An Updated Review on Prebiotics: Insights on Potentials of Food Seeds Waste as Source of Potential Prebiotics
Abstract
:1. Background
2. Prebiotics as Natural Supplement
2.1. What Are Prebiotics?
2.2. Criteria for Classifying Compounds as Prebiotics
2.3. Types of Prebiotics
2.3.1. Fructans
2.3.2. Galactooligosaccharides
2.3.3. Starch- and Glucose-Derived Oligosaccharides
2.3.4. Pectin Oligosaccharides
2.3.5. Miscellaneous
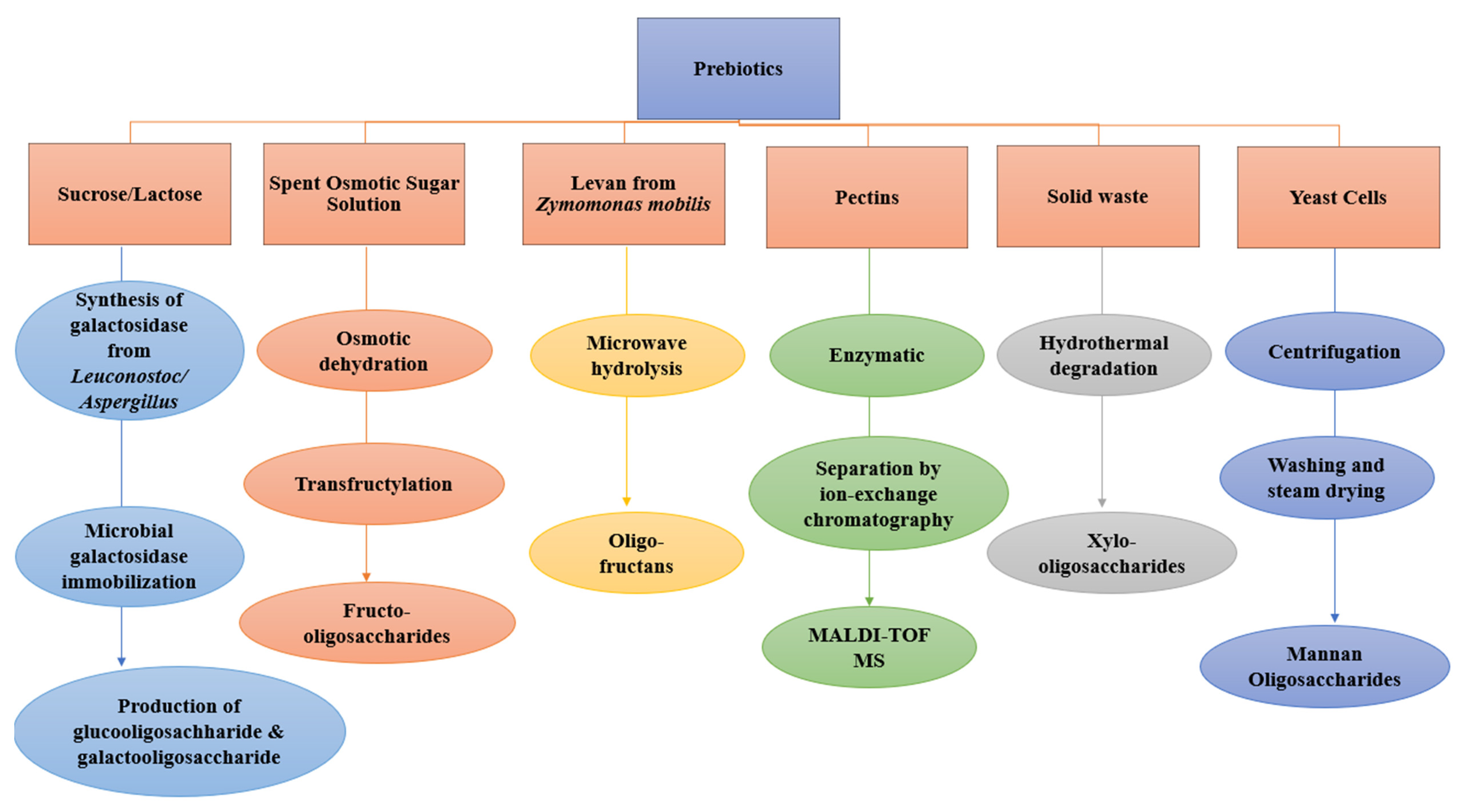
2.4. The Sources and Production of Prebiotics
2.4.1. Production of Fructo-Oligosaccharides
2.4.2. Galactooligosaccharides
2.5. Assessment of Prebiotic Efficacy
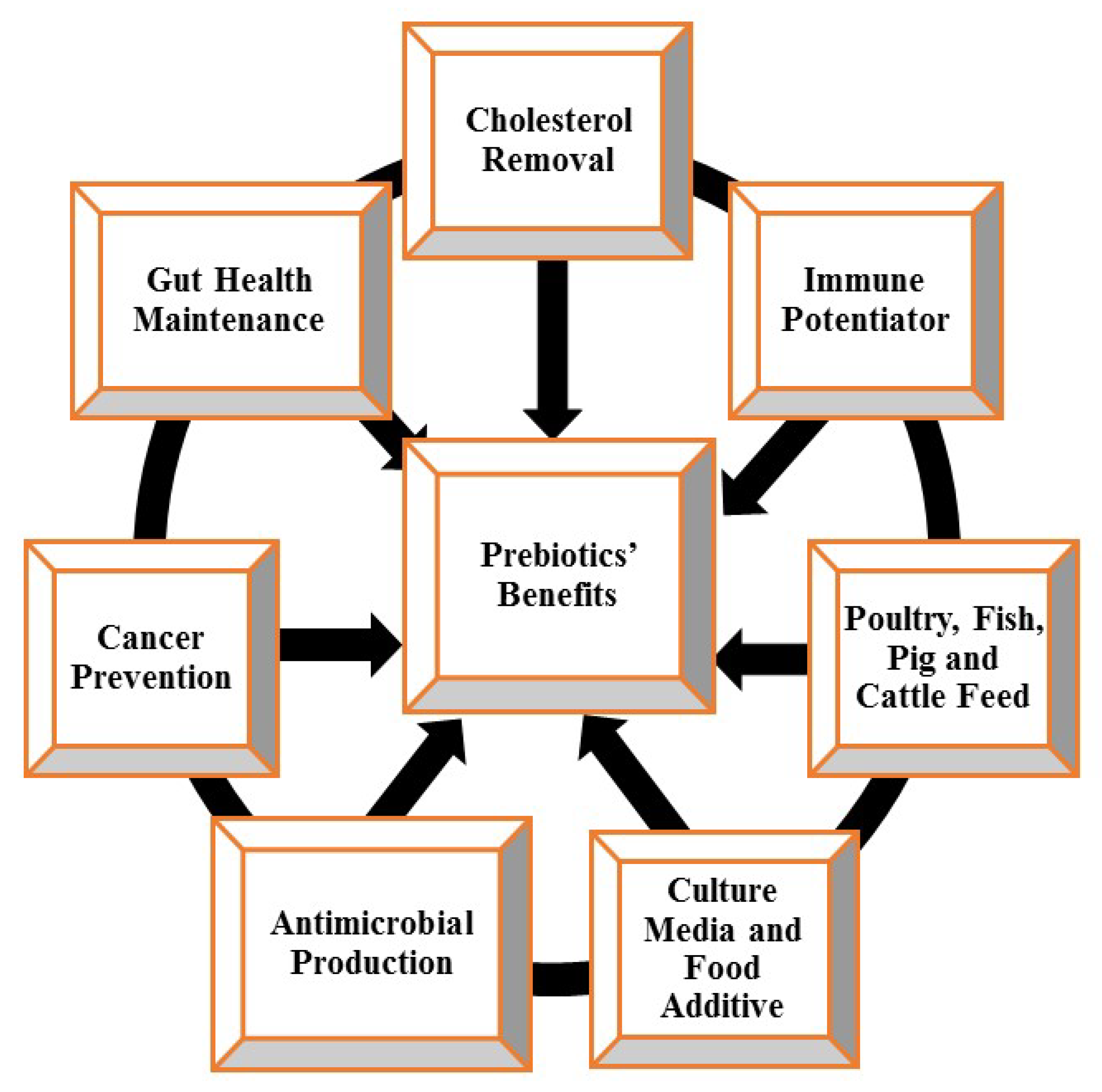
2.6. Health Benefits and Mechanisms of Prebiotics
2.6.1. As Starter Culture Media and Food Additives
2.6.2. Gastro-Intestinal Tract (GIT) Improvement
2.6.3. Anticancer Agents and Immune Potentiators
2.6.4. Removal of Cholesterol, Cardiovascular Disease Reduction, and Obesity Prevention
2.6.5. Vaginal Ecosystem Restoration
2.6.6. Production of Antimicrobials
2.6.7. Production of Environmentally Friendly Agricultural Feeds
2.6.8. Prebiotic Supplementation Provides Nutritional Value
3. Seed Waste as a Source of Prebiotics
3.1. Fruit Seeds
3.1.1. Date Seeds
3.1.2. Grape Seeds
3.1.3. Mango Seeds
3.1.4. Tamarind Seeds
3.2. Cereals
3.2.1. Brewer’s Spent Grains (BSG)
3.2.2. Coffee Spent Grounds (SCGs)
3.2.3. Buckwheat
3.3. Pulses and Legumes
3.3.1. Pulses
3.3.2. Legumes
3.4. Oil Seeds
Sesame Seeds
4. Insights on Prebiotics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, S.; Goyal, A. The current trends and future perspectives of prebiotics research: A review. 3 Biotech 2012, 2, 115–125. [Google Scholar] [CrossRef]
- Gibson, G.R.; Probert, H.M.; Loo, J.V.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Sabater-Molina, M.; Larque, E.; Torrella, F.; Zamora, S. Dietary fructooligosaccharides and potential benefits on health. J. Physiol. Biochem. 2009, 65, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wang, Y.; Li, J.; Lin, Q. Effect of prebiotic xylooligosaccharides on growth performances and digestive enzyme activities of allogynogenetic crucian carp (Carassius auratus gibelio). Fish. Physiol. Biochem. 2009, 35, 351–357. [Google Scholar] [CrossRef]
- Femia, A.P.; Salvadori, M.; Broekaert, W.F.; Francois, I.E.; Delcour, J.A.; Courtin, C.M.; Caderni, G. Arabinoxylan-oligosaccharides (AXOS) reduce preneoplastic lesions in the colon of rats treated with 1,2-dimethylhydrazine (DMH). Eur. J. Nutr. 2010, 49, 127–132. [Google Scholar] [CrossRef]
- Figueroa-González, I.; Quijano, G.; Ramirez, G.; Cruz-Guerrero, A. Probiotics and prebiotics—Perspectives and challenges. J. Sci. Food Agric. 2011, 91, 1341–1348. [Google Scholar] [CrossRef]
- Yeo, S.K.; Liong, M.T. Effect of prebiotics on viability and growth characteristics of probiotics in soymilk. J. Sci. Food Agric. 2010, 90, 267–275. [Google Scholar] [CrossRef]
- Vamanu, E.; Vamanu, A. The influence of prebiotics on bacteriocin synthesis using the strain Lactobacillus paracasei CMGB16. Afr. J. Microbiol. Res. 2010, 4, 534–537. [Google Scholar]
- Mandal, V.; Sen, S.K.; Mandal, N.C. Effect of prebiotics on bacteriocin production and cholesterol lowering activity of Pediococcus acidilactici LAB5. World J. Microbiol. Biotechnol. 2009, 25, 1837–1847. [Google Scholar] [CrossRef]
- Vaidya, R.H.; Sheth, M.K. Processing and storage of Indian cereal and cereal products alters its resistant starch content. J. Food Sci. Technol. 2011, 48, 622–627. [Google Scholar] [CrossRef]
- Ahmdifar, E.; Akrami, R.; Ghelichi, A.; Mohammadi Zarejabad, A. Effects of different dietary prebiotic inulin levels on blood serum enzymes, hematologic, and biochemical parameters of great sturgeon (Huso huso) juveniles. Comp. Clin. Pathol. 2010, 20, 447–451. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Scott, K.P.; Grimaldi, R.; Cunningham, M.; Sarbini, S.R.; Wijeyesekera, A.; Tang, M.L.; Lee, J.Y.; Yau, Y.F.; Ansell, J.; Theis, S. Developments in understanding and applying prebiotics in research and practice—An ISAPP conference paper. J. Appl. Microbiol. 2020, 128, 934–949. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Canani, R.B.; Fairweather-Tait, S.; Heinonen, M.; Korhonen, H.; La Vieille, S.; Marchelli, R.; Martin, A.; Naska, A.; Neuhäuser-Berthold, M.; et al. Scientific Opinion on the substantiation of a health claim related to “native chicory inulin” and maintenance of normal defecation by increasing stool frequency pursuant to Article 13.5 of Regulation (EC) No 1924/2006. EFSA J. 2015, 13, 3951. [Google Scholar] [CrossRef]
- Pineiro, M.; Asp, N.G.; Reid, G.; Macfarlane, S.; Morelli, L.; Brunser, O.; Tuohy, K. FAO Technical meeting on prebiotics. J. Clin. Gastroenterol. 2008, 42, S156–S159. [Google Scholar] [CrossRef]
- Kodagoda, K.; Marapana, R. Utilization of fruit processing by-products for industrial applications: A review. Int. J. Food Sci. Technol. 2017, 2, 24–30. [Google Scholar]
- Ordoudi, S.A.; Bakirtzi, C.; Tsimidou, M.Z. The potential of tree fruit stone and seed wastes in Greece as sources of bioactive ingredients. Recycling 2018, 3, 9. [Google Scholar] [CrossRef]
- Swaroopa, C.; Kashmira, L.; Vikas, G.; Rajan, W. Assessment of the prebiotic potential of seed coats from green gram (Vigna radiata) and black gram (Vigna mungo). J. Food Sci. Technol. 2022, 59, 583–588. [Google Scholar] [CrossRef]
- Shahidi, F. Nutraceuticals and functional foods: Whole versus processed foods. Trends Food Sci. Technol. 2009, 20, 376–387. [Google Scholar] [CrossRef]
- Arulnathan, N.; Murugan, M.; Balakrishnan, V. Proximate principles, Fibre fraction and Mineral content of Black gram husk (Vigna mungo). Int. J. Livest. Res. 2013, 3, 24–30. [Google Scholar]
- Palaniappan, A.; Balasubramaniam, V.G.; Antony, U. Prebiotic potential of xylooligosaccharides derived from finger millet seed coat. Food Biotechnol. 2017, 31, 264–280. [Google Scholar] [CrossRef]
- Saman, P.; Tuohy, K.M.; Vazquez, J.A.; Gibson, G.; Pandiella, S.S. In vitro evaluation of prebiotic properties derived from rice bran obtained by debranning technology. Int. J. Food Sci. Nutr. 2017, 68, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Olivo, G.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Prebiotic compounds from agro-industrial by-products. J. Food Biochem. 2019, 43, e12711. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef]
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G. Dietary prebiotics: Current status and new definition. Food Sci. Technol. Bull. Funct Foods 2010, 7, 1–19. [Google Scholar] [CrossRef]
- Slavin, J.L. Carbohydrates, dietary fiber, and resistant starch in white vegetables: Links to health outcomes. Adv. Nutr. 2013, 4, 351S–355S. [Google Scholar] [CrossRef]
- Howlett, J.F.; Betteridge, V.A.; Champ, M.; Craig, S.A.S.; Meheust, A.; Jones, J.M. The definition of dietary fiber—Discussions at the Ninth Vahouny Fiber Symposium: Building scientific agreement. Food Nutr. Res. 2010, 54, 5750. [Google Scholar] [CrossRef]
- Gullón, B.; Gómez, B.; Martínez-Sabajanes, M.; Yáñez, R.; Parajó, J.; Alonso, J. Pectic oligosaccharides: Manufacture and functional properties. Trends Food Sci. Technol. 2013, 30, 153–161. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J.; Michel, C. How to Manipulate the Microbiota: Prebiotics. Adv. Exp. Med. Biol. 2016, 902, 119–142. [Google Scholar] [CrossRef]
- Scott, K.P.; Martin, J.C.; Duncan, S.H.; Flint, H.J. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol. Ecol. 2014, 87, 30–40. [Google Scholar] [CrossRef]
- Fuentes-Zaragoza, E.; Sánchez-Zapata, E.; Sendra, E.; Sayas, E.; Navarro, C.; Fernández-López, J.; Pérez-Alvarez, J.A. Resistant starch as prebiotic: A review. Starch-Stärke 2011, 63, 406–415. [Google Scholar] [CrossRef]
- Johnson, C.R.; Thavarajah, D.; Combs, G.F.; Thavarajah, P. Lentil (Lens culinaris L.): A prebiotic-rich whole food legume. Food Res. Int. 2013, 51, 107–113. [Google Scholar] [CrossRef]
- Yoo, H.D.; Kim, D.; Paek, S.H.; Oh, S.E. Plant Cell Wall Polysaccharides as Potential Resources for the Development of Novel Prebiotics. Biomol. Ther. 2012, 20, 371–379. [Google Scholar] [CrossRef]
- Sorrenti, V.; Ali, S.; Mancin, L.; Davinelli, S.; Paoli, A.; Scapagnini, G. Cocoa Polyphenols and Gut Microbiota Interplay: Bioavailability, Prebiotic Effect, and Impact on Human Health. Nutrients 2020, 12, 1908. [Google Scholar] [CrossRef]
- Al-Thubiani, A.S.; Khan, M.S.A. The Prebiotic Properties of Date Palm (Phoenix dactylifera L.) Seeds in Stimulating Probiotic Lactobacillus. J. Pure Appl. Microbiol. 2017, 11, 1675–1686. [Google Scholar] [CrossRef]
- Havenaar, R.; Bonnin-Marol, S.; Van Dokkum, W.; Petitet, S.; Schaafsma, G. Inulin: Fermentation and microbial ecology in the intestinal tract. Food Rev. Int 1999, 15, 109–120. [Google Scholar] [CrossRef]
- Torres, D.P.M.; Gonçalves, M.d.P.F.; Teixeira, J.A.; Rodrigues, L.R. Galacto-Oligosaccharides: Production, Properties, Applications, and Significance as Prebiotics. Compr. Rev. Food Sci. Food Saf. 2010, 9, 438–454. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Steed, H.; Macfarlane, S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J. Appl. Microbiol. 2008, 104, 305–344. [Google Scholar] [CrossRef] [PubMed]
- Pranami, D.; Sharma, R.; Pathak, H. Lactulose: A prebiotic, laxative and detoxifying agent. Drugs Ther. Perspect. 2017, 33, 228–233. [Google Scholar] [CrossRef]
- Whelan, K. Mechanisms and effectiveness of prebiotics in modifying the gastrointestinal microbiota for the management of digestive disorders. Proc. Nutr. Soc. 2013, 72, 288–298. [Google Scholar] [CrossRef]
- Herfel, T.M.; Jacobi, S.K.; Lin, X.; Fellner, V.; Walker, D.C.; Jouni, Z.E.; Odle, J. Polydextrose enrichment of infant formula demonstrates prebiotic characteristics by altering intestinal microbiota, organic acid concentrations, and cytokine expression in suckling piglets. J. Nutr. 2011, 141, 2139–2145. [Google Scholar] [CrossRef] [Green Version]
- Ze, X.L.; Duncan, S.H.; Louis, P.; Flint, H.J. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 2012, 6, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P.E. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, J.B.; Ding, E.L.; Dixon, R.; Pasinetti, G.M.; Villarreal, F. The Science of Cocoa Flavanols: Bioavailability, Emerging Evidence, and Proposed Mechanisms. Adv. Nutr. 2014, 5, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Varzakas, T.; Kandylis, P.; Dimitrellou, D.; Salamoura, C.; Zakynthinos, G.; Proestos, C. Innovative and fortified food: Probiotics, prebiotics, GMOs, and superfood. In Preparation and Processing of Religious and Cultural Foods; Ali, E., Nizar, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 67–129. [Google Scholar]
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Yusof, R.M.; Hassan, F.A. Prebiotics as functional foods: A review. J. Funct. Foods 2013, 5, 1542–1553. [Google Scholar] [CrossRef]
- Panesar, P.S.; Kumari, S.; Panesar, R. Biotechnological approaches for the production of prebiotics and their potential applications. Crit. Rev. Biotechnol. 2013, 33, 345–364. [Google Scholar] [CrossRef]
- Holck, J.; Hjerno, K.; Lorentzen, A.; Vigsnaes, L.K.; Hemmingsen, L.; Licht, T.R.; Mikkelsen, J.D.; Meyer, A.S. Tailored enzymatic production of oligosaccharides from sugar beet pectin and evidence of differential effects of a single DP chain length difference on human faecal microbiota composition after in vitro fermentation. Process. Biochem. 2011, 46, 1039–1049. [Google Scholar] [CrossRef]
- Huerta, L.M.; Vera, C.; Guerrero, C.; Wilson, L.; Illanes, A. Synthesis of galacto-oligosaccharides at very high lactose concentrations with immobilized β-galactosidases from Aspergillus oryzae. Process. Biochem. 2011, 46, 245–252. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Jiang, Y.; Duan, Z.-Y.; Shao, W.-L.; Li, H.-Z. Expression and characterization of an α-glucosidase from Thermoanaerobacter ethanolicus JW200 with potential for industrial application. Biologia 2009, 64, 1053–1057. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Rezessy-Szabo, J.M.; Czukor, B.; Hoschke, A. Continuous production of oligofructose syrup from Jerusalem artichoke juice by immobilized endo-inulinase. Process. Biochem. 2011, 46, 298–303. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Xu, X.; Ning, Y.; Jin, Z.; Tian, Y. Biochemical characterization of an intracellular 6G-fructofuranosidase from Xanthophyllomyces dendrorhous and its use in production of neo-fructooligosaccharides (neo-FOSs). Bioresour. Technol. 2011, 102, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Nimpiboon, P.; Nakapong, S.; Pichyangkura, R.; Ito, K.; Pongsawasdi, P. Synthesis of a novel prebiotic trisaccharide by a type I α-glucosidase from B. licheniformis strain TH4-2. Process. Biochem. 2011, 46, 448–457. [Google Scholar] [CrossRef]
- De Paula, V.C.; Pinheiro, I.O.; Lopes, C.E.; Calazans, G.C. Microwave-assisted hydrolysis of Zymomonas mobilis levan envisaging oligofructan production. Bioresour. Technol. 2008, 99, 2466–2470. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, P.T.; Ramesh, M.N.; Prapulla, S.G. Recent trends in the microbial production, analysis and application of Fructooligosaccharides. Trends Food Sci. Technol. 2005, 16, 442–457. [Google Scholar] [CrossRef]
- Yun, J.W. Fructooligosaccharides—Occurrence, preparation, and application. Enzyme Microb. Technol. 1996, 19, 107–117. [Google Scholar] [CrossRef]
- Prapulla, S.G.; Subhaprada, V.; Karanth, N.G. Microbial production of oligosaccharides: A review. Adv. Appl. Microbiol. 2000, 47, 299–343. [Google Scholar] [CrossRef]
- Barreteau, H.; Delattre, C.; Michaud, P. Production of oligosaccharides as promising new food additive generation. Food Technol. Biotech. 2006, 44, 323–333. [Google Scholar]
- Sangeetha, P.T.; Ramesh, M.N.; Prapulla, S.G. Production of fructo-oligosaccharides by fructosyl transferase from Aspergillus oryzae CFR 202 and Aureobasidium pullulans CFR 77. Process. Biochem. 2004, 39, 753–758. [Google Scholar] [CrossRef]
- Caicedo, L.; Silva, E.; Sanchez, O. Semibatch and continuous fructooligosaccharides production by Aspergillus sp. N74 in a mechanically agitated airlift reactor. J. Chem. Technol. Biotechnol. 2009, 84, 650–656. [Google Scholar] [CrossRef]
- Chen, W.-c.; Liu, C.-h. Production of β-fructofuranosidase by Aspergillus japonicus. Enzyme Microb. Technol. 1996, 18, 153–160. [Google Scholar] [CrossRef]
- Mohkam, M.; Nezafat, N.; Berenjian, A.; Negahdaripour, M.; Behfar, A.; Ghasemi, Y. Role of bacillus genus in the production of value-added compounds. In Bacilli and Agrobiotechnology; Islam, M.T., Rahman, M., Pandey, P., Jha, C.K., Aeron, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–33. [Google Scholar]
- Prata, M.B.; Mussatto, S.I.; Rodrigues, L.R.; Teixeira, J.A. Fructooligosaccharide production by Penicillium expansum. Biotechnol. Lett. 2010, 32, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, A.E.; Piccoli, R.M.; da Silva, E.S.; de Andrade Rodrigues, M.F. Microbial production of fructosyltransferases for synthesis of pre-biotics. Biotechnol. Lett. 2008, 30, 1867–1877. [Google Scholar] [CrossRef]
- Jong, W.Y.; Seung, K.S. The production of high-content fructo-oligosaccharides from sucrose by the mixed-enzyme system of fructosyltransferase and glucose oxidase. Biotechnol. Lett. 1993, 15, 573–576. [Google Scholar] [CrossRef]
- Aachary, A.A.; Prapulla, S.G. Value addition to spent osmotic sugar solution (SOS) by enzymatic conversion to fructooligosaccharides (FOS), a low calorie prebiotic. Innov. Food Sci. Emerg. 2009, 10, 284–288. [Google Scholar] [CrossRef]
- Sheu, D.C.; Duan, K.J.; Cheng, C.Y.; Bi, J.L.; Chen, J.Y. Continuous production of high-content fructooligosaccharides by a complex cell system. Biotechnol. Prog. 2002, 18, 1282–1286. [Google Scholar] [CrossRef]
- Crittenden, R.G.; Playne, M.J. Purification of food-grade oligosaccharides using immobilised cells of Zymomonas mobilis. Appl. Microbiol. Biotechnol. 2002, 58, 297–302. [Google Scholar] [CrossRef]
- Goulas, A.; Tzortzis, G.; Gibson, G.R. Development of a process for the production and purification of α-and β-galactooligosaccharides from Bifidobacterium bifidum NCIMB 41171. Int. Dairy J. 2007, 17, 648–656. [Google Scholar] [CrossRef]
- Hernández, O.; Ruiz-Matute, A.I.; Olano, A.; Moreno, F.J.; Sanz, M.L. Comparison of fractionation techniques to obtain prebiotic galactooligosaccharides. Int. Dairy J. 2009, 19, 531–536. [Google Scholar] [CrossRef]
- Yoon, S.H.; Mukerjea, R.; Robyt, J.F. Specificity of yeast (Saccharomyces cerevisiae) in removing carbohydrates by fermentation. Carbohydr. Res. 2003, 338, 1127–1132. [Google Scholar] [CrossRef]
- Palcic, M.M. Biocatalytic synthesis of oligosaccharides. Curr. Opin. Biotechnol. 1999, 10, 616–624. [Google Scholar] [CrossRef]
- Weijers, C.A.; Franssen, M.C.; Visser, G.M. Glycosyltransferase-catalyzed synthesis of bioactive oligosaccharides. Biotechnol. Adv. 2008, 26, 436–456. [Google Scholar] [CrossRef]
- Koizumi, S.; Endo, T.; Tabata, K.; Ozaki, A. Large-scale production of UDP-galactose and globotriose by coupling metabolically engineered bacteria. Nat. Biotechnol. 1998, 16, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Albermann, C.; Piepersberg, W.; Wehmeier, U.F. Synthesis of the milk oligosaccharide 2′-fucosyllactose using recombinant bacterial enzymes. Carbohydr. Res. 2001, 334, 97–103. [Google Scholar] [CrossRef]
- Priem, B.; Gilbert, M.; Wakarchuk, W.W.; Heyraud, A.; Samain, E. A new fermentation process allows large-scale production of human milk oligosaccharides by metabolically engineered bacteria. Glycobiology 2002, 12, 235–240. [Google Scholar] [CrossRef]
- Monsan, P.; Paul, F. Enzymatic-Synthesis of Oligosaccharides. FEMS Microbiol. Rev. 1995, 16, 187–192. [Google Scholar] [CrossRef]
- Osman, A.; Tzortzis, G.; Rastall, R.A.; Charalampopoulos, D. BbgIV is an important Bifidobacterium β-galactosidase for the synthesis of prebiotic galactooligosaccharides at high temperatures. J. Agric. Food Chem. 2012, 60, 740–748. [Google Scholar] [CrossRef]
- Rabiu, B.A.; Jay, A.J.; Gibson, G.R.; Rastall, R.A. Synthesis and fermentation properties of novel galacto-oligosaccharides by beta-galactosidases from Bifidobacterium species. Appl. Environ. Microbiol. 2001, 67, 2526–2530. [Google Scholar] [CrossRef]
- Zarate, S.; Lopez-Leiva, M.H. Oligosaccharide Formation During Enzymatic Lactose Hydrolysis: A Literature Review. J. Food Prot. 1990, 53, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Prenosil, J.E.; Stuker, E.; Bourne, J.R. Formation of oligosaccharides during enzymatic lactose: Part I: State of art. Biotechnol. Bioeng. 1987, 30, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Nguyen, T.H.; Nguyen, H.A.; Nguyen, T.T.; Maischberger, T.; Kittl, R.; Haltrich, D. Characterization of a heterodimeric GH2 beta-galactosidase from Lactobacillus sakei Lb790 and formation of prebiotic galacto-oligosaccharides. J. Agric. Food Chem. 2011, 59, 3803–3811. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Nguyen, T.H.; Nguyen, T.T.; Maischberger, T.; Haltrich, D. beta-Galactosidase from Lactobacillus plantarum WCFS1: Biochemical characterization and formation of prebiotic galacto-oligosaccharides. Carbohydr. Res. 2010, 345, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Angioloni, A.; Collar, C. Physicochemical and nutritional properties of reduced-caloric density high-fibre breads. LWT 2011, 44, 747–758. [Google Scholar] [CrossRef]
- Fukuda, H.; Hama, S.; Tamalampudi, S.; Noda, H. Whole-cell biocatalysts for biodiesel fuel production. Trends Biotechnol. 2008, 26, 668–673. [Google Scholar] [CrossRef]
- Burton, S.G.; Cowan, D.A.; Woodley, J.M. The search for the ideal biocatalyst. Nat. Biotechnol. 2002, 20, 37–45. [Google Scholar] [CrossRef]
- Schmid, A.; Dordick, J.S.; Hauer, B.; Kiener, A.; Wubbolts, M.; Witholt, B. Industrial biocatalysis today and tomorrow. Nature 2001, 409, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Onishi, N.; Kira, I.; Yokozeki, K. Galacto-oligosaccharide production from lactose by Sirobasidium magnum CBS6803. Lett. Appl. Microbiol. 1996, 23, 253–256. [Google Scholar] [CrossRef]
- Onishi, N.; Tanaka, T. Purification and properties of a galacto-and gluco-oligosaccharide-producing β-glycosidase from Rhodotorula minuta IFO879. J. Ferment. Bioeng. 1996, 82, 439–443. [Google Scholar] [CrossRef]
- Onishi, N.; Tanaka, T. Purification and characterization of galacto-oligosaccharide-producing β-galactosidase from Sirobasidium magnum. Lett. Appl. Microbiol. 1997, 24, 82–86. [Google Scholar] [CrossRef]
- Onishi, N.; Yamashiro, A.; Yokozeki, K. Production of galacto-oligosaccharide from lactose by Sterigmatomyces elviae CBS8119. Appl. Environ. Microbiol. 1995, 61, 4022–4025. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Tzortzis, G.; Rastall, R.A.; Charalampopoulos, D. A comprehensive investigation of the synthesis of prebiotic galactooligosaccharides by whole cells of Bifidobacterium bifidum NCIMB 41171. J. Biotechnol. 2010, 150, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Ji, E.-S.; Park, N.-H.; Oh, D.-K. Galacto-oligosaccharide production by a thermostable recombinant β-galactosidase from Thermotoga maritima. World J. Microbiol. Biotechnol. 2005, 21, 759–764. [Google Scholar] [CrossRef]
- Terpe, K. Overview of bacterial expression systems for heterologous protein production: From molecular and biochemical fundamentals to commercial systems. Appl. Microbiol. Biotechnol. 2006, 72, 211–222. [Google Scholar] [CrossRef]
- Demain, A.L.; Vaishnav, P. Production of recombinant proteins by microbes and higher organisms. Biotechnol. Adv. 2009, 27, 297–306. [Google Scholar] [CrossRef]
- Porro, D.; Sauer, M.; Branduardi, P.; Mattanovich, D. Recombinant protein production in yeasts. Mol. Biotechnol. 2005, 31, 245–259. [Google Scholar] [CrossRef]
- Buckholz, R.G.; Gleeson, M.A. Yeast systems for the commercial production of heterologous proteins. Biotechnology 1991, 9, 1067–1072. [Google Scholar] [CrossRef]
- Bajury, D.M.; Nashri, S.M.; King Jie Hung, P.; Sarbini, S.R. Evaluation of potential prebiotics: A review. Food Rev. Int. 2018, 34, 639–664. [Google Scholar] [CrossRef]
- Dey, M. Toward a Personalized Approach in Prebiotics Research. Nutrients 2017, 9, 92. [Google Scholar] [CrossRef]
- Doo, E.-H.; Schwab, C.; Chassard, C.; Lacroix, C. Cumulative effect of yeast extract and fructooligosaccharide supplementation on composition and metabolic activity of elderly colonic microbiota in vitro. J. Funct. Foods 2019, 52, 43–53. [Google Scholar] [CrossRef]
- Garcia-Mazcorro, J.F.; Barcenas-Walls, J.R.; Suchodolski, J.S.; Steiner, J.M. Molecular assessment of the fecal microbiota in healthy cats and dogs before and during supplementation with fructo-oligosaccharides (FOS) and inulin using high-throughput 454-pyrosequencing. PeerJ 2017, 5, e3184. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D.; Holzapfel, W.; Nero, L.A. In Vitro Evaluation of Beneficial Properties of Bacteriocinogenic Lactobacillus plantarum ST8Sh. Probiotics Antimicrob. Proteins 2017, 9, 194–203. [Google Scholar] [CrossRef]
- Khangwal, I.; Shukla, P. Prospecting prebiotics, innovative evaluation methods, and their health applications: A review. 3 Biotech 2019, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, W.; Huang, G.; Zhang, W.; Ni, L. In vitro and in vivo evaluation of the prebiotic effect of raw and roasted almonds (Prunus amygdalus). J. Sci. Food Agric. 2016, 96, 1836–1843. [Google Scholar] [CrossRef] [PubMed]
- Takagi, R.; Sasaki, K.; Sasaki, D.; Fukuda, I.; Tanaka, K.; Yoshida, K.-I.; Kondo, A.; Osawa, R. A Single-Batch Fermentation System to Simulate Human Colonic Microbiota for High-Throughput Evaluation of Prebiotics. PLoS ONE 2016, 11, e0160533. [Google Scholar] [CrossRef]
- Evdokimova, S.A.; Karetkin, B.A.; Guseva, E.V.; Gordienko, M.G.; Khabibulina, N.V.; Panfilov, V.I.; Menshutina, N.V.; Gradova, N.B. A Study and Modeling of Bifidobacterium and Bacillus Coculture Continuous Fermentation under Distal Intestine Simulated Conditions. Microorganisms 2022, 10, 929. [Google Scholar] [CrossRef]
- Blanquet-Diot, S.; Denis, S.; Chalancon, S.; Chaira, F.; Cardot, J.-M.; Alric, M. Use of Artificial Digestive Systems to Investigate the Biopharmaceutical Factors Influencing the Survival of Probiotic Yeast During Gastrointestinal Transit in Humans. Pharm. Res. 2012, 29, 1444–1453. [Google Scholar] [CrossRef]
- Molly, K.; Vande Woestyne, M.; Verstraete, W. Development of a 5-step multi-chamber reactor as a simulation of the human intestinal microbial ecosystem. Appl. Microbiol. Biotechnol. 1993, 39, 254–258. [Google Scholar] [CrossRef]
- Samal, L.; Chaturvedi, V.B.; Saikumar, G.; Somvanshi, R.; Pattanaik, A.K. Prebiotic potential of Jerusalem artichoke (Helianthus tuberosus L.) in Wistar rats: Effects of levels of supplementation on hindgut fermentation, intestinal morphology, blood metabolites and immune response. J. Sci. Food Agric. 2015, 95, 1689–1696. [Google Scholar] [CrossRef]
- Oliveira, D.; Vidal, L.; Ares, G.; Walter, E.H.M.; Rosenthal, A.; Deliza, R. Sensory, microbiological and physicochemical screening of probiotic cultures for the development of non-fermented probiotic milk. LWT-Food Sci. Technol. 2017, 79, 234–241. [Google Scholar] [CrossRef]
- Inoue, H.; Takayama, K.; Takahara, C.; Tabuchi, N.; Okamura, N.; Narahara, N.; Kojima, E.; Date, Y.; Tsuruta, Y. Determination of Short-Chain Fatty Acids in Mouse Feces by High-Performance Liquid Chromatography Using 2-Nitrophenylhydrazine as a Labeling Reagent. Biol. Pharm. Bull. 2019, 42, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Zhao, X.; Xiao, L.; Zhao, H. Development and validation of a HILIC-ELSD method for simultaneous analysis of non-substituted and acetylated xylo-oligosaccharides. J. Pharm. Biomed. Anal. 2017, 139, 232–237. [Google Scholar] [CrossRef]
- Xiao, X.; Wen, J.-Y.; Wang, Y.-Y.; Bian, J.; Li, M.-F.; Peng, F.; Sun, R.-C. NMR and ESI–MS spectrometry characterization of autohydrolysis xylo-oligosaccharides separated by gel permeation chromatography. Carbohydr. Polym. 2018, 195, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Castaño, G.P.; Caro-Quintero, A.; Reyes, A.; Lizcano, F. Advances in Gut Microbiome Research, Opening New Strategies to Cope with a Western Lifestyle. Front. Genet. 2017, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, C.; García-Villanova, B.; Guerra-Hernández, E.; Verardo, V. Grape Seeds Proanthocyanidins: An Overview of In Vivo Bioactivity in Animal Models. Nutrients 2019, 11, 2435. [Google Scholar] [CrossRef] [PubMed]
- Reid, G. Probiotics and prebiotics—Progress and challenges. Int. Dairy J. 2008, 18, 969–975. [Google Scholar] [CrossRef]
- Foschi, C.; Laghi, L.; Parolin, C.; Giordani, B.; Compri, M.; Cevenini, R.; Marangoni, A.; Vitali, B. Novel approaches for the taxonomic and metabolic characterization of lactobacilli: Integration of 16S rRNA gene sequencing with MALDI-TOF MS and 1H-NMR. PLoS ONE 2017, 12, e0172483. [Google Scholar] [CrossRef]
- Chunchai, T.; Thunapong, W.; Yasom, S.; Wanchai, K.; Eaimworawuthikul, S.; Metzler, G.; Lungkaphin, A.; Pongchaidecha, A.; Sirilun, S.; Chaiyasut, C.; et al. Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J. Neuroinflamm. 2018, 15, 11. [Google Scholar] [CrossRef]
- Valdés-Varela, L.; Ruas-Madiedo, P.; Gueimonde, M. In vitro fermentation of different fructo-oligosaccharides by Bifidobacterium strains for the selection of synbiotic combinations. Int. J. Food Microbiol. 2017, 242, 19–23. [Google Scholar] [CrossRef]
- Aryantini, N.P.D.; Yamasaki, E.; Kurazono, H.; Sujaya, I.N.; Urashima, T.; Fukuda, K. In vitro safety assessments and antimicrobial activities of Lactobacillus rhamnosus strains isolated from a fermented mare’s milk. Anim. Sci. J. 2017, 88, 517–525. [Google Scholar] [CrossRef]
- Yadav, R.; Kumar, V.; Baweja, M.; Shukla, P. Gene editing and genetic engineering approaches for advanced probiotics: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1735–1746. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.P.D.; Perego, P.; De Oliveira, M.N.; Converti, A. Effect of inulin as a prebiotic to improve growth and counts of a probiotic cocktail in fermented skim milk. LWT-Food Sci. Technol. 2011, 44, 520–523. [Google Scholar] [CrossRef]
- Rodrigues, D.; Rocha-Santos, T.A.P.; Gomes, A.M.; Goodfellow, B.J.; Freitas, A.C. Lipolysis in probiotic and synbiotic cheese: The influence of probiotic bacteria, prebiotic compounds and ripening time on free fatty acid profiles. Food Chem. 2012, 131, 1414–1421. [Google Scholar] [CrossRef]
- MacDonald, A.; Cochrane, B.; Wopereis, H.; Loveridge, N. Specific prebiotics in a formula for infants with Phenylketonuria. Mol. Genet. Metab. 2011, 104, S55–S59. [Google Scholar] [CrossRef]
- Bodera, P. Influence of prebiotics on the human immune system (GALT). Recent Pat. Inflamm. Allergy Drug Discov. 2008, 2, 149–153. [Google Scholar] [CrossRef]
- Srinivasjois, R.; Rao, S.; Patole, S. Prebiotic supplementation of formula in preterm neonates: A systematic review and meta-analysis of randomised controlled trials. Clin. Nutr. 2009, 28, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Nishimukai, M.; Taguchi, H.; Senoura, T.; Hamada, S.; Matsui, H.; Yamamoto, T.; Wasaki, J.; Hara, H.; Ito, S. Prebiotic properties of epilactose. J. Dairy Sci. 2008, 91, 4518–4526. [Google Scholar] [CrossRef]
- Nurmi, J.T.; Puolakkainen, P.A.; Rautonen, N.E. Bifidobacterium Lactis sp. 420 up-regulates cyclooxygenase (Cox)-1 and down-regulates Cox-2 gene expression in a Caco-2 cell culture model. Nutr. Cancer 2005, 51, 83–92. [Google Scholar] [CrossRef]
- Delgado, G.T.C.; Tamashiro, W.M.D.S.C.; Marostica, M.R.; Moreno, Y.M.F.; Pastore, G.M. The putative effects of prebiotics as immunomodulatory agents. Food Res. Int. 2011, 44, 3167–3173. [Google Scholar] [CrossRef]
- Stam, J.; van Stuijvenberg, M.; Garssen, J.; Knipping, K.; Sauer, P.J. A mixture of three prebiotics does not affect vaccine specific antibody responses in healthy term infants in the first year of life. Vaccine 2011, 29, 7766–7772. [Google Scholar] [CrossRef]
- Harris, K.A.; Kris-Etherton, P.M. Effects of whole grains on coronary heart disease risk. Curr. Atheroscler. Rep. 2010, 12, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Costabile, A.; Martin-Pelaez, S.; Vitaglione, P.; Klinder, A.; Gibson, G.R.; Fogliano, V. Potential prebiotic activity of oligosaccharides obtained by enzymatic conversion of durum wheat insoluble dietary fibre into soluble dietary fibre. Nutr. Metab. Cardiovasc. 2009, 19, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.R.; Birkett, A.M.; Thomas, W.; Slavin, J.L. Effects of short-chain fructooligosaccharides on satiety responses in healthy men and women. Appetite 2011, 56, 128–134. [Google Scholar] [CrossRef]
- Cani, P.D.; Lecourt, E.; Dewulf, E.M.; Sohet, F.M.; Pachikian, B.D.; Naslain, D.; De Backer, F.; Neyrinck, A.M.; Delzenne, N.M. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am. J. Clin. Nutr. 2009, 90, 1236–1243. [Google Scholar] [CrossRef]
- Pliszczak, D.; Bourgeois, S.; Bordes, C.; Valour, J.P.; Mazoyer, M.A.; Orecchioni, A.M.; Nakache, E.; Lanteri, P. Improvement of an encapsulation process for the preparation of pro- and prebiotics-loaded bioadhesive microparticles by using experimental design. Eur. J. Pharm. Sci. 2011, 44, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Ibuki, M.; Kovacs-Nolan, J.; Fukui, K.; Kanatani, H.; Mine, Y. beta 1-4 mannobiose enhances Salmonella-killing activity and activates innate immune responses in chicken macrophages. Vet. Immunol. Immunopathol. 2011, 139, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, A.J.; Jones, C.M.; Elizondo-Salazar, J.A.; Terrill, S.J. Effects of a prebiotic supplement on health of neonatal dairy calves. Livest. Sci. 2009, 125, 149–154. [Google Scholar] [CrossRef]
- Baines, D.; Erb, S.; Lowe, R.; Turkington, K.; Sabau, E.; Kuldau, G.; Juba, J.; Masson, L.; Mazza, A.; Roberts, R. A prebiotic, Celmanax, decreases Escherichia coli O157:H7 colonization of bovine cells and feed-associated cytotoxicity in vitro. BMC Res. Notes 2011, 4, 110. [Google Scholar] [CrossRef]
- De Caire, G.Z.; Parada, J.L.; Zaccaro, M.C.; de Cano, M.M.S. Effect of Spirulina platensis biomass on the growth of lactic acid bacteria in milk. World J. Microbiol. Biotechnol. 2000, 16, 563–565. [Google Scholar] [CrossRef]
- Damen, B.; Pollet, A.; Dornez, E.; Broekaert, W.F.; Van Haesendonck, I.; Trogh, I.; Arnaut, F.; Delcour, J.A.; Courtin, C.M. Xylanase-mediated in situ production of arabinoxylan oligosaccharides with prebiotic potential in whole meal breads and breads enriched with arabinoxylan rich materials. Food Chem. 2012, 131, 111–118. [Google Scholar] [CrossRef]
- Vergara, C.M.D.C.; Honorato, T.L.; Maia, G.A.; Rodrigues, S. Prebiotic effect of fermented cashew apple (Anacardium occidentale L.) juice. LWT-Food Sci. Technol. 2010, 43, 141–145. [Google Scholar] [CrossRef]
- Uchida, M.; Mogami, O.; Matsueda, K. Characteristic of milk whey culture with Propionibacterium freudenreichii ET-3 and its application to the inflammatory bowel disease therapy. Inflammopharmacology 2007, 15, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Di Criscio, T.; Fratianni, A.; Mignogna, R.; Cinquanta, L.; Coppola, R.; Sorrentino, E.; Panfili, G. Production of functional probiotic, prebiotic, and synbiotic ice creams. J. Dairy Sci. 2010, 93, 4555–4564. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Nueno Palop, C.; Tuohy, K.; Gibson, G.R.; Bennett, R.N.; Waldron, K.W.; Bisignano, G.; Narbad, A.; Faulds, C.B. In vitro evaluation of the prebiotic activity of a pectic oligosaccharide-rich extract enzymatically derived from bergamot peel. Appl. Microbiol. Biotechnol. 2007, 73, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Tenorio, M.D.; Espinosa-Martos, I.; Prestamo, G.; Ruperez, P. Soybean whey enhance mineral balance and caecal fermentation in rats. Eur. J. Nutr. 2010, 49, 155–163. [Google Scholar] [CrossRef]
- Madhukumar, M.S.; Muralikrishna, G. Structural characterisation and determination of prebiotic activity of purified xylo-oligosaccharides obtained from Bengal gram husk (Cicer arietinum L.) and wheat bran (Triticum aestivum). Food Chem. 2010, 118, 215–223. [Google Scholar] [CrossRef]
- Wang, S.-L.; Liang, Y.-C.; Liang, T.-W. Purification and characterization of a novel alkali-stable α-amylase from Chryseobacterium taeanense TKU001, and application in antioxidant and prebiotic. Process. Biochem. 2011, 46, 745–750. [Google Scholar] [CrossRef]
- Gullon, P.; Gonzalez-Munoz, M.J.; Parajo, J.C. Manufacture and prebiotic potential of oligosaccharides derived from industrial solid wastes. Bioresour. Technol. 2011, 102, 6112–6119. [Google Scholar] [CrossRef]
- Smith, S.C.; Choy, R.; Johnson, S.K.; Hall, R.S.; Wildeboer-Veloo, A.C.M.; Welling, G.W. Lupin kernel fiber consumption modifies fecal microbiota in healthy men as determined by rRNA gene fluorescent in situ hybridization. Eur. J. Nutr. 2006, 45, 335–341. [Google Scholar] [CrossRef]
- Platat, C.; Habib, H.M.; Hashim, I.B.; Kamal, H.; AlMaqbali, F.; Souka, U.; Ibrahim, W.H. Production of functional pita bread using date seed powder. J. Food Sci. Technol. 2015, 52, 6375–6384. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.; Ali, H. Chemical and technological properties of improved snacks from oat and date seeds composite flour. Am. J. Food Technol. 2017, 12, 201–208. [Google Scholar] [CrossRef]
- Nancib, N.; Nancib, A.; Boudrant, J. Use of waste date products in the fermentative formation of baker’s yeast biomass by Saccharomyces cerevisiae. Bioresour. Technol. 1997, 60, 67–71. [Google Scholar] [CrossRef]
- Engelbrecht, A.M.; Mattheyse, M.; Ellis, B.; Loos, B.; Thomas, M.; Smith, R.; Peters, S.; Smith, C.; Myburgh, K. Proanthocyanidin from grape seeds inactivates the PI3-kinase/PKB pathway and induces apoptosis in a colon cancer cell line. Cancer Lett. 2007, 258, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Oda, E.; Yamashita, T.; Naemura, A.; Ijiri, Y.; Yamakoshi, J.; Yamamoto, J. Anti-thrombotic effect of proanthocyanidin, a purified ingredient of grape seed. Thromb. Res. 2005, 115, 115–121. [Google Scholar] [CrossRef]
- Maisuthisakul, P.; Gordon, M.H. Antioxidant and tyrosinase inhibitory activity of mango seed kernel by product. Food Chem. 2009, 117, 332–341. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2022, 14, 137. [Google Scholar] [CrossRef]
- Jahurul, M.H.A.; Zaidul, I.S.M.; Ghafoor, K.; Al-Juhaimi, F.Y.; Nyam, K.-L.; Norulaini, N.A.N.; Sahena, F.; Mohd Omar, A.K. Mango (Mangifera indica L.) by-products and their valuable components: A review. Food Chem. 2015, 183, 173–180. [Google Scholar] [CrossRef]
- Utami, M.M.D.; Dewi, A.C.; Ningsih, N. Potential of tamarind seeds (Tamarindus indica L.) as prebiotics on the growth of lactic acid bacteria. IOP Conf. Ser. Earth Environ. Sci. 2022, 980, 012017. [Google Scholar] [CrossRef]
- Joseph, J.; Kanchalochana, S.; Rajalakshmi, G.; Hari, V.; Durai, R. Tamarind seed polysaccharide: A promising natural excipient for pharmaceuticals. Int. J. Green Pharm. 2012, 6, 270–278. [Google Scholar] [CrossRef]
- Harris, S.; Powers, S.; Monteagudo-Mera, A.; Kosik, O.; Lovegrove, A.; Shewry, P.; Charalampopoulos, D. Determination of the prebiotic activity of wheat arabinogalactan peptide (AGP) using batch culture fermentation. Eur. J. Nutr. 2020, 59, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Brunschwiler, C.; Heine, D.; Kappeler, S.; Conde-Petit, B.; Nystrom, L. Direct measurement of rice bran lipase activity for inactivation kinetics and storage stability prediction. J. Cereal Sci. 2013, 58, 272–277. [Google Scholar] [CrossRef]
- Gomez, B.; Miguez, B.; Veiga, A.; Parajo, J.C.; Alonso, J.L. Production, Purification, and in Vitro Evaluation of the Prebiotic Potential of Arabinoxylooligosaccharides from Brewer’s Spent Grain. J. Agric. Food Chem. 2015, 63, 8429–8438. [Google Scholar] [CrossRef]
- Santos, M.; Jimemez, J.J.; Bartolome, B.; Gomez-Cordoves, C.; del Nozal, M.J. Variability of brewer’s spent grain within a brewery. Food Chem. 2003, 80, 17–21. [Google Scholar] [CrossRef]
- Khalid, S.; ur Rehman, A.; Mansha, M.A.; Mukhtar, A.; Rehman, M. Exploring phyto-biochemical and nutracetical profiling of buckwheat: A. J. Agric. Environ. Food Secur. 2020, 2, 49–89. [Google Scholar]
- Nguyen, Q.A.; Cho, E.J.; Lee, D.-S.; Bae, H.-J. Development of an advanced integrative process to create valuable biosugars including manno-oligosaccharides and mannose from spent coffee grounds. Bioresour. Technol. 2019, 272, 209–216. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Pastoriza, S.; Fernández-Arteaga, A.; Luzón, G.; Jiménez-Hernández, N.; D’Auria, G.; Francino, M.P.; Rufián-Henares, J.Á. Spent Coffee Grounds Extract, Rich in Mannooligosaccharides, Promotes a Healthier Gut Microbial Community in a Dose-Dependent Manner. J. Agric. Food Chem. 2019, 67, 2500–2509. [Google Scholar] [CrossRef]
- Amarowicz, R. Legume Seeds as an Important Component of Human Diet. Foods 2020, 9, 1812. [Google Scholar] [CrossRef] [PubMed]
- Cichońska, P.; Ziarno, M. Legumes and Legume-Based Beverages Fermented with Lactic Acid Bacteria as a Potential Carrier of Probiotics and Prebiotics. Microorganisms 2022, 10, 91. [Google Scholar] [CrossRef]
- Aziz, A.H.; Aboeleinen, K.A. Use of sesame and probiotic lactobacilli in making nutraceutical fermented dairy products. J. Food Dairy Sci. 2010, 1, 555–565. [Google Scholar] [CrossRef]
- Michailidis, D.; Angelis, A.; Aligiannis, N.; Mitakou, S.; Skaltsounis, L. Recovery of Sesamin, Sesamolin, and Minor Lignans From Sesame Oil Using Solid Support-Free Liquid-Liquid Extraction and Chromatography Techniques and Evaluation of Their Enzymatic Inhibition Properties. Front. Pharm. 2019, 10, 723. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Food and Agriculture Organization of the United Nations; FAO: Rome, Italy, 2016. [Google Scholar]
- Besbes, S.; Blecker, C.; Deroanne, C.; Drira, N.E.; Attia, H. Date seeds: Chemical composition and characteristic profiles of the lipid fraction. Food Chem. 2004, 84, 577–584. [Google Scholar] [CrossRef]
- Al-Shahib, W.; Marshall, R.J. The fruit of the date palm: Its possible use as the best food for the future? Int. J. Food Sci. Nutr. 2003, 54, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Al-Farsi, M.A.; Lee, C.Y. Nutritional and Functional Properties of Dates: A Review. Crit. Rev. Food Sci. Nutr. 2008, 48, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Al-Farsi, M.; Alasalvar, C.; Al-Abid, M.; Al-Shoaily, K.; Al-Amry, M.; Al-Rawahy, F. Compositional and functional characteristics of dates, syrups, and their by-products. Food Chem. 2007, 104, 943–947. [Google Scholar] [CrossRef]
- Hamada, J.S.; Hashim, I.B.; Sharif, F.A. Preliminary analysis and potential uses of date pits in foods. Food Chem. 2002, 76, 135–137. [Google Scholar] [CrossRef]
- Nehdi, I.; Omri, S.; Khalil, M.I.; Al-Resayes, S.I. Characteristics and chemical composition of date palm (Phoenix canariensis) seeds and seed oil. Ind. Crops Prod. 2010, 32, 360–365. [Google Scholar] [CrossRef]
- Ghnimi, S.; Umer, S.; Karim, A.; Kamal-Eldin, A. Date fruit (Phoenix dactylifera L.): An underutilized food seeking industrial valorization. NFS J. 2017, 6, 1–10. [Google Scholar] [CrossRef]
- Thouri, A.; Chahdoura, H.; El Arem, A.; Omri Hichri, A.; Ben Hassin, R.; Achour, L. Effect of solvents extraction on phytochemical components and biological activities of Tunisian date seeds (var. Korkobbi and Arechti). BMC Complement. Altern. Med. 2017, 17, 248. [Google Scholar] [CrossRef]
- Vandepopuliere, J.M.; Alyousef, Y.; Lyons, J.J. Dates and Date Pits as Ingredients in Broiler Starting and Coturnix Quail Breeder Diets. Poult. Sci 1995, 74, 1134–1142. [Google Scholar] [CrossRef]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadenti, A. Grape seed oil compounds: Biological and chemical actions for health. Nutr. Metab. Insights 2016, 9, S32910. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in grape seeds—Biochemistry and functionality. J. Med. Food 2003, 6, 291–299. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, J.; Deng, Z.; Pan, X.; Xie, X.; Peng, C. Effective utilization of food wastes: Bioactivity of grape seed extraction and its application in food industry. J. Funct. Foods 2020, 73, 104113. [Google Scholar] [CrossRef]
- Karaman, S.; Karasu, S.; Tornuk, F.; Toker, O.S.; Gecgel, U.; Sagdic, O.; Ozcan, N.; Gul, O. Recovery potential of cold press byproducts obtained from the edible oil industry: Physicochemical, bioactive, and antimicrobial properties. J. Agric. Food Chem. 2015, 63, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Özvural, E.B.; Vural, H. Grape seed flour is a viable ingredient to improve the nutritional profile and reduce lipid oxidation of frankfurters. Meat Sci. 2011, 88, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Akca, S.; Akpinar, A. The Effects of Grape, pomegranate, Sesame Seed Powder and Their Oils on Probiotic Ice Cream: Total phenolic contents, antioxidant activity and probiotic viability. Food Biosci. 2021, 42, 101203. [Google Scholar] [CrossRef]
- Varzakas, T.; Zakynthinos, G.; Verpoort, F. Plant Food Residues as a Source of Nutraceuticals and Functional Foods. Foods 2016, 5, 88. [Google Scholar] [CrossRef]
- Leanpolchareanchai, J.; Padois, K.; Falson, F.; Bavovada, R.; Pithayanukul, P. Microemulsion System for Topical Delivery of Thai Mango Seed Kernel Extract: Development, Physicochemical Characterisation and Ex Vivo Skin Permeation Studies. Molecules 2014, 19, 17107–17129. [Google Scholar] [CrossRef]
- Torres-León, C.; Rojas, R.; Contreras-Esquivel, J.C.; Serna-Cock, L.; Belmares-Cerda, R.E.; Aguilar, C.N. Mango seed: Functional and nutritional properties. Trends Food Sci. Technol. 2016, 55, 109–117. [Google Scholar] [CrossRef]
- Fowomola, M. Some nutrients and antinutrients contents of mango (Magnifera indica) seed. Afr. J. Food Sci. 2010, 4, 472–476. [Google Scholar] [CrossRef]
- Saito, K.; Kohno, M.; Yoshizaki, F.; Niwano, Y. Extensive Screening for Edible Herbal Extracts with Potent Scavenging Activity against Superoxide Anions. Plant. Foods Hum. Nutr. 2008, 63, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Bishnoi, R.S.; Kumar, M.; Fenin, V.; Jain, C.P. Applications of tamarind seeds polysaccharide-based copolymers in controlled drug delivery: An overview. Asian J. Pharm. Pharmacol. 2018, 4, 23–30. [Google Scholar] [CrossRef]
- Abiraami, R.; Palanidorai, R.; Pugazhenthi, T.; Prabu, M. Physicochemical and sensory evaluation of functional yoghurt enriched with tamarind seed kernel powder. J. Pharmacogn. Phytochem. 2021, 10, 1852–1855. [Google Scholar]
- Sharma, P.; Gaur, V.K.; Kim, S.H.; Pandey, A. Microbial strategies for bio-transforming food waste into resources. Bioresour. Technol. 2020, 299, 122580. [Google Scholar] [CrossRef] [PubMed]
- Anal, A.K. Food Processing By-Products and their Utilization: Introduction. In Food Processing By-Products and Their Utilization; Anil, K.A., Ed.; Wiley: Hoboken, NJ, USA, 2017; Volume 1, pp. 1–10. [Google Scholar]
- Jones, J.M. CODEX-aligned dietary fiber definitions help to bridge the ‘fiber gap’. Nutr. J. 2014, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kolida, S.; Gibson, G.R. Prebiotic capacity of inulin-type fructans. J. Nutr. 2007, 137, 2503S–2506S. [Google Scholar] [CrossRef] [Green Version]
- Broekaert, W.F.; Courtin, C.M.; Verbeke, K.; Van de Wiele, T.; Verstraete, W.; Delcour, J.A. Prebiotic and other health-related effects of cereal-derived arabinoxylans, arabinoxylan-oligosaccharides, and xylooligosaccharides. Crit. Rev. Food Sci. Nutr. 2011, 51, 178–194. [Google Scholar] [CrossRef]
- Carlson, J.L.; Erickson, J.M.; Hess, J.M.; Gould, T.J.; Slavin, J.L. Prebiotic Dietary Fiber and Gut Health: Comparing the in Vitro Fermentations of Beta-Glucan, Inulin and Xylooligosaccharide. Nutrients 2017, 9, 1361. [Google Scholar] [CrossRef]
- Hughes, S.A.; Shewry, P.R.; Gibson, G.R.; McCleary, B.V.; Rastall, R.A. In vitro fermentation of oat and barley derived beta-glucans by human faecal microbiota. FEMS Microbiol. Ecol. 2008, 64, 482–493. [Google Scholar] [CrossRef]
- Vergara, B.S. Rice plant growth and development. In Rice; Springer: Berlin/Heidelberg, Germany, 1991; pp. 13–22. [Google Scholar]
- Hettiarachchy, N.S.; Ju, Z.Y.; Siebenmorgen, T.; Sharp, R.N. Rice: Production, processing, and utilization. In Handbook of Cereal Science and Technology; Kulp, K., Ponte, J.G., Eds.; CRC Press: Boca Raton, FL, USA, 2000; Volume 1, pp. 203–222. [Google Scholar]
- Alvarez, E.E.; Sanchez, P.G. Dietary fibre. Nutr. Hosp. 2006, 21, 60–71. [Google Scholar]
- Beards, E.; Tuohy, K.; Gibson, G. Bacterial, SCFA and gas profiles of a range of food ingredients following in vitro fermentation by human colonic microbiota. Anaerobe 2010, 16, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Macfarlane, G.T. The control and consequences of bacterial fermentation in the human colon. J. Appl. Bacteriol. 1991, 70, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Hallfrisch, J.; FACN; Behall, K.M. Mechanisms of the effects of grains on insulin and glucose responses. J. Am. Coll. Nutr. 2000, 19, 320S–325S. [Google Scholar] [CrossRef] [PubMed]
- Kahlon, T.S.; Chow, F.I.; Sayre, R.N. Cholesterol-Lowering Properties of Rice Bran. Cereal Food World 1994, 39, 99–103. [Google Scholar]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Treimo, J.; Westereng, B.; Horn, S.J.; Forssell, P.; Robertson, J.A.; Faulds, C.B.; Waldron, K.W.; Buchert, J.; Eijsink, V.G. Enzymatic solubilization of brewers’ spent grain by combined action of carbohydrases and peptidases. J. Agric. Food Chem. 2009, 57, 3316–3324. [Google Scholar] [CrossRef]
- Robertson, J.A.; I’Anson, K.J.A.; Treimo, J.; Faulds, C.B.; Brocklehurst, T.F.; Eijsink, V.G.H.; Waldron, K.W. Profiling brewers’ spent grain for composition and microbial ecology at the site of production. LWT-Food Sci. Technol. 2010, 43, 890–896. [Google Scholar] [CrossRef]
- Kabel, M.A.; Schols, H.A.; Voragen, A.G.J. Complex xylo-oligosaccharides identified from hydrothermally treated Eucalyptus wood and brewery’s spent grain. Carbohydr. Polym. 2002, 50, 191–200. [Google Scholar] [CrossRef]
- Pabbathi, N.P.P.; Velidandi, A.; Pogula, S.; Gandam, P.K.; Baadhe, R.R.; Sharma, M.; Sirohi, R.; Thakur, V.K.; Gupta, V.K. Brewer’s spent grains-based biorefineries: A critical review. Fuel 2022, 317, 123435. [Google Scholar] [CrossRef]
- Ioannidou, S.M.; Pateraki, C.; Ladakis, D.; Papapostolou, H.; Tsakona, M.; Vlysidis, A.; Kookos, I.K.; Koutinas, A. Sustainable production of bio-based chemicals and polymers via integrated biomass refining and bioprocessing in a circular bioeconomy context. Bioresour. Technol. 2020, 307, 123093. [Google Scholar] [CrossRef]
- Ladakis, D.; Papapostolou, H.; Vlysidis, A.; Koutinas, A. Inventory of food processing side streams in European Union and prospects for biorefinery development. In Food Industry Wastes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 181–199. [Google Scholar]
- Mussatto, S.I. Brewer’s spent grain: A valuable feedstock for industrial applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef]
- Yu, D.; Sun, Y.; Wang, W.; O’Keefe, S.F.; Neilson, A.P.; Feng, H.; Wang, Z.; Huang, H. Recovery of protein hydrolysates from brewer’s spent grain using enzyme and ultrasonication. Int. J. Food Sci. Technol. 2020, 55, 357–368. [Google Scholar] [CrossRef]
- Waters, D.M.; Jacob, F.; Titze, J.; Arendt, E.K.; Zannini, E. Fibre, protein and mineral fortification of wheat bread through milled and fermented brewer’s spent grain enrichment. Eur. Food Res. Technol. 2012, 235, 767–778. [Google Scholar] [CrossRef]
- Jaeger, A.; Zannini, E.; Sahin, A.W.; Arendt, E.K. Barley Protein Properties, Extraction and Applications, with a Focus on Brewers’ Spent Grain Protein. Foods 2021, 10, 1389. [Google Scholar] [CrossRef]
- Brennan, C.S.; Cleary, L.J. The potential use of cereal (1→ 3, 1→ 4)-β-d-glucans as functional food ingredients. J. Cereal Sci. 2005, 42, 1–13. [Google Scholar] [CrossRef]
- Fastnaught, C.E. Barley fiber. In Handbook of Dietary Fiber; Cho, S.S., Dreher, M.L., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 519–542. [Google Scholar]
- Salazar-López, N.J.; López-Rodríguez, C.V.; Hernández-Montoya, D.A.; Campos-Vega, R. Health Benefits of Spent Coffee Grounds. In Food Wastes and By-Products; Wiley: Hoboken, NJ, USA, 2020; pp. 327–351. [Google Scholar]
- Desai, N.M.; Martha, G.S.; Harohally, N.V.; Murthy, P.S. Non-digestible oligosaccharides of green coffee spent and their prebiotic efficiency. LWT 2020, 118, 108784. [Google Scholar] [CrossRef]
- Wongsiridetchai, C.; Jonjaroen, V.; Sawangwan, T.; Charoenrat, T.; Chantorn, S. Evaluation of prebiotic mannooligosaccharides obtained from spent coffee grounds for nutraceutical application. LWT 2021, 148, 111717. [Google Scholar] [CrossRef]
- Wijngaard, H.; Arendt, E.K. Buckwheat. Cereal Chem. 2006, 83, 391–401. [Google Scholar] [CrossRef]
- Krkošková, B.; Mrazova, Z. Prophylactic components of buckwheat. Food Res. Int. 2005, 38, 561–568. [Google Scholar] [CrossRef]
- Pomeranz, Y.; Lorenz, K. Buckwheat: Structure, composition, and utilization. Crit. Rev. Food Sci. Nutr. 1983, 19, 213–258. [Google Scholar] [CrossRef]
- Dorrell, D. Fatty acid composition of buckwheat seed. J. Am. Oil Chem. Soc. 1971, 48, 693–696. [Google Scholar] [CrossRef]
- Gulpinar, A.R.; Orhan, I.E.; Kan, A.; Senol, F.S.; Celik, S.A.; Kartal, M. Estimation of in vitro neuroprotective properties and quantification of rutin and fatty acids in buckwheat (Fagopyrum esculentum Moench) cultivated in Turkey. Food Res. Int. 2012, 46, 536–543. [Google Scholar] [CrossRef]
- Préstamo, G.; Rupérez, P.; Espinosa-Martos, I.; Villanueva, M.J.; Lasunción, M.A. The effects of okara on rat growth, cecal fermentation, and serum lipids. Eur. Food Res. Technol. 2007, 225, 925–928. [Google Scholar] [CrossRef]
- Parate, V.R.; Talib, M.I. Characterization of tur dal (Cajanus cajan) husk carbon and its kinetics and isotherm study for removing Cu (II) ions. IOSR J. Environ. Sci. 2015, 9, 27–41. [Google Scholar] [CrossRef]
- Mualikrishna, G.; Tharanathan, R.N. Characterization of pectic polysaccharides from pulse husks. Food Chem. 1994, 50, 87–89. [Google Scholar] [CrossRef]
- Bose, D.; Shams-Ud-Din, M. The effect of chickpea (Cicer arietinim) husk on the properties of cracker biscuits. J. Bangladesh Agric. Univ. 2010, 8, 147–152. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Oomah, B.D. Minor components of pulses and their potential impact on human health. Food Res. Int. 2010, 43, 461–482. [Google Scholar] [CrossRef]
- Tunde-Akintunde, T.Y.; Akintunde, B.O. Some Physical Properties of Sesame Seed. Biosyst. Eng. 2004, 88, 127–129. [Google Scholar] [CrossRef]
- Fukuda, Y.; Osawa, T.; Namiki, M.; Ozaki, T. Studies on Antioxidative Substances in Sesame Seed. Agric. Biol. Chem. 1985, 49, 301–306. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Appelqvist, L.Å.; Yousif, G. Lignan analysis in seed oils from four Sesamum species: Comparison of different chromatographic methods. J. Am. Oil Chem. Soc. 1994, 71, 141–147. [Google Scholar] [CrossRef]
- Moazzami, A.A.; Haese, S.L.; Kamal-Eldin, A. Lignan contents in sesame seeds and products. Eur. J. Lipid Sci. Technol. 2007, 109, 1022–1027. [Google Scholar] [CrossRef]
- Moazzami, A.A.; Kamal-Eldin, A. Sesame seed is a rich source of dietary lignans. J. Am. Oil Chem. Soc. 2006, 83, 719. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.G.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; Francois, P.; de Vos, W.M.; et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef] [PubMed]
- Bockmuhl, D. Prebiotic cosmetics: An alternative to antibacterial products. IFSSC Mag 2006, 9, 1–5. [Google Scholar] [CrossRef]
- Grüber, C.; Van Stuijvenberg, M.; Mosca, F.; Moro, G.; Chirico, G.; Braegger, C.P.; Riedler, J.; Boehm, G.; Wahn, U.; Group, M.W. Reduced occurrence of early atopic dermatitis because of immunoactive prebiotics among low-atopy-risk infants. J. Allergy Clin. Immunol. 2010, 126, 791–797. [Google Scholar] [CrossRef] [PubMed]

| Types | Examples | Functions | Reference |
|---|---|---|---|
| Fructans | Inulin FOS | Selective stimulation of LAB | [31,32] |
| Galactooligosaccharide | Lactose-based GOS Galactose-based GOS Raffinose family Oligosaccharide | Stimulation of Bifidobacteria and Lactobacilli | [24,27,31] |
| Starch- and Glucose-derived Oligosaccharides | Resistant starch Polydextrose | Butyrate production Stimulation of Bifidobacteria | [33,34] |
| Pectin Oligosaccharide | [24,35] | ||
| Miscellaneous | Cocoa-derived Polyphenols | Modulation of microbial diversity. Cell membrane integrity | [36] |
| Glycoproteins | [37] | ||
| Glycolipids | [37] |
| Assessment Type | Techniques | Phases | Types of System | Type of Models | Conditions of Assessment | Samples to Be Evaluated | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (h) | pH | Temp (°C) | Detector | Salts | Enzymes | |||||||
| Digestion of prebiotics | in vitro | Oral | 5 | 7 | 37 | NaCl | Alpha-amylase | [104] | ||||
| Gastric | 2 | 2.5 | 37 | Pepsin, Gastric Lipase | [105] | |||||||
| Intestinal | 2 | 6 | 37 | Chymotrypsin, Trypsin, Colipase and Pancreatic Lipase | [106] | |||||||
| In vivo | Animals | 672 | 18–29 | Fecal sample | [106,107] | |||||||
| Human clinical | 336 | Undigested prebiotics | [106] | |||||||||
| Fermentation of prebiotics | In vitro | Batch culture | 24 | 6.5 | 37 | Na2HPO4, NaH2PO4 | Fecal sample | [22,108] | ||||
| Continuous culture | Single staged, multi-staged | 16 | 6.8 | 37 | Fecal sample | [109] | ||||||
| Artificial gut | TIM | 20 | 6 | 37 | Fecal sample | [110] | ||||||
| SHIME | 24–72 | 6.5 7.0 7.5 | 37 | Na2Co3 | Fecal sample | [111] | ||||||
| In vivo | Animals | 672 | 22–29 | Fecal sample | [112,113] | |||||||
| Human Clinical | Fecal sample or Breathed air | [113] | ||||||||||
| Analysis of Prebiotics | SCFAs Analysis | HPLC | 1 | 2.5 | 40 | UV-Vis | [106,114] | |||||
| GC | 0.5 | 250 | FID | [106] | ||||||||
| HILIC | 0.5 | 35 | ELSD | [106,115] | ||||||||
| LC–ESI–MS. | 0.5 | 35 | DAD | [115,116] | ||||||||
| Gut Microbiota Enumeration | Culturing | [117] | ||||||||||
| Molecular Methods | RT-PCR | [117] | ||||||||||
| qPCR | [118] | |||||||||||
| DGGE | [106] | |||||||||||
| T-RFLP | [119] | |||||||||||
| DNA Microarray | [106] | |||||||||||
| 16s rRNA | [106,120] | |||||||||||
| Pyrosequencing | [106,120] | |||||||||||
| Health Benefits | Functionality | Prebiotic Type | Industries | Product | Reference |
|---|---|---|---|---|---|
| Growth medium for starter culture | Reduction in generation time Reduction of linoleic acid content | Inulin FOS | Dairy | Cheese | [125,126] |
| GIT improvement | Constipation reduction Colitis prevention GIT microflora improvement | FOS Maltodextrin Mannooligosaccharide Arabinogalactans | Food Pharmaceutical | Infant formula | [1,3,7] |
| Anticancer and immune potentiator | Carcinogen reduction Primary bile acids to secondary bile acids conversion Promotion of T Helper 1 and regulatory T cells Regulation of IgE-mediated allergic responses | AXOS Epilactose GOS FOS, Pectins | Pharmaceutical | Supplemented diets Antibody Vaccines | [1,130,133] |
| Cardiovascular disease and obesity reduction | Satiety induction | FOS | Pharmaceutical | Supplements | [1,136] |
| Vaginal Health | Vagina microbiota restoration | Pectinate | Pharmaceutical | Bioadhesive | [1,138] |
| Agricultural feed | Mycotoxin reduction Growth enhancement Disease resistance | MNB XOS Inulin | Poultry Animal husbandry | Animal feeds Poultry feeds | [1,4,139] |
| Antibiotic Production | Inhibition of E. coli | Sorbitol Inulin Raffinose Lactulose | Pharmaceutical | Bacteriocin | [8,9] |
| Supplementations | Increased nutritional value Induction of LAB growth | AXOS | Dairy Baking | Milk Bread | [143,145] |
| Seed Groups | Seed Sub-Groups | Extraction Methods | Active Components | Uses | References |
|---|---|---|---|---|---|
| Fruit seeds | Date seed | Microbial fermentation | Dietary fibers | Cultivation of Saccharomyces cerevisiae | [153,154,155] |
| Grape seeds | Aqueous | Proanthocyanidins | Antithrombotic Antitumor Anti-mutagenic | [156,157] | |
| Mango seeds | Ethanolic | Polyphenols | Modulation of gut microbiota | [158,159,160] | |
| Tamarind | Alcoholic | Monomers of glucose, galactose and xylose | Stimulation of LAB growth Anti-diabetic | [161,162] | |
| Cereals | Rice | Aqueous | Dietary fibers | Satiety regulation Reduction in the glycemic index of food Prevention of diseases | [163,164] |
| Brewer’s spent grains | Acid hydrolysis | Xylose | Increase in fat excretion Reduction of gallstones and plasma cholesterol | [165,166] | |
| Buckwheat | Ethanolic | Resistant starch | Decrease in cholesterol level Colon health improvement | [1,167] | |
| Coffee spent | Enzymatic hydrolysis | MOS | Stimulate growth of microbiota | [168,169] | |
| Legumes and pulses | Beans | Acid and alkaline hydrolysis | Dietary fibers Phenolics | Support growth of LAB Antioxidant potential Anti-diabetic | [170,171] |
| Oil seeds | Sesame seeds | Organic solvent | Sesamin and Sesamolin | Anti-hypertensive Lowering of Cholesterols Anti-cancer Stimulation of LAB growth | [172,173] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bamigbade, G.B.; Subhash, A.J.; Kamal-Eldin, A.; Nyström, L.; Ayyash, M. An Updated Review on Prebiotics: Insights on Potentials of Food Seeds Waste as Source of Potential Prebiotics. Molecules 2022, 27, 5947. https://doi.org/10.3390/molecules27185947
Bamigbade GB, Subhash AJ, Kamal-Eldin A, Nyström L, Ayyash M. An Updated Review on Prebiotics: Insights on Potentials of Food Seeds Waste as Source of Potential Prebiotics. Molecules. 2022; 27(18):5947. https://doi.org/10.3390/molecules27185947
Chicago/Turabian StyleBamigbade, Gafar Babatunde, Athira Jayasree Subhash, Afaf Kamal-Eldin, Laura Nyström, and Mutamed Ayyash. 2022. "An Updated Review on Prebiotics: Insights on Potentials of Food Seeds Waste as Source of Potential Prebiotics" Molecules 27, no. 18: 5947. https://doi.org/10.3390/molecules27185947
APA StyleBamigbade, G. B., Subhash, A. J., Kamal-Eldin, A., Nyström, L., & Ayyash, M. (2022). An Updated Review on Prebiotics: Insights on Potentials of Food Seeds Waste as Source of Potential Prebiotics. Molecules, 27(18), 5947. https://doi.org/10.3390/molecules27185947








