Comparison on Protein Bioaccessibility of Soymilk Gels Induced by Glucono-δ-Lactone and Lactic Acid Bacteria
Abstract
:1. Introduction
2. Results and Discussion
2.1. Proximate Analysis
2.2. Protein Degradation during GIS Digestion
2.3. The DH and Protein Digestibility
2.4. Bioaccessible Peptides
2.5. SDS-PAGE
2.6. Amino Acids Analysis
3. Materials and Methods
3.1. Materials
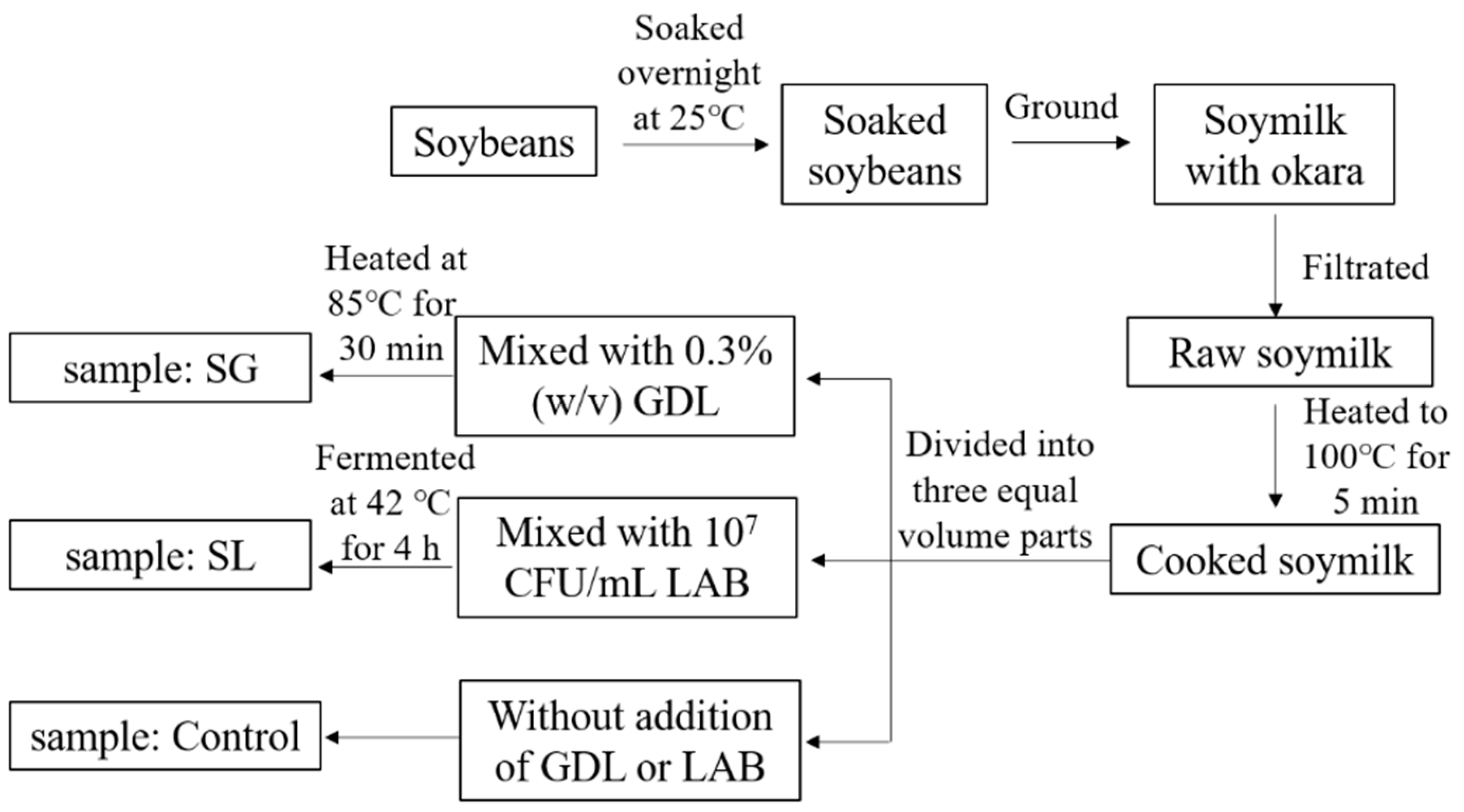
3.2. Preparation of Soymilk Gels
3.3. Proximate Analysis of Soymilk Gels
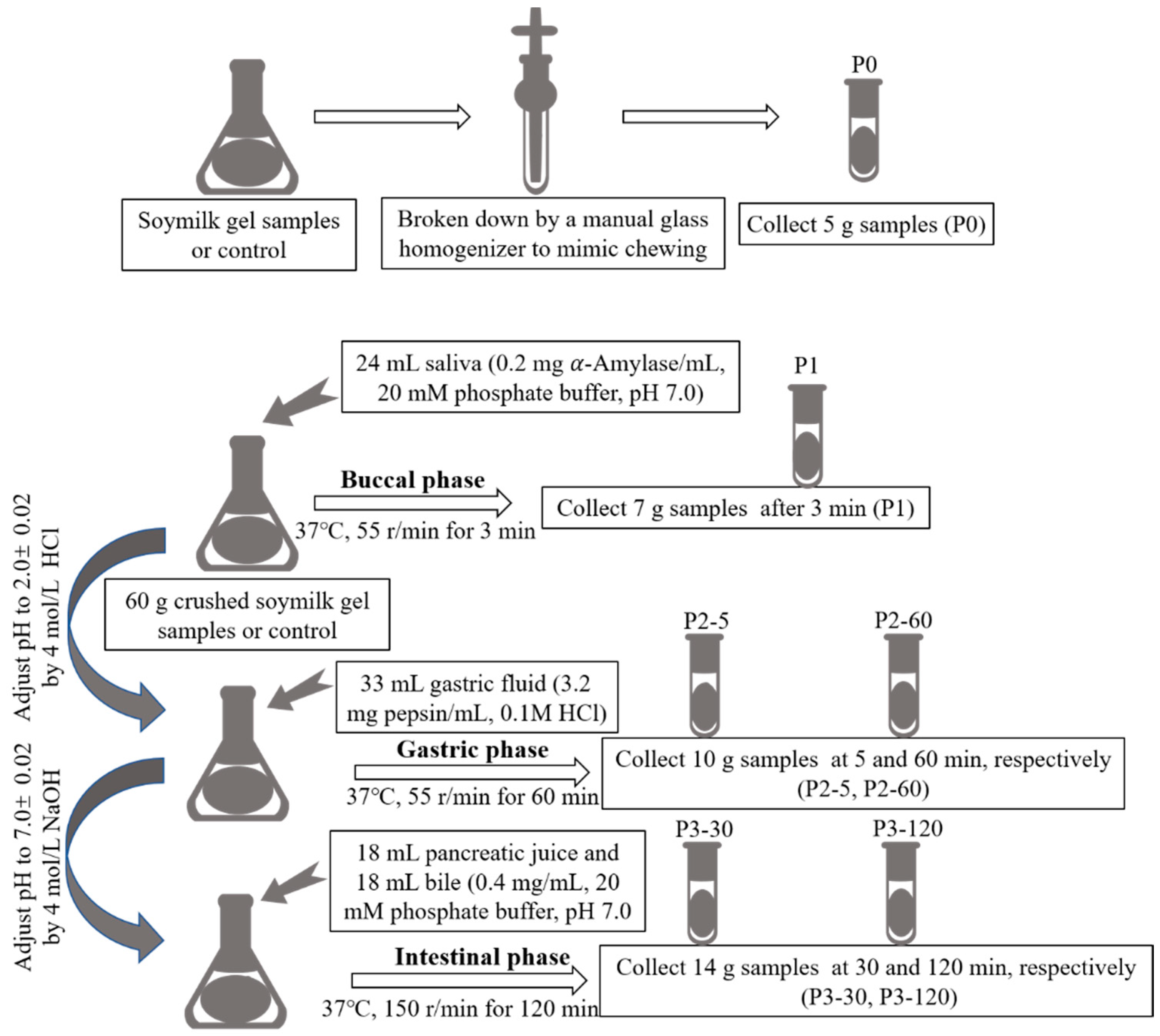
3.4. In Vitro Gastrointestinal Simulated Digestion
3.5. Soluble Protein Content Determination
3.6. The Degree of Hydrolysis and In Vitro Protein Digestibility
3.7. Peptide Content Measurement
3.8. SDS-PAGE
3.9. Free Amino Acids Determination
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ali, F.; Tian, K.; Wang, Z.X. Modern techniques efficacy on tofu processing: A review. Trends Food Sci. Technol. 2021, 116, 766–785. [Google Scholar] [CrossRef]
- Shi, Y.G.; Yang, Y.; Piekoszewski, W.; Zeng, J.H.; Guan, H.N.; Wang, B.; Liu, L.L.; Zhu, X.Q.; Chen, F.L.; Zhang, N. Influence of four different coagulants on the physicochemical properties, textural characteristics and flavour of tofu. Int. J. Food Sci. Technol. 2020, 55, 1218–1229. [Google Scholar] [CrossRef]
- Zheng, L.; Regenstein, J.M.; Teng, F.; Li, Y. Tofu products: A review of their raw materials, processing conditions, and packaging. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3683–3714. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Rui, X.; Zhang, Y.; Cai, F.; Chen, X.; Jiang, M. Production of tofu by lactic acid bacteria isolated from naturally fermented soy whey and evaluation of its quality. LWT-Food Sci. Technol. 2017, 82, 227–234. [Google Scholar] [CrossRef]
- Xing, G.; Giosafatto, C.V.L.; Carpentieri, A.; Pasquino, R.; Dong, M.; Mariniello, L. Gelling behavior of bio-tofu coagulated by microbial transglutaminase combined with lactic acid bacteria. Food Res. Int. 2020, 134, 109200. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, S.; Li, L. The gel properties and gastric digestion kinetics of a novel lactic acid bacteria fermented tofu: Focusing on the effects of transglutaminase. LWT-Food Sci. Technol. 2021, 143, 110998. [Google Scholar] [CrossRef]
- Kumari, M.; Kokkiligadda, A.; Dasriya, V.; Naithani, H. Functional relevance and health benefits of soymilk fermented by lactic acid bacteria. J. Appl. Microbiol. 2022, 133, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, L.; Hebert, E.M.; Garro, M.S.; de Giori, G.S. Proteolytic activity of Lactobacillus strains on soybean proteins. LWT-Food Sci. Technol. 2014, 59, 780–785. [Google Scholar] [CrossRef]
- Undhad Trupti, J.; Das, S.; Solanki, D.; Kinariwala, D.; Hati, S. Bioactivities and ACE-inhibitory peptides releasing potential of lactic acid bacteria in fermented soy milk. Food Prod. Process. Nutr. 2021, 3, 1–14. [Google Scholar] [CrossRef]
- Sanjukta, S.; Rai, A.K. Production of bioactive peptides during soybean fermentation and their potential health benefits. Trends Food Sci. Technol. 2016, 50, 1–10. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Chen, X.; Feng, M.; Rui, X.; Jiang, M.; Dong, M. Microbiological, physicochemical and rheological properties of fermented soymilk produced with exopolysaccharide (EPS) producing lactic acid bacteria strains. LWT-Food Sci. Technol. 2014, 57, 477–485. [Google Scholar] [CrossRef]
- Pang, Z.; Xu, R.; Zhu, Y.; Li, H.; Bansal, N.; Liu, X. Comparison of rheological, tribological, and microstructural properties of soymilk gels acidified with glucono-δ-lactone or culture. Food Res. Int. 2019, 121, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Ayivi, R.D.; Gyawali, R.; Krastanov, A.; Aljaloud, S.O.; Worku, M.; Tahergorabi, R.; Silva, R.C.; Ibrahim, S.A. Lactic acid bacteria: Food safety and human health applications. Dairy 2020, 1, 202–232. [Google Scholar] [CrossRef]
- Ringgenberg, E.; Alexander, M.; Corredig, M. Effect of concentration and incubation temperature on the acid induced aggregation of soymilk. Food Hydrocoll. 2013, 30, 463–469. [Google Scholar] [CrossRef]
- Lu, Y.; Cui, Z.; Guan, X.; Lin, J.; Zhong, X.; Zhang, M. Effect of magnesium chloride concentration on soymilk coagulation mechanism. LWT-Food Sci. Technol. 2021, 150, 112000. [Google Scholar] [CrossRef]
- Grygorczyk, A.; Corredig, M. Acid induced gelation of soymilk, comparison between gels prepared with lactic acid bacteria and glucono-δ-lactone. Food Chem. 2013, 141, 1716–1721. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability and bioaccessibility of food bioactive compounds; overview and assessment by in vitro methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2862–2884. [Google Scholar] [CrossRef]
- Lucas-González, R.; Viuda-Martos, M.; Pérez-Alvarez, J.A.; Fernández-López, J. In vitro digestion models suitable for foods: Opportunities for new fields of application and challenges. Food Res. Int. 2018, 107, 423–436. [Google Scholar] [CrossRef]
- Xing, G.; Rui, X.; Wang, D.; Liu, M.; Chen, X.; Dong, M. Effect of fermentation pH on protein bioaccessibility of soymilk curd with added tea polyphenols as assessed by in vitro gastrointestinal digestion. J. Agric. Food Chem. 2017, 65, 11125–11132. [Google Scholar] [CrossRef]
- Rui, X.; Zhang, Q.; Huang, J.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Does lactic fermentation influence soy yogurt protein digestibility: A comparative study between soymilk and soy yogurt at different pH. J. Sci. Food Agric. 2019, 99, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Safdar, B.; Wang, Y.; Sun, M.; Liu, X. Improvement of tribo-rheological properties of acid soymilk gels by reinforcement of 7S or 11S proteins. Food Hydrocoll. 2021, 110, 106173. [Google Scholar] [CrossRef]
- Wan, Y.; Li, Y.; Guo, S. Characteristics of soy protein isolate gel induced by glucono-δ-lactone: Effects of the protein concentration during preheating. Food Hydrocoll. 2021, 113, 106525. [Google Scholar] [CrossRef]
- Rui, X.; Xing, G.; Zhang, Q.; Zare, F.; Li, W.; Dong, M. Protein bioaccessibility of soymilk and soymilk curd prepared with two Lactobacillus plantarum strains as assessed by in vitro gastrointestinal digestion. Innov. Food Sci. Emerg. Technol. 2016, 38, 155–159. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Bhandari, B.; Cichero, J.; Prakash, S. Gastrointestinal digestion of dairy and soy proteins in infant formulas: An in vitro study. Food Res. Int. 2015, 76, 348–358. [Google Scholar] [CrossRef]
- Xu, X.; Qiao, Y.; Shi, B.; Dia, V.P. Alcalase and bromelain hydrolysis affected physicochemical and functional properties and biological activities of legume proteins. Food Struct. 2021, 27, 100178. [Google Scholar] [CrossRef]
- Eberhardt, A.; López, E.C.; Marino, F.; Mammarella, E.J.; Manzo, R.M.; Sihufe, G.A. Whey protein hydrolysis with microbial proteases: Determination of kinetic parameters and bioactive properties for different reaction conditions. Int. J. Dairy Technol. 2021, 74, 489–504. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Qing, S.; Zhang, Q.; Li, W.; Azarpazhooh, E.; Simpson, B.K.; Rui, X. Effects of different satiety levels on the fate of soymilk protein in gastrointestinal digestion and antigenicity assessed by an in vitro dynamic gastrointestinal model. Food Funct. 2019, 10, 7855–7864. [Google Scholar] [CrossRef]
- Yang, X.; Ren, Y.; Liu, H.; Huo, C.; Li, L. Differences in the physicochemical, digestion and microstructural characteristics of soy protein gel acidified with lactic acid bacteria, glucono-δ-lactone and organic acid. Int. J. Biol. Macromol. 2021, 185, 462–470. [Google Scholar] [CrossRef]
- Jiménez-Munoz, L.; Tsochatzis, E.D.; Corredig, M. Impact of the structural modifications of potato protein in the digestibility process under semi-dynamic simulated human gastrointestinal in vitro system. Nutrients 2022, 14, 2505. [Google Scholar] [CrossRef] [PubMed]
- Meinlschmidt, P.; Sussmann, D.; Schweiggert-Weisz, U.; Eisner, P. Enzymatic treatment of soy protein isolates: Effects on the potential allergenicity, technofunctionality, and sensory properties. Food Sci. Nutr. 2016, 4, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.E.; Moraru, C.I. Effect of High Pressure Processing and heat treatment on in vitro digestibility and trypsin inhibitor activity in lentil and faba bean protein concentrates. LWT-Food Sci. Technol. 2021, 152, 112342. [Google Scholar] [CrossRef]
- Carbonaro, M.; Maselli, P.; Nucara, A. Structural aspects of legume proteins and nutraceutical properties. Food Res. Int. 2015, 76, 19–30. [Google Scholar] [CrossRef]
- Peng, Y.; Kyriakopoulou, K.; Ndiaye, M.; Bianeis, M.; Keppler, J.K.; van der Goot, A.J. Characteristics of soy protein prepared using an aqueous ethanol washing process. Foods 2021, 10, 2222. [Google Scholar] [CrossRef] [PubMed]
- Lou, M.; Ritzoulis, C.; Liu, J.; Zhang, X.; Han, J.; Liu, W. In vitro digestion of tofu with different textures using an artificial gastric digestive system. Food Res. Int. 2022, 157, 111458. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Ritzoulis, C.; Han, J.; Han, F.; Jin, W.; Liu, W. Particle degradation and nutrient bioavailability of soybean milk during in vitro digestion. Food Biophys. 2021, 16, 58–69. [Google Scholar] [CrossRef]
- AACC. Approved Methods of the American Association of Cereal Chemists, 10th ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 2000. [Google Scholar]
- Hui, T.; Xing, G. Effect of transglutaminase pre-crosslinking treatment incorporated with glucono-δ-lactone on the physicochemical and digestive properties of tofu. Polymers 2022, 14, 2364. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford method for protein quantitation. In Basic Protein and Peptide Protocols. Methods in Molecular Biology; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 1994; Volume 32, pp. 9–15. [Google Scholar]
- Ajibola, C.F.; Aluko, R.E. Physicochemical and functional properties of 2s, 7s, and 11s enriched hemp seed protein fractions. Molecules 2022, 27, 1059. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, Y.; Liu, Q.; Simpson, B.K.; Azarpazhooh, E.; Dong, M.; Rui, X. Effect of lactic fermentation on soy protein digestive pattern assessed by an in vitro dynamic gastrointestinal digestion model and the influence on human faecal microbiota. J. Sci. Food Agric. 2021, 101, 871–879. [Google Scholar] [CrossRef]
- Aro, J.M.A.; Nyam-Osor, P.; Tsuji, K.; Shimada, K.I.; Fukushima, M.; Sekikawa, M. The effect of starter cultures on proteolytic changes and amino acid content in fermented sausages. Food Chem. 2010, 119, 279–285. [Google Scholar] [CrossRef]
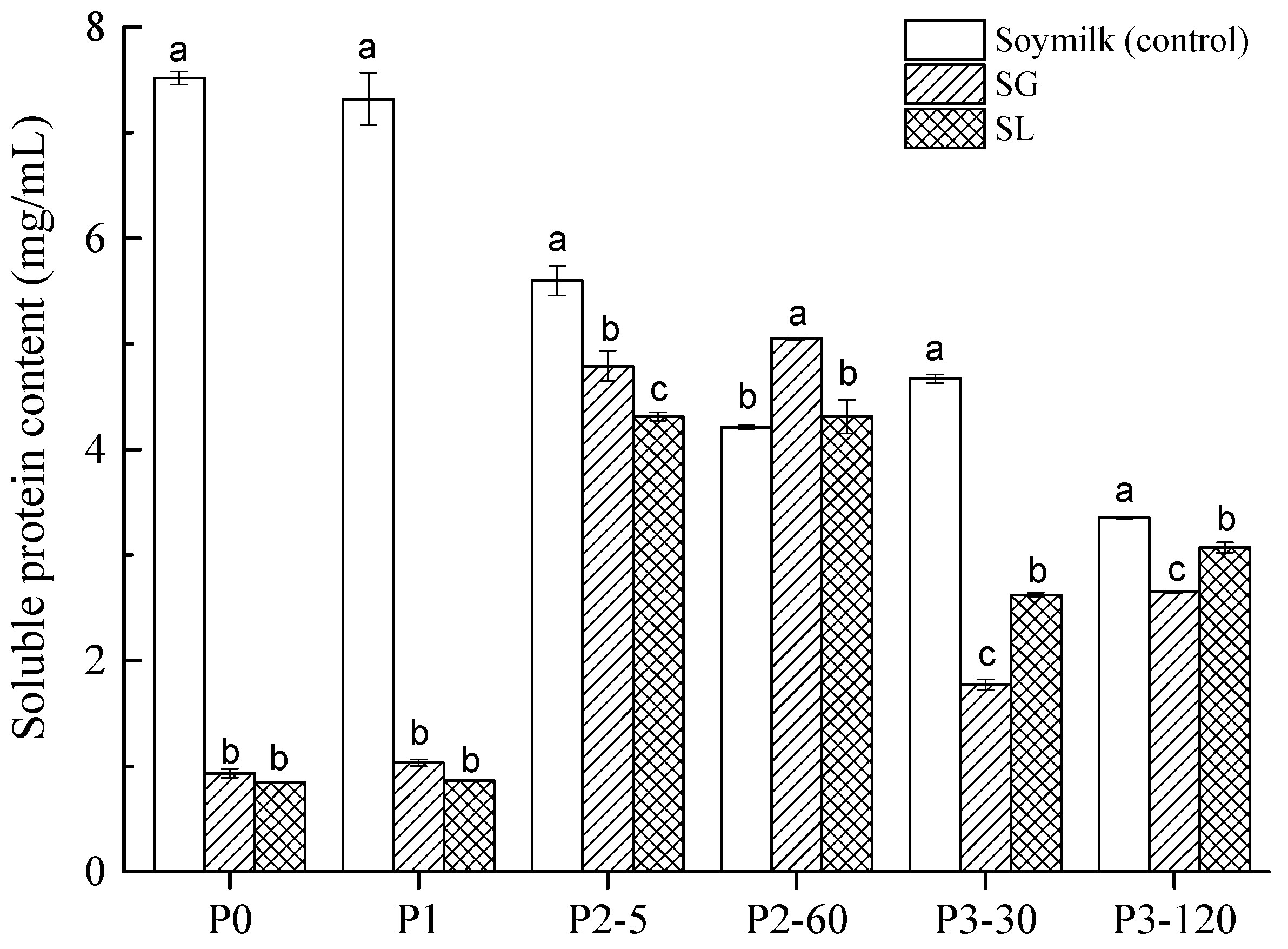

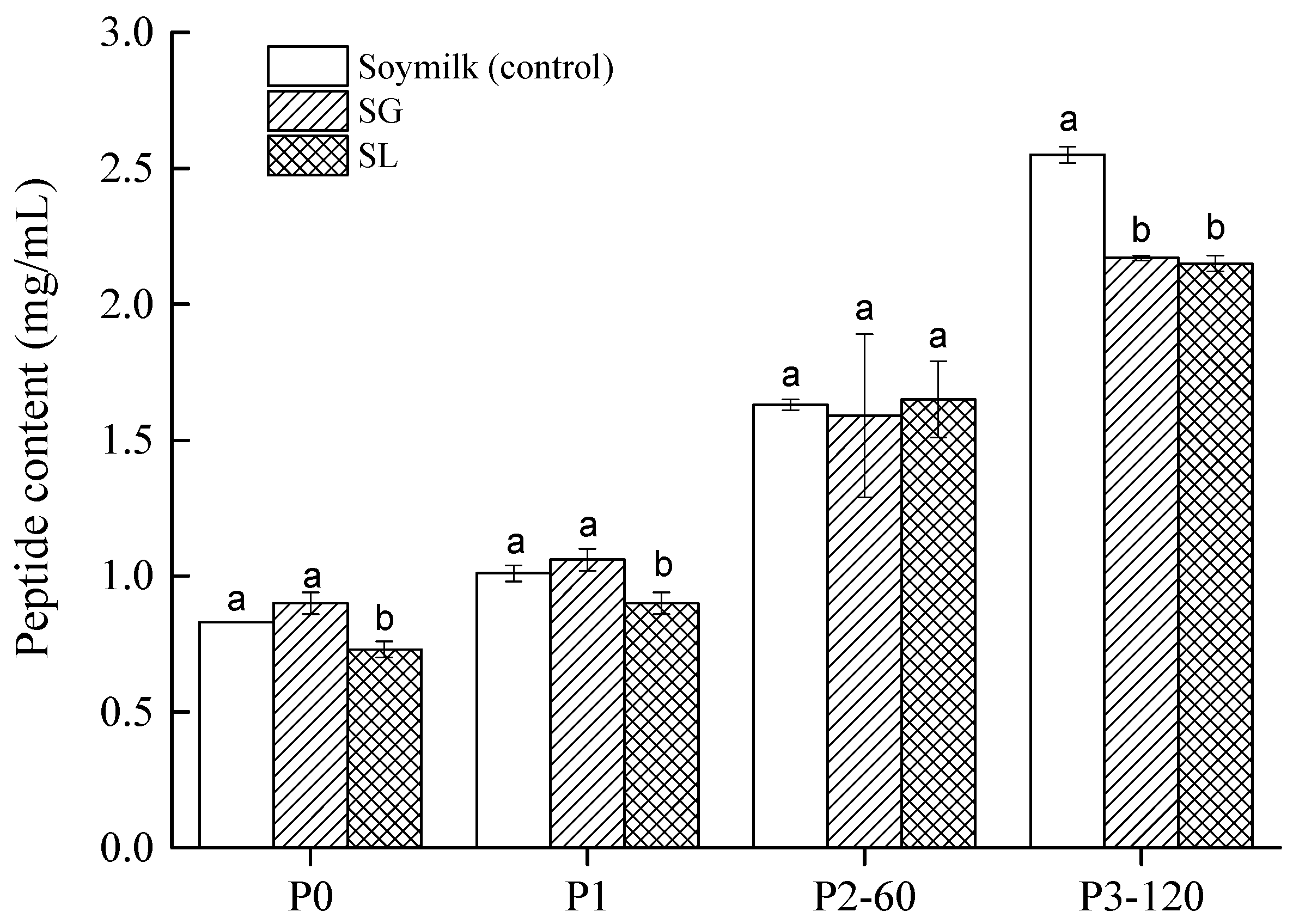
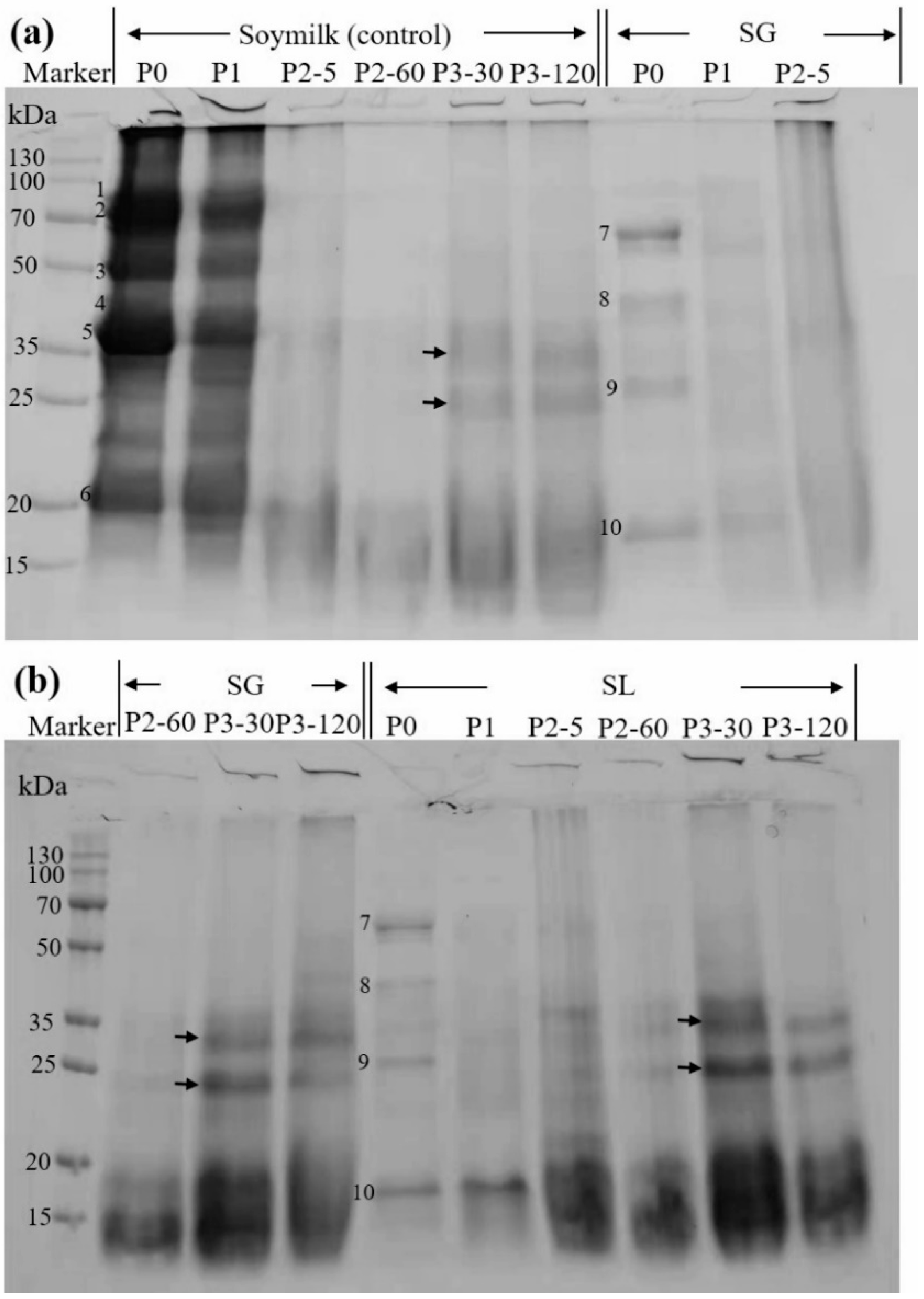
| Sample | In Vitro Digestibility (%) |
|---|---|
| Soymilk (control) | 82.16 ± 1.12 a |
| SG | 75.33 ± 1.00 c |
| SL | 80.57 ± 1.53 b |
| Amino Acid Types | Soymilk (Control) | SG | SL |
|---|---|---|---|
| Asp | 0.160 | 0.140 | 0.146 |
| Thr | 0.090 | 0.070 | 0.083 |
| Ser | 0.121 | 0.125 | 0.127 |
| Glu | 0.823 | 0.667 | 0.824 |
| Gly | 0.135 | 0.091 | 0.104 |
| Ala | 0.622 | 0.463 | 0.491 |
| Cys | 0.026 | 0.011 | 0.030 |
| Val | 0.074 | 0.067 | 0.070 |
| Met | 0.005 | 0.003 | 0.004 |
| Ile | 0.000 | 0.000 | 0.002 |
| Leu | 2.753 | 2.523 | 2.476 |
| Tyr | 5.077 | 4.578 | 4.638 |
| Phe | 1.788 | 1.731 | 1.764 |
| Lys | 4.749 | 5.483 | 5.550 |
| His | 0.280 | 0.287 | 0.278 |
| Trp | 4.040 | 3.528 | 3.961 |
| Pro | 0.111 | 0.084 | 0.089 |
| Essential amino acids | 13.499 | 13.405 | 13.910 |
| Total | 20.854 | 19.851 | 20.637 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hui, T.; Tang, T.; Gu, X.; Yuan, Z.; Xing, G. Comparison on Protein Bioaccessibility of Soymilk Gels Induced by Glucono-δ-Lactone and Lactic Acid Bacteria. Molecules 2022, 27, 6202. https://doi.org/10.3390/molecules27196202
Hui T, Tang T, Gu X, Yuan Z, Xing G. Comparison on Protein Bioaccessibility of Soymilk Gels Induced by Glucono-δ-Lactone and Lactic Acid Bacteria. Molecules. 2022; 27(19):6202. https://doi.org/10.3390/molecules27196202
Chicago/Turabian StyleHui, Tianran, Ting Tang, Xuan Gu, Zhen Yuan, and Guangliang Xing. 2022. "Comparison on Protein Bioaccessibility of Soymilk Gels Induced by Glucono-δ-Lactone and Lactic Acid Bacteria" Molecules 27, no. 19: 6202. https://doi.org/10.3390/molecules27196202
APA StyleHui, T., Tang, T., Gu, X., Yuan, Z., & Xing, G. (2022). Comparison on Protein Bioaccessibility of Soymilk Gels Induced by Glucono-δ-Lactone and Lactic Acid Bacteria. Molecules, 27(19), 6202. https://doi.org/10.3390/molecules27196202