Abstract
Natural and synthetic β-lactam derivatives constitute an interesting class of compounds due to their diverse biological activity. Mostly used as antibiotics, they were also found to have antitubercular, anticancer and antidiabetic activities, among others. In this investigation, six new 3,3-dichloro-β-lactams prepared in a previous work were evaluated for their hemolytic and cytotoxic properties. The results showed that the proposed compounds have non-hemolytic properties and exhibited an interesting cytotoxic activity toward squamous cell carcinoma (A431 cell line), which was highly dependent on the structure and concentration of these β-lactams. Among the molecules tested, 2b was the most cytotoxic, with the lowest IC50 values (30–47 µg/mL) and a promising selectivity against the tumor cells compared with non-tumoral cells.
1. Introduction
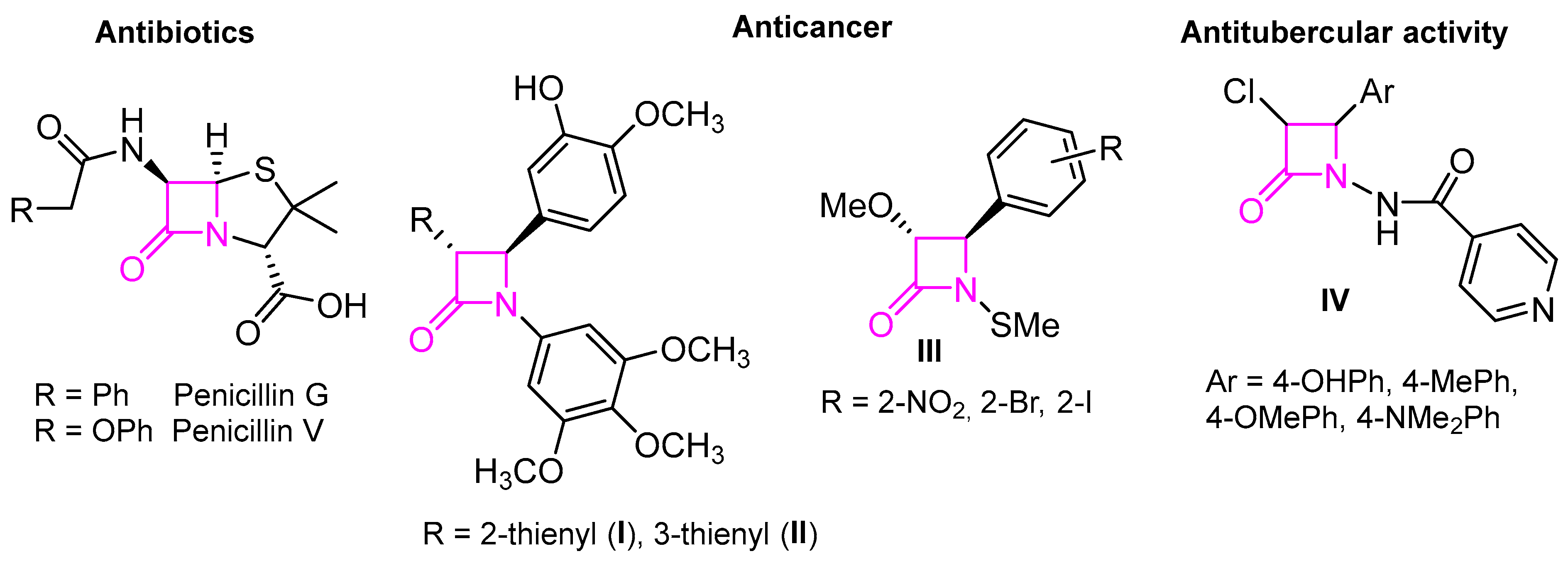
β-Lactam derivatives are the most commonly used bactericidal agents worldwide [1]. Moreover, the β-lactam ring is also found in many other natural and unnatural compounds, with a wide spectrum of activities [2] (Figure 1). For example, in 2011, Meegan et al. reported the synthesis of a series of β-lactams via the Staudinger reaction [3]. Among these, 3-(2-thienyl) I and 3-(3-thienyl) II displayed the highest potency in human MCF-7 breast cancer cells, with IC50 values of 7 nM and 10 nM, respectively, comparable to combretastatin A. Dou et al. have also demonstrated that N-thiolated β-lactams III have a tumor cell-killing ability through the induction of DNA damage and subsequent apoptosis [4]. Additionally, Hussain et al. reported the synthesis of β-lactam derivatives IV with significant antimycobacterial (antitubercular) activity [5]. β-Lactams have been prepared using a wide range of strategies [6,7]. Among these are the Staudinger procedure, also called the Staudinger ketene–imine non-photochemical 2 + 2 cycloaddition [8,9]; the carbonylative ring-opening of aziridines [10]; the Kinugasa reaction, which takes place between terminal alkynes and a nitrone in the presence of copper(I) [11,12,13,14]; and metal-catalyzed C-H insertion of diazoamide carbenes activated by rhodium and ruthenium complexes, among others [15].
Figure 1.
Some β-lactam derivatives with biological activities.
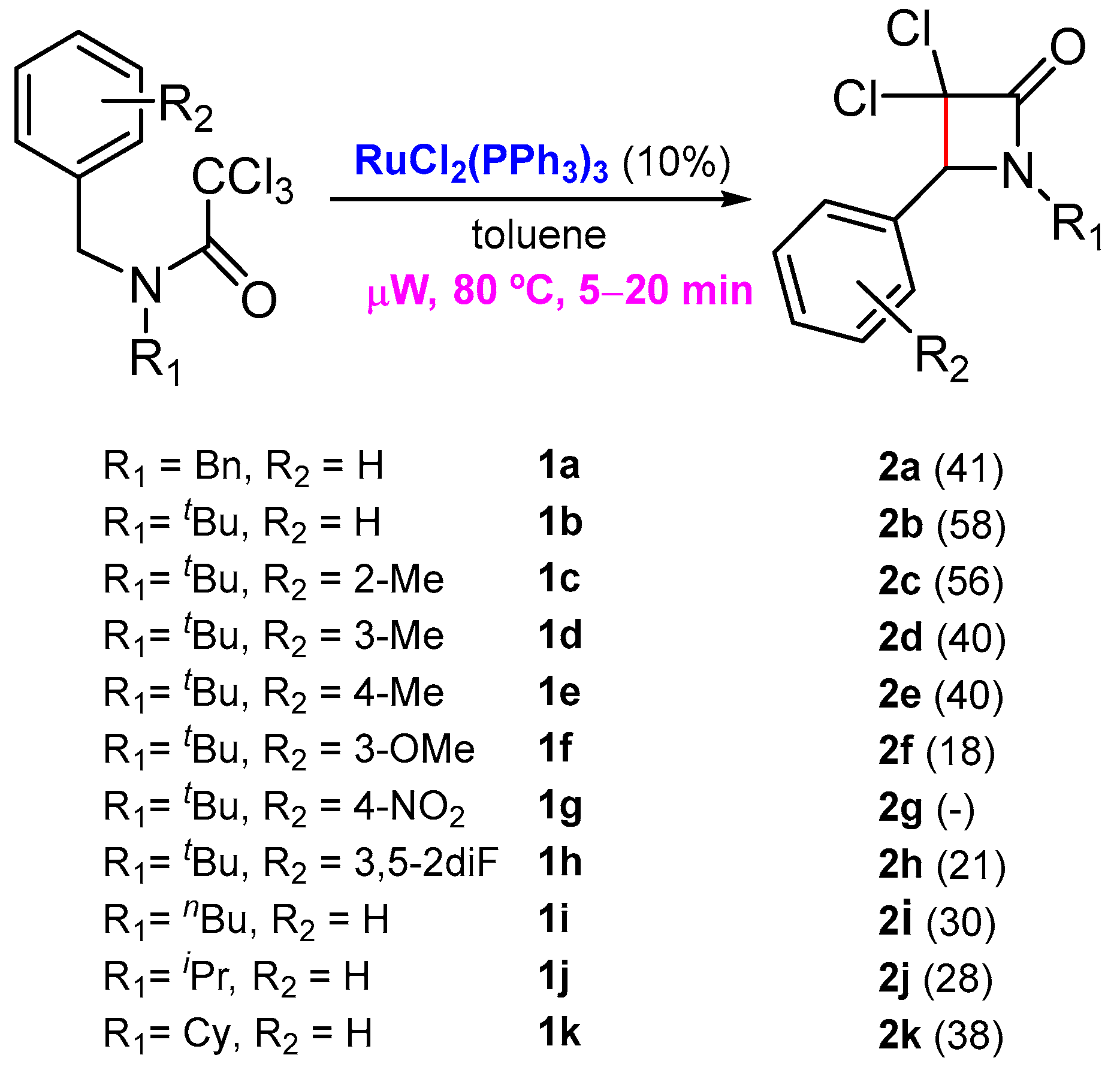
As part of our efforts to prepare new nitrogen-containing heterocycles with cytotoxic activity, a few years ago, we reported an unprecedented ruthenium-catalyzed synthesis of β-lactams from readily available N-benzyltrichloroacetamides through benzylic C-H activation [16]. This work was an extension of a previous investigation where we reported the first Grubbs’ second-generation catalyst-promoted intramolecular dearomative ATRC, with trichloroacetamides embodying an electron-rich arene for the preparation of 2-azaspirodecadienes [17,18]. At that time, there was only one example reported by Cramer and co-workers, where a similar saturated C(sp3)-C(sp3) bond formation was achieved through an asymmetric C-H functionalization from chloroacetamides, catalyzed by palladium(0) [19]. In our investigation, the best results were attained in the presence of a catalytic amount of RuCl2(PPh3)3 under microwave activation, in a very short reaction time (Scheme 1) [16].
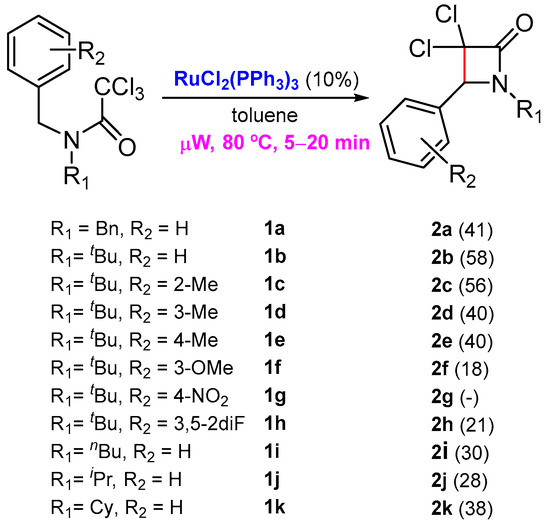
Scheme 1.
Synthesis of β-lactams through a benzylic C-H activation.
Even if the β-lactams prepared using our strategy were isolated with moderate yields, the simplicity of the process and the challenging reaction involved are unprecedented (For NMR spectra of the products involved in this synthetic study see Supplementary Materials). The next step was to study the biological properties of these lactams. In this work, in vitro experiments were performed to determine the biocompatible characterization of six new 3,3-dichloro-β-lactams derivatives 2b, 2c, 2e, 2h, 2j and 2k, regarding their hemolytic and cytotoxic properties in red blood cells (RBCs) and representative skin cell lines, respectively. In view of their putative selective toxicity, both tumoral (A431 cell line) and non-tumoral (HaCaT) cell lines were investigated.
2. Results and Discussion
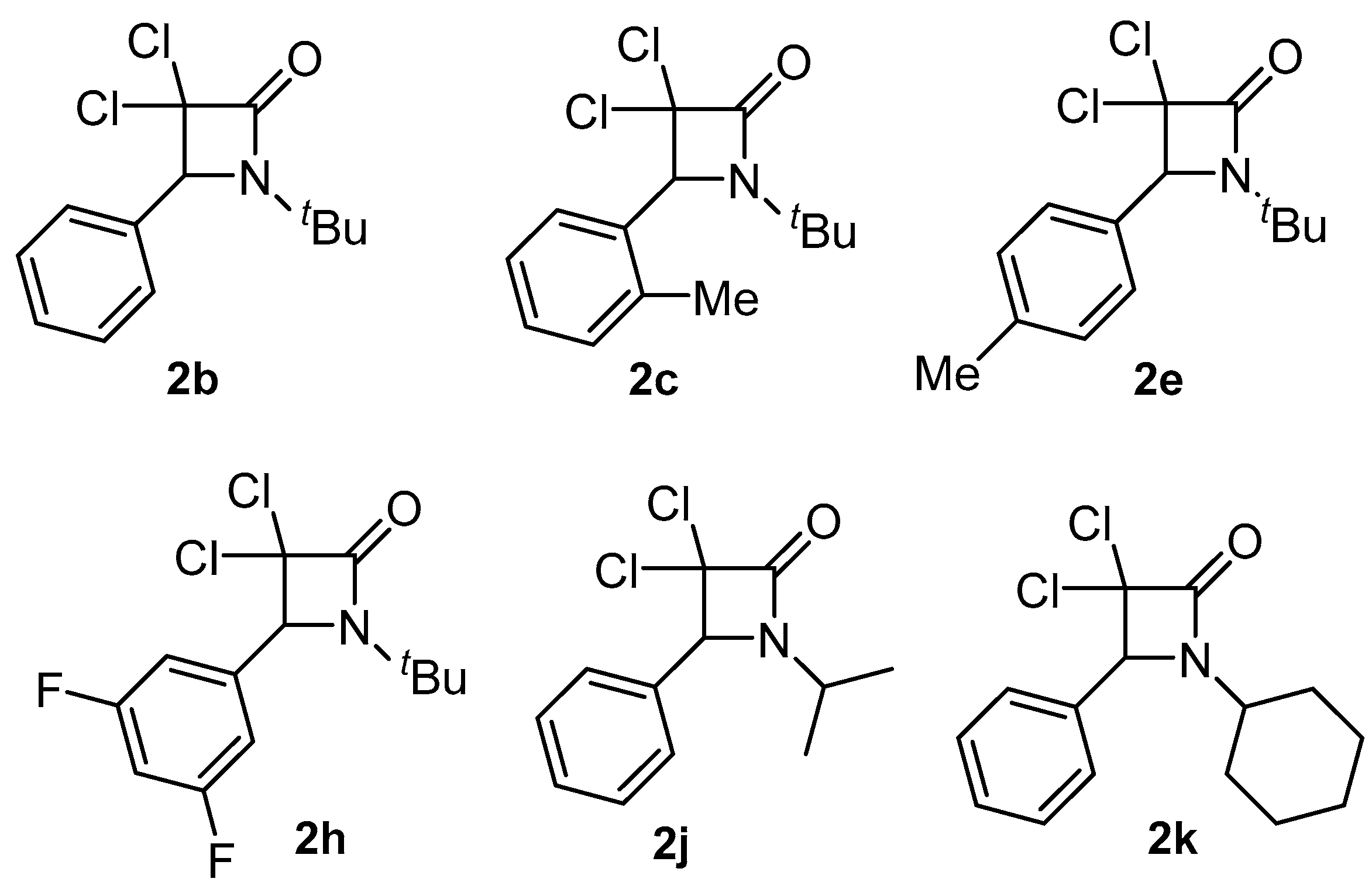
As was mentioned before with the background reported in the Introduction section, it was of great interest to investigate and characterize some of the β-lactams prepared in our previous investigation, namely 2b, 2c, 2e, 2h, 2j and 2k, for their hemocompatibility and cytotoxic activity (Figure 2).
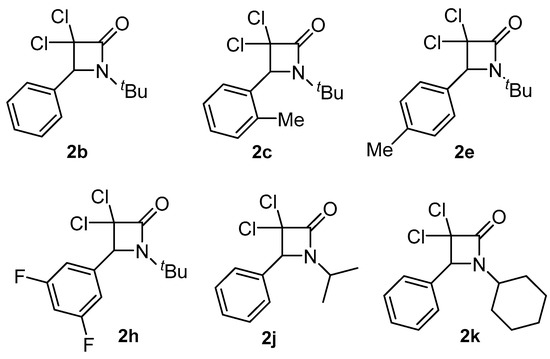
Figure 2.
Selected β-lactams for hemocompatibility and cytotoxicity studies.
2.1. Hemocompatibility Studies
Following the ISO 10993-4 concerning the biological evaluation of medical devices and their interactions with blood, an in vitro hemocompatibility assay was carried out. Under the assayed conditions, the degree of hemolysis produced by the different compounds by incubation with RBC suspension was determined. The chemical modification of the hemolytic response was evaluated at 2 different concentrations (10 and 80 µg/mL). Table 1 shows representative results as a function of the chemical structure. The degree of hemolysis fluctuated slightly, with values ranging between 0.01 and 0.05% for compounds 2b–2j at concentrations equal to 10 µg/mL. The maximum degree of hemolysis was achieved with compound 2k, with hemolysis close to 0.25%. By increasing the concentration up to 80 µg/mL, no appreciable increase in the hemolytic response was observed. Only under discrete conditions, hemolysis increases to 2.5% (compound 2b).

Table 1.
Percentage of hemolysis induced by compounds 2b–2k as a function of concentration.
Considering the criteria for which compounds are classified as non-hemolytic (values < 2%), slightly hemolytic (with values 2– 5%) and hemolytic (values >5%), it could be concluded that the proposed compounds showed non-hemolytic properties [20].
2.2. Cell Viability-Screening Assays
The interaction between the biological systems and the new materials is of fundamental importance for the study of new materials. After ensuring that the selected β-lactams have a non-hemolytic character, we were very interested in investigating the cytotoxic activity they induced. In vitro cell culture assays are of principal interest in the assessment of the biocompatibility of new compounds. In addition, such initial in vitro analyses provide an excellent way to screen materials before performing in-depth in vivo-based analyses. Among the putative cell lines suitable to perform the present study, commercially available cell lines that include representative cells of the skin were chosen. For this purpose, we used the immortal human keratinocyte (HaCaT) and the squamous cell carcinoma (A431) lines as closely representative skin cell lines with non-tumoral and tumoral characteristics, respectively. The function of the skin includes acting as a barrier, providing protection from physical, chemical and microbiological agents. Keratinocytes represent 95% of the epidermal cells, acting as a structural and barrier function of the epidermis. Their role in skin inflammatory and immunological responses, as well as in wound repair are well-recognized [21]. The spontaneously immortalized human keratinocyte HaCaT cell line from adult skin has been proposed as a model for the study of keratinocyte functions. This work includes another keratinocyte cell line with tumoral characteristics, such as those of squamous cell carcinoma (SCC), namely the A431 cell line, which is important considering that SCC is by far the most common skin cancer and is actually more common than any other form of cancer [22].
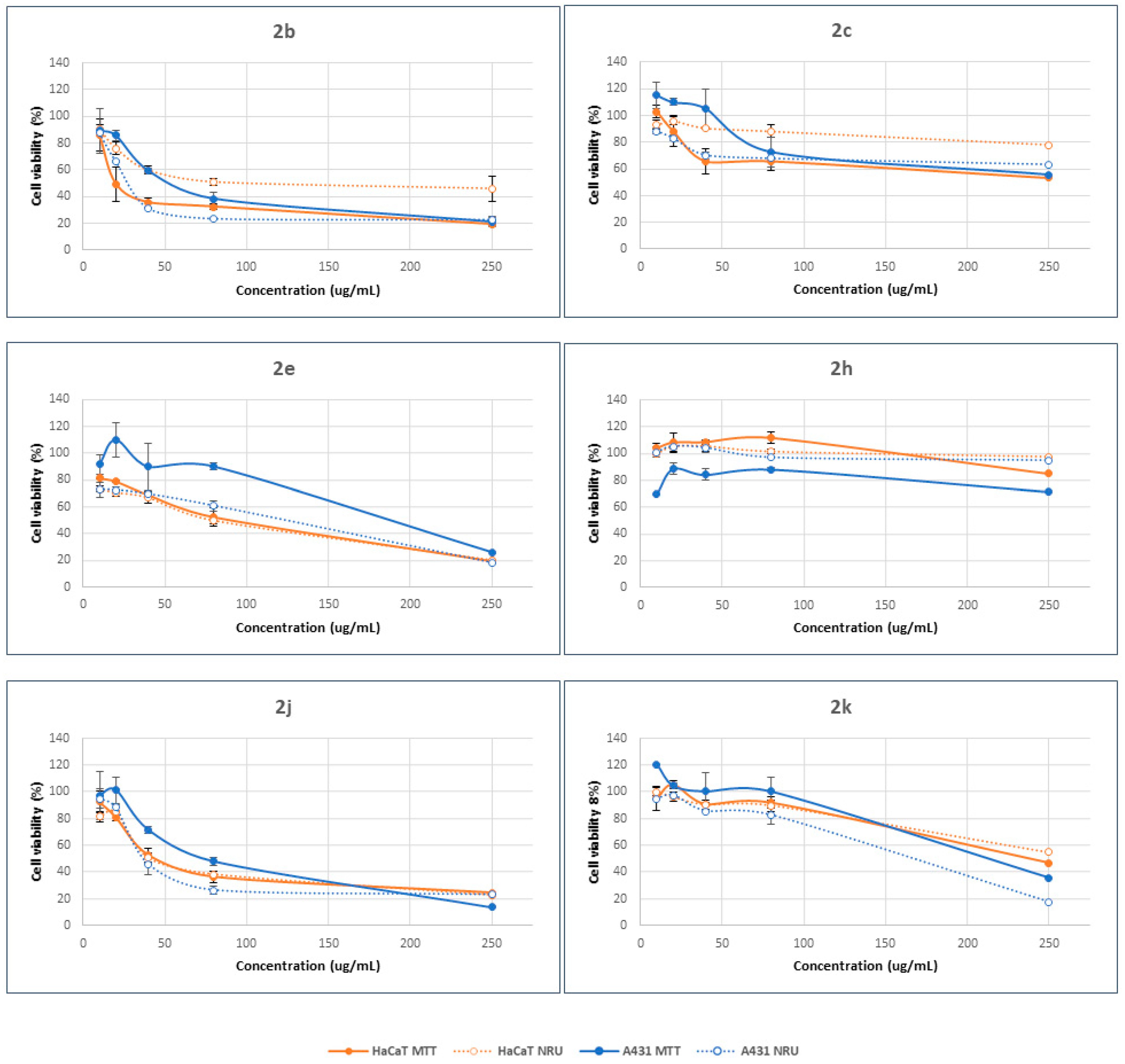
Two different endpoints, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) and neutral red uptake (NRU), were used to assess differences in cell-induced cytotoxicity. The former method offers details about the modification of the metabolic activity of mitochondria inside the cells. In the latter case, however, the information derived is related to the interaction with the plasmatic membrane. Dose–response curves were determined by the MTT [23] and NRU [24] assays using HaCaT and A431 cell lines. Cytotoxicity assays were performed at concentrations ranging between 10 and 250 µg/mL. The results demonstrated that the cytotoxic response is highly dependent on the structural characteristics of β-lactam compounds and their concentration. In general, cell viability decreases with increasing concentration, showing a dose–concentration response (Figure 3). However, the final response seems to be a function of the β-lactam derivative.
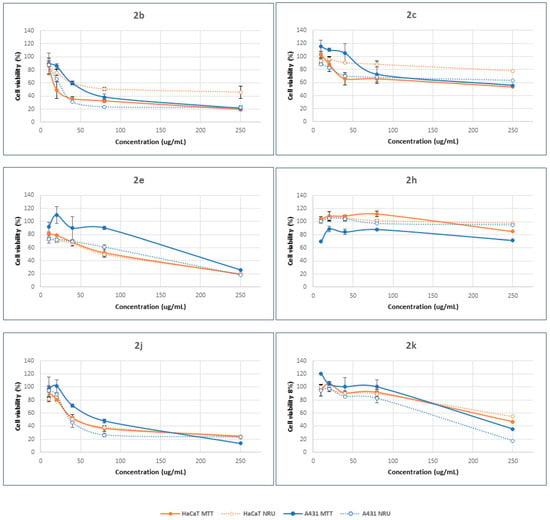
Figure 3.
Concentration-dependent viability of HaCaT and A431 cells treated with β-lactam derivatives for 24 h, determined by MTT (solid lines) and NRU (dotted lines) assays. The data correspond to the average of three independent experiments ± standard deviation.
Compounds 2b, 2e, 2j and 2k were able to decrease the percentage viability compared to control cells, with values lower than 20%, as a function of cell type and endpoint method. Compounds 2c and 2h, however, promoted cell viability by up to 50% in all cases. Moreover, the structure of the β-lactam determined the selectivity against the cell type and the mode of action. Hence, compound 2e promoted higher cytotoxicity in HaCaT cells than in A431 cells, regardless of the concentration range. Meanwhile, the opposite results were observed in the case of compound 2h. There were no big differences between the cellular responses when the other β-lactams were considered. As a general trend, these results suggest minor differences between the mode of action of the tested compounds in relation with the plasma membrane and lysosomal accumulation (NRU method) and the modification of the metabolic activity of mitochondria inside the cells (MTT method). In all cases, these compounds can achieve the mitochondrial compartment after alteration of the plasma membrane.
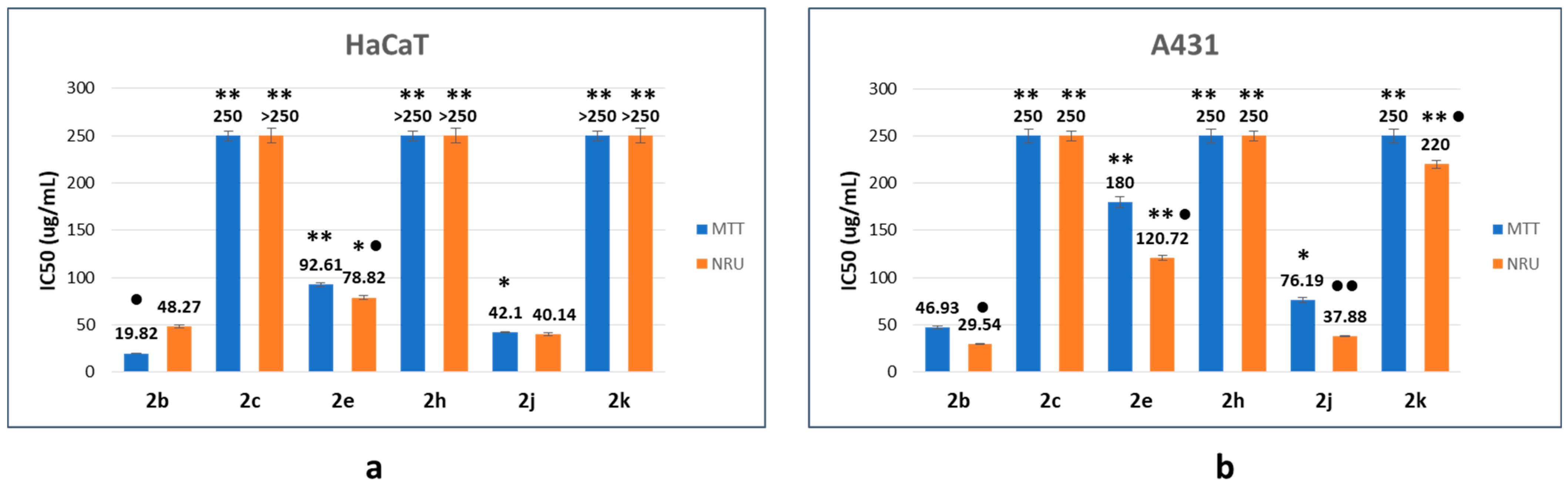
From the fitting of concentration-dependent viability curves, the corresponding half-maximal inhibitory concentration (IC50) was determined. The results obtained are summarized in Figure 4 and Table 2. The structural characteristics of β-lactam derivatives seem to be the main factor in the tested compounds’ cytotoxic response. Lactam 2b, as a reference compound, was the most cytotoxic product, showing the lowest IC50 values (20–49 µg/mL and 30–47 µg/mL for HaCaT and A431 cell lines, respectively).
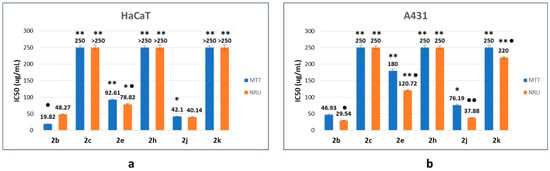
Figure 4.
Half-maximal inhibitory concentration (IC50) values of the corresponding β-lactams on HaCaT (a) and A431 (b) cell lines as a function of the endpoint method. The data correspond to the average of three independent experiments ± standard deviation. * p < 0.005 and ** p < 0.001 indicate significant differences in comparison with compound 2b. ● p < 0.005 and ●● p < 0.001 indicate significant differences between endpoint methods for the same compound.

Table 2.
Cell viability assessment: IC50 values of the corresponding β-lactams as a function of cell line and endpoint method. Selectivity index against the tumor cell line (A431) in comparison with the non-tumor cell line (HaCaT).
The cellular response depends on the nature of the substituent on the benzene ring and its location. The introduction of two fluorine atoms (compound 2h) generates a more biocompatible compound, with IC50 values close to 250 µg/mL (A431 cell line) or higher than 250 µg/mL (HaCaT cell line), which correspond to the highest tested concentration, in a manner independent of the endpoint method. The location of the methyl group in the benzene ring is also an important parameter concerning cell response. Thus, when the methyl group is in the ortho position (compound 2c), IC50 values are close to 250 µg/mL (HaCaT and A431, MTT endpoint) and higher than 250 µg/mL (HaCaT and A431, NRU endpoint). However, when the methyl substituent is in the para position (compound 2e), the compound preserves the cytotoxic activity, with IC50 values between 78 and 93 µg/mL (HaCaT), and between 120 and 180 µg/mL (A431). Switching from t-Bu to i-Pr (compound 2j) has a poor influence on the cytotoxic response, since IC50 values between 40 and 42 µg/mL (HaCaT), and between 38 and 76 µg/mL (A431) were found. However, in the case of the cyclohexyl derivative 2k, limited interaction with the proposed cells was observed, as is indicated by the IC50 values close to 220 µg/mL (HaCaT, NRU) or higher than 250 µg/mL (HaCaT, MTT and A431 in all cases).
When the IC50 values as a function of the endpoint method were compared, the results obtained suggested that, in general, the interaction with the cell membrane is favored. In almost all cases, IC50 values corresponding to the NRU method were lower than those obtained with the MTT endpoint. Moreover, based on the values displayed in Table 2, the selectivity index (SI) seems to depend on the endpoint method. In the case of the MTT method, poor selectivity was detected. In all cases, SI values are equal to or lower than 1, demonstrating no selectivity in the mode of action. However, some selectivity among the proposed cell lines was observed when the NRU endpoint was considered, demonstrating discrete selectivity in the mode of action of almost all compounds (except for compound 2e).
Such initial in vitro analyses provide an excellent way to screen materials before performing in-depth in vivo-based analyses. In addition, preclinical models such as human cell line model systems may be particularly useful to help predict anticancer drug response and to further improve our understanding of the mechanisms of drug action in cases where there is limited access to clinical samples and/or the cost of obtaining clinical samples to study the drug response is too high. Based on the results obtained, we can confirm that the synthesized β-lactams represent new compounds with antitumoral properties which can be modulated as a function of the synthesized β-lactams’ structure.
3. Materials and Methods for the Biological Assays
3.1. Materials
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), L-glutamine solution (200 mM), trypsin–EDTA solution (170,000 U/L trypsin and 0.2 g/L EDTA), penicillin–streptomycin solution (10,000 U/mL penicillin and 10 mg/mL streptomycin) and phosphate-buffered saline (PBS) were obtained from Lonza (Verviers, Belgium). 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) and neutral red dye (NR) were from Sigma–Aldrich (St. Louis, MO, USA). The 75 cm2 flasks and 96-well plates were obtained from TPP (Trasadingen, Switzerland). All other reagents were of analytical grade.
3.2. Methods
3.2.1. In Vitro Assay with Human Erythrocytes
Acquisition and Extraction of the Erythrocytes
Human blood samples were obtained from the Banc de Sang i Teixits de Barcelona (Spain) from the Catalan Department of Health. Blood was deposited in tubes with the anticoagulant EDTA-K3. Blood samples were centrifuged at 3000 rpm at 4 °C for 10 min (Megafuge 2.0 R. Heraeus Instruments, Hanau, Germany) to induce sedimentation. Plasma was extracted with a Pasteur pipette. Next, the residual pellet was washed with PBS at pH 7.4. This procedure was repeated three times to remove residual leukocytes and platelets and to concentrate the erythrocytes. Following the last wash, the erythrocyte suspension was diluted (1:1) in PBS at pH 7.4 to obtain a suitable erythrocyte suspension (cell density of 8 × 109 cell/mL).
Hemolysis Assay
The hemolysis assay determined the capability of the different compounds to induce hemolysis of the erythrocyte membrane. Stock solutions of each compound at 1 mg/mL in PBS at pH 7.4 were prepared. Different volumes (10–80 µL) were placed in polystyrene tubes, and an aliquot of 25 µL of the erythrocyte suspensions was added to each tube. The final volume was 1 mL. The tubes were incubated at room temperature under rotatory conditions. Next, the tubes were centrifuged at 10,000 rpm for 5 min. The supernatants’ absorbance at 540 nm (Shimadzu UV-160A, Shimadzu, Duisburg, Germany) was compared with that of the control samples hemolyzed with distilled water (positive control). The negative control was obtained by incubating an aliquot of 25 µL of the erythrocyte suspension with PBS at pH 7.4.
The degree of hemolysis was determined with the following equation:
where Abs, Abs0 and Abs100 are the absorbance of test samples, of the suspension treated with isotonic physiological buffer saline (PBS) and of the suspension of complete hemolysis treated with distilled water, respectively.
Hemolysis (%) = 100 × (Abs − Ab0)/(Abs100 − Abs0)
3.3. Cell Cultures
The immortal human keratinocyte (HaCaT) and the squamous cell carcinoma (A431) cell lines were obtained from Celltec UB. Cells were grown in DMEM medium (4.5 g/L glucose) supplemented with 10% (v/v) FBS, 2 mM L-glutamine, 100 U/mL penicillin and 100 µg/mL streptomycin at 37 °C, 5% CO2. Cells were routinely cultured in 75 cm2 culture flasks and were trypsinized using trypsin–EDTA when the cells reached approximately 80% confluence. The trypan blue assay, which allows a direct identification and enumeration of live (unstained) and dead (blue) cells in a given population, was used to evaluate the viability of the cells in the cell suspension obtained.
3.4. Cell Viability-Screening Assays
HaCaT cells (1 × 105 cells/mL) and A431 cells (5 × 104 cells/mL) were grown at the defined densities in the 60 central wells of a 96-well plate. Cells were incubated for 24 h in 5% CO2 at 37 °C. Then, the spent medium was removed, and cells were incubated for 24 h with the corresponding compound solutions (1 mg/mL) previously diluted in the minimum amount of DMF (dimethylformamide) and then in DMEM medium supplemented with 5% FBS (100 µL) at the required concentration range (10–250 µg/mL). The viability of the cells upon incubation with the β-lactam derivatives was assayed using 2 different endpoints: NRU and MTT.
3.4.1. NRU Assay
The neutral red uptake (NRU) assay is based on the accumulation of the dye in the lysosomes of viable cells. After the cells were incubated for 24 h with the corresponding systems, the medium was removed, and the solutions were incubated with the NR dye (Sigma-Aldrich, St. Louis, MO, USA) solution (50 µg/mL) dissolved in the medium without FBS and without phenol red (Lonza, Verviers, Belgium) for 3 h. Cells were then washed with sterile PBS, followed by the addition of 100 µL of a solution containing 50% absolute ethanol and 1% acetic acid in distilled water to extract the dye. To promote total dissolution of the dye, plates were placed in a microtiter-plate shaker for 5 min at room temperature. The absorbance of the resulting solutions was measured at 550 nm (Bio-Rad 550 microplate reader, Bio-Rad California, Hercules, CA, USA). Finally, the effect of each treatment was calculated as the percentage of dye uptake by viable cells relative to the control cells (cells without any treatment).
3.4.2. MTT Assay
Only living cells can reduce the yellow tetrazolium salt 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) to insoluble purple formazan crystals. After 24 h of incubation of the cells with the corresponding NPs, the medium was removed, and 100 µL of MTT (Sigma-Aldrich, St. Louis, United States) in PBS (5 mg/mL) diluted 1:10 in culture medium without phenol red and without FBS (Lonza, Verviers, Belgium) was added to the cells. After 3 h of incubation, the medium was removed. Thereafter, 100 µL of DMSO (Sigma-Aldrich, St. Louis, MO, USA) was added to each well to dissolve the purple formazan crystals. Agitation and determination of the absorbance of the extracted solution were performed under the same conditions, as described in Section 3.4.1. Finally, the effect of each treatment was calculated as the percentage of tetrazolium salt reduction by viable cells relative to the control cells (cells without any treatment).
3.4.3. Selectivity towards Cancer Cells
The corresponding half-maximal inhibitory concentration (IC50) values for the different formulations as a function of cell line and endpoint were determined from the fitting of concentration-dependent viability curves.
The corresponding selectivity indexes toward tumoral cells were calculated as the following ratio:
where HaCaT keratinocytes were used as close representatives of a skin model cell line under non-tumoral conditions.
SI = IC50 (non-tumoral cell line)/IC50 (tumoral cell line)
3.5. Statistical Analyses
Experiments were performed three times on independent occasions unless otherwise stated. The results are expressed as means ± standard deviation. One-way analysis of variance (ANOVA) was used to determine statistical differences between data sets, followed by Scheffé post hoc tests for multiple comparisons. IBM SPSS Statistics software version 27.0 (New York, NY, USA) was used to execute statistical analyses. Differences were considered statistically significant at p < 0.005 and 0.001. Significant differences were illustrated in the figures with an asterisk or other superscript symbols.
4. Conclusions
In summary, 3,3-dichloro-β-lactams 2b, 2c, 2e, 2h, 2j and 2k, prepared through an unprecedented RuCl2(PPh3)3-catalyzed benzylic C-H activation from benzyl-tethered trichloroacetamides, were evaluated for their hemolytic and cytotoxic properties. The results showed that the proposed compounds have non-hemolytic properties and that the cytotoxic response is highly dependent on the structure and concentration of these β-lactams. Compound 2b was found to be the most cytotoxic of the list, showing the lowest IC50 values (20–49 µg/mL and 30–47 µg/mL for HaCaT and A431 cell lines, respectively), with moderate selective toxicity against tumoral cells.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules27185975/s1, Figure S1: 1H and 13C spectra of 1e, Figure S2: 1H and 13C spectra of 1f, Figure S3: 1H and 13C spectra of 1g, Figure S4: 1H and 13C spectra of 2a, Figure S5: 1H and 13C spectra of 2b, Figure S6: 1H and 13C spectra of 2c, Figure S7: 1H and 13C spectra of 2d, Figure S8: 1H and 13C spectra of 2e, Figure S9: 1H and 13C spectra of 2f, Figure S10: 1H and 13C spectra of 2h, Figure S11: 1H and 13C spectra of 2i, Figure S12: 1H and 13C spectra of 2j, Figure S13: 1H and 13C spectra of 2k.
Author Contributions
Conceptualization F.D.; methodology, F.D. and M.d.C.M.; investigation, F.D., A.G.S. and M.d.C.M.; writing—original draft preparation, F.D. and M.d.C.M.; writing—review and editing F.D. and M.d.C.M.; project administration, F.D.; funding acquisition, F.D. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support for this research was provided mainly by projects Fundació Bosch i Gimpera 310959 and 309959 (IP: F. Diaba), and by project PID2019-104188GB-I00 in which F. Diaba is participating.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available within the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fernandes, R.; Amador, P.; Prudêncio, C. β-lactams: Chemical structure, mode of action and mechanisms of resistance. Rev. Med. Microbiol. 2013, 24, 7–17. [Google Scholar] [CrossRef]
- Gupta, A.; Halve, A.K. β-Lactams: A mini review of their biological activity. Int. J. Pharmaceut. Sci. Res. 2015, 6, 978–987. [Google Scholar]
- O’Boyle, N.M.; Greene, L.M.; Bergin, O.; Fichet, J.B.; McCabe, T.; Lloyd, D.G.; Zisterer, D.M.; Meegan, M.J. Synthesis, evaluation and structural studies of antiproliferative tubulin-targeting azetidin-2-ones. Bioorg. Med. Chem. 2011, 19, 2306–2325. [Google Scholar] [CrossRef]
- Smith, D.M.; Kazi, A.; Smith, L.; Long, T.E.; Heldreth, B.; Turos, E.; Dou, Q.P. A novel β-lactam antibiotic activates tumor cell apoptotic program by inducing DNA damage. Mol. Pharmacol. 2002, 61, 1348–1358. [Google Scholar] [CrossRef]
- Hussain, S.; Jadhav, S.; Rai, M.; Farooqui, M. Synthesis, characterization and biological evaluation of N [3-Chloro-2 (aryl)-4-oxoazitidin-1-y] pyridine-4-carboxamide. Int. J. Drug Des. Discov. 2011, 2, 527–532. [Google Scholar]
- Hosseyni, S.; Jarrahpour, A. Recent advances in β-lactam synthesis. Org. Biomol. Chem. 2018, 16, 6840–6852. [Google Scholar] [CrossRef]
- Pitts, C.R.; Lectka, T. Chemical synthesis of β-lactams: Asymmetric catalysis and other recent advances. Chem. Rev. 2014, 114, 7930–7953. [Google Scholar] [CrossRef]
- Staudinger, H. Zur kenntniss der ketene. Diphenylketen. Justus Liebig’s Ann. Chem. 1907, 356, 51–123. [Google Scholar] [CrossRef]
- Palomo, C.; Aizpurua, J.M.; Ganboa, I.; Oiarbide, M. Asymmetric synthesis of β-lactams through the Staudinger reaction and their use as building blocks of natural and nonnatural products. Curr. Med. Chem. 2004, 11, 1837–1872. [Google Scholar] [CrossRef]
- Khumtaveeporn, K.; Alper, H. Transition metal mediated carbonylative ring expansion of heterocyclic compounds. Acc. Chem. Res. 1995, 28, 414–422. [Google Scholar] [CrossRef]
- Kinugasa, M.; Hashimoto, S. The reactions of copper(I) phenylacetylide with nitrones. J. Chem. Soc. Chem. Commun. 1972, 8, 466–467. [Google Scholar] [CrossRef]
- Ahn, C.; Kenninghton, J.W.; DeShong, P.A. New approach to the synthesis of monocyclic β-lactam derivatives. J. Org. Chem. 1994, 59, 6282–6286. [Google Scholar] [CrossRef]
- Marco-Contelles, J. β-Lactam synthesis by the Kinugasa reaction. Angew. Chem. Int. Ed. 2004, 43, 2198–2200. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Singh, P.; Bhargava, G. Cu(I) mediated Kinugasa reactions of α,β-unsaturated nitrones: A facile, diastereoselective route to 3-(hydroxy/bromo)methyl-1-aryl-4-(-styryl)azetidin-2-ones. New J. Chem. 2016, 40, 8216–8219. [Google Scholar] [CrossRef]
- Dong, K.; Qiu, L.; Xu, X. Selective synthesis of β-lactams via catalytic metal carbene C-H insertion reactions. Curr. Org. Chem. 2016, 20, 29–40. [Google Scholar] [CrossRef]
- Sandor, A.G.; Diaba, F. Microwave-assisted benzylic C-H activation using ruthenium catalysts. Synthesis of β-lactams. Int. J. Curr. Res. 2019, 11, 6930–6936. [Google Scholar]
- Diaba, F.; Martínez-Laporta, A.; Bonjoch, J. Atom transfer radical cyclization of trichloroacetamides to electron-rich acceptors using Grubbs’ catalysts: Synthesis of the tricyclic framework of FR901483. J. Org. Chem. 2014, 79, 9365–9372. [Google Scholar] [CrossRef]
- Diaba, F.; Montiel, J.A.; Martínez-Laporta, A.; Bonjoch, J. Dearomative radical spirocyclization from N-benzyltrichloroacetamides revisited using a copper(I)-mediated atom transfer reaction leading to 2-azaspiro [4.5]decanes. Tetrahedron Lett. 2013, 54, 2619–2622. [Google Scholar] [CrossRef]
- Pedroni, J.; Boghi, M.; Saget, T.; Cramer, N. Access to β-lactams by enantioselective palladium(0)-catalyzed C(sp3)-H alkylation. Angew. Chem. Int. Ed. 2014, 53, 9064–9067. [Google Scholar] [CrossRef]
- Repetto, G.; Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Schurer, N.; Kohne, A.; Schliep, V.; Barlag, K.; Goerz, G. Lipid composition and synthesis of HaCaT cells, an immortalized human keratinocyte line, in comparison with normal human adult keratinocytes. Exp. Dermatol. 1993, 2, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Samarasinghe, V.; Madan, V. Nonmelanoma skin cancer. J. Cutan. Aesthet. Surg. 2012, 5, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insight into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar]
- Dobrovolskaia, M.A.; Clogston, J.D.; Neun, B.W.; Hall, J.B.; Patri, A.K.; McNeil, S.E. Method for analysis of nanoparticle hemolytic properties in vitro. Nano Lett. 2008, 8, 2180–2187. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).