Recent Progress on Techniques in the Detection of Aflatoxin B1 in Edible Oil: A Mini Review
Abstract
:1. Introduction
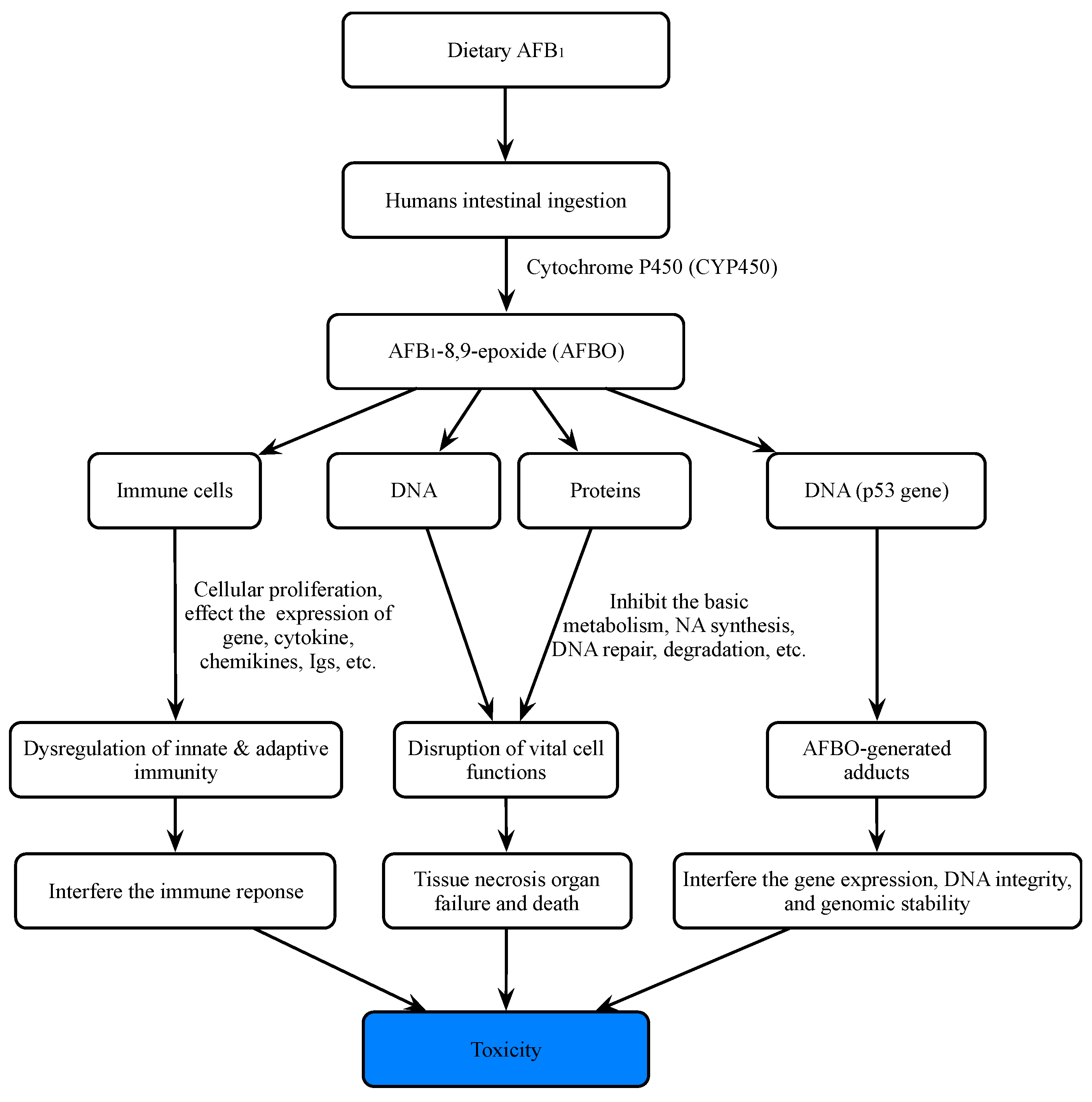
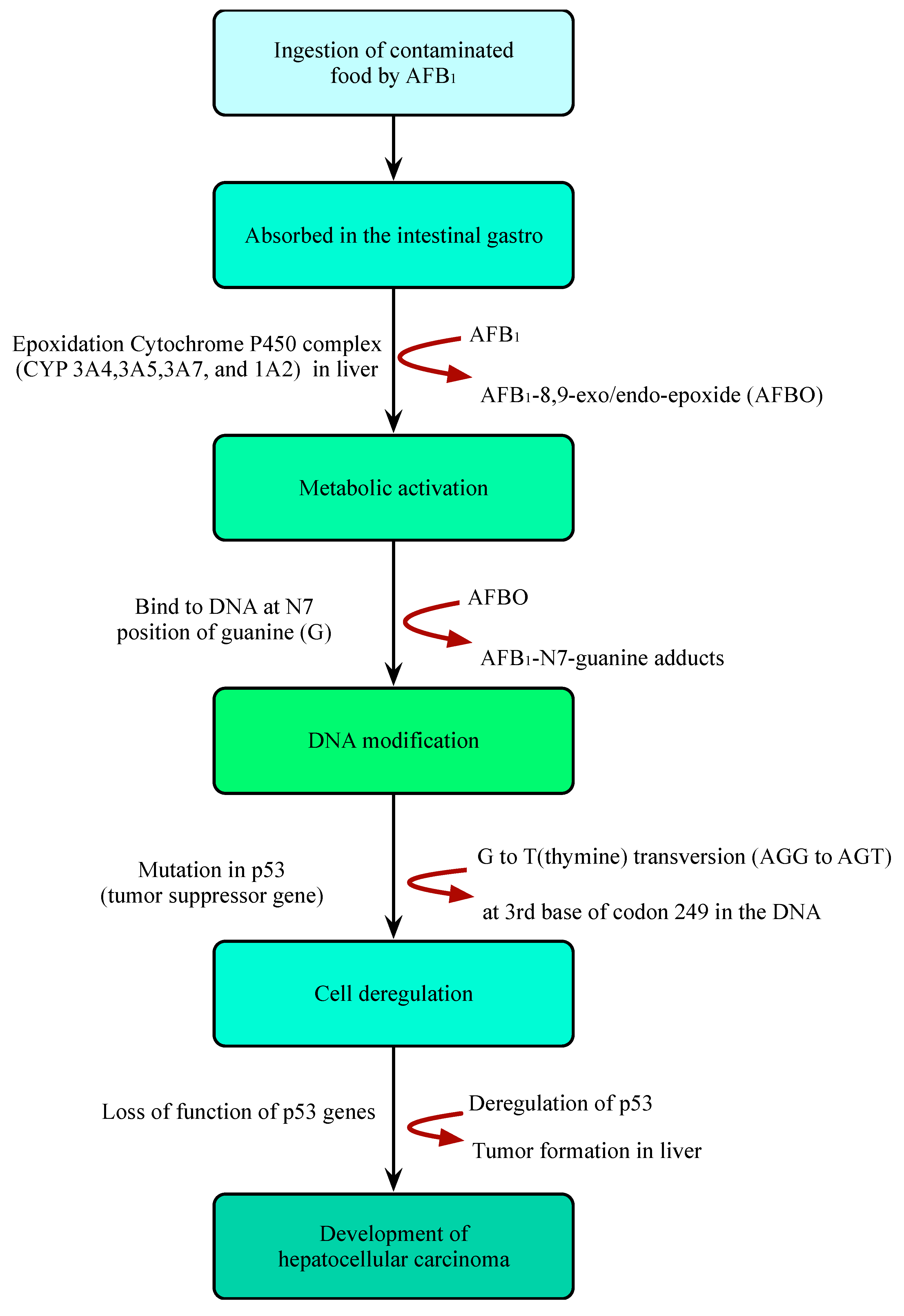
2. Importance of Aflatoxins
3. AFB1 Regulations on Edible Oil
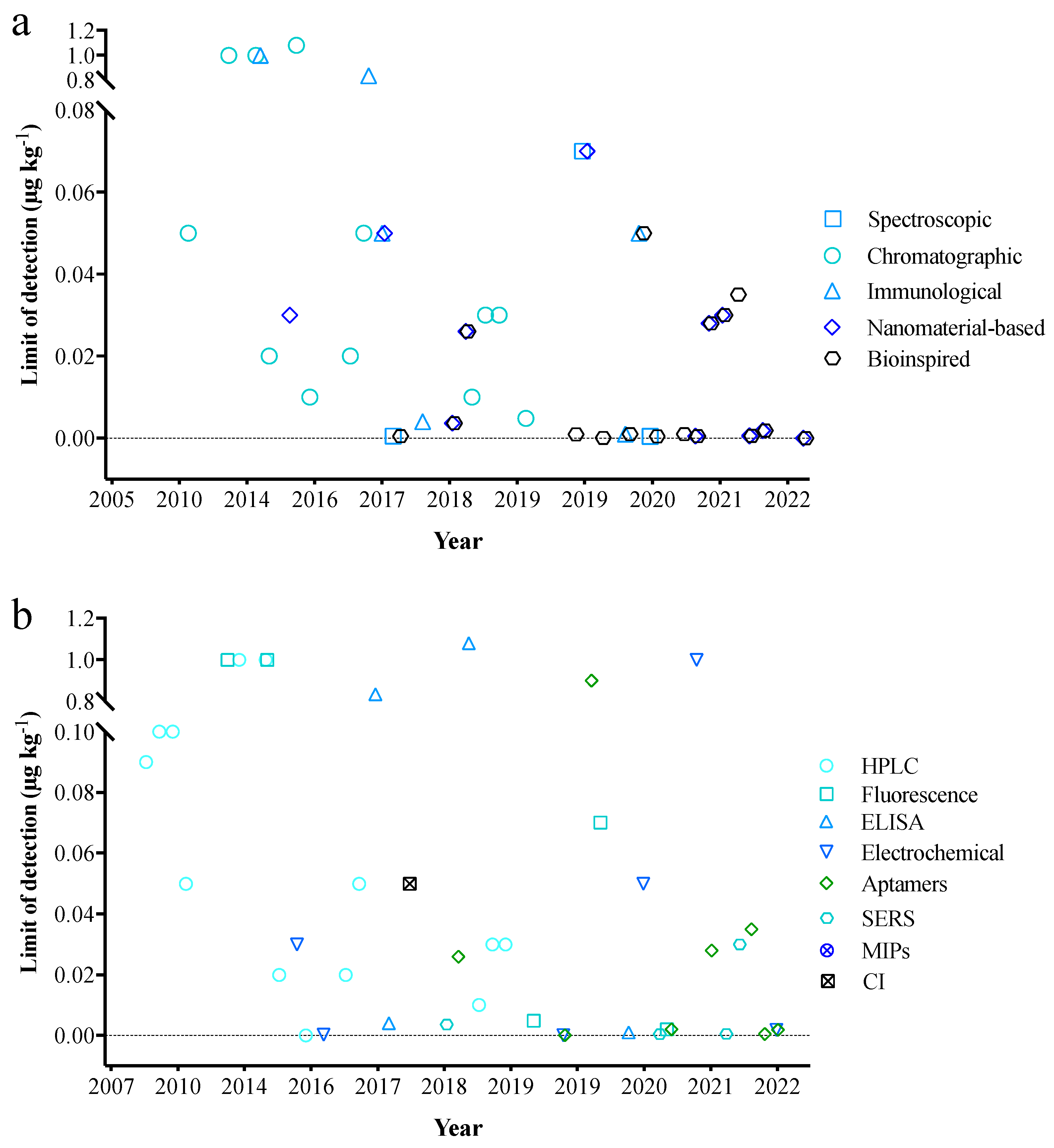
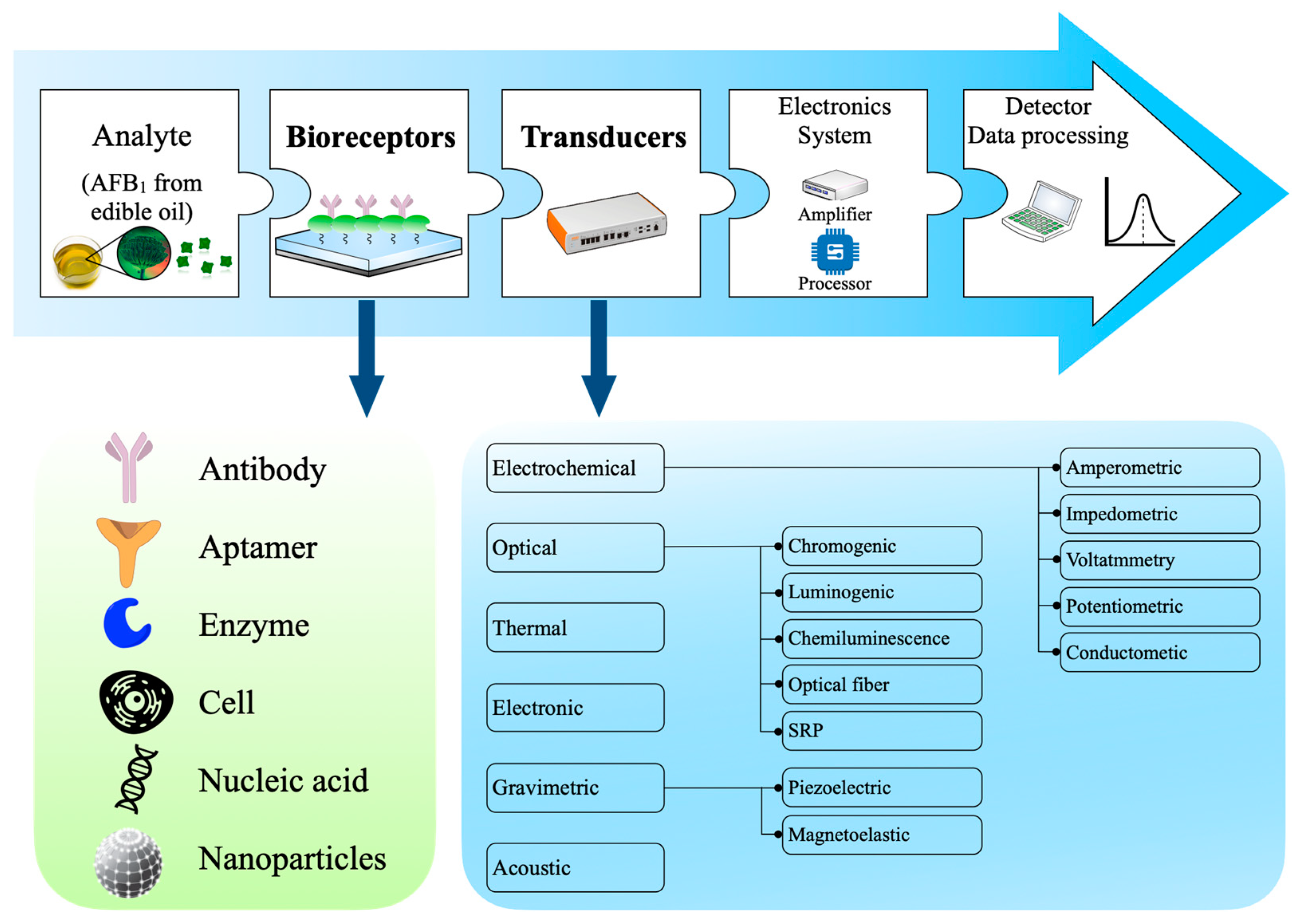
4. Methods for Detecting AFB1 in Edible Oil
4.1. Chromatographic Technology
4.1.1. High Performance Liquid Chromatography (HPLC)
4.1.2. Thin-Layer Chromatography (TLC)
4.2. Spectroscopic Technology
4.2.1. Fluorescence Spectrophotometry
4.2.2. Infrared (IR) Spectroscopy
4.2.3. Terahertz (THz) Spectroscopy
4.2.4. Surface-Enhanced Raman Spectroscopy (SERS)
4.3. Immunological Technology
Enzyme-Linked Immunosorbent Assay (ELISA)
4.4. Electrochemical Biosensing Technology
4.4.1. Amperometric Biosensors
4.4.2. Electrochemical Impedance Spectroscopy (EIS)
4.4.3. Voltammetry Biosensors
4.4.4. Nanomaterial-Based Biosensors
Characteristic of Nanomaterials Based Electrochemical Biosensors
Signal Generator
Fluorescent Label
Signal Amplification
4.5. Bioinspired Recognition Elements for Biosensors
4.5.1. Antibody
4.5.2. Aptamers
SERS
4.5.3. Molecularly Imprinted Polymers (MIPs)
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AChE | Acetylcholinesterase |
| Adanrs | Aunr@DNTB@Ag Nanorods |
| AFB1 | Aflatoxin B1 |
| AFB2 | Aflatoxin B2 |
| AFBO | Aflatoxin B1–8,9-Epoxide |
| AFG1 | Aflatoxin G1 |
| AFG2 | Aflatoxin G2 |
| AFM1 | Aflatoxin M1 |
| AFM2 | Aflatoxin M2 |
| Afs | Aflatoxins |
| AIE | Induced Emission |
| AOAC | Association of Analytical Communities |
| AuNPs | Gold Nanoparticles |
| BPNSs | Black phosphorus nanosheets |
| CBNs | Carbon-Based Nanomaterials |
| CEN | European Committee for Standardization |
| CODEX | European Commission and The Codex Alimentarius Commission |
| CV | Cyclic Voltammetry |
| CQDS | Carbon Quantum Dots |
| CSNPs | Core-Shell Nanoparticles |
| CYP | Cytochrome P |
| DLLME Dispersive | Liquid-Liquid Microextraction |
| DNTB | 5,5′-Dithiobis (2-Nitrobenzoic Acid) |
| DS | dual-terminal stemmed |
| DTNs | DNA tetrahedral nanostructures |
| ECL | Electrochemiluminescence |
| EIS | Electrochemical Impedance Spectroscopy |
| ELISA | Enzyme-Linked Immunoassay |
| EU | European Union |
| EXO I | Exonuclease I |
| FAO | Food and Agriculture Organization |
| FRET | Fluorescence Resonance Energy Transfer |
| GCEs | Glassy Carbon Electrodes |
| Gdadnts | Gold Nanotriangles (Gnts)-DTNB@Ag-DTNB Nanotriangles |
| GO | Graphene Oxide |
| GOD | Graphene Quantum Dots |
| GPC | Gel Permeation Chromatography |
| GST | Glutathione-S-Transferase |
| HCR | Hybridization Chain Reaction |
| HBV | Hepatitis B Virus |
| HPLC-FD | High-Performance Liquid Chromatography Coupled with A Fluorescence Detector |
| HPLC-MS | High-Performance Liquid Chromatography Coupled with Mass Spectrometry |
| Iacs | Immunoaffinity Columns |
| IMSPE | Immuno magnetic solid phase extraction |
| IRs | Infrared spectroscopy |
| ITC | Isothermal Titration Calorimetry |
| KCN | Editpotassium Cyanide |
| LC-MS-MS | Liquid Chromatography-Tandem Mass Spectrometry |
| LIF | Laser-Induced Fluorescence |
| LLE | Liquid-Liquid Extraction or Partitioning |
| LOD | Limits of Detection |
| LTC | Immune Assay Extraction and Low Temperature Cleanup |
| MIPs | Molecularly Imprinted Polymers |
| MNPs | Metal Nanoparticles |
| MST | Microthermophoresis |
| NH2-DNA1 | Amino-Terminal AFB1 Aptamer |
| NH2-Rh | 5-aminotetramethylrhodamine |
| NIR | Near Infrared |
| PABA-R-GO | Hybrid 4-Aminobenzoic Acid-Reduced Graphene Oxide |
| PB | Prussian Blue |
| QDs | Quantum Dots |
| QuEChERS | Quick, Easy, Cheap, Effective, Rugged, and Safe |
| RAbs | Recombinant Antibodies |
| RS | Raman spectroscopy |
| SELEX | Systematic Evolution of Ligand by the Exponential Enrichment Process |
| SERS | Surface-Enhanced Raman Spectroscopy |
| SH-DNA2 | Complementary Aptamer |
| SPCE | Screen-Printed Carbon Electrode |
| SPE | Solid Phase Extraction |
| THz | Terahertz |
| TLC | Thin-Layer Chromatography |
| TMB | 3,3,5,5-Tetramethylbenzidine |
| UCNPs | Upconversion Nanoparticles |
| UHPLC/UPLC | Ultra-High Performance Liquid Chromatography |
| UHPLC-Qqq-MS/MS | Ultra-High Performance Liquid Chromatography Coupled to A Triple Quadrupole Analyzer |
| US-FDA | US Food and Drugs Administration |
| WHO | The World Health Organization |
| β-CD | β-cyclodextrin |
References
- Shabeer, S.; Asad, S.; Jamal, A.; Ali, A. Aflatoxin Contamination, Its Impact and Management Strategies: An Updated Review. Toxins 2022, 14, 307. [Google Scholar] [CrossRef] [PubMed]
- Skrzydlewski, P.; Twaruzek, M.; Grajewski, J. Cytotoxicity of Mycotoxins and Their Combinations on Different Cell Lines: A Review. Toxins 2022, 14, 244. [Google Scholar] [CrossRef]
- Holban, A.M.; Grumezescu, A.M. Microbial Contamination and Food Degradation; Academic Press: London, UK, 2018; Volume 10. [Google Scholar]
- Pickova, D.; Ostry, V.; Malir, F. A Recent Overview of Producers and Important Dietary Sources of Aflatoxins. Toxins 2021, 13, 186. [Google Scholar] [CrossRef] [PubMed]
- Anater, A.; Manyes, L.; Meca, G.; Ferrer, E.; Luciano, F.B.; Pimpão, C.T.; Font, G. Mycotoxins and their consequences in aquaculture: A review. Aquaculture 2016, 451, 1–10. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, J.; Yan, Z.; Li, Z.; Cheng, Y.; Chang, W. Occurrence and co-occurrence of mycotoxins in nuts and dried fruits from China. Food Control 2018, 88, 181–189. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, D. Worldwide Occurrence of Mycotoxins in Cereals and Cereal-Derived Food Products: Public Health Perspectives of Their Co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef]
- Azam, M.S.; Ahmed, S.; Islam, M.N.; Maitra, P.; Islam, M.M.; Yu, D. Critical Assessment of Mycotoxins in Beverages and Their Control Measures. Toxins 2021, 13, 323. [Google Scholar] [CrossRef]
- Mahato, D.K.; Lee, K.E.; Kamle, M.; Devi, S.; Dewangan, K.N.; Kumar, P.; Kang, S.G. Aflatoxins in Food and Feed: An Overview on Prevalence, Detection and Control Strategies. Front. Microbiol. 2019, 10, 2266. [Google Scholar] [CrossRef]
- Ünüsan, N. Systematic review of mycotoxins in food and feeds in Turkey. Food Control 2019, 97, 1–14. [Google Scholar] [CrossRef]
- Ahlberg, S.H.; Joutsjoki, V.; Korhonen, H.J. Potential of lactic acid bacteria in aflatoxin risk mitigation. Int. J. Food. Microbiol. 2015, 207, 87–102. [Google Scholar] [CrossRef]
- Jard, G.; Liboz, T.; Mathieu, F.; Guyonvarc’h, A.; Lebrihi, A. Review of mycotoxin reduction in food and feed: From prevention in the field to detoxification by adsorption or transformation. Food Addit. Contam. Part A 2011, 28, 1590–1609. [Google Scholar] [CrossRef] [PubMed]
- OECD/FAO. OECD-FAO Agricultural Outlook 2022–2031; OECD Publishing: Paris, France, 2022. [Google Scholar] [CrossRef]
- Mao, X.; Yan, A.; Wan, Y.; Luo, D.; Yang, H. Dispersive Solid-Phase Extraction Using Microporous Sorbent UiO-66 Coupled to Gas Chromatography-Tandem Mass Spectrometry: A QuEChERS-Type Method for the Determination of Organophosphorus Pesticide Residues in Edible Vegetable Oils without Matrix Interference. J. Agric. Food Chem. 2019, 67, 1760–1770. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I.; Taniwaki, M.H.; Cole, M.B. Mycotoxin production in major crops as influenced by growing, harvesting, storage and processing, with emphasis on the achievement of Food Safety Objectives. Food Control 2013, 32, 205–215. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Moradi, M.; Dragoi, E.-N.; Khaneghah, A.M. A review on mycotoxins detection techniques in edible oils. Int. J. Environ. Anal. Chem. 2020, 102, 2125–2139. [Google Scholar] [CrossRef]
- Javanmardi, F.; Khodaei, D.; Sheidaei, Z.; Bashiry, M.; Nayebzadeh, K.; Vasseghian, Y.; Mousavi Khaneghah, A. Decontamination of Aflatoxins in Edible Oils: A Comprehensive Review. Food Rev. Int. 2020, 38, 1–17. [Google Scholar] [CrossRef]
- Shavakhi, F.; Rahmani, A.; Piravi-Vanak, Z. A global systematic review and meta-analysis on prevalence of the aflatoxin B1 contamination in olive oil. J. Food. Sci. Technol. 2022, 1–10. [Google Scholar] [CrossRef]
- He, X.; Zhang, Y.; Yang, X.; Chen, M.; Pang, Y.; Shen, F.; Fang, Y.; Liu, Q.; Hu, Q. Estimating bulk optical properties of AFB1 contaminated edible oils in 300-900 nm by combining double integrating spheres technique with laser induced fluorescence spectroscopy. Food Chem. 2022, 375, 131666. [Google Scholar] [CrossRef]
- Qi, N.; Yu, H.; Yang, C.; Gong, X.; Liu, Y.; Zhu, Y. Aflatoxin B1 in peanut oil from Western Guangdong, China, during 2016-2017. Food Addit. Contam. Part B 2019, 12, 45–51. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Zhang, Q. Advances in the development of detection techniques for mycotoxins in vegetable oil. Chin. J. Chromatogr. 2019, 37, 569–580. [Google Scholar] [CrossRef]
- Ji, J.; Jiang, M.; Zhang, Y.; Hou, J.; Sun, S. Co-occurrence of aflatoxins in plant oil products from China. Food Addit. Contam. Part B 2022, 1–8. [Google Scholar] [CrossRef]
- Einolghozati, M.; Talebi-Ghane, E.; Ranjbar, A.; Mehri, F. Concentration of aflatoxins in edible vegetable oils: A systematic meta-analysis review. Eur. Food Res. Technol. 2021, 247, 2887–2897. [Google Scholar] [CrossRef]
- Bordin, K.; Sawada, M.M.; Rodrigues, C.E.d.C.; da Fonseca, C.R.; Oliveira, C.A.F. Incidence of Aflatoxins in Oil Seeds and Possible Transfer to Oil: A Review. Food Eng. Rev. 2014, 6, 20–28. [Google Scholar] [CrossRef]
- Shephard, G.S. Aflatoxins in peanut oil: Food safety concerns. World Mycotoxin J. 2018, 11, 149–158. [Google Scholar] [CrossRef]
- Wu, L.X.; Ding, X.X.; Li, P.W.; Du, X.H.; Zhou, H.Y.; Bai, Y.Z.; Zhang, L.X. Aflatoxin contamination of peanuts at harvest in China from 2010 to 2013 and its relationship with climatic conditions. Food Control 2016, 60, 117–123. [Google Scholar] [CrossRef]
- Wu, L.; Li, G.; Xu, X.; Zhu, L.; Huang, R.; Chen, X. Application of nano-ELISA in food analysis: Recent advances and challenges. TrAC, Trends Anal. Chem. 2019, 113, 140–156. [Google Scholar] [CrossRef]
- Hayashi, Y.; Matsuda, R.; Maitani, T.; Imai, K.; Nishimura, W.; Ito, K.; Maeda, M. Precision, limit of detection and range of quantitation in competitive ELISA. Anal. Chem. 2004, 76, 1295–1301. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, Y.; Yu, W.; Zhang, J.; Wang, J.; Wan, F.; Kim, Y.; Liu, Y.; Kou, X. Recent advances in aflatoxin B1 detection based on nanotechnology and nanomaterials-A review. Anal. Chim. Acta. 2019, 1069, 1–27. [Google Scholar] [CrossRef]
- Goud, K.Y.; Reddy, K.K.; Satyanarayana, M.; Kummari, S.; Gobi, K.V. A review on recent developments in optical and electrochemical aptamer-based assays for mycotoxins using advanced nanomaterials. Mikrochim. Acta 2019, 187, 29. [Google Scholar] [CrossRef]
- Bhardwaj, H.; Sumana, G.; Marquette, C.A. Gold nanobipyramids integrated ultrasensitive optical and electrochemical biosensor for Aflatoxin B1 detection. Talanta 2021, 222, 121578. [Google Scholar] [CrossRef] [PubMed]
- Danesh, N.M.; Bostan, H.B.; Abnous, K.; Ramezani, M.; Youssefi, K.; Taghdisi, S.M.; Karimi, G. Ultrasensitive detection of aflatoxin B1 and its major metabolite aflatoxin M1 using aptasensors: A review. TrAC Trends Anal. Chem. 2018, 99, 117–128. [Google Scholar] [CrossRef]
- Castillo, G.; Poturnayová, A.; Šnejdárková, M.; Hianik, T.; Spinella, K.; Mosiello, L. Development of electrochemical aptasensor using dendrimers as an immobilization platform for detection of Aflatoxin B1 in food samples. In Proceedings of the 2015 XVIII AISEM Annual Conference, Trento, Italy, 3–5 February 2015; pp. 1–4. [Google Scholar]
- Zheng, M.Z.; Richard, J.L.; Binder, J. A review of rapid methods for the analysis of mycotoxins. Mycopathologia 2006, 161, 261–273. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Robbins, C.A.; Swenson, L.J.; Nealley, M.L.; Gots, R.E.; Kelman, B.J. Health effects of mycotoxins in indoor air: A critical review. Appl. Occup. Environ. Hyg. 2000, 15, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Catanante, G.; Rhouati, A.; Hayat, A.; Marty, J.L. An Overview of Recent Electrochemical Immunosensing Strategies for Mycotoxins Detection. Electroanalysis 2016, 28, 1750–1763. [Google Scholar] [CrossRef]
- Yao, H.; Hruska, Z.; Di Mavungu, J.D. Developments in detection and determination of aflatoxins. World Mycotoxin J. 2015, 8, 181–191. [Google Scholar] [CrossRef]
- Liu, D.; Li, W.; Zhu, C.; Li, Y.; Shen, X.; Li, L.; Yan, X.; You, T. Recent progress on electrochemical biosensing of aflatoxins: A review. TrAC Trends Anal. Chem. 2020, 133, 115966. [Google Scholar] [CrossRef]
- Hui, Y.; Wang, B.; Ren, R.; Zhao, A.; Zhang, F.; Song, S.; He, Y. An electrochemical aptasensor based on DNA-AuNPs-HRP nanoprobes and exonuclease-assisted signal amplification for detection of aflatoxin B1. Food Control 2020, 109, 106902. [Google Scholar] [CrossRef]
- Luan, Y.; Chen, Z.; Xie, G.; Chen, J.; Lu, A.; Li, C.; Fu, H.; Ma, Z.; Wang, J. Rapid Visual Detection of Aflatoxin B1 by Label-Free Aptasensor Using Unmodified Gold Nanoparticles. J. Nanosci. Nanotechnol. 2015, 15, 1357–1361. [Google Scholar] [CrossRef]
- Nguyen, B.H.; Tran, L.D.; Do, Q.P.; Nguyen, H.L.; Tran, N.H.; Nguyen, P.X. Label-free detection of aflatoxin M1 with electrochemical Fe3O4/polyaniline-based aptasensor. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2229–2234. [Google Scholar] [CrossRef]
- Taghdisi, S.M.; Danesh, N.M.; Ramezani, M.; Abnous, K. A new amplified fluorescent aptasensor based on hairpin structure of G-quadruplex oligonucleotide-Aptamer chimera and silica nanoparticles for sensitive detection of aflatoxin B1 in the grape juice. Food Chem. 2018, 268, 342–346. [Google Scholar] [CrossRef]
- Henry, S.H.; Bosch, F.X.; Troxell, T.C.; Bolger, P.M. Reducing liver cancer--global control of aflatoxin. Science 1999, 286, 2453–2454. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; He, B.; Zhang, L.; Li, P.; Zhang, Q.; Ding, X.; Zhang, W. A Structure Identification and Toxicity Assessment of the Degradation Products of Aflatoxin B(1) in Peanut Oil under UV Irradiation. Toxins 2016, 8, 332. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Eş, I.; Raeisi, S.; Fakhri, Y. Aflatoxins in cereals: State of the art. J. Food Saf. 2018, 38, 12532. [Google Scholar] [CrossRef]
- Qureshi, H. Is Aflatoxin B1 A Biomarker for Pathogenic Potential of Aspergillus flavus? J. Cell Sci. 2014, 5, 1000188. [Google Scholar] [CrossRef]
- Ali, N. Aflatoxins in rice: Worldwide occurrence and public health perspectives. Toxicol. Rep. 2019, 6, 1188–1197. [Google Scholar] [CrossRef]
- Sun, C.; Liao, X.; Jia, B.; Shi, L.; Zhang, D.; Wang, R.; Zhou, L.; Kong, W. Development of a ZnCdS@ZnS quantum dots-based label-free electrochemiluminescence immunosensor for sensitive determination of aflatoxin B1 in lotus seed. Mikrochim. Acta 2020, 187, 236. [Google Scholar] [CrossRef]
- Bhardwaj, H.; Sumana, G.; Marquette, C.A. A label-free ultrasensitive microfluidic surface Plasmon resonance biosensor for Aflatoxin B1 detection using nanoparticles integrated gold chip. Food Chem. 2020, 307, 125530. [Google Scholar] [CrossRef]
- Ramalho, L.N.Z.; Porta, L.D.; Rosim, R.E.; Petta, T.; Augusto, M.J.; Silva, D.M.; Ramalho, F.S.; Oliveira, C.A.F. Aflatoxin B1 residues in human livers and their relationship with markers of hepatic carcinogenesis in Sao Paulo, Brazil. Toxicol. Rep. 2018, 5, 777–784. [Google Scholar] [CrossRef]
- Kensler, T.W.; Roebuck, B.D.; Wogan, G.N.; Groopman, J.D. Aflatoxin: A 50-year odyssey of mechanistic and translational toxicology. Toxicol. Sci. 2011, 120 (Suppl. 1), S28–S48. [Google Scholar] [CrossRef] [PubMed]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.J.; Yang, H.I.; Wu, H.C.; Liu, J.; Wang, L.Y.; Lu, S.N.; Lee, M.H.; Jen, C.L.; You, S.L.; Santella, R.M.; et al. Aflatoxin B1 exposure increases the risk of cirrhosis and hepatocellular carcinoma in chronic hepatitis B virus carriers. Int. J. Cancer 2017, 141, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Wild, C.P.; Turner, P.C. The toxicology of aflatoxins as a basis for public health decisions. Mutagenesis 2002, 17, 471–481. [Google Scholar] [CrossRef]
- Zhou, Q.; Tang, D. Recent advances in photoelectrochemical biosensors for analysis of mycotoxins in food. TrAC Trends Anal. Chem. 2020, 124, 115814. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Pashazadeh, P.; Hejazi, M.; de la Guardia, M.; Mokhtarzadeh, A. Recent advances in Nanomaterial-mediated Bio and immune sensors for detection of aflatoxin in food products. TrAC Trends Anal. Chem. 2017, 87, 112–128. [Google Scholar] [CrossRef]
- Keenan, J.; Jolly, P.; Preko, P.; Baidoo, J.; Wang, J.S.; Phillips, T.D.; Williams, J.H.; McGwin, G., Jr. Association Between Aflatoxin B1 Albumin Adduct Levels and Tuberculosis Infection Among HIV+ Ghanaians. Arch. Clin. Microbiol. 2011, 2, 3. [Google Scholar]
- Williams, R.; Airey, M.; Baxter, H.; Forrester, J.; Kennedy-Martin, T.; Girach, A. Epidemiology of diabetic retinopathy and macular oedema: A systematic review. Eye 2004, 18, 963–983. [Google Scholar] [CrossRef]
- Gong, Y.; Hounsa, A.; Egal, S.; Turner, P.C.; Sutcliffe, A.E.; Hall, A.J.; Cardwell, K.; Wild, C.P. Postweaning exposure to aflatoxin results in impaired child growth: A longitudinal study in Benin, West Africa. Environ. Health Persp. 2004, 112, 1334–1338. [Google Scholar] [CrossRef]
- Abdolmaleki, K.; Khedri, S.; Alizadeh, L.; Javanmardi, F.; Oliveira, C.A.F.; Mousavi Khaneghah, A. The mycotoxins in edible oils: An overview of prevalence, concentration, toxicity, detection and decontamination techniques. Trends Food Sci. Technol. 2021, 115, 500–511. [Google Scholar] [CrossRef]
- Claeys, L.; Romano, C.; De Ruyck, K.; Wilson, H.; Fervers, B.; Korenjak, M.; Zavadil, J.; Gunter, M.J.; De Saeger, S.; De Boevre, M.; et al. Mycotoxin exposure and human cancer risk: A systematic review of epidemiological studies. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1449–1464. [Google Scholar] [CrossRef] [PubMed]
- Adeyeye, S.A.O.; Yildiz, F. Fungal mycotoxins in foods: A review. Cogent Food Agric. 2016, 2, 1213127. [Google Scholar] [CrossRef]
- Selamat, J.; Iqbal, S.Z. Food Safety: Basic Concepts, Recent Issues, and Future Challenges; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Directorate-General for Health and Consumers. The Rapid Alert System for Food and Feed (RASFF): Annual Report 2005; Publications Office: Luxembourg, 2006. [Google Scholar]
- Papachristou, A.; Markaki, P. Determination of ochratoxin A in virgin olive oils of Greek origin by immunoaffinity column clean-up and high-performance liquid chromatography. Food Addit. Contam. 2004, 21, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Reddy, K.R. Challenges and issues concerning mycotoxins contamination in oil seeds and their edible oils: Updates from last decade. Food Chem. 2017, 215, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, P.M.; Sasmal, D. Mycotoxins–limits and regulations. Anc. Sci. Life 2001, 20, 1. [Google Scholar]
- Zinedine, A.; Mañes, J. Occurrence and legislation of mycotoxins in food and feed from Morocco. Food Control 2009, 20, 334–344. [Google Scholar] [CrossRef]
- Selvaraj, J.N.; Wang, Y.; Zhou, L.; Zhao, Y.; Xing, F.; Dai, X.; Liu, Y. Recent mycotoxin survey data and advanced mycotoxin detection techniques reported from China: A review. Food Addit. Contam. Part A 2015, 32, 440–452. [Google Scholar] [CrossRef]
- Anukul, N.; Vangnai, K.; Mahakarnchanakul, W. Significance of regulation limits in mycotoxin contamination in Asia and risk management programs at the national level. J. Food Drug Anal. 2013, 21, 227–241. [Google Scholar] [CrossRef]
- Ji, F.; He, D.; Olaniran, A.O.; Mokoena, M.P.; Xu, J.; Shi, J. Occurrence, toxicity, production and detection of Fusarium mycotoxin: A review. Food Prod. Process. Nutr. 2019, 1, 1–14. [Google Scholar] [CrossRef]
- Aiko, V.; Mehta, A. Occurrence, detection and detoxification of mycotoxins. J. Biosci. 2015, 40, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Le, V.T.; Vasseghian, Y.; Dragoi, E.N.; Moradi, M.; Mousavi Khaneghah, A. A review on graphene-based electrochemical sensor for mycotoxins detection. Food Chem. Toxicol. 2021, 148, 111931. [Google Scholar] [CrossRef]
- Turner, N.W.; Bramhmbhatt, H.; Szabo-Vezse, M.; Poma, A.; Coker, R.; Piletsky, S.A. Analytical methods for determination of mycotoxins: An update (2009–2014). Anal. Chim. Acta. 2015, 901, 12–33. [Google Scholar] [CrossRef]
- Ingenbleek, L.; Sulyok, M.; Adegboye, A.; Hossou, S.E.; Kone, A.Z.; Oyedele, A.D.; Kisito, C.; Dembele, Y.K.; Eyangoh, S.; Verger, P.; et al. Regional Sub-Saharan Africa Total Diet Study in Benin, Cameroon, Mali and Nigeria Reveals the Presence of 164 Mycotoxins and Other Secondary Metabolites in Foods. Toxins 2019, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Liu, D.; Li, Y.; Shen, X.; Ma, S.; Liu, Y.; You, T. Ratiometric electrochemical aptasensor for ultrasensitive detection of Ochratoxin A based on a dual signal amplification strategy: Engineering the binding of methylene blue to DNA. Biosens. Bioelectron. 2020, 150, 111814. [Google Scholar] [CrossRef] [PubMed]
- Karunarathna, N.B.; Fernando, C.J.; Munasinghe, D.M.S.; Fernando, R. Occurrence of aflatoxins in edible vegetable oils in Sri Lanka. Food Control 2019, 101, 97–103. [Google Scholar] [CrossRef]
- Wang, N.; Duan, C.; Geng, X.; Li, S.; Ding, K.; Guan, Y. One step rapid dispersive liquid-liquid micro-extraction with in-situ derivatization for determination of aflatoxins in vegetable oils based on high performance liquid chromatography fluorescence detection. Food Chem. 2019, 287, 333–337. [Google Scholar] [CrossRef]
- Nabizadeh, S.; Shariatifar, N.; Shokoohi, E.; Shoeibi, S.; Gavahian, M.; Fakhri, Y.; Azari, A.; Mousavi Khaneghah, A. Prevalence and probabilistic health risk assessment of aflatoxins B1, B2, G1, and G2 in Iranian edible oils. Environ. Sci. Pollut. Res. Int. 2018, 25, 35562–35570. [Google Scholar] [CrossRef]
- Ma, F.; Chen, R.; Li, P.; Zhang, Q.; Zhang, W.; Hu, X. Preparation of an immunoaffinity column with amino-silica gel microparticles and its application in sample cleanup for aflatoxin detection in agri-products. Molecules 2013, 18, 2222–2235. [Google Scholar] [CrossRef]
- Afzali, D.; Ghanbarian, M.; Mostafavi, A.; Shamspur, T.; Ghaseminezhad, S. A novel method for high preconcentration of ultra trace amounts of B(1), B(2), G(1) and G(2) aflatoxins in edible oils by dispersive liquid-liquid microextraction after immunoaffinity column clean-up. J. Chromatogr. A 2012, 1247, 35–41. [Google Scholar] [CrossRef]
- Elzupir, A.O.; Suliman, M.A.; Ibrahim, I.A.; Fadul, M.H.; Elhussein, A.M. Aflatoxins levels in vegetable oils in Khartoum State, Sudan. Mycotoxin Res. 2010, 26, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xu, D. Application of Stable Isotope Dilution and Liquid Chromatography Tandem Mass Spectrometry for Multi-Mycotoxin Analysis in Edible Oils. J. AOAC Int. 2019, 102, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Eom, T.; Cho, H.D.; Kim, J.; Park, M.; An, J.; Kim, M.; Kim, S.H.; Han, S.B. Multiclass mycotoxin analysis in edible oils using a simple solvent extraction method and liquid chromatography with tandem mass spectrometry. Food Addit. Contam. Part A 2017, 34, 2011–2022. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, X.; Wang, Y.; Zhu, F.; Jiang, R.; Ouyang, G. High enrichment and ultra-trace analysis of aflatoxins in edible oils by a modified hollow-fiber liquid-phase microextraction technique. Chem. Commun. 2017, 53, 8988–8991. [Google Scholar] [CrossRef] [PubMed]
- Sharmili, K.; Jinap, S.; Sukor, R. Development, optimization and validation of QuEChERS based liquid chromatography tandem mass spectrometry method for determination of multimycotoxin in vegetable oil. Food Control 2016, 70, 152–160. [Google Scholar] [CrossRef]
- Pereira, V.L.; Fernandes, J.O.; Cunha, S.C. Comparative assessment of three cleanup procedures after QuEChERS extraction for determination of trichothecenes (type A and type B) in processed cereal-based baby foods by GC-MS. Food Chem. 2015, 182, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Nathanail, A.V.; Syvahuoko, J.; Malachova, A.; Jestoi, M.; Varga, E.; Michlmayr, H.; Adam, G.; Sievilainen, E.; Berthiller, F.; Peltonen, K. Simultaneous determination of major type A and B trichothecenes, zearalenone and certain modified metabolites in Finnish cereal grains with a novel liquid chromatography-tandem mass spectrometric method. Anal. Bioanal. Chem. 2015, 407, 4745–4755. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Cavaliere, C.; Foglia, P.; Samperi, R.; Stampachiacchiere, S.; Ventura, S.; Lagana, A. Multiclass analysis of mycotoxins in biscuits by high performance liquid chromatography-tandem mass spectrometry. Comparison of different extraction procedures. J. Chromatogr. A 2014, 1343, 69–78. [Google Scholar] [CrossRef]
- Malachova, A.; Sulyok, M.; Beltran, E.; Berthiller, F.; Krska, R. Optimization and validation of a quantitative liquid chromatography-tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices. J. Chromatogr. A 2014, 1362, 145–156. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Anagnostopoulos, C.; Liapis, K.; Haroutounian, S.A.; Zervas, G. Determination of mycotoxins in feedstuffs and ruminant’s milk using an easy and simple LC-MS/MS multiresidue method. Talanta 2014, 130, 8–19. [Google Scholar] [CrossRef]
- Yang, L.X.; Liu, Y.P.; Miao, H.; Dong, B.; Yang, N.J.; Chang, F.Q.; Yang, L.X.; Sun, J.B. Determination of aflatoxins in edible oil from markets in Hebei Province of China by liquid chromatography-tandem mass spectrometry. Food Addit. Contam. Part B 2011, 4, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Trucksess, M.W.; White, K.D. Determination of aflatoxins B1, B2, G1, and G2 in olive oil, peanut oil, and sesame oil. J. AOAC Int. 2010, 93, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Junsai, T.; Poapolathep, S.; Sutjarit, S.; Giorgi, M.; Zhang, Z.; Logrieco, A.F.; Li, P.; Poapolathep, A. Determination of Multiple Mycotoxins and Their Natural Occurrence in Edible Vegetable Oils Using Liquid Chromatography-Tandem Mass Spectrometry. Foods 2021, 10, 2795. [Google Scholar] [CrossRef]
- Deng, H.; Su, X.; Wang, H. Simultaneous Determination of Aflatoxin B1, Bisphenol A, and 4-Nonylphenol in Peanut Oils by Liquid-Liquid Extraction Combined with Solid-Phase Extraction and Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry. Food Anal. Methods 2017, 11, 1303–1311. [Google Scholar] [CrossRef]
- Hidalgo-Ruiz, J.L.; Romero-Gonzalez, R.; Martinez Vidal, J.L.; Garrido Frenich, A. A rapid method for the determination of mycotoxins in edible vegetable oils by ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem. 2019, 288, 22–28. [Google Scholar] [CrossRef]
- Zhu, Z.; Feng, M.; Zuo, L.; Zhu, Z.; Wang, F.; Chen, L.; Li, J.; Shan, G.; Luo, S.Z. An aptamer based surface plasmon resonance biosensor for the detection of ochratoxin A in wine and peanut oil. Biosens. Bioelectron. 2015, 65, 320–326. [Google Scholar] [CrossRef]
- Idris, Y.M.; Mariod, A.A.; Elnour, I.A.; Mohamed, A.A. Determination of aflatoxin levels in Sudanese edible oils. Food Chem. Toxicol. 2010, 48, 2539–2541. [Google Scholar] [CrossRef]
- Majerus, P.; Graf, N.; Kramer, M. Rapid determination of zearalenone in edible oils by HPLC with fluorescence detection. Mycotoxin Res. 2009, 25, 117–121. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, L.; Yang, H.; Guo, Q.; Shi, H.; Pan, H.; Zhao, L.; Qian, C. Determination of benzo(a)pyrene and aflatoxins (B1, B2, G1, G2) in vegetable oil by GPC-HPLC-FLD. Anal. Methods 2014, 6, 1545–1549. [Google Scholar] [CrossRef]
- Xie, J.; Peng, T.; He, J.L.; Shao, Y.; Fan, C.L.; Chen, Y.; Jiang, W.X.; Chen, M.; Wang, Q.; Pei, X.Y.; et al. Preparation and characterization of an immunoaffinity column for the selective extraction of aflatoxin B1 in 13 kinds of foodstuffs. J. Chromatogr. B 2015, 998–999, 50–56. [Google Scholar] [CrossRef]
- Han, X.; Xu, W.; Zhang, J.; Xu, J.; Li, F. Co-Occurrence of Beauvericin and Enniatins in Edible Vegetable Oil Samples, China. Toxins 2019, 11, 100. [Google Scholar] [CrossRef] [Green Version]
- Drzymala, S.S.; Weiz, S.; Heinze, J.; Marten, S.; Prinz, C.; Zimathies, A.; Garbe, L.A.; Koch, M. Automated solid-phase extraction coupled online with HPLC-FLD for the quantification of zearalenone in edible oil. Anal. Bioanal. Chem. 2015, 407, 3489–3497. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yang, H. Pyrethroid residue determination in organic and conventional vegetables using liquid-solid extraction coupled with magnetic solid phase extraction based on polystyrene-coated magnetic nanoparticles. Food Chem. 2017, 217, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Zuo, J.; Liu, K.; Ma, L.; Li, C.; Li, Y.; Kong, D. Highly selective enrichment of aflatoxin B1 from edible oil using polydopamine-modified magnetic nanomaterials. Food Sci. Technol. 2021, 41, 321–327. [Google Scholar] [CrossRef]
- Ferracane, R.; Tafuri, A.; Logieco, A.; Galvano, F.; Balzano, D.; Ritieni, A. Simultaneous determination of aflatoxin B1 and ochratoxin A and their natural occurrence in Mediterranean virgin olive oil. Food Addit. Contam. 2007, 24, 173–180. [Google Scholar] [CrossRef]
- Giménez, I.; Herrera, M.; Escobar, J.; Ferruz, E.; Lorán, S.; Herrera, A.; Ariño, A. Distribution of deoxynivalenol and zearalenone in milled germ during wheat milling and analysis of toxin levels in wheat germ and wheat germ oil. Food Control 2013, 34, 268–273. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Jiang, Y.; Li, J.; Pan, C. Determination of multiresidues in rapeseed, rapeseed oil, and rapeseed meal by acetonitrile extraction, low-temperature cleanup, and detection by liquid chromatography with tandem mass spectrometry. J. Agric. Food Chem. 2012, 60, 5089–5098. [Google Scholar] [CrossRef]
- Payanan, T.; Leepipatpiboon, N.; Varanusupakul, P. Low-temperature cleanup with solid-phase extraction for the determination of polycyclic aromatic hydrocarbons in edible oils by reversed phase liquid chromatography with fluorescence detection. Food Chem. 2013, 141, 2720–2726. [Google Scholar] [CrossRef]
- Urusov, A.E.; Petrakova, A.V.; Vozniak, M.V.; Zherdev, A.V.; Dzantiev, B.B. Rapid immunoenzyme assay of aflatoxin B1 using magnetic nanoparticles. Sensors 2014, 14, 21843–21857. [Google Scholar] [CrossRef]
- Yu, X.; Li, Z.; Zhao, M.; Lau, S.C.S.; Ru Tan, H.; Teh, W.J.; Yang, H.; Zheng, C.; Zhang, Y. Quantification of aflatoxin B1 in vegetable oils using low temperature clean-up followed by immuno-magnetic solid phase extraction. Food Chem. 2019, 275, 390–396. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, X.; Shen, C.; Qu, B. Determination of 16 mycotoxins in vegetable oils using a QuEChERS method combined with high-performance liquid chromatography-tandem mass spectrometry. Food Addit. Contam. Part A 2017, 34, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Zhang, H.; Wu, L.; Jin, N.; Wang, J.; Jiang, K. Simultaneous determination of zearalenone and its derivatives in edible vegetable oil by gel permeation chromatography and gas chromatography-triple quadrupole mass spectrometry. Food Chem. 2015, 166, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Alshannaq, A.; Yu, J.H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public. Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Vaz, A.; Rodrigues, P.; Veloso, A.; Venâncio, A.; Peres, A. Thin Films Sensor Devices for Mycotoxins Detection in Foods: Applications and Challenges. Chemosensors 2019, 7, 3. [Google Scholar] [CrossRef]
- Shephard, G.S. Current Status of Mycotoxin Analysis: A Critical Review. J. AOAC Int. 2016, 99, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Schwack, W.; Pellissier, E.; Morlock, G. Analysis of unauthorized Sudan dyes in food by high-performance thin-layer chromatography. Anal. Bioanal. Chem. 2018, 410, 5641–5651. [Google Scholar] [CrossRef]
- Gauthier, M.S.; O’Brien, E.L.; Bigornia, S.; Mott, M.; Cacicedo, J.M.; Xu, X.J.; Gokce, N.; Apovian, C.; Ruderman, N. Decreased AMP-activated protein kinase activity is associated with increased inflammation in visceral adipose tissue and with whole-body insulin resistance in morbidly obese humans. Biochem. Biophys. Res. Commun. 2011, 404, 382–387. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.; Wang, W.; Hu, B.; Zhou, L.; Ye, H.; Zeng, X. Changes in molecular structure of chickpea starch during processing treatments: A thin layer chromatography study. Food Chem. 2018, 243, 186–191. [Google Scholar] [CrossRef]
- Villani, T.S.; Reichert, W.; Ferruzzi, M.G.; Pasinetti, G.M.; Simon, J.E.; Wu, Q. Chemical investigation of commercial grape seed derived products to assess quality and detect adulteration. Food Chem. 2015, 170, 271–280. [Google Scholar] [CrossRef]
- Wacoo, A.P.; Wendiro, D.; Vuzi, P.C.; Hawumba, J.F. Methods for Detection of Aflatoxins in Agricultural Food Crops. J. Appl. Chem. 2014, 2014, 706291. [Google Scholar] [CrossRef]
- Cabezudo, I.; Salazar, M.O.; Ramallo, I.A.; Furlan, R.L.E. Effect-directed analysis in food by thin-layer chromatography assays. Food Chem. 2022, 390, 132937. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhang, J.; Wang, P.; Wang, Y.; Chen, J. Thin-layer chromatography of mycotoxins and comparison with other chromatographic methods. J. Chromatogr. A 1998, 815, 3–20. [Google Scholar] [CrossRef]
- Sereshti, H.; Poursorkh, Z.; Aliakbarzadeh, G.; Zarre, S. Quality control of saffron and evaluation of potential adulteration by means of thin layer chromatography-image analysis and chemometrics methods. Food Control 2018, 90, 48–57. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, C.; Lu, C.; Zhou, S.; Tian, G.; He, L.; Bao, Y.; Fauconnier, M.L.; Xiao, H.; Zheng, J. Simultaneous determination of 14 bioactive citrus flavonoids using thin-layer chromatography combined with surface enhanced Raman spectroscopy. Food Chem. 2021, 338, 128115. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G.E.; Klingelhofer, I. Liquid chromatography-bioassay-mass spectrometry for profiling of physiologically active food. Anal. Chem. 2014, 86, 8289–8295. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Yao, H.; Hruska, Z.; Burger, L.W.; Rajasekaran, K.; Bhatnagar, D. Recent development of optical methods in rapid and non-destructive detection of aflatoxin and fungal contamination in agricultural products. TrAC Trends Anal. Chem. 2018, 100, 65–81. [Google Scholar] [CrossRef]
- Wu, Q.; Xie, L.; Xu, H. Determination of toxigenic fungi and aflatoxins in nuts and dried fruits using imaging and spectroscopic techniques. Food Chem. 2018, 252, 228–242. [Google Scholar] [CrossRef]
- Yadav, N.; Yadav, S.S.; Chhillar, A.K.; Rana, J.S. An overview of nanomaterial based biosensors for detection of Aflatoxin B1 toxicity in foods. Food Chem. Toxicol. 2021, 152, 112201. [Google Scholar] [CrossRef]
- Karoui, R.; Blecker, C. Fluorescence Spectroscopy Measurement for Quality Assessment of Food Systems—A Review. Food Bioprocess Technol. 2010, 4, 364–386. [Google Scholar] [CrossRef]
- Ma, H.; Ran, C.; Li, M.; Gao, J.; Wang, X.; Zhang, L.; Bian, J.; Li, J.; Jiang, Y. Graphene oxide-coated stir bar sorptive extraction of trace aflatoxins from soy milk followed by high performance liquid chromatography-laser-induced fluorescence detection. Food Addit. Contam. Part A 2018, 35, 772–781. [Google Scholar] [CrossRef]
- Hruska, Z.; Yao, H.; Kincaid, R.; Brown, R.; Cleveland, T.; Bhatnagar, D. Fluorescence Excitation–Emission Features of Aflatoxin and Related Secondary Metabolites and Their Application for Rapid Detection of Mycotoxins. Food Bioprocess Technol. 2014, 7, 1195–1201. [Google Scholar] [CrossRef]
- Chen, M.; He, X.; Pang, Y.; Shen, F.; Fang, Y.; Hu, Q. Laser induced fluorescence spectroscopy for detection of Aflatoxin B1 contamination in peanut oil. J. Food Meas. Charact. 2021, 15, 2231–2239. [Google Scholar] [CrossRef]
- Qu, J.H.; Liu, D.; Cheng, J.H.; Sun, D.W.; Ma, J.; Pu, H.; Zeng, X.A. Applications of near-infrared spectroscopy in food safety evaluation and control: A review of recent research advances. Crit. Rev. Food Sci. Nutr. 2015, 55, 1939–1954. [Google Scholar] [CrossRef] [PubMed]
- Rohman, A. Infrared spectroscopy for quantitative analysis and oil parameters of olive oil and virgin coconut oil: A review. Int. J. Food Prop. 2016, 20, 1447–1456. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Uddin, M.; Okazaki, E. Classification of Fresh and Frozen-thawed Fish by Near-infrared Spectroscopy. J. Food Sci. 2004, 69, C665–C668. [Google Scholar] [CrossRef]
- Kurz, C.; Leitenberger, M.; Carle, R.; Schieber, A. Evaluation of fruit authenticity and determination of the fruit content of fruit products using FT-NIR spectroscopy of cell wall components. Food Chem. 2010, 119, 806–812. [Google Scholar] [CrossRef]
- Kuligowski, J.; Carrión, D.; Quintás, G.; Garrigues, S.; de la Guardia, M. Direct determination of polymerised triacylglycerides in deep-frying vegetable oil by near infrared spectroscopy using Partial Least Squares regression. Food Chem. 2012, 131, 353–359. [Google Scholar] [CrossRef]
- Balabin, R.M.; Smirnov, S.V. Melamine detection by mid- and near-infrared (MIR/NIR) spectroscopy: A quick and sensitive method for dairy products analysis including liquid milk, infant formula, and milk powder. Talanta 2011, 85, 562–568. [Google Scholar] [CrossRef]
- Ravikanth, L.; Jayas, D.S.; White, N.D.G.; Fields, P.G.; Sun, D.-W. Extraction of Spectral Information from Hyperspectral Data and Application of Hyperspectral Imaging for Food and Agricultural Products. Food Bioprocess Technol. 2016, 10, 1–33. [Google Scholar] [CrossRef]
- Morsy, N.; Sun, D.W. Robust linear and non-linear models of NIR spectroscopy for detection and quantification of adulterants in fresh and frozen-thawed minced beef. Meat Sci. 2013, 93, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liu, T.; Liu, Y. FT-NIR and Confocal Microscope Raman Spectroscopic Studies of Sesame Oil Adulteration. In Proceedings of the Computer and Computing Technologies in Agriculture V, Berlin, Heidelberg, 29 October 2011; pp. 24–31. [Google Scholar]
- Mignani, A.G.; Ciaccheri, L.; Ottevaere, H.; Thienpont, H.; Conte, L.; Marega, M.; Cichelli, A.; Attilio, C.; Cimato, A. Visible and near-infrared absorption spectroscopy by an integrating sphere and optical fibers for quantifying and discriminating the adulteration of extra virgin olive oil from Tuscany. Anal. Bioanal. Chem. 2011, 399, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Sinelli, N.; Cerretani, L.; Egidio, V.D.; Bendini, A.; Casiraghi, E. Application of near (NIR) infrared and mid (MIR) infrared spectroscopy as a rapid tool to classify extra virgin olive oil on the basis of fruity attribute intensity. Food Res. Int. 2010, 43, 369–375. [Google Scholar] [CrossRef]
- Luna, A.S.; da Silva, A.P.; Pinho, J.S.; Ferre, J.; Boque, R. Rapid characterization of transgenic and non-transgenic soybean oils by chemometric methods using NIR spectroscopy. Spectrochim. Acta Part A 2013, 100, 115–119. [Google Scholar] [CrossRef]
- Yao, W.; Liu, R.; Xu, Z.; Zhang, Y.; Deng, Y.; Guo, H.; Cozzolino, D. Rapid Determination of Aflatoxin B1 Contamination in Peanut Oil by Fourier Transform Near-Infrared Spectroscopy. J. Spectro. 2022, 2022, 1–9. [Google Scholar] [CrossRef]
- Song, H.; Li, F.; Guang, P.; Yang, X.; Pan, H.; Huang, F. Detection of Aflatoxin B1 in Peanut Oil Using Attenuated Total Reflection Fourier Transform Infrared Spectroscopy Combined with Partial Least Squares Discriminant Analysis and Support Vector Machine Models. J. Food Prot. 2021, 84, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.; He, C.; Xie, M.; Luo, H.; Wang, Y.; Zhang, J. Rapid screen of aflatoxin-contaminated peanut oil using Fourier transform infrared spectroscopy combined with multivariate decision tree. Int. J. Food Sci. Technol. 2018, 53, 2386–2393. [Google Scholar] [CrossRef]
- Rawson, A.; Sunil, C.K. Recent Advances in Terahertz Time-Domain Spectroscopy and Imaging Techniques for Automation in Agriculture and Food Sector. Food Anal. Methods 2021, 15, 498–526. [Google Scholar] [CrossRef]
- Jan, A.; Pandiselvam, R.; Kothakota, A.; Sruthi, N.; Ramesh, S. Terahertz Spectroscopy Imaging Technique: Non-Destructive Tool For Evaluation Of Quality And Safety Of Food Products. In Handbook of Research on Food Processing and Preservation Technologies; Apple Academic Press: New York, NY, USA, 2021; pp. 141–157. [Google Scholar]
- Feng, C.H.; Otani, C. Terahertz spectroscopy technology as an innovative technique for food: Current state-of-the-Art research advances. Crit. Rev. Food Sci. Nutr. 2021, 61, 2523–2543. [Google Scholar] [CrossRef]
- Afsah-Hejri, L.; Hajeb, P.; Ara, P.; Ehsani, R.J. A Comprehensive Review on Food Applications of Terahertz Spectroscopy and Imaging. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1563–1621. [Google Scholar] [CrossRef]
- Kawano, Y.; Ishibashi, K. An on-chip near-field terahertz probe and detector. Nat. Photonics 2008, 2, 618–621. [Google Scholar] [CrossRef]
- McIntosh, A.I.; Yang, B.; Goldup, S.M.; Watkinson, M.; Donnan, R.S. Terahertz spectroscopy: A powerful new tool for the chemical sciences? Chem. Soc. Rev. 2012, 41, 2072–2082. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Sun, D.-W.; Pu, H. Emerging non-destructive terahertz spectroscopic imaging technique: Principle and applications in the agri-food industry. Trends Food Sci. Technol. 2017, 67, 93–105. [Google Scholar] [CrossRef]
- Chen, M.; Lijuan, X. A Preliminary Study of Aflatoxin B1 Detection in Peanut Oil by Terahertz Time-Domain Spectroscopy. Trans. ASABE 2014, 57, 1793–1799. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, P.; Wu, C.; Liu, C.; Yang, J.; Zheng, L. Rapid determination of aflatoxin B1 concentration in soybean oil using terahertz spectroscopy with chemometric methods. Food Chem. 2019, 293, 213–219. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, L.; Ying, Y. Overview of imaging methods based on terahertz time-domain spectroscopy. Appl. Spectrosc. Rev. 2021, 57, 249–264. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, J.; Sun, D.W. Raman spectroscopic techniques for detecting structure and quality of frozen foods: Principles and applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 2623–2639. [Google Scholar] [CrossRef]
- Wu, Z.; Pu, H.; Sun, D.-W. Fingerprinting and tagging detection of mycotoxins in agri-food products by surface-enhanced Raman spectroscopy: Principles and recent applications. Trends Food Sci. Technol. 2021, 110, 393–404. [Google Scholar] [CrossRef]
- He, H.; Sun, D.W.; Pu, H.; Chen, L.; Lin, L. Applications of Raman spectroscopic techniques for quality and safety evaluation of milk: A review of recent developments. Crit. Rev. Food Sci. Nutr. 2019, 59, 770–793. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, X.; Li, M.; Jiang, H.; Chen, Q. Feasibility study on Raman spectra-based deep learning models for monitoring the contamination degree and level of aflatoxin B1 in edible oil. Microchem. J. 2022, 180, 107613. [Google Scholar] [CrossRef]
- Zhu, C.; Jiang, H.; Chen, Q. High Precisive Prediction of Aflatoxin B1 in Pressing Peanut Oil Using Raman Spectra Combined with Multivariate Data Analysis. Foods 2022, 11, 1565. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, G.; Mehedi, H.M.; Ouyang, Q.; Chen, Q. A universal SERS aptasensor based on DTNB labeled GNTs/Ag core-shell nanotriangle and CS-Fe3O4 magnetic-bead trace detection of Aflatoxin B1. Anal. Chim. Acta. 2017, 986, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yang, M.; Yang, X.; Li, H.; Guo, Z.; Rahma, M. A large Raman scattering cross-section molecular embedded SERS aptasensor for ultrasensitive Aflatoxin B1 detection using CS-Fe3O4 for signal enrichment. Spectrochim. Acta Part A 2018, 189, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Hou, Q.; Waterhouse, G.I.N.; Hou, J.; Ai, S.; Li, X. A simple aptamer-based fluorescent aflatoxin B1 sensor using humic acid as quencher. Talanta 2019, 205, 120131. [Google Scholar] [CrossRef]
- Chen, Q.; Jiao, T.; Yang, M.; Li, H.; Ahmad, W.; Hassan, M.M.; Guo, Z.; Ali, S. Pre etched Ag nanocluster as SERS substrate for the rapid quantification of AFB1 in peanut oil via DFT coupled multivariate calibration. Spectrochim. Acta Part A 2020, 239, 118411. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Xiong, C.; Deng, Q.; Zhang, X.; Wang, S.; Chen, M.M. An ultrasensitive CH3NH3PbBr3 quantum dots@SiO2-based electrochemiluminescence sensing platform using an organic electrolyte for aflatoxin B1 detection in corn oil. Food Chem. 2022, 390, 133200. [Google Scholar] [CrossRef]
- He, H.; Sun, D.W.; Pu, H.; Huang, L. Bridging Fe3O4@Au nanoflowers and Au@Ag nanospheres with aptamer for ultrasensitive SERS detection of aflatoxin B1. Food Chem. 2020, 324, 126832. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, D.-W.; Pu, H.; Wei, Q. A simple and sensitive aptasensor based on SERS for trace analysis of kanamycin in milk. J. Food Meas. Charact. 2020, 14, 3184–3193. [Google Scholar] [CrossRef]
- Toh, S.Y.; Citartan, M.; Gopinath, S.C.; Tang, T.H. Aptamers as a replacement for antibodies in enzyme-linked immunosorbent assay. Biosens. Bioelectron. 2015, 64, 392–403. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, D.W.; Pu, H.; Wei, Q. Ultrasensitive analysis of kanamycin residue in milk by SERS-based aptasensor. Talanta 2019, 197, 151–158. [Google Scholar] [CrossRef]
- Azadniya, E.; Goldoni, L.; Bandiera, T.; Morlock, G.E. Same analytical method for both (bio)assay and zone isolation to identify/quantify bioactive compounds by quantitative nuclear magnetic resonance spectroscopy. J. Chromatogr. A 2020, 1628, 461434. [Google Scholar] [CrossRef] [PubMed]
- Malu, S.P.; Donatus, R.B.; Ugye, J.T.; Imarenezor, E.P.K.; Leubem, A. Determination of Aflaxtoxin in Some Edible Oils Obtained from Makurdi Metropolis, North Central Nigeria. Am. J. Chem. Appl. 2017, 4, 36–40. [Google Scholar]
- Zhang, Y.; Li, M.; Cui, Y.; Hong, X.; Du, D. Using of Tyramine Signal Amplification to Improve the Sensitivity of ELISA for Aflatoxin B1 in Edible Oil Samples. Food Anal. Methods 2018, 11, 2553–2560. [Google Scholar] [CrossRef]
- Pundir, C.S.; Yadav, N.; Chhillar, A.K. Occurrence, synthesis, toxicity and detection methods for acrylamide determination in processed foods with special reference to biosensors: A review. Trends Food Sci. Technol. 2019, 85, 211–225. [Google Scholar] [CrossRef]
- Wang, X.; Shan, Y.; Gong, M.; Jin, X.; Lv, l.; Jiang, M.; Xu, J. A novel electrochemical sensor for ochratoxin A based on the hairpin aptamer and double report DNA via multiple signal amplification strategy. Sens. Actuators B 2019, 281, 595–601. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, B.; Shen, C.; Cheong, L.Z. Direct, selective and ultrasensitive electrochemical biosensing of methyl parathion in vegetables using Burkholderia cepacia lipase@MOF nanofibers-based biosensor. Talanta 2019, 197, 356–362. [Google Scholar] [CrossRef]
- Lin, T.; Shen, Y. Fabricating electrochemical aptasensors for detecting aflatoxin B1 via layer-by-layer self-assembly. J. Electroanal. Chem. 2020, 870, 114247. [Google Scholar] [CrossRef]
- Xuan, Z.; Liu, H.; Ye, J.; Li, L.; Tian, W.; Wang, S. Reliable and disposable quantum dot-based electrochemical immunosensor for aflatoxin B1 simplified analysis with automated magneto-controlled pretreatment system. Anal. Bioanal. Chem. 2020, 412, 7615–7625. [Google Scholar] [CrossRef]
- Ben Rejeb, I.; Arduini, F.; Arvinte, A.; Amine, A.; Gargouri, M.; Micheli, L.; Bala, C.; Moscone, D.; Palleschi, G. Development of a bio-electrochemical assay for AFB1 detection in olive oil. Biosens. Bioelectron. 2009, 24, 1962–1968. [Google Scholar] [CrossRef]
- Kim, M.; Iezzi, R., Jr.; Shim, B.S.; Martin, D.C. Impedimetric Biosensors for Detecting Vascular Endothelial Growth Factor (VEGF) Based on Poly(3,4-ethylene dioxythiophene) (PEDOT)/Gold Nanoparticle (Au NP) Composites. Front. Chem. 2019, 7, 234. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, B.; Tian, Y.; Guo, Q.; Yang, X.; Nie, G. An enhanced photoelectrochemical sensor for aflatoxin B1 detection based on organic-inorganic heterojunction nanomaterial: Poly(5-formylindole)/NiO. Mikrochim. Acta 2020, 187, 467. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Panchal, A.; Yadav, N.; Narang, J. Analytical techniques for the detection of glycated haemoglobin underlining the sensors. Int. J. Biol. Macromol. 2020, 155, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, Y.; Hu, C.; Wu, H.; Yang, Y.; Huang, C.; Jia, N. Highly sensitive electrochemical impedance spectroscopy immunosensor for the detection of AFB1 in olive oil. Food Chem. 2015, 176, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Perumal, V.; Hashim, U. Advances in biosensors: Principle, architecture and applications. J. Appl. Biomed. 2014, 12, 1–15. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Chu, Y.; Ma, H.; Li, Y.; Wu, D.; Du, B.; Wei, Q. Disposable competitive-type immunoassay for determination of aflatoxin B1 via detection of copper ions released from Cu-apatite. Talanta 2016, 147, 556–560. [Google Scholar] [CrossRef]
- Farka, Z.; Jurik, T.; Kovar, D.; Trnkova, L.; Skladal, P. Nanoparticle-Based Immunochemical Biosensors and Assays: Recent Advances and Challenges. Chem. Rev. 2017, 117, 9973–10042. [Google Scholar] [CrossRef]
- Wang, X.; Wu, X.; Lu, Z.; Tao, X. Comparative Study of Time-Resolved Fluorescent Nanobeads, Quantum Dot Nanobeads and Quantum Dots as Labels in Fluorescence Immunochromatography for Detection of Aflatoxin B1 in Grains. Biomolecules 2020, 10, 575. [Google Scholar] [CrossRef]
- Perez-Fernandez, B.; de la Escosura-Muniz, A. Electrochemical biosensors based on nanomaterials for aflatoxins detection: A review (2015–2021). Anal. Chim. Acta. 2022, 1212, 339658. [Google Scholar] [CrossRef]
- Sun, C.; Li, H.; Koidis, A.; Chen, Q. Quantifying Aflatoxin B1 in peanut oil using fabricating fluorescence probes based on upconversion nanoparticles. Spectrochim. Acta Part A 2016, 165, 120–126. [Google Scholar] [CrossRef]
- Xie, G.; Zhu, M.; Liu, Z.; Zhang, B.; Shi, M.; Wang, S. Development and evaluation of the magnetic particle-based chemiluminescence immunoassay for rapid and quantitative detection of Aflatoxin B1 in foodstuff. Food Agric. Immunol. 2017, 29, 564–576. [Google Scholar] [CrossRef]
- Neelam; Chhillar, A.K.; Rana, J.S. Enzyme nanoparticles and their biosensing applications: A review. Anal. Biochem. 2019, 581, 113345. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Bond, A.M.; Zhai, J.; Zhang, J. Utilization of nanoparticle labels for signal amplification in ultrasensitive electrochemical affinity biosensors: A review. Anal. Chim. Acta. 2013, 797, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Peltomaa, R.; Benito-Pena, E.; Moreno-Bondi, M.C. Bioinspired recognition elements for mycotoxin sensors. Anal. Bioanal. Chem. 2018, 410, 747–771. [Google Scholar] [CrossRef] [PubMed]
- Miklos, G.; Angeli, C.; Ambrus, A.; Nagy, A.; Kardos, V.; Zentai, A.; Kerekes, K.; Farkas, Z.; Jozwiak, A.; Bartok, T. Detection of Aflatoxins in Different Matrices and Food-Chain Positions. Front. Microbiol. 2020, 11, 1916. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Q.; Wu, W. Progress on Structured Biosensors for Monitoring Aflatoxin B1 From Biofilms: A Review. Front. Microbiol. 2020, 11, 408. [Google Scholar] [CrossRef]
- Langone, J.J.; Van Vunakis, H. Aflatoxin B1: Specific antibodies and their use in radioimmunoassay. J. Natl. Cancer Inst. 1976, 56, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Bazin, I.; Tria, S.A.; Hayat, A.; Marty, J.L. New biorecognition molecules in biosensors for the detection of toxins. Biosens. Bioelectron. 2017, 87, 285–298. [Google Scholar] [CrossRef]
- Ma, H.; Sun, J.; Zhang, Y.; Bian, C.; Xia, S.; Zhen, T. Label-free immunosensor based on one-step electrodeposition of chitosan-gold nanoparticles biocompatible film on Au microelectrode for determination of aflatoxin B1 in maize. Biosens. Bioelectron. 2016, 80, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Tian, Y.; Shen, Q.; Liu, R.; Shi, R.; Wang, H.; Yang, Z. A novel nanobody and mimotope based immunoassay for rapid analysis of aflatoxin B1. Talanta 2019, 195, 55–61. [Google Scholar] [CrossRef]
- Shi, L.; Wang, Z.; Yang, G.; Yang, H.; Zhao, F. A novel electrochemical immunosensor for aflatoxin B1 based on Au nanoparticles-poly 4-aminobenzoic acid supported graphene. Appl. Surf. Sci. 2020, 527, 146934. [Google Scholar] [CrossRef]
- Pfeiffer, F.; Mayer, G. Selection and Biosensor Application of Aptamers for Small Molecules. Front. Chem. 2016, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.Y.; Byun, J. Nucleic Acid aptamers: New methods for selection, stabilization, and application in biomedical science. Biomol. Ther. 2013, 21, 423–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinjung, F.; Klussmann, S.; Erdmann, V.; Scheller, F.; Fürste, J.; Bier, F. High-affinity RNA as a recognition element in a biosensor. Anal. Chem. 1998, 70, 328–331. [Google Scholar] [CrossRef]
- Ma, X.; Wang, W.; Chen, X.; Xia, Y.; Wu, S.; Duan, N.; Wang, Z. Selection, identification, and application of Aflatoxin B1 aptamer. Eur. Food Res. Technol. 2014, 238, 919–925. [Google Scholar] [CrossRef]
- Vidal, J.C.; Bonel, L.; Ezquerra, A.; Hernandez, S.; Bertolin, J.R.; Cubel, C.; Castillo, J.R. Electrochemical affinity biosensors for detection of mycotoxins: A review. Biosens. Bioelectron. 2013, 49, 146–158. [Google Scholar] [CrossRef]
- Rivas, L.; Mayorga-Martinez, C.C.; Quesada-Gonzalez, D.; Zamora-Galvez, A.; de la Escosura-Muniz, A.; Merkoci, A. Label-free impedimetric aptasensor for ochratoxin-A detection using iridium oxide nanoparticles. Anal. Chem. 2015, 87, 5167–5172. [Google Scholar] [CrossRef]
- McKeague, M.; Bradley, C.R.; De Girolamo, A.; Visconti, A.; Miller, J.D.; Derosa, M.C. Screening and initial binding assessment of fumonisin b(1) aptamers. Int. J. Mol. Sci. 2010, 11, 4864–4881. [Google Scholar] [CrossRef]
- Sabet, F.S.; Hosseini, M.; Khabbaz, H.; Dadmehr, M.; Ganjali, M.R. FRET-based aptamer biosensor for selective and sensitive detection of aflatoxin B1 in peanut and rice. Food Chem. 2017, 220, 527–532. [Google Scholar] [CrossRef]
- Chen, X.; Bai, X.; Li, H.; Zhang, B. Aptamer-based microcantilever array biosensor for detection of fumonisin B-1. RSC Adv. 2015, 5, 35448–35452. [Google Scholar] [CrossRef]
- Guo, X.; Wen, F.; Zheng, N.; Li, S.; Fauconnier, M.L.; Wang, J. A qPCR aptasensor for sensitive detection of aflatoxin M1. Anal. Bioanal. Chem. 2016, 408, 5577–5584. [Google Scholar] [CrossRef]
- Samokhvalov, A.V.; Safenkova, I.V.; Eremin, S.A.; Zherdev, A.V.; Dzantiev, B.B. Use of anchor protein modules in fluorescence polarisation aptamer assay for ochratoxin A determination. Anal. Chim. Acta 2017, 962, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lu, Z.; Tan, X.; Xiao, X.; Wang, P.; Wu, L.; Shao, K.; Yin, W.; Han, H. Ultrasensitive detection of aflatoxin B1 by SERS aptasensor based on exonuclease-assisted recycling amplification. Biosens. Bioelectron. 2017, 97, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Abnous, K.; Danesh, N.M.; Alibolandi, M.; Ramezani, M.; Sarreshtehdar Emrani, A.; Zolfaghari, R.; Taghdisi, S.M. A new amplified pi-shape electrochemical aptasensor for ultrasensitive detection of aflatoxin B1. Biosens. Bioelectron. 2017, 94, 374–379. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Pang, G.; Zhang, Y.; Guo, S. Tuning the Aggregation/Disaggregation Behavior of Graphene Quantum Dots by Structure-Switching Aptamer for High-Sensitivity Fluorescent Ochratoxin A Sensor. Anal. Chem. 2017, 89, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shen, Y.; Shen, G.; Wang, S.; Shen, G.; Yu, R. Electrochemical immunosensor based on Pd-Au nanoparticles supported on functionalized PDDA-MWCNT nanocomposites for aflatoxin B1 detection. Anal. Biochem. 2016, 494, 10–15. [Google Scholar] [CrossRef]
- Ren, S.; Li, Y.; Guo, Q.; Peng, Y.; Bai, J.; Ning, B.; Gao, Z. Turn-on fluorometric immunosensor for diethylstilbestrol based on the use of air-stable polydopamine-functionalized black phosphorus and upconversion nanoparticles. Mikrochim. Acta 2018, 185, 429. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ali, S.; Ouyang, Q.; Wang, L.; Rong, Y.; Chen, Q. Highly specific and sensitive detection of aflatoxin B1 in food based on upconversion nanoparticles-black phosphorus nanosheets aptasensor. Microchem. J. 2021, 171, 106847. [Google Scholar] [CrossRef]
- Xia, X.; Wang, H.; Yang, H.; Deng, S.; Deng, R.; Dong, Y.; He, Q. Dual-Terminal Stemmed Aptamer Beacon for Label-Free Detection of Aflatoxin B1 in Broad Bean Paste and Peanut Oil via Aggregation-Induced Emission. J. Agric. Food Chem. 2018, 66, 12431–12438. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, Y.; Li, X.; Wang, S.; Dong, Y. Development of a chimeric aptamer and an AuNPs aptasensor for highly sensitive and specific identification of Aflatoxin B1. Sens. Actuators B 2020, 319, 128250. [Google Scholar] [CrossRef]
- Zhong, T.; Li, S.; Li, X.; JiYe, Y.; Mo, Y.; Chen, L.; Zhang, Z.; Wu, H.; Li, M.; Luo, Q. A label-free electrochemical aptasensor based on AuNPs-loaded zeolitic imidazolate framework-8 for sensitive determination of aflatoxin B1. Food Chem. 2022, 384, 132495. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Q.; Hu, X.; Wang, F.; Hu, M.; Yu, Q.; Zhang, G. Electrochemical immunosensor based on AuNPs/Zn/Ni-ZIF-8-800@graphene for rapid detection of aflatoxin B1 in peanut oil. Anal. Biochem. 2022, 650, 114710. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, F.; Li, Q.; Wang, L.; Fan, C. Isothermal Amplification of Nucleic Acids. Chem. Rev. 2015, 115, 12491–12545. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cecconello, A.; Idili, A.; Ricci, F.; Willner, I. Triplex DNA Nanostructures: From Basic Properties to Applications. Angew. Chem. Int. Ed. Engl. 2017, 56, 15210–15233. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhang, H.; Zhao, L.; Wang, Y.; Chen, X.; Zhai, H.; Tian, M.; Zhao, R.; Wang, T.; Xu, H.; et al. A novel aptasensor based on HCR and G-quadruplex DNAzyme for fluorescence detection of Carcinoembryonic Antigen. Talanta 2021, 221, 121451. [Google Scholar] [CrossRef]
- Zhang, Q. Application of Hybridization Chain Reaction (HCR) in Electrochemical Analysis. Int. J. Electrochem. Sci. 2022, 17, 220227. [Google Scholar] [CrossRef]
- Dirks, R.M.; Pierce, N.A. Triggered amplification by hybridization chain reaction. Proc. Natl. Acad. Sci. USA 2004, 101, 15275–15278. [Google Scholar] [CrossRef]
- Evanko, D. Hybridization chain reaction. Nat. Methods 2004, 1, 186. [Google Scholar] [CrossRef]
- Zhou, R.; Zeng, Z.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Chen, C. Traditional and new applications of the HCR in biosensing and biomedicine. Analyst 2021, 146, 7087–7103. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, F.; Yang, Q.; Wu, W. Graphene oxide quantum dots based nanotree illuminates AFB1: Dual signal amplified aptasensor detection AFB1. Sens. Actuators B 2021, 345, 130387. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, M.; Li, M.; Xiao, Z.; Ling, L. Hybridization chain reaction and DNAzyme-based dual signal amplification strategy for sensitive fluorescent sensing of aflatoxin B1 by using the pivot of triplex DNA. Food Res. Int. 2022, 158, 111538. [Google Scholar] [CrossRef]
- Molaei, M.J. Principles, mechanisms, and application of carbon quantum dots in sensors: A review. Anal. Methods 2020, 12, 1266–1287. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Tajik, S.; Dourandish, Z.; Zhang, K.; Beitollahi, H.; Le, Q.V.; Jang, H.W.; Shokouhimehr, M. Carbon and graphene quantum dots: A review on syntheses, characterization, biological and sensing applications for neurotransmitter determination. RSC Adv. 2020, 10, 15406–15429. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zheng, M.; Ma, H.; Xuan, Z.; Tian, W.; Liu, H.; Wang, S.; Zhang, Y. Development and Validation of an Automated Magneto-Controlled Pretreatment for Chromatography-Free Detection of Aflatoxin B1 in Cereals and Oils through Atomic Absorption Spectroscopy. Toxins 2022, 14, 454. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, X.; Wang, Y.; Zheng, X.; Li, C.M. Aptamer based fluorescence recovery assay for aflatoxin B1 using a quencher system composed of quantum dots and graphene oxide. Microchim. Acta 2014, 182, 571–578. [Google Scholar] [CrossRef]
- Hussain, A.; Sun, D.W.; Pu, H. Bimetallic core shelled nanoparticles (Au@AgNPs) for rapid detection of thiram and dicyandiamide contaminants in liquid milk using SERS. Food Chem. 2020, 317, 126429. [Google Scholar] [CrossRef]
- Jiao, T.; Ahmad, W.; Zhu, J.; Hassan, M.M.; Wang, J.; Rong, Y.; Guo, Z.; Li, H.; Ding, Z.; Lv, C.; et al. Aggregation triggered aflatoxin B1 determination in foodstuff employing 5-aminotetramethylrhodamine decorated gold–silver core–shell nanoparticles in surface enhanced Raman scattering. Sens. Actuators B 2021, 331, 129424. [Google Scholar] [CrossRef]
- Xiong, Z.; Wang, Q.; Xie, Y.; Li, N.; Yun, W.; Yang, L. Simultaneous detection of aflatoxin B1 and ochratoxin A in food samples by dual DNA tweezers nanomachine. Food Chem. 2021, 338, 128122. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.S.; Wei, M.; Wei, W.; Liu, Y.; Liu, S. Electrochemical aptasensor for aflatoxin B1 based on smart host-guest recognition of β-cyclodextrin polymer. Biosens. Bioelectron. 2019, 129, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, O.S.; Bedwell, T.S.; Esen, C.; Garcia-Cruz, A.; Piletsky, S.A. Molecularly Imprinted Polymers in Electrochemical and Optical Sensors. Trends Biotechnol. 2019, 37, 294–309. [Google Scholar] [CrossRef]
- Uzun, L.; Turner, A.P. Molecularly-imprinted polymer sensors: Realising their potential. Biosens. Bioelectron. 2016, 76, 131–144. [Google Scholar] [CrossRef] [PubMed]
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ge, Y.; Piletsky, S.A.; Lunec, J. Molecularly Imprinted Sensors: Overview and Applications; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Haupt, K. Molecular Imprinting; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 325. [Google Scholar]
- Mirsky, V.M.; Yatsimirsky, A. Artificial Receptors for Chemical Sensors; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Sergey, A.; Piletsky, M.J.W. Designing Receptors for the Next Generation of Biosensors, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 12, p. 264. [Google Scholar]
- Lee, S.-W.; Kunitake, T. Handbook of Molecular Imprinting: Advanced Sensor Applications; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Wang, S.; Zhu, X.; Zhao, M.; Li, S.; Le, Y.; Li, H. Optical sensors based on molecularly imprinted nanomaterials. In Smart Nanomaterials for Sensor Application; Bentham: Oak Park, IL, USA, 2012; pp. 60–75. [Google Scholar]
- Alvarez-Lorenzo, C. Handbook of Molecularly Imprinted Polymers; Smithers Rapra: Shawbury, UK, 2013. [Google Scholar]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef]
- Sergeyeva, T.; Yarynka, D.; Piletska, E.; Linnik, R.; Zaporozhets, O.; Brovko, O.; Piletsky, S.; El’skaya, A. Development of a smartphone-based biomimetic sensor for aflatoxin B1 detection using molecularly imprinted polymer membranes. Talanta 2019, 201, 204–210. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, Y.; Wu, X.; Pan, M.; Hu, N.; Wang, J.; Wang, S. Quartz crystal microbalance sensor based on covalent organic framework composite and molecularly imprinted polymer of poly(o-aminothiophenol) with gold nanoparticles for the determination of aflatoxin B1. Sens. Actuators B 2019, 291, 293–297. [Google Scholar] [CrossRef]
- Gui, R.; Jin, H.; Guo, H.; Wang, Z. Recent advances and future prospects in molecularly imprinted polymers-based electrochemical biosensors. Biosens. Bioelectron. 2018, 100, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, C.; Foglia, P.; Guarino, C.; Nazzari, M.; Samperi, R.; Lagana, A. Determination of aflatoxins in olive oil by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2007, 596, 141–148. [Google Scholar] [CrossRef]
- Tan, H.; Ma, L.; Guo, T.; Zhou, H.; Chen, L.; Zhang, Y.; Dai, H.; Yu, Y. A novel fluorescence aptasensor based on mesoporous silica nanoparticles for selective and sensitive detection of aflatoxin B1. Anal. Chim. Acta 2019, 1068, 87–95. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Yang, H.; Dong, Y.; Zhang, K.; Lu, Y.; Deng, R.; He, Q. Enzyme-free amplified and ultrafast detection of aflatoxin B1 using dual-terminal proximity aptamer probes. Food Chem. 2019, 283, 32–38. [Google Scholar] [CrossRef]
- Xiao, M.W.; Bai, X.L.; Liu, Y.M.; Yang, L.; Liao, X. Simultaneous determination of trace Aflatoxin B1 and Ochratoxin A by aptamer-based microchip capillary electrophoresis in food samples. J. Chromatogr. A 2018, 1569, 222–228. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.; Zhou, T.; Yang, D.; Wang, Q.; Zhou, Y. Occurrence of four mycotoxins in cereal and oil products in Yangtze Delta region of China and their food safety risks. Food Control 2014, 35, 117–122. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Wang, Y.; Peng, Y.; Nie, J.; Gao, G.; Zhi, J. Development of an Escherichia coli-based electrochemical biosensor for mycotoxin toxicity detection. Bioelectrochemistry 2020, 133, 107453. [Google Scholar] [CrossRef] [PubMed]


| Countries/Agencies | Food Products | Edible Vegetable Oil | Total of AFs (μg kg−1) | AFB1 (μg kg−1) | Refs. |
|---|---|---|---|---|---|
| EU | Oil seeds | - | 15 | 8 | [32,69] |
| EU | - | Peanut oil Others oil | 4 | 2 | [32,69] |
| China | - | Maize oil Peanut oil Others oils | - - - | 20 20 10 | [73] |
| Greece | - | Olive oil | - | - | [69] |
| Russia | - | Vegetable oil | - | - | [64] |
| France | - | Vegetable oil | - | 5 | [64] |
| Kenya | - | Vegetable oil | 20 | - | [64,71] |
| Taiwan | - | Edible oil | 10 | - | [65] |
| Morocco | - | Vegetable oil | - | 5 | [72] |
| Thailand | All foods | Oil and fats | 20 | - | [64,65,74] |
| USA | All foods | - | 20 | - | [64,71,75] |
| Brazil | All foods | - | - | 15 | [76] |
| India | All foods | - | - | 30 | [65] |
| Chile | All foods | - | - | - | [76] |
| Indonesia | All foods | - | 35 | 20 | [65] |
| Singapore Australia | All foods | - | 5 | - | [64,65] |
| Malaysia | All foods | - | 35 | - | [65] |
| Japan Vietnam | All foods | - | 10 | - | [65] |
| Sri Lanka | All foods | - | 30 | - | [65] |
| Matrix | Analytical Method | Sample Preparation Method | Linear Range | Recovery | LOD | Ref. |
|---|---|---|---|---|---|---|
| Peanut oil | ELISA | Immunoaffinity column cleanup | - | 84.40–92.60% | 1.08 μg kg−1 | [20] |
| Coconut oil | HPLC-FLD | Immunoaffinity chromatography | - | - | 0.01–0.04 μg kg−1 | [81] |
| Peanut oil Corn oil Soybean oil | HPLC-FLD | DLLME with in situ derivatization | 0.1–100 ng mL−1 | 106.90–121.50% | 0.03 ng mL−1 | [82] |
| Sunflower oil Olive oil Canola oil Frying oil Blend oil | HPLC-FLD | Immunoaffinity column cleanup | 0.04–0.16 ng g−1 | 95.56–102.13% | 0.16 ng g−1 | [83] |
| Vegetable oil | HPLC-FLD | Immunoaffinity chromatography - Solid phase extraction | - | 95.20–99.00% | 0.25 μg kg−1 | [84] |
| Coconut oil Almond oil Soybean oil Olive oil | HPLC -FLD | Immuno Affinity chromatography combined with DLLME | 0.005–10.00 ng mL−1 | 96.00–109.90% | 830 ng mL−1 | [85] |
| Sesame oil Sunflower oil Peanut oil Mixed oil | HPLC-FLD | Liquid-Liquid Extraction | 0.34–109.2 µg kg−1 | 83.00–96.00% | 0.09–1.5 µg kg−1 | [86] |
| Canola oil Corn oil Olive oil Peanut oil Soybean oil | LC-MS/MS | Liquid-Partitioning | - | 101.00–111.00% | 0.030 μg kg−1 | [87] |
| Soybean oil Corn oil Rice bran oil | LC-MS/MS | QuEChERS × DLLME | - | 70.70–76.00% | – | [88] |
| Blend oil Peanut oil Maize oil | LC-MS/MS | Hollow fiber liquid phase microextraction | 0.1–500 μg kg−1 | 78.59–80.61% | 0.02 μg kg−1 | [89] |
| Sunflower oil Palm oil Corn oil | LC-ESI-MS/MS | QuEChERS | 0.04–2000 ng g−1 | 87.90–106.60% | 0.01 ng kg−1 | [90] |
| Soybean oil Corn oil Peanut oil Blended oil | LC–MS/MS | Immunoaffinity chromatography | 0.16 μg kg−1 | 87.40–97.30% | 0.05 μg kg−1 | [96] |
| Olive oil Peanut oil Sesame oil | LC-MS/MS | Immunoaffinity column cleanup | 2–20 mg kg−1 | 87.70–102.20% | 0.1 μg kg−1 | [97] |
| Olive oil | LC/ESI-MS/MS | Matrix Solid Phase Dispersion | 0.2–0.4 (pg inj) | 95.00–98.00% | 0.2 pg inj | [261] |
| Olive oil Sunflower oil Soybean oil Corn oil | UHPLC-QqQ-MS/MS | QuEChERS | 0.5–25 μg kg−1 | 96.00–107.90% | 0.5 μg kg−1 | [100] |
| Sesame oil Groundnut oil Cottonseed oil | HPLC | Liquid-Liquid extraction | 0.2–0.8 μg kg−1 | - | 0.1 μg kg−1 | [102] |
| Vegetable oils | GPC-HPLC-FLD | Liquid-Liquid Extraction | 1.0–30.0 μg kg−1 | 82.60–90.60% | 1.0 μg kg−1 | [104] |
| Peanut oil Sunflower oil Olive oil | IAC-LC-ESI–MS/MS | Liquid-Liquid Extraction | 0.02–10 μg kg−1 | 84.00–99.00% | 0.02 μg kg−1 | [105] |
| Virgin olive oil | HPLC-FLD | Solid Phase Extraction | 65.50–87.50% | 0.25 ng g−1 | [110] | |
| Canola oil Soybean oil Corn oil Olive oil Peanut oil | FL | LTC-IMSPE | 0.0048–0.0126 ng·g−1 | 79.60–117.90% | 0.0048 ng·g−1 | [115] |
| Rapeseed oil Peanut oil Blended oil Blended olive oil Sunflower oil Tea oil Rice oil Corn oil Sesame oil Soybean oil | HPLC-MS/MS | QuEChERS | 0.2–20 ng mL−1 | 87.80–98.60% | 0.05 ng g−1 | [116] |
| Soya bean oil Groundnut oil Beniseed oil Palm kernel oil Melon oil Coconut oil | ELISA | Immunoaffinity column cleanup (226 Aflatoxin clean-up Column) | - | - | ≤0.8352 μg L−1 | [179] |
| Peanut oil Virgin olive oil | ELISA& TSA-ELISA | Liquid-Liquid Extraction | - | 81.40–118.80% | 0.004 ng mL−1 | [180] |
| Olive oil | Amperometric biosensor coupled with AChE enzyme | Liquid-Liquid Extraction | 10–60 ppb | 76.00–78.00% | 2 ppb | [186] |
| Olive oil | EIS based on MWCNTs/RTIL composite films | Liquid-partitioning | 0.1–10 ng mL−1 | 96.00–116.00% | 0.03 ng mL−1 | [190] |
| Peanut oil | Disposable electrochemical immunosensor with Au NPs modified SPCE | - | 0.001–100 ng mL−1 | 90.00–102.00% | 0.2 pg mL−1 | [192] |
| Peanut oil | Fluorescence spectroscopy based on UCNPs upconversion nanoparticles (UCNPs) | Liquid-partitioning | 0.2–100 ng mL−1 | 92.80–113.40% | 0.2 ng mL−1 | [196] |
| Oil | Chemiluminescence immunoassay combined with the magnetic particles (MPCLIA) | Liquid-partitioning | 0.1–100 ng mL−1 | 85.67–108.67% | 0.05 ng mL−1 | [197] |
| Corn germ oil Peanut oil | An immunoassay based on both recombinant antibody and its mimotope | Liquid-Liquid extraction | 0.242.21 ng mL−1 | 86.70–116.20% | 0.13 ng mL−1 | [206] |
| Vegetable oil | Immobilized immunosensor based on the hybrid gold nanoparticles-poly 4-aminobenzoic acid supported graphene | - | 0.01–25 ng mL−1 | - | 0.001 ng mL−1 | [207] |
| Peanut oil | UCNPs-BPNSs aptamer | - | 0.2–500 ng mL−1 | 92.89–99.24% | 0.028 ng mL−1 | [224] |
| Peanut oil | Dual-terminal stemmed aptamer beacon, aggregation-induced emission | Liquid-Liquid Extraction | 40–300 ng mL−1 | 93.59–109.30% | 27.3 ng mL−1 | [225] |
| Corn oil | A chimeric aptamer-based gold nanoparticles aptasensor | - | 5–5120 nM | 91.50–117.60% | 1.88 nM | [226] |
| Corn oil Peanut oil | An electrochemical aptasensor base on an AuNPs/ZIF-8 nanocomposite | - | 10.0–1.0 × 105 pg mL−1 | 93.49–106.90% | 1.82 pg mL−1 | [227] |
| Peanut oil | An electrochemical aptasensor base on an AuNPs/Zn/Ni-ZIF-8–800@ graphene nanocomposite | - | 0.18–100 ng mL−1 | 80.26–109.60% | 0.18 ng mL−1 | [228] |
| Oil | An aptasensor of hybridization chain reaction and Zn2+-dependent DNAzyme catalyzed cleavage | - | 0.4–16 nmol L−1 | 92.20–107.80% | 0.22 nmol L−1 | [237] |
| Oil | Fabricating electrochemical aptasensors | - | 0.04–0.10 ng m L-1 | 94.5–103.3% | 0.002 ng m L−1 | [184] |
| Peanut oil | Electrochemical immunosensor base on AFB1-BSA-QDs | - | 0.08–80 μg kg−1 | 102.70–113.30% | 0.05 μg kg−1 | [185] |
| Peanut oil | SERS aptasensor | - | 0.0001–100 ng·mL−1 | 96.60–115.00% | 0.40 pg·mL−1 | [174] |
| Peanut oil | SERS aptasensor | - | 0.001–10 ng mL−1 | 94.70–109.00% | 0.54 pg mL−1 | [169] |
| Peanut oil | Q-dots-aptamer-GO fluorescence quenching system | - | 1.6–160 μM | - | 1.4 nM | [242] |
| Peanut oil | Atomic absorption spectroscopy | - | 2.5–240 μg kg−1 | - | 0.04 μg kg−1 | [241] |
| Peanut oil | SERS aptasensor | – | 0.01–100 ng mL−1 | 91.09–105.73% | 0.0036 ng mL−1 | [170] |
| Peanut oil | SERS aptasensor with NH2-Rh-Au@Ag CSNPs | Solid Phase Extraction | 0.1–5.0 ng mL−1 | - | 0.03 ng mL−1 | [244] |
| Olive oil Peanut oil | Dual DNA tweezers nanomachine | - | 0.08–10 ppb | 90.00–110.00% | 0.035 ppb | [245] |
| Peanut oil | Electrochemical aptasensor based on smart host-guest recognition of β-cyclodextrin polymer | - | 0.1 × 10−4–10 ng mL−1 | 94.50–106.70% | 0.049 pg mL−1 | [246] |
| Peanut oil | A dual signal amplified aptasensor based on DNA walker, (DTNs) and network (HCR) | - | 1−1000 pg mL−1 | 87.56–105.28% | 0.492 pg mL−1 | [236] |
| Corn oil | A novel fluorescence aptasensor based on mesoporous silica nanoparticles | - | 0.5–50 ng mL−1 | 90.30–92.40% | 0.13 ng mL−1 | [262] |
| Peanut oil | Dual-terminal proximity aptamer probes | - | 1.0–200 ng mL−1 | 90.30–102.91% | 0.9 ng mL−1 | [263] |
| Sesame oil Olive oil Peanut oil Soybean oil | An aptamer-based MCE-LIF | - | 0.05–5.0 ng mL−1 | 95.29–109.19% | 0.026 ng mL−1 | [264] |
| Peanut oil | A simple fluorescent AFB1 sensor based on a humic acid/carbon dots system | - | 0.1–0.8 ng mL−1 | 103.80–108.00% | 70 pg mL−1 | [171] |
| Peanut oil | SERS aptasensor | - | 0.01–100 ng mL−1 | 90.40–113.10% | 5.0 ng mL−1 | [172] |
| Edible oil | Immunoaffinity chromatography fluorometer | Immunoaffinity column clean-up | 1.0–32.2 μg kg−1 | - | 1 μg kg−1 | [265] |
| Corn oil | An MIP-ECP-ECL sensing platform based on CH3NH3PbBr3 quantum dots (MAPB QDs)@SiO2 | - | 10−5–10 ng mL−1 | 102.00–110.00% | 8.5 fg mL−1 | [173] |
| Soybean oil | Terahertz spectroscopy (photoelectric techniques) | - | - | - | 2 μg kg−1 | [162] |
| Peanut oil Corn oil | ELC based on Escherichia coli | - | 0.01–0.3 μg mL−1 | 90.00–112.00% | 1 μg mL−1 | [266] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, S.; Niu, L.; Liu, Y. Recent Progress on Techniques in the Detection of Aflatoxin B1 in Edible Oil: A Mini Review. Molecules 2022, 27, 6141. https://doi.org/10.3390/molecules27196141
Yin S, Niu L, Liu Y. Recent Progress on Techniques in the Detection of Aflatoxin B1 in Edible Oil: A Mini Review. Molecules. 2022; 27(19):6141. https://doi.org/10.3390/molecules27196141
Chicago/Turabian StyleYin, Shipeng, Liqiong Niu, and Yuanfa Liu. 2022. "Recent Progress on Techniques in the Detection of Aflatoxin B1 in Edible Oil: A Mini Review" Molecules 27, no. 19: 6141. https://doi.org/10.3390/molecules27196141
APA StyleYin, S., Niu, L., & Liu, Y. (2022). Recent Progress on Techniques in the Detection of Aflatoxin B1 in Edible Oil: A Mini Review. Molecules, 27(19), 6141. https://doi.org/10.3390/molecules27196141







