Bioavailability of Celecoxib Formulated with Mesoporous Magnesium Carbonate—An In Vivo Evaluation
Abstract
1. Introduction
2. Results
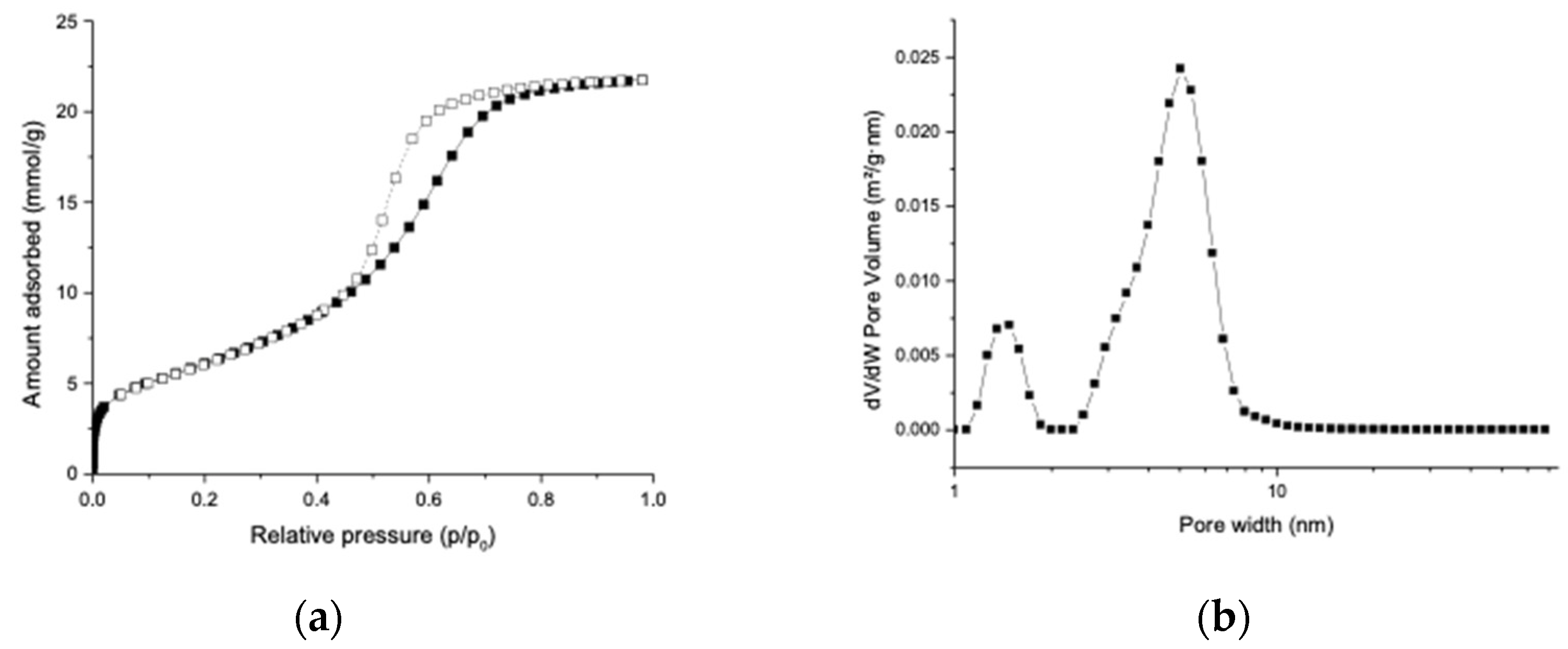
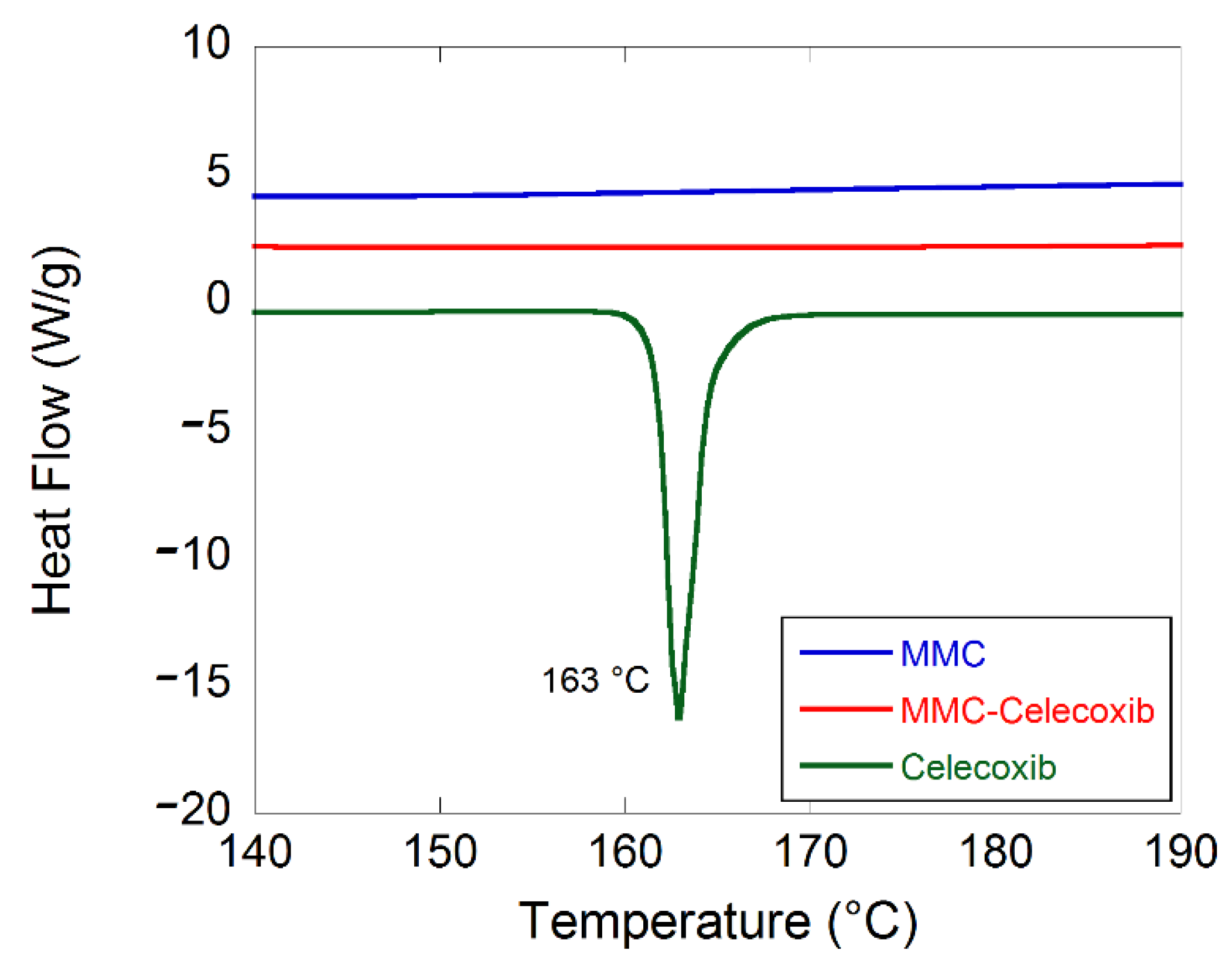
2.1. Material Characterization
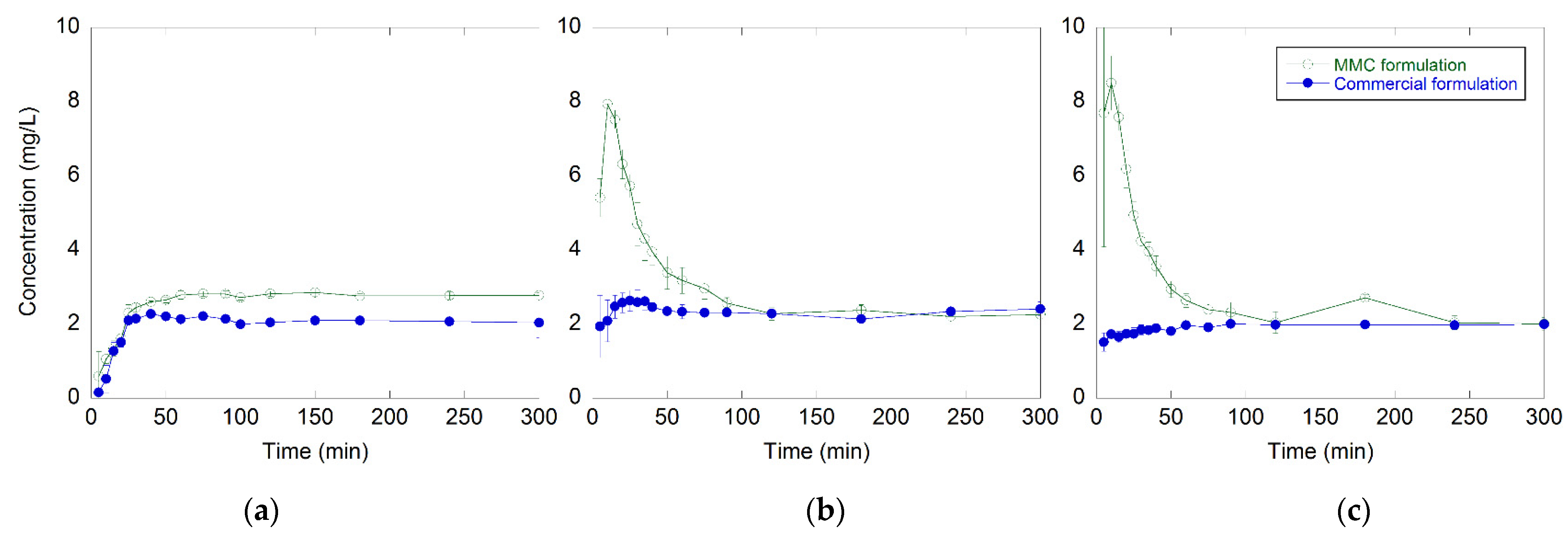
2.2. In Vitro Drug Release Test
2.3. In Vivo Absorption of Celecoxib
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of MMC
4.3. N2 Sorption Analysis
4.4. Drug-Loading Procedure
4.5. Drug-Loading Analysis
4.6. Differential Scanning Calorimetry
4.7. In Vitro Drug Release Test
4.8. In Vivo Drug Absorption of Celecoxib
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J.H. Strategies to Address Low Drug Solubility in Discovery and Development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef] [PubMed]
- Ghadi, R.; Dand, N. Review article BCS class IV drugs: Highly notorious candidates for formulation development. J. Control. Release 2017, 248, 71–95. [Google Scholar] [CrossRef] [PubMed]
- Kambayashi, A.; Kiyota, T.; Fujiwara, M.; Dressman, J.B. PBPK modeling coupled with biorelevant dissolution to forecast the oral performance of amorphous solid dispersion formulations. Eur. J. Pharm. Sci. 2019, 135, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.J.; Bergström, C.A.S.; Vinarov, Z.; Kuentz, M.; Brouwers, J.; Augustijns, P.; Brandl, M.; Bernkop-Schnürch, A.; Shrestha, N.; Préat, V.; et al. Successful oral delivery of poorly water-soluble drugs both depends on the intraluminal behavior of drugs and of appropriate advanced drug delivery systems. Eur. J. Pharm. Sci. 2019, 137, 104967. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Bedi, N.; Tiwary, A.K. Enhancing solubility of poorly aqueous soluble drugs: Critical appraisal of techniques. J. Pharm. Investig. 2018, 48, 509–526. [Google Scholar] [CrossRef]
- Bremmell, K.E.; Prestidge, C.A. Enhancing oral bioavailability of poorly soluble drugs with mesoporous silica based systems: Opportunities and challenges. Drug Dev. Ind. Pharm. 2019, 45, 349–358. [Google Scholar] [CrossRef]
- Shen, S.C.; Dong, Y.C.; Letchmanan, K.; Ng, W.K. Mesoporous Materials and Technologies for Development of Oral Medicine. In Nanostructures for Oral Medicine, 1st ed.; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 699–749. [Google Scholar]
- Katsiotis, C.S.; Åhlén, M.; Strømme, M.; Welch, K. 3D-Printed Mesoporous Carrier System for Delivery of Poorly Soluble Drugs. Pharmaceutics 2021, 13, 1096. [Google Scholar] [CrossRef]
- Sinclair, W.; Leane, M.; Clarke, G.; Dennis, A.; Tobyn, M.; Timmis, P. Physical stability and recrystallization kinetics of amorphous ibipinabant drug product by fourier transform raman spectroscopy. J. Pharm. Sci. 2011, 100, 4687–4699. [Google Scholar] [CrossRef]
- Zhang, P.; Forsgren, J.; Strømme, M. Stabilisation of amorphous ibuprofen in Upsalite, a mesoporous magnesium carbonate, as an approach to increasing the aqueous solubility of poorly soluble drugs. Int. J. Pharm. 2014, 472, 185–191. [Google Scholar] [CrossRef]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef]
- Ricarte, R.G.; Van Zee, N.J.; Li, Z.; Johnson, L.M.; Lodge, T.P.; Hillmyer, M.A. Recent Advances in Understanding the Micro- and Nanoscale Phenomena of Amorphous Solid Dispersions. Mol. Pharm. 2019, 16, 4089–4103. [Google Scholar] [CrossRef] [PubMed]
- Pandi, P.; Bulusu, R.; Kommineni, N.; Khan, W.; Singh, M. Amorphous solid dispersions: An update for preparation, characterization, mechanism on bioavailability, stability, regulatory considerations and marketed products. Int. J. Pharm. 2020, 586, 119560. [Google Scholar] [CrossRef] [PubMed]
- Forsgren, J.; Frykstrand, S.; Grandfield, K.; Mihranyan, A.; Strømme, M. A Template-Free, Ultra-Adsorbing, High Surface Area Carbonate Nanostructure. PLoS ONE 2013, 8, e68486. [Google Scholar] [CrossRef] [PubMed]
- Frykstrand, S.; Forsgren, J.; Mihranyan, A.; Strømme, M. On the pore forming mechanism of Upsalite, a micro- and mesoporous magnesium carbonate. Microporous Mesoporous Mater. 2014, 190, 99–104. [Google Scholar] [CrossRef]
- Cheung, O.; Zhang, P.; Frykstrand, S.; Zheng, H.; Yang, T.; Sommariva, M.; Zou, X.; Strømme, M. Nanostructure and pore size control of template-free synthesised mesoporous magnesium carbonate. RSC Adv. 2016, 6, 74241–74249. [Google Scholar] [CrossRef]
- Yang, J.; Alvebratt, C.; Zhang, P.; Zardán Gómez de la Torre, T.; Strømme, M.; Bergström, C.A.S.; Welch, K. Enhanced release of poorly water-soluble drugs from synergy between mesoporous magnesium carbonate and polymers. Int. J. Pharm. 2017, 525, 183–190. [Google Scholar] [CrossRef]
- Gomez de la Torre, J.; Bergström, C.; Zardan Gomez de la Torre, T. Increasing the Transport of Celecoxib over a Simulated Intestine Cell Membrane Model Using Mesoporous Magnesium Carbonate. Molecules 2021, 26, 6553. [Google Scholar] [CrossRef]
- Zhang, P.; Zardán Gómez de la Torre, T.; Welch, K.; Bergström, C.; Strømme, M. Supersaturation of poorly soluble drugs induced by mesoporous magnesium carbonate. Eur. J. Pharm. Sci. 2016, 93, 468–474. [Google Scholar] [CrossRef]
- Zhang, P.; Zardán Gómez De La Torre, T.; Forsgren, J.; Bergström, C.A.S.; Strømme, M. Diffusion-Controlled Drug Release from the Mesoporous Magnesium Carbonate Upsalite®. J. Pharm. Sci. 2016, 105, 657–663. [Google Scholar] [CrossRef]
- Vall, M.; Strømme, M.; Cheung, O. Amine-Modified Mesoporous Magnesium Carbonate as an Effective Adsorbent for Azo Dyes. ACS Omega 2019, 4, 2973–2979. [Google Scholar] [CrossRef]
- Trzeciak, K.; Chotera-ouda, A.; Bak-sypien, I.I.; Potrzebowski, M.J. Mesoporous silica particles as drug delivery systems—The state of the art in loading methods and the recent progress in analytical techniques for monitoring these processes. Pharmaceutics 2021, 13, 950. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous silica nanoparticles for drug delivery: Current insights. Molecules 2018, 23, 47. [Google Scholar] [CrossRef] [PubMed]
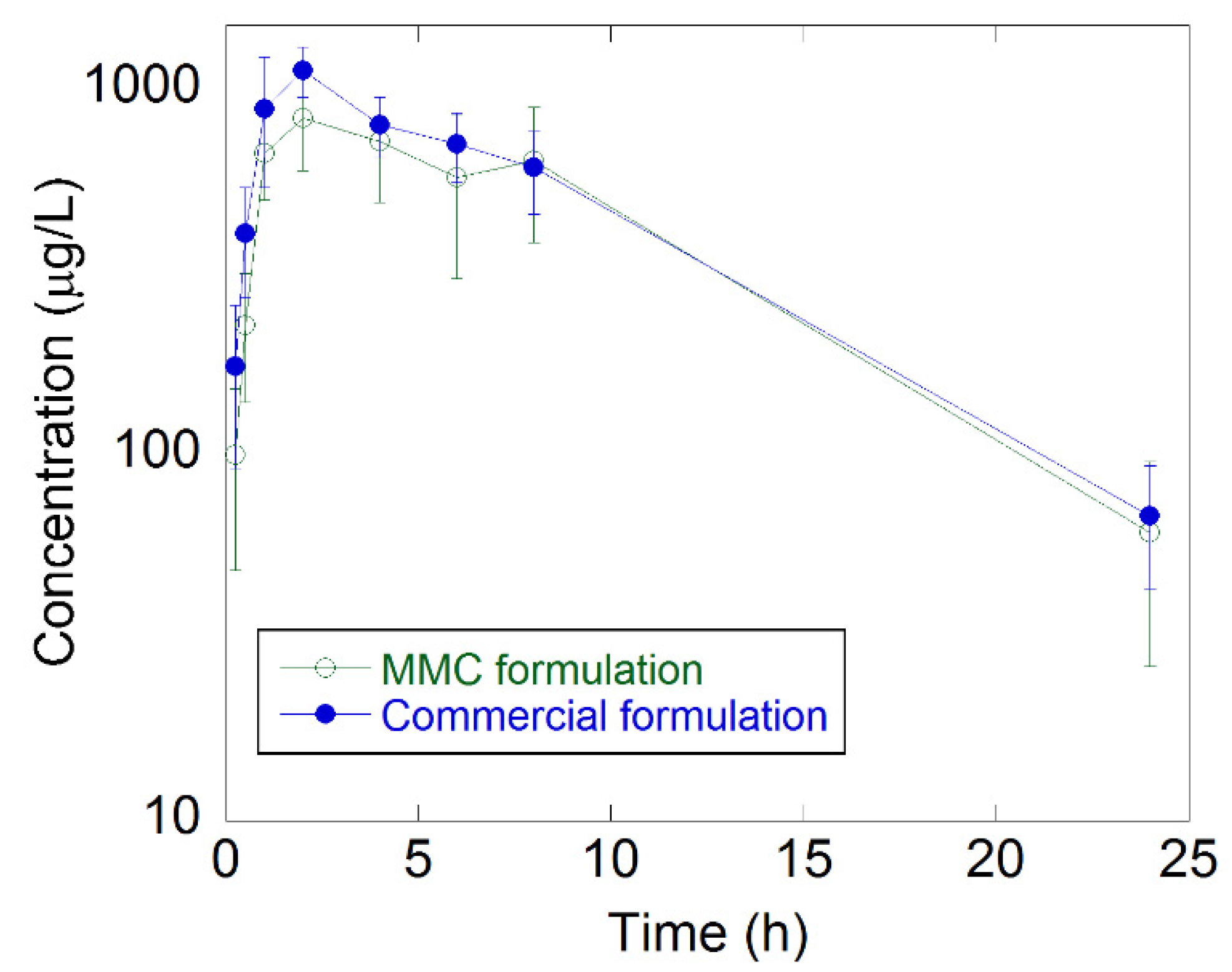
| Group | Dose (mg/kg) | Cmax (μg/L) | Tmax (h) | T1/2 (h) | AUC (h μg L−1) | Frel (%) |
|---|---|---|---|---|---|---|
| Commercial formulation | 6 | 1160 ± 182 | 1.67 ± 0516 | 6.4 ± 1.19 | 10700 ± 2160 | - |
| MMC formulation | 6 | 875 ± 220 | 2.33 ± 0.816 | 6.12 ± 1.37 | 9480 ± 3470 | 89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zardán Gómez de la Torre, T.; Lindmark, T.; Cheung, O.; Bergström, C.; Strømme, M. Bioavailability of Celecoxib Formulated with Mesoporous Magnesium Carbonate—An In Vivo Evaluation. Molecules 2022, 27, 6188. https://doi.org/10.3390/molecules27196188
Zardán Gómez de la Torre T, Lindmark T, Cheung O, Bergström C, Strømme M. Bioavailability of Celecoxib Formulated with Mesoporous Magnesium Carbonate—An In Vivo Evaluation. Molecules. 2022; 27(19):6188. https://doi.org/10.3390/molecules27196188
Chicago/Turabian StyleZardán Gómez de la Torre, Teresa, Tuulikki Lindmark, Ocean Cheung, Christel Bergström, and Maria Strømme. 2022. "Bioavailability of Celecoxib Formulated with Mesoporous Magnesium Carbonate—An In Vivo Evaluation" Molecules 27, no. 19: 6188. https://doi.org/10.3390/molecules27196188
APA StyleZardán Gómez de la Torre, T., Lindmark, T., Cheung, O., Bergström, C., & Strømme, M. (2022). Bioavailability of Celecoxib Formulated with Mesoporous Magnesium Carbonate—An In Vivo Evaluation. Molecules, 27(19), 6188. https://doi.org/10.3390/molecules27196188







