
- Ruggiero, M.T.; Bardon, T.; Strlicč, M.; Taday, P.F.; Korter, T.M. Assignment of the Terahertz Spectra of Crystalline Copper Sulfateand Its Hydrates via Solid-State Density Functional Theory. J. Phys. Chem. A 2014, 118, 10101–10108. [Google Scholar] [CrossRef]
- Absher, M. Chapter 1- Hemocytometer Counting. In Tissue Culture: Methods and Applications; Academic Press: New York, NY, USA, 1973; pp. 395–397. [Google Scholar]
- Xu, Y.; Havenith, M. Perspective: Watching low-frequency vibrations of water in biomolecular recognition by THz spectroscopy. J. Chem. Phys. 2015, 143, 170901. [Google Scholar] [CrossRef]
- Andersen, R.; Kawachi, M. Traditional Microalgae Isolation Techniques; Elsevier Academic Press: Burlington, MA, USA, 2005. [Google Scholar]
- Kooistra, W.; Sarno, D.; Balzano, S.; Gu, H.F.; Andersen, R.A.; Zingone, A. Global diversity and biogeography of Skeletonema species (Bacillariophyta). Protist 2008, 159, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Sarno, D.; Kooistra, W.; Balzano, S.; Hargraves, P.E.; Zingone, A. Diversity in the genus Skeletonema (Bacillariophyceae): III. Phylogenetic position and morphological variability of Skeletonema costatum and Skeletonema grevillei, with the description of Skeletonema ardens sp nov. J. Phycol. 2007, 43, 156–170. [Google Scholar] [CrossRef]
- Sarno, D.; Kooistra, W.; Medlin, L.K.; Percopo, I.; Zingone, A. Diversity in the genus Skeletonema (Bacillariophyceae). II. An assesment of the taxonomy of S. costatum-like species with the description of four new species. J. Physicol. 2005, 41, 151–176. [Google Scholar]
- Guillard, R.R.L.; Ryther, J.H. Studies on Marine Planktonic Diatoms, I. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Sinyukov, A.M.; Hayden, L.M. Efficient Electrooptic Polymers for THz Applications. J. Phys. Chem. B 2004, 108, 8515–8522. [Google Scholar] [CrossRef]
- Press, W.H.; Flannery, B.P.; Teukolsky, S.A.; Vetterling, W.T. Numerical Recipes in Pascal; Cambridge University Press: New York, NY, USA, 1989. [Google Scholar]
| Technique | Optical Process | Vibrational Mode | Real-Time Capability | Harmful | Water Absorption |
|---|---|---|---|---|---|
| Raman | two-photon | single-bond | YES | YES | LOW |
| IAS | one-photon | single-bond | YES | LOW | MEDIUM |
| THz-TDS | one-photon | Collective | YES | NO | STRONG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Paturzo, M.; Sardo, A.; Orefice, I.; Yu, Q.; Rubano, A.; Paparo, D. Toxic Effect of Metal Doping on Diatoms as Probed by Broadband Terahertz Time-Domain Spectroscopy. Molecules 2022, 27, 5897. https://doi.org/10.3390/molecules27185897
Kumar R, Paturzo M, Sardo A, Orefice I, Yu Q, Rubano A, Paparo D. Toxic Effect of Metal Doping on Diatoms as Probed by Broadband Terahertz Time-Domain Spectroscopy. Molecules. 2022; 27(18):5897. https://doi.org/10.3390/molecules27185897
Chicago/Turabian StyleKumar, Rohit, Melania Paturzo, Angela Sardo, Ida Orefice, Qiucheng Yu, Andrea Rubano, and Domenico Paparo. 2022. "Toxic Effect of Metal Doping on Diatoms as Probed by Broadband Terahertz Time-Domain Spectroscopy" Molecules 27, no. 18: 5897. https://doi.org/10.3390/molecules27185897


.jpg)



