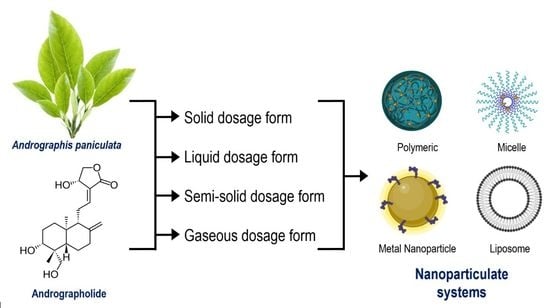
Andrographis paniculata Dosage Forms and Advances in Nanoparticulate Delivery Systems: An Overview
Abstract
:1. Introduction
1.1. Botanical Description of A. paniculata
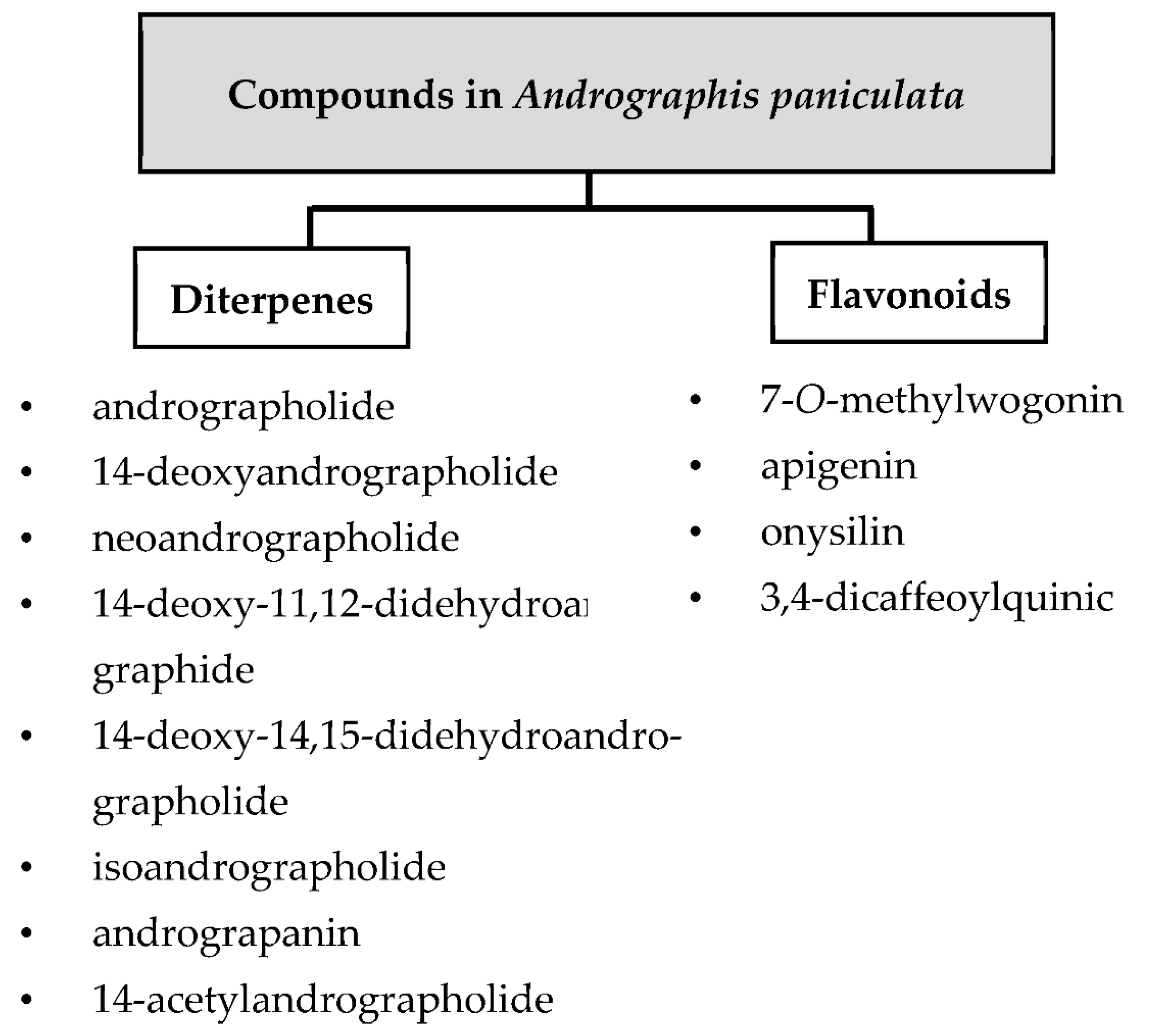
1.2. Major Phytochemicals
2. Conventional Dosage Forms
2.1. Solid Dosage Forms: Tablets, Capsules, and Pills
2.2. Liquid Dosage Forms: Extracts, Syrups, Tinctures, and Injections
2.3. Semi-Solid Dosage Forms: Creams, Pastes, Ointments, Gels, Poultices, and Foams
2.4. Gaseous Dosage Forms: Aerosols, Sprays, and Inhalers
3. Advances in A. paniculata Nano-Formulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, Y.; Chen, S.R.; Chai, L.; Zhao, J.; Wang, Y.; Wang, Y. Overview of pharmacological activities of Andrographis paniculata and its major compound andrographolide. Crit. Rev. Food Sci. Nutr. 2019, 59, S17–S29. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Urbi, Z.; Sule, A.; Rahman, K.M.H. Andrographis paniculata (Burm. f.) Wall. Ex Nees: A review of ethnobotany, phytochemistry, and pharmacology. Sci. World J. 2014, 2014, 274905. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.; Rajalakshmi, S.; Mehta, P.; Shaikh, K.; Bothiraja, C. Strategies for formulation development of andrographolide. RSC Adv. 2016, 73, 69282–69300. [Google Scholar] [CrossRef]
- Liu, X.Y.; Niu, X.; Feng, Q.J.; Yang, X.Z.; Wang, D.W.; Zhao, T.; Li, L.; Du, H. A new biocompatible microemulsion increases extraction yield and bioavailability of Andrographis paniculata. Chin. J. Nat. Med. 2016, 14, 683–691. [Google Scholar] [CrossRef]
- Sivananthan, M.; Elamaran, M. Medicinal and pharmacological properties of Andrographis paniculata. Int. J. Biomol. Biomed. 2013, 3, 1–12. [Google Scholar]
- Thokchom, A.; Talukdar, A.; Chingakham, B.; Ningthoujam, S.; Choudhury, M. Traditional usage of ethnomedicinal plants in treating liver disorders at Manipur, North East India. Eur. J. Med. Plants 2018, 23, 1–10. [Google Scholar] [CrossRef]
- Hu, X.Y.; Wu, R.H.; Logue, M.; Blondel, C.; Lai, L.Y.W.; Stuart, B.; Flower, A.; Fei, Y.T.; Moore, M.; Shepherd, J.; et al. Andrographis paniculata (Chuān Xīn Lián) for symptomatic relief of acute respiratory tract infections in adults and children: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0181780. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, T.; Guo, Q. Characterization of a traditional Chinese medicine plant the chloroplast genome of Andrographis paniculata. Mitochondrial DNA Part B Res. 2020, 5, 1949–1951. [Google Scholar] [CrossRef]
- Hidalgo, M.A.; Hancke, J.L.; Bertoglio, J.C.; Burgos, R.A. Andrographolide a new potential drug for the long term treatment of rheumatoid arthritis disease. In Innovative Rheumatology; InTech: London, UK, 2013. [Google Scholar] [CrossRef]
- Banerjee, S.; Kar, A.; Mukherjee, P.K.; Haldar, P.K.; Sharma, N.; Katiyar, C.K. Immunoprotective potential of Ayurvedic herb Kalmegh (Andrographis paniculata) against respiratory viral infections—LC–MS/MS and network pharmacology analysis. Phytochem. Anal. 2021, 32, 629–639. [Google Scholar] [CrossRef]
- Mehta, S.; Sharma, A.K.; Singh, R.K. Therapeutic journey of Andrographis paniculata (Burm.f.) Nees from natural to synthetic and nanoformulations. Mini-Rev. Med. Chem. 2021, 21, 1556–1577. [Google Scholar] [CrossRef]
- Mehta, S.; Sharma, A.K.; Singh, R.K. Ethnobotany, pharmacological activities and bioavailability studies of “King of Bitter” (Kalmegh): A review (2010–2020). Comb. Chem. High Throughput Screen. 2021, 24, 788–807. [Google Scholar] [CrossRef]
- Chao, W.W.; Lin, B.F. Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian). Chin. Med. 2010, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Okhuarobo, A.; Ehizogie Falodun, J.; Erharuyi, O.; Imieje, V.; Falodun, A.; Langer, P. Harnessing the medicinal properties of Andrographis paniculata for diseases and beyond: A review of its phytochemistry and pharmacology. Asian Pac. J. Trop. Dis. 2014, 4, 213–222. [Google Scholar] [CrossRef]
- Bokelmann, J.M. Andrographis (Andrographis paniculata): Whole plant. In Medicinal Herbs in Primary Care; Elsevier: Amsterdam, The Netherlands, 2022; pp. 189–194. [Google Scholar] [CrossRef]
- Aminah, N.S.; Tun, K.N.W.; Kristanti, A.N.; Aung, H.T.; Takaya, Y.; Choudhary, M.I. Chemical constituents and their biological activities from Taunggyi (Shan State) medicinal plants. Heliyon 2021, 7, 60173. [Google Scholar] [CrossRef] [PubMed]
- Deiva Suga, S.S. Molecular docking analysis of phytocompounds from Andrographis paniculata binding with proteins in the notch-signaling pathway. Bioinformation 2020, 16, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Mussard, E.; Cesaro, A.; Lespessailles, E.; Legrain, B.; Berteina-Raboin, S.; Toumi, H. Andrographolide, a natural antioxidant: An update. Antioxidants 2019, 20, 571. [Google Scholar] [CrossRef]
- Samy, R.P.; Thwin, M.M.; Gopalakrishnakone, P. Phytochemistry, pharmacology and clinical use of Andrographis paniculata. Nat. Product Commun. 2007, 2, 1934578X0700200519. [Google Scholar] [CrossRef]
- Thakur, A.K.; Chatterjee, S.S.; Kumar, V. Andrographolides and traditionally used Andrographis paniculata as potential adaptogens: Implications for therapeutic innovation. Tang 2014, 4, 15.1–15.14. [Google Scholar] [CrossRef]
- Ashalatha, M.; Vasundara Devi, B.; Padmavathi, D. Drug dosage forms-expanding horizon. IOSR J. Dental Med. Sci. 2016, 15, 41–49. [Google Scholar] [CrossRef]
- Sohail Arshad, M.; Zafar, S.; Yousef, B.; Alyassin, Y.; Ali, R.; AlAsiri, A.; Chang, M.W.; Ahmad, Z.; Ali Elkordy, A.; Faheem, A.; et al. A review of emerging technologies enabling improved solid oral dosage form manufacturing and processing. Adv. Drug Deliv. Rev. 2021, 178, 113840. [Google Scholar] [CrossRef]
- Kalaria, D.R.; Parker, K.; Reynolds, G.K.; Laru, J. An industrial approach towards solid dosage development for first-in-human studies: Application of predictive science and Lean principles. Drug Discov. Today 2020, 25, 505–518. [Google Scholar] [CrossRef] [PubMed]
- EMA. European Medicines Agency. Available online: http://www.ema.europa.eu/contact (accessed on 29 June 2022).
- Hancke, J.; Burgos, R.; Caceres, D.; Wikman, G. A double-blind study with a new monodrug Kan Jang: Decrease of symptoms and improvement in the recovery from common colds. Phytother. Res. 1995, 9, 559–562. [Google Scholar] [CrossRef]
- Gabrielian, E.S.; Shukarian, A.K.; Goukasova, G.I.; Chandanian, G.L.; Panossian, A.G.; Wikman, G.; Wagner, H. A double blind, placebo-controlled study of Andrographis paniculata fixed combination Kan Jang in the treatment of acute upper respiratory tract infections including sinusitis. Phytomedicine 2002, 9, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, G.; Liu, H.; Wang, D.; Song, X.; Chen, Y. Determination of andrographolide, deoxyandrographolide and neoandrographolide in the Chinese herb Andrographis paniculata by micellar electrokinetic capillary chromatography. Phytochem. Anal. 2002, 13, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Solomon Jeeva, J.J. Andrographis paniculata: A review of its traditional uses, phytochemistry and pharmacology. Med. Aromat. Plants 2014, 3, 169. [Google Scholar] [CrossRef]
- Chturvedi, G.N.; Tomar, G.S.; Tiwari, S.K.; Singh, K.P. Clinical studies on Kalmegh (Andrographis paniculata Nees) in infective hepatitis. Anc. Sci. Life 1983, 2, 208–215. [Google Scholar]
- Kulichenko, L.L.; Kireyeva, L.V.; Malyshkina, E.N.; Wikman, G. A randomized, controlled study of Kan Jang versus amantadine in the treatment of influenza in Volgograd. J. Herb. Pharmacother. 2003, 3, 77–93. [Google Scholar] [CrossRef]
- Narimanyan, M.; Jamalyan, K.; Balyan, A.; Barth, A.; Palm, S.; Wikman, G.; Panossian, A. Early intervention with Kan Jang® to treat upper-respiratory tract infections: A randomized, quadruple-blind study. J. Tradit. Complement. Med. 2021, 11, 552–562. [Google Scholar] [CrossRef]
- Cáceres, D.D.; Hancke, J.L.; Burgos, R.A.; Wikman, G.K. Prevention of common colds with Andrographis paniculata dried extract. A pilot double blind trial. Phytomedicine 1997, 4, 101–104. [Google Scholar] [CrossRef]
- Saxena, R.C.; Singh, R.; Kumar, P.; Yadav, S.C.; Negi, M.P.S.; Saxena, V.S.; Joshua, A.J.; Vijayabalaji, V.; Goudar, K.S.; Venkateshwarlu, K.; et al. A randomized double-blind placebo controlled clinical evaluation of extract of Andrographis paniculata (KalmColdTM) in patients with uncomplicated upper respiratory tract infection. Phytomedicine 2010, 17, 178–185. [Google Scholar] [CrossRef]
- Cáceres, D.D.; Hancke, J.L.; Burgos, R.A.; Sandberg, F.; Wikman, G.K. Use of visual analogue scale measurements (VAS) to assess the effectiveness of standardized Andrographis paniculata extract SHA-10 in reducing the symptoms of common cold. A randomized double blind-placebo study. Phytomedicine 1999, 6, 217–223. [Google Scholar] [CrossRef]
- Calabrese, C.; Berman, S.H.; Babish, J.G.; Ma, X.; Shinto, L.; Dorr, M.; Wells, K.; Wenner, C.A.; Standish, L.J. A phase I trial of andrographolide in HIV positive patients and normal volunteers. Phytother. Res. 2000, 14, 333–338. [Google Scholar] [CrossRef]
- Spasov, A.A.; Ostrovskij, O.V.; Chernikov, M.V.; Wikman, G. Comparative controlled study of Andrographis paniculata fixed combination, Kan Jang® and an Echinacea preparation as adjuvant, in the treatment of uncomplicated respiratory disease in children. Phytother. Res. 2004, 18, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Melchior, J.; Spasov, A.A.; Ostrovskij, O.V.; Bulanov, A.E.; Wikman, G. Double-blind, placebo-controlled pilot and phase III study of activity of standardized Andrographis paniculata herba Nees extract fixed combination (Kan Jang) in the treatment of uncomplicated upper-respiratory tract infection. Phytomedicine 2000, 7, 341–350. [Google Scholar] [CrossRef]
- Ratiani, L.; Pachkoria, E.; Mamageishvili, N.; Shengelia, R.; Hovhannisyan, A.; Panossian, A. Efficacy of Kan Jang® in patients with mild COVID-19: Interim analysis of a randomized, quadruple-blind, placebo-controlled trial. Pharmaceuticals 2022, 15, 1013. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Urbi, Z.; Karuniawati, H.; Mohiuddin, R.B.; Qrimida, A.M.; Allzrag, A.M.M.; Ming, L.C.; Pagano, E.; Capasso, R. Andrographis paniculata (Burm. f.) Wall. Ex Nees: An updated review of phytochemistry, antimicrobial pharmacology, and clinical safety and efficacy. Life 2021, 11, 348. [Google Scholar] [CrossRef]
- Yoopan, N.; Thisoda, P.; Rangkadilok, N.; Sahasitiwat, S.; Pholphana, N.; Ruchirawat, S.; Satayavivad, J. Cardiovascular effects of 14-deoxy-11,12-didehydroandrographolide and Andrographis paniculata extracts. Planta Med. 2007, 73, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Therapeutic Goods Administration. Safety Review of Andrographis paniculata and Anaphylactic/Allergic Reactions; Australian Government, Department of Health: Canberra, Australia, 2015.
- Panossian, A.; Hovhannisyan, A.; Mamikonyan, G.; Abrahamian, H.; Hambardzumyan, E.; Gabrielian, E.; Goukasova, G.; Wikman, G.; Wagner, H. Pharmacokinetic and oral bioavailability of andrographolide from Andrographis paniculata fixed combination Kan Jang in rats and human. Phytomedicine 2000, 7, 351–364. [Google Scholar] [CrossRef]
- Adiguna, S.P.; Panggabean, J.A.; Atikana, A.; Untari, F.; Izzati, F.; Bayu, A.; Rosyidah, A.; Rahmawati, S.I.; Putra, M.Y. Antiviral activities of andrographolide and its derivatives: Mechanism of action and delivery system. Pharmaceuticals 2021, 14, 1102. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Yang, X.; Li, H.; Han, L.; Li, X.; Dong, X.; Zhu, Q.; Ye, M.; Feng, Q.; Niu, X. Preparation and evaluation of andrographolide-loaded microemulsion. J. Microencapsul. 2012, 29, 657–665. [Google Scholar] [CrossRef]
- Shariff, Z.B.; Dahmash, D.T.; Kirby, D.J.; Missaghi, S.; Rajabi-Siahboomi, A.; Maidment, I.D. Does the formulation of oral solid dosage forms affect acceptance and adherence in older patients? A mixed methods systematic review. J. Am. Med. Directors Assoc. 2020, 21, 1015–1023.e8. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.V.; Ansel, H.C. Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems; Wolters Kluwer: Philadelphia, PA, USA, 2014; ISBN 978-1-4511-8876-9. [Google Scholar]
- Awad, A.; Madla, C.M.; Gavins, F.K.H.; Allahham, N.; Trenfield, S.J.; Basit, A.W. Liquid dosage forms. In Remington; Academic Press: Cambridge, MA, USA, 2021; pp. 359–379. [Google Scholar] [CrossRef]
- Gautami, J. Liquid dosage forms. Nano Sci. Nano Technol. Indian J. 2016, 10, 101. [Google Scholar]
- Mennella, J.A.; Reed, D.R.; Mathew, P.S.; Roberts, K.M.; Mansfield, C.J. A spoonful of sugar helps the medicine go down: Bitter masking by sucrose among children and adults. Chem. Sens. 2015, 40, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Chapter 9: Flavors, Sweeteners, and Colors. In The Art, Science, and Technology of Pharmaceutical Compounding, 6th ed.; The American Pharmacists Association: Washington, DC, USA, 2020. [CrossRef]
- Widyawaruyanti, A.; Asrory, M.; Ekasari, W.; Setiawan, D.; Radjaram, A.; Tumewu, L.; Hafid, A.F. In vivo antimalarial activity of Andrographis paniculata tablets. Procedia Chem. 2014, 13, 101–104. [Google Scholar] [CrossRef]
- Haque, M.Z.; Rouf, A.; Jalil, A.; Islam, B. Production of Kalmegh syrup and studies on its toxic activities. J. Adv. Sci. Res. 2012, 3, 45–48. [Google Scholar]
- Suhas Kshirsagar, P.; Sanjay Mote, D.; Pramod Patil, S. Design, formulation and evaluation of Oxymel containing Andrographis paniculata extract. Int. J. Pharm. Investig. 2020, 10, 456–459. [Google Scholar] [CrossRef]
- Yang, T.; Xu, C.; Wang, Z.T.; Wang, C.H. Comparative pharmacokinetic studies of andrographolide and its metabolite of 14-deoxy-12-hydroxy-andrographolide in rat by ultra-performance liquid chromatography-mass spectrometry. Biomed. Chromatogr. 2013, 27, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Lajoinie, A.; Janiaud, P.; Henin, E.; Gleize, J.C.; Berlion, C.; Nguyen, K.A.; Nony, P.; Gueyffier, F.; Maucort-Boulch, D.; Kassaï Koupaï, B. Assessing the effects of solid versus liquid dosage forms of oral medications on adherence and acceptability in children. Cochrane Database Syst. Rev. 2017, 1–13. [Google Scholar] [CrossRef]
- Yue, C.S.; Scarsi, C.; Ducharme, M.P. Pharmacokinetics and potential advantages of a new oral solution of Levothyroxine Vsother available dosage forms. Arzneim.-Forsch. Drug Res. 2012, 62, 631–636. [Google Scholar] [CrossRef]
- Shukla, J. Recent Advances in Semisolid Dosage Forms. Available online: https://www.researchgate.net/publication/320923384_Recent_Advances_in_Semisolid_Dosage_Forms (accessed on 2 January 2022).
- Brown, T.L.; Petrovski, S.; Chan, H.T.; Angove, M.J.; Tucci, J. Semi-solid and solid dosage forms for the delivery of phage therapy to epithelia. Pharmaceuticals 2018, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- Bora, A.; Deshmukh, S.; Swain, K. Recent advances in semisolid dosage form. Int. J. Pharm. Sci. Res. 2014, 5, 3594. [Google Scholar] [CrossRef]
- Chauhan, L.; Gupta, S. Creams: A review on classification, preparation methods, evaluation and its applications. J. Drug Deliv. Ther. 2020, 10, 281–289. [Google Scholar] [CrossRef]
- Thawornrungroj, S.; Kuphasuk, Y.; Petmitr, S.; Srisatjaluk, R.; Kitkumthorn, N. The application of Andrographis paniculata gel as an adjunct in the treatment of chronic periodontitis: Clinical and microbiological effects. Naresuan Univ. J. Sci. Technol. NUJST. 2011, 19, 38–49. [Google Scholar]
- Tuan Kub, T.N.; Ab Manaf, N.A.; Che Ibrahim, A.S. Comparison of well diffusion, disc diffusion and broth dilution methods for antimicrobial activity of Andrographis paniculata herbal gel against acne-associated pathogen. Malays. J. Microbiol. 2021, 17, 90–96. [Google Scholar] [CrossRef]
- Samuel, A.J.; Mulla, N. Formulation and evaluation of herbal topical gel containing leaves extract of Andrographis paniculata. J. Drug Deliv. Ther. 2020, 10, 48–51. [Google Scholar] [CrossRef]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P.; Household, S.L. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Dewi, I.K.; Lestari, T.; Rofi’ah, S.N. Formulation and physical test of ethanolic extract Sambiloto leaves (Andrographis paniculata) Ointment. Medicine 2015, 6, 56–60. [Google Scholar] [CrossRef]
- Olutayo, A.A.; Babalola, C.O.; Femi-Oyewo, M.N.; Balogun, G.Y. Antimicrobial activity and stability of Andrographis paniculata cream containing Shea Butter. Nig. J. Pharm. Res. 2019, 15, 9–18. [Google Scholar]
- Sharadha, M.; Gowda, D.V.; Vishal Gupta, N.; Akhila, A.R. An overview on topical drug delivery system—Updated review. Int. J. Res. Pharm. Sci. 2020, 11, 368–385. [Google Scholar] [CrossRef]
- Stability Considerations of Dosage Forms. Available online: http://www.pharmacopeia.cn/v29240/usp29nf24s0_c1191.html (accessed on 18 February 2022).
- Stein, S.W.; Thiel, C.G. The history of therapeutic aerosols: A chronological review. J. Aerosol Med. Pulm. Drug Deliv. 2017, 30, 20–41. [Google Scholar] [CrossRef] [PubMed]
- Chapter 8 Respiratory Dosage Forms—Pharmaceutical Press. Available online: https://www.pharmpress.com/files/docs/chp8-9780857110787.pdf (accessed on 30 March 2022).
- Khan, I.; Yousaf, S.; Alhnan, M.A.; Ahmed, W.; Elhissi, A.; Jackson, M.J. Design characteristics of inhaler devices used for pulmonary delivery of medical aerosols. In Surgical Tools and Medical Devices, 2nd ed.; Springer International Publishing: New York, NY, USA, 2016; pp. 573–591. [Google Scholar] [CrossRef]
- Bao, Z.; Guan, S.; Cheng, C.; Wu, S.; Wong, S.H.; Michael Kemeny, D.; Leung, B.P.; Fred Wong, W.S. A novel antiinflammatory role for andrographolide in asthma via inhibition of the nuclear factor-kb pathway. Am. J. Respir. Crit. Care Med. 2009, 179, 657–665. [Google Scholar] [CrossRef] [PubMed]
- A Guide To Aerosol Delivery Devices for Respiratory Therapists; American Association for Respiratory Care: Irving, TX, USA, 2017.
- Chapter 24: Inhalation Preparations. In The Art, Science, and Technology of Pharmaceutical Compounding, 6th ed.; The American Pharmacists Association: Washington, DC, USA, 2020. [CrossRef]
- Hill, N.S.; Preston, I.R.; Roberts, K.E. Inhaled therapies for pulmonary hypertension. Respir. Care 2015, 60, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Zhang, X. Andrographis Extract Formulations. U.S. Patent No. US 20070202164A1, 2007. Available online: https://patents.google.com/patent/US20070202164A1/en (accessed on 20 February 2022).
- Raman, S.; Mahmood, S.; Hilles, A.R.; Javed, M.N.; Azmana, M.; Al-Japairai, K.A.S. Polymeric nanoparticles for brain drug delivery—A review. Curr. Drug Metab. 2020, 21, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.; Mahmood, S.; Rahman, A. A review on lipid-polymer hybrid nanoparticles and preparation with recent update. Mater. Sci. Forum 2020, 981, 322–327. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Oseni, B.A.; Azubuike, C.P.; Okubanjo, O.O.; Igwilo, C.I.; Panyam, J. Encapsulation of andrographolide in poly(lactide-co-glycolide) nanoparticles: Formulation, optimization and in vitro efficacy studies. Front. Bioeng. Biotechnol. 2021, 9, 639409. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, S.; Ahmad, F.; Rathaur, S. Antifilarial efficacy of green silver nanoparticles synthesized using Andrographis paniculata. J. Drug Deliv. Sci. Technol. 2020, 56, 101557. [Google Scholar] [CrossRef]
- Li, M.; Zhang, T.; Zhu, L.; Wang, R.; Jin, Y. Liposomal andrographolide dry powder inhalers for treatment of bacterial pneumonia via anti-inflammatory pathway. Int. J. Pharm. 2017, 528, 163–171. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Jamaludin, R.; Mohd Daud, N.; Raja Sulong, R.S.; Yaakob, H.; Abdul Aziz, A.; Khamis, S.; Md Salleh, L. Andrographis paniculata-loaded niosome for wound healing application: Characterisation and in vivo analyses. J. Drug Deliv. Sci. Technol. 2021, 63, 102427. [Google Scholar] [CrossRef]
- Kaur, J.; Gulati, M.; Jha, N.K.; Disouza, J.; Patravale, V.; Dua, K.; Singh, S.K. Recent advances in developing polymeric micelles for treating cancer: Breakthroughs and bottlenecks in their clinical translation. Drug Discov. Today 2022, 27, 1495–1512. [Google Scholar] [CrossRef]
- Kaur, J.; Gulati, M.; Kapoor, B.; Jha, N.K.; Gupta, P.K.; Gupta, G.; Chellappan, D.K.; Devkota, H.P.; Prasher, P.; Ansari, M.S.; et al. Advances in designing of polymeric micelles for biomedical application in brain related diseases. Chem.-Biol. Interact. 2022, 361, 109960. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Song, S.; Miao, X.; Liu, X.; Zhao, J.; Wang, Z.; Shao, X.; Zhang, Y.; Han, G. MPEG-PLA micelle for delivery of effective parts of Andrographis paniculata. Curr. Drug Deliv. 2018, 15, 532–540. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef]
- Jesus, S.; Schmutz, M.; Som, C.; Borchard, G.; Wick, P.; Borges, O. Hazard assessment of polymeric nanobiomaterials for drug delivery: What can we learn from literature so far. Front. Bioeng. Biotechnol. 2019, 7, 261. [Google Scholar] [CrossRef]
- Kulsirirat, T.; Sathirakul, K.; Kamei, N.; Takeda-Morishita, M. The in vitro and in vivo study of novel formulation of andrographolide PLGA nanoparticle embedded into gelatin-based hydrogel to prolong delivery and extend residence time in joint. Int. J. Pharm. 2021, 602, 120618. [Google Scholar] [CrossRef]
- Chakraborty, S.; Ehsan, I.; Mukherjee, B.; Mondal, L.; Roy, S.; das Saha, K.; Paul, B.; Debnath, M.C.; Bera, T. Therapeutic potential of andrographolide-loaded nanoparticles on a Murine asthma model. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102006. [Google Scholar] [CrossRef]
- Das, S.; Halder, A.; Mandal, S.; Mazumder, M.A.J.; Bera, T.; Mukherjee, A.; Roy, P. Andrographolide engineered gold nanoparticle to overcome drug resistant visceral leishmaniasis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 751–762. [Google Scholar] [CrossRef]
- Kotakadi, V.S.; Gaddam, S.A.; Subba Rao, Y.; Prasad, T.N.V.K.V.; Varada Reddy, A.; Sai Gopal, D.V.R. Biofabrication of silver nanoparticles using Andrographis paniculata. Eur. J. Med. Chem. 2014, 73, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Syukri, Y.; Martien, R.; Lukitaningsih, E.; Nugroho, A.E. Novel self-nano emulsifying drug delivery system (SNEDDS) of andrographolide isolated from Andrographis paniculata Nees: Characterization, in-vitro and in-vivo assessment. J. Drug Deliv. Sci. Technol. 2018, 47, 514–520. [Google Scholar] [CrossRef]
- Rajakumar, G.; Thiruvengadam, M.; Mydhili, G.; Gomathi, T.; Chung, I.M. Green approach for synthesis of zinc oxide nanoparticles from Andrographis paniculata leaf extract and evaluation of their antioxidant, anti-diabetic, and anti-inflammatory activities. Bioprocess Biosyst. Eng. 2018, 41, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Zhongyu, X.; Jiangmeng, R.; Qiufang, J.; Fuzheng, R.; Mengting, H.; Wenrui, D.; Bubing, Z. Andrographolide-loaded silk fibroin nanoparticles. RSC Adv. 2018, 8, 34726–34732. [Google Scholar] [CrossRef] [PubMed]
- Karthik, K.; Shashank, M.; Revathi, V.; Tatarchuk, T. Facile microwave-assisted green synthesis of NiO nanoparticles from Andrographis paniculata leaf extract and evaluation of their photocatalytic and anticancer activities. Mol. Cryst. Liq. Cryst. 2018, 673, 70–80. [Google Scholar] [CrossRef]
- Jiang, C.; Jiang, Z.; Zhu, S.; Amulraj, J.; Deenadayalan, V.K.; Jacob, J.A.; Qian, J. Biosynthesis of silver nanoparticles and the identification of possible reductants for the assessment of in vitro cytotoxic and in vivo antitumor effects. J. Drug Deliv. Sci. Technol. 2021, 63, 102444. [Google Scholar] [CrossRef]
- Selvam, P.; Wadwani, A.; Raman, S.C.V.; Krishnan, K.S. Design and synthesis of biogenic silver nanoparticles from Andragraphis paniculata as potential anticancer agents. Indian J. Pharm. Sci. 2019, 81, 173–176. [Google Scholar] [CrossRef]
- Verma, V.K.; Zaman, K.; Verma, S.; Verma, S.K.; Sarwa, K.K. Role of semi-purified andrographolide from Andrographis paniculata extract as nano-phytovesicular carrier for enhancing oral absorption and hypoglycemic activity. Chin. Herb. Med. 2020, 12, 142–155. [Google Scholar] [CrossRef]
- Guccione, C.; Oufir, M.; Piazzini, V.; Eigenmann, D.E.; Jähne, E.A.; Zabela, V.; Faleschini, M.T.; Bergonzi, M.C.; Smiesko, M.; Hamburger, M.; et al. Andrographolide-loaded nanoparticles for brain delivery: Formulation, characterisation and in vitro permeability using HCMEC/D3 cell line. Eur. J. Pharm. Biopharm. 2017, 119, 253–263. [Google Scholar] [CrossRef]
- Kansom, T.; Sajomsang, W.; Saeeng, R.; Rojanarata, T.; Ngawhirunpat, T.; Patrojanasophon, P.; Opanasopit, P. Fabrication and characterization of andrographolide analogue (3A.1) nanosuspensions stabilized by amphiphilic chitosan derivatives for colorectal cancer therapy. J. Drug Deliv. Sci. Technol. 2019, 54, 101287. [Google Scholar] [CrossRef]
- Bothiraja, C.; Pawar, A.P.; Shaikh, K.S.; Sher, P. Eudragit® EPO based nanoparticle suspension of andrographolide: In vitro and in vivo. Nanosci. Nanotechnol. Lett. 2011, 1, 156–164. [Google Scholar] [CrossRef]
- Graverini, G.; Piazzini, V.; Landucci, E.; Pantano, D.; Nardiello, P.; Casamenti, F.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Bergonzi, M.C. Solid lipid nanoparticles for delivery of andrographolide across the blood-brain barrier: In vitro and in vivo evaluation. Colloids Surf. B Biointerfaces 2018, 161, 302–313. [Google Scholar] [CrossRef]
- Sahlan, M.; Evelyn, K.; Pratami, D.K.; Mulia, K. In vitro release study of Sambiloto (Andrographis paniculata) extract encapsulated by casein micelle as anti-diabetic herbal drug. AIP Conf. Proc. 2019, 2175, 020042. [Google Scholar] [CrossRef]
- Sari, R.; Widyawaruyanti, A.; Anindita, F.B.T.; Astuti, S.K.; Setyawan, D. Development of andrographolide-carboxymethyl chitosan nanoparticles: Characterization, in vitro release and in vivo antimalarial activity study. Turk. J. Pharm. Sci. 2018, 15, 136–141. [Google Scholar] [CrossRef]
- Indrati, O.; Martien, R.; Rohman, A.; Nugroho, A.K. Development of nanoemulsion-based hydrogel containing andrographolide: Physical properties and stability evaluation. J. Pharm. Bioallied Sci. 2020, 12, S816–S820. [Google Scholar] [CrossRef]
- Uma Devi, A.; Khanam, S. Preparation and characterization of herbal nanoformulation containing Andrographis paniculata extract. Int. J. Pharm. Sci. Res. 2019, 10, 5380. [Google Scholar] [CrossRef]

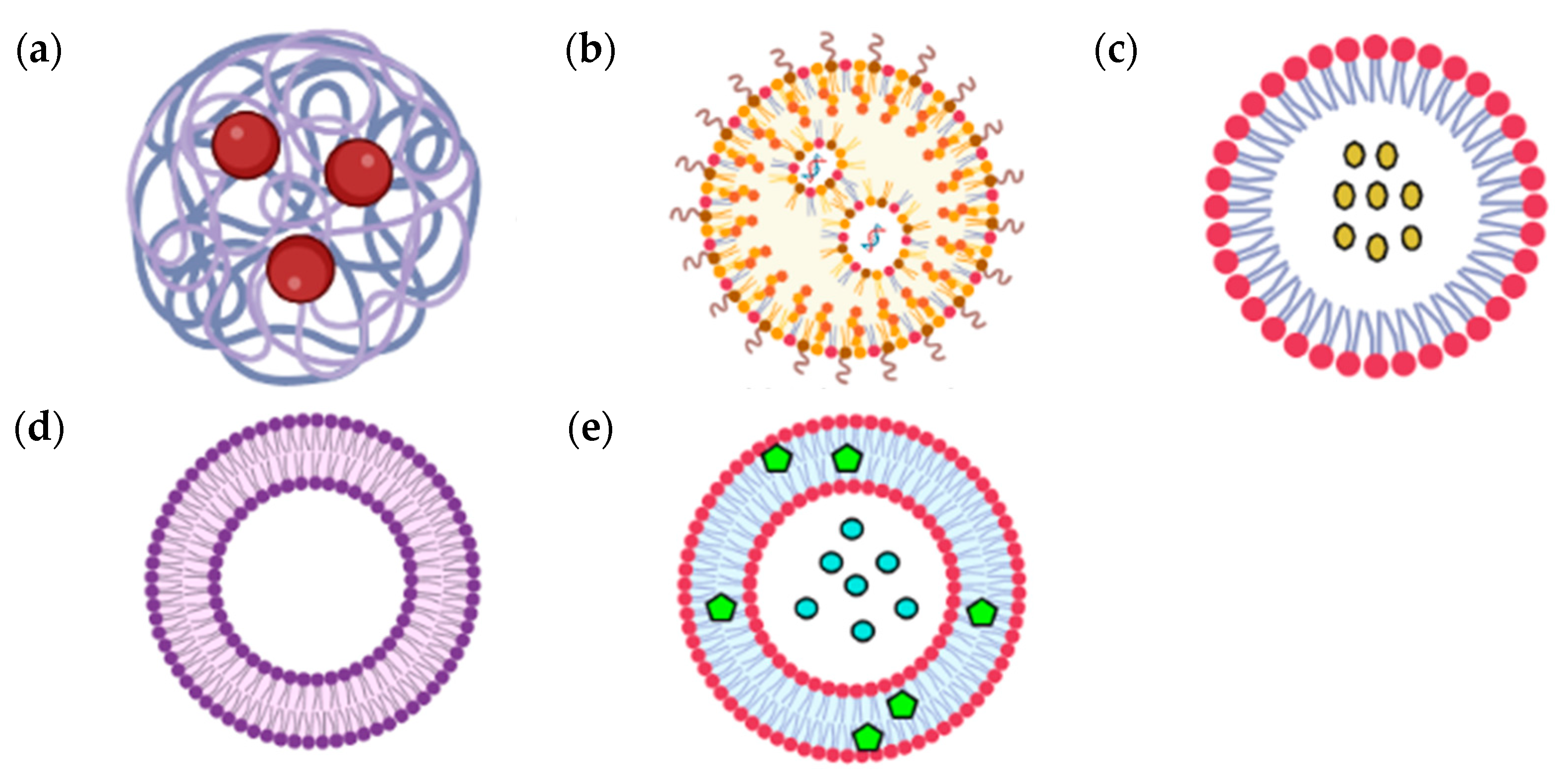
| Type of Nanoparticles | Comp | Size and Shape | Formulation Technique | Results | Target Activity/Disease | Ref |
|---|---|---|---|---|---|---|
| PLGA nanoparticle embedded into gelatin-based hydrogel | AG | 494.00 ± 4.28 nm (acid end group) and 529.30 ± 7.36 nm (ester end group) (Spherical shape) | Single emulsion solvent evaporation method | The intra-articular drug delivery results in a long-standing sustained release injection (≥2 months) and implantation (≈2 months) for osteoarthritis disease’s local treatment. | Osteoarthritis disease | [92] |
| PLGA nanoparticles | AG | 250 nm (spherical with no visual crevices) | Multiple-emulsion solvent evaporation technique | AG’s bioavailability is enhanced through oral (AUC0-t: 5.37 times and 6.38 times higher in plasma and lung than free-AG) or pulmonary (AUC0-t: 3.2 times and 3.6 times higher in plasma and lung than free-AG) administration. The content of serum IgE, cell numbers, broncho-alveolar lavage fluid levels, NF-κβ suppression demonstrates greatly improved results, with sustained release AG NPs compared to free AG. | Asthma | [93] |
| Gold nanoparticles | AG | 543 nm from plasmon response and 14 nm in TEM (crystalline and spherical) | Facile one-pot technique | The formulation exhibits strong anti-leishmanicidal effects, with macrophage uptake completed within 2 h of introduction, resulting in an IC 50 value of 19 ± 1.7 µM for wild life, and 41 ± 6 µM for paromomycin or 55 ± 7.3 µM for sodium stibogluconate resistant strains. | Drug resistant VL strains | [94] |
| Silver Nanoparticles | AP Extract (APE) | 53.2 ± 2 nm (Crystalline and spherical) | Priosynthes/green synthesis | APE silver nanoparticles exhibited promising antifungal activity in both Aspergillus niger and Penicillium sp. (Inhibition diameter: 12 mm and 14 mm) | Antifungal Activity | [95] |
| Novel Self-Nano Emulsifying Drug Delivery System (SNEDDS) | AG | 8.4 ± 0.6 nm to 115.0 ± 2.9 nm (globule) | Shake-flask method, followed by ternary phase diagrams. | AG SNEDDS had a better efficacy in comparison to plain AG, with a 1.26-fold rise in Cmax, 1.2-fold improvement in AUC, and 1.72-fold decline in Tmax in an animal model (New Zealand white rabbits). | NA | [96] |
| Liposomes | AG | 77.91 nm | Injection method | In vivo studies showed rats sprayed using liposome inhalers demonstrated a powerful anti-S. aureus effect of pneumonic instead of AG with 10x higher dose or of penicillin. It reduced pro-inflammatory cytokines (TNF-α, IL-1) and restricted IκB-α phosphorylation, to modulate the immune response against antibacterial effect and downregulate the inflammatory response. | Bacterial pneumonia/Antibacterial and anti-inflammatory activity | [83] |
| Zinc oxide nanoparticles (nanocrystal) | AP Extract (APE) | 96 to 115 nm (spherical, oval, hexagonal) | Biosynthesis/green synthesis method | AP green ZnO nanoparticles have excellent potentials vs. AP leaf extract alone in antidiabetic (IC50: 121.42 lg/mL vs. 149.65 lg/mL), anti-inflammatory (IC50: 66.78 lg/mL vs. 75.42 lg/mL) and antioxidant (62%) activity. | Antidiabetic, anti-inflammatory, antioxidant activity | [97] |
| Regenerated silk fibroin (RSF) nanoparticles | AP Extract (APE) | 200 to 1000 nm (spherical structure) | Green and mild method with additions of ethanol, mPEG-NH2, and freezing RSF-ethanol solution. | For an in vitro drug release profile, 90.9% of APE was released from APE-loaded RSFNPs up to 72 h against APE suspension and APE-saturated solution, which showed a two-fold lower drug release profile. No cytotoxicity was observed and it can attach easily to MDA-MB-231 and Hela cells, demonstrating its anti-proliferative activity. | Lymphatic chemotherapy/anti-proliferative activity. | [98] |
| NiO nanoparticles | AP Extract (APE) | 24 nm (cubic structure) | Microwave-assisted biogenic | Green NiO nanoparticles were evaluated for photodegradation, which resulted in a 88.13% degradation efficiency. It expressed the the lowest IC50 value in an MCF-7 cell line study compared with other metal oxide nanoparticles. | Breast Cancer/anticancer activity. | [99] |
| Silver nanoparticles | AP Aqueous Extract | 123.1 nm | Biosynthesis method | HeLa cell line were used to test the impact of cytotoxicity, which is known to be dose-dependent, with 7.285 μg/mL as the half maximal inhibitory concentration. A total of 24 male mice were injected intraperitoneally with AgNPs (350 g/kg BW) and plant extract (80 mg/kg BW) for 10 days, following tumor formation with a calculated dose (40 µL). The biochemical and hematological indicators studied were restored to near-normal levels in all of the therapy groups, showing AgNPs’ effectiveness against carcinoma cells. | Antitumor activity. | [100] |
| Silver nanoparticles | AP Methanol Extract | 18–70 nm (spherical) | Biosynthesis method | The anticancer efficacy was investigated against neuroblastoma cells and normal Vero cells, which resulted in IC 50 values of 32 and 60 g/mL, respectively. No cytotoxicity (CC 50 value of 329.29 and 368 µg/mL) was induced by nanoparticles to Vero cell lines. | Anticancer activity. | [101] |
| Nano-phytovesicular system | Semi-purified AG | 395.5 ± 5.80 nm (spherical, unilamellar vesicles with globular shapes) | Liquid dispersion technique with slight modifications | The hyperglycemic condition of rats was significantly protected by the nano-phyto vesicles of semi-purified AP extract, corresponding to 25 mg/kg AG. Compared to free AG (50 mg/kg), the AG vesicles produced superior results in body weight development, oral glucose tolerance test, as well as blood glucose level. Therefore, increased oral absorption, bioavailability, and improved antihyperglycemic action were shown in in vitro and in vivo studies. | Hypoglycaemic activity | [102] |
| Human serum albumin-based nanoparticles (HSA NPs) and poly ethylcyanoacrylate nanoparticles (PECA NPs) | AG | 151.7 to 335.1 nm (spherical) | Thermal and chemical cross-linking method for HSAT AG NPs and HSAC AG NPs and Emulsion polymerization method to yield PECA AG NPs. | An in vitro BBB model based on the hCMEC/D3 cell line was used to examine the potential of free AG and AG-loaded in HSAT and PECA NPs to cross the blood–brain barrier (BBB). In silico research anticipated that free AG would not permeate the BBB model. HSAT NPs enhanced the AG penetration by two-fold, while retaining cell layer integrity, whereas PECA NPs momentarily damaged the BBB integrity. | Inflammation-related pathologies/neurodegenerative disorders | [103] |
| Niosome | AP Extract (water and ethanol) | 125–226 nm (spherical) | Proniosome-derived niosomal dispersion | Ethanol AP extract niosomal gel had a 100% wound recovery rate while protecting tissue from oxidative stress. Towards the end of 14 days, prominent collagen fibers and the development of hair follicles were detected in fibroblasts cells. | Wound healing/antioxidant activity. | [85] |
| Nanosuspensions | 19-tert-butyldiphenylsilyl-8,17-epoxy AG (3A.1) (AG analogue) | less than 300 nm (spherical) | Single step nanoprecipitation (bottom-up) technique | The anticancer activity of NSC derivatives stabilized 3A.1 nanosuspension against colorectal cancer (HCT116) cells was substantially enhanced regarding in vitro cytotoxicity. | Colorectal cancer/anticancer activity. | [104] |
| Eudragit (R) EPO Based Nanoparticle Suspension | AG | 255.9 nm | Nanoprecipitation and lyophilization technique | Compared to pure AG, there was a substantial increase in drug dissolution, with total drug release within 10 min in NPs suspension and re-dispersed in NPs suspension. However, lyophilization slowed the release of AG. In contrast to AG, the NP suspension and re-dispersed NPs suspension showed better hepatoprotectivity for CCl4-induced hepatotoxicity in rats. Histopathological research on liver lesions confirmed the findings. | Hepatic lesions/Hepatoprotective activity. | [105] |
| Solid Lipid Nanoparticles (SLN) | AG | 262 to 278 nm (Spherical) | Emulsion/evaporation/solidifying technique | In vitro findings using hCMEC/D3 cells, an in vitro BBB model, and a permeation test (PAMPA) showed that AG-SLN increased AG permeability and sustainability compared to free AG. Fluorescent SLN were found in brain parenchyma after intravenous delivery in vivo, indicating their ability to transcend the BBB. | Oxidative stress mediated neurotoxicity, inflammation-mediated neurodegeneration, and cerebral ischemia | [106] |
| Casein Micelle | AP Extract (APE) | - | - | Casein breakdown tends to produce a burst release profile in less than 30 min of simulated gastric fluid digestion (500 of substrate ratio: 1 enzyme optimal (w/w)). In digestion of simulated gastric fluid, the incorporation of APE in casein micelles delays casein breakdown, leading to a sustained release profile of casein degradation in around 4 h of digestion. | Antidiabetic Activity | [107] |
| Chitosan Nanoparticles | AG | 500 nm to 3000 nm (crystalline) | Ionic gelation technique (spray drying) | The amount of AG dissolved from chitosan nanoparticles during in vitro drug release was enhanced by 6.5-fold compared to AG alone, meanwhile the in vivo antimalarial activity was 1.65-times higher in Plasmodium berghei infected mice. | Antimalarial activity | [108] |
| Nanoemulsion-based Hydrogel | AG | 56.5 ± 1.92 nm (droplet) | Ultrasonication Method | An optimum nanoemulsion of 1.35% of triethanolamine, 9% of propylene glycol, and 34.65% of carbopol was incorporated into a hydrogel base (1:1). A pH of 6.50 ± 0.02 and viscosity cP of 2492.33 ± 36.91 showed the optimum results of the AG-nano-emulsion-based hydrogel. | NA | [109] |
| Eudragit S 100 nanoparticles | AP Methanolic Extract | 300–400 nm (smooth and spherical shape) | Solvent displacement method | It was noted to have an encapsulation efficiency > 75%, as well as an in vitro drug release of >55%, after 8 h. | NA | [110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raman, S.; Murugaiyah, V.; Parumasivam, T. Andrographis paniculata Dosage Forms and Advances in Nanoparticulate Delivery Systems: An Overview. Molecules 2022, 27, 6164. https://doi.org/10.3390/molecules27196164
Raman S, Murugaiyah V, Parumasivam T. Andrographis paniculata Dosage Forms and Advances in Nanoparticulate Delivery Systems: An Overview. Molecules. 2022; 27(19):6164. https://doi.org/10.3390/molecules27196164
Chicago/Turabian StyleRaman, Subashini, Vikneswaran Murugaiyah, and Thaigarajan Parumasivam. 2022. "Andrographis paniculata Dosage Forms and Advances in Nanoparticulate Delivery Systems: An Overview" Molecules 27, no. 19: 6164. https://doi.org/10.3390/molecules27196164
APA StyleRaman, S., Murugaiyah, V., & Parumasivam, T. (2022). Andrographis paniculata Dosage Forms and Advances in Nanoparticulate Delivery Systems: An Overview. Molecules, 27(19), 6164. https://doi.org/10.3390/molecules27196164