Abstract
The medicinal plant Artabotrys hexapetalus (synonyms: A. uncinatus and A. odoratissimus) is known as yingzhao in Chinese. Extracts of the plant have long been used in Asian folk medicine to treat various symptoms and diseases, including fevers, microbial infections, ulcers, hepatic disorders and other health problems. In particular, extracts from the roots and fruits of the plant are used for treating malaria. Numerous bioactive natural products have been isolated from the plant, mainly aporphine (artabonatines, artacinatine) and benzylisoquinoline (hexapetalines) alkaloids, terpenoids (artaboterpenoids), flavonoids (artabotrysides), butanolides (uncinine, artapetalins) and a small series of endoperoxides known as yingzhaosu A-to-D. These natural products confer antioxidant, anti-inflammatory and antiproliferative properties to the plant extracts. The lead compound yingzhaosu A displays marked activities against the malaria parasites Plasmodium falciparum and P. berghei. Total syntheses have been developed to access yingzhaosu compounds and analogues, such as the potent compound C14-epi-yingzhaosu A and simpler molecules with a dioxane unit. The mechanism of action of yingzhaosu A points to an iron(II)-induced degradation leading to the formation of two alkylating species, an unsaturated ketone and a cyclohexyl radical, which can then react with vital parasitic proteins. A bioreductive activation of yingzhaosu A endoperoxide can also occur with the heme iron complex. The mechanism of action of yingzhaosu endoperoxides is discussed, to promote further chemical and pharmacological studies of these neglected, but highly interesting bioactive compounds. Yingzhaosu A/C represent useful templates for designing novel antimalarial drugs.
1. Introduction
Despite new treatment modalities, malaria remains a major public health threat worldwide, causing more than 400,000 deaths per year, predominantly in children in sub-Saharan Africa and tropical regions [1]. Recently, the malaria vaccine RTS,S (Mosquirix™) has received regulatory approval for the prevention of malaria infection. The launch of this vaccine represents a major achievement in limiting the transmission of the parasite, but it shows a modest efficacy against malaria illness [2]. New treatments are still needed to combat the disease, notably the drug-resistant forms, which develop rapidly. There is a significant need for efficient, affordable and well-tolerated antimalarial drugs [3].
The first-line treatment of Plasmodium falciparum mild malaria generally relies on combination therapy, including artemisinin (ART) and/or chloroquine, whereas intravenous artesunate is often preferred in cases of severe malaria, at least in developed countries [4]. The combination of artesunate and pyronaridine (Pyramax®) is also approved to treat uncomplicated malaria [5,6]. ART and derivatives have been extensively studied as antimalarial drugs. The discovery of artemisinin was largely based on traditional Chinese medicine (TCM), as recognized by Dr. Youyou Tu, who was awarded the Nobel Prize in Physiology and Medicine in 2015 for her discovery of ART and its therapeutic effects on malaria [7]. Herbs of the Artemisia family (qinghao in Chinese) have been extensively studied for their pharmacological properties and phytochemical content.
In contrast, the plant yingzhao (Artabotrys unciatus (L.) Meer.) has been considerably less investigated. Moreover, the main phytochemical compounds found in yingzhao, known as yingzhaosu, have been essentially neglected, at least from a pharmacological viewpoint (Figure 1). The present review offers a survey of the current knowledge and recent research about this plant and its bioactive constituents.
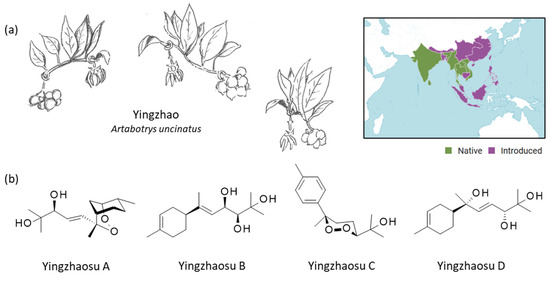
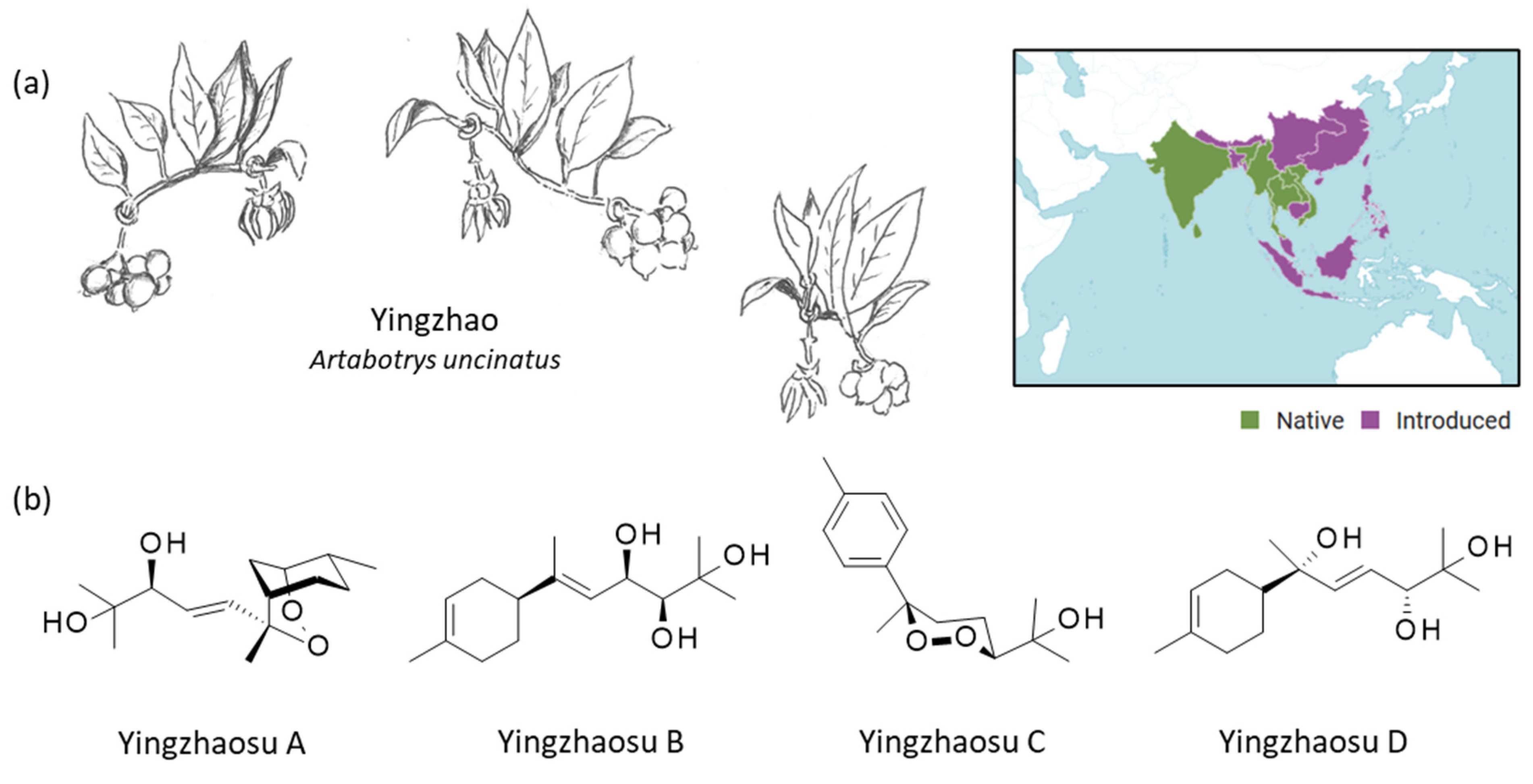
Figure 1.
Yingzhao plant and yingzhaosu compounds. (a) The plant yingzhao (Artabotrys uncinatus (L.) Meer.) with a view of the young fruits, the leaves and the flower (drawing: Prof. J.-P. Hénichart). The plant is largely distributed in Southeast Asia. The plant is native to countries such as India, Thailand, Vietnam, and Sri Lanka (and other counties in green) and has been introduced in Indonesoia, China, Japan (and other countries in purple) (https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:72395-1, accessed on 14 September 2022). (b) Structure of the four yingzhaosu compounds, all isolated from yingzhao.
Specifically, the review presents the origin and use of the plant yingzhao, with a detailed analysis of the phytochemicals isolated from the different parts of the plant. Their mechanisms of action are discussed, focusing on the yingzhaosu products to highlight the reactivity of the endoperoxide compounds yingzhaosu A and C. The significance of the work reported here is high considering the need for new drugs to treat malaria but also a novel targeted approach to treating other parasitic diseases and cancers. Artemisinin-type drugs are increasingly considered to treat hematological malignancies, viral infections and other human diseases [8,9,10]. The importance of the reactive endoperoxide function in chemistry is well recognized, and the use of naturally occurring endoperoxides to design new drugs has often been underlined [11,12]. In this context, it is timely and essential to promote yingzhaosu compounds.
2. The Plant Yingzhao
The Chinese medicine yingzhao is usually associated with the plant name Artabotrys uncinatus (L.) Meer. (Annonaceae), from which yingzhaosu compounds have been isolated, as discussed above (Figure 1). However, and in fact, A. unciatus is one of the synonyms for the plant Artabotrys hexapetalus (L.f.) Bhandari, which is the accepted botanical name [13]. Other synonyms are used, such as A. odoratissimus R.Brown. (Table 1). This plant, commonly called tail grape or climbing ylang-ylang, can be found in China, India, Malaysia, Indonesia, Vietnam and other countries of Southeast Asia. It is native to Sri Lanka and Southern India and can be grown in many places [14]. For example, two established plants of A. hexapetalus (L.f.) Bhand. growing at the Fairchild Tropical Garden in Miami (US) have been used to study plant architecture during growth [15]. It can also be found in some tropical African countries, like Tanzania [16].

Table 1.
The plant yingzhao, accepted botanical names and synonyms.
Yingzhao behaves as a woody climber, possibly growing up to 10 m in height. It should not be confused with Cananga odorata (ylang-ylang) used in perfumery. A. hexapetalus is a woody scandent climbing shrub. The leaves are oblong to broadly lanceolate in shape and the flowers are fragrant, with yellow petals (Figure 1). The plant is recognizable from the flower stalks, which are shaped like hooks. With beautiful and aromatic flowers, it is an ornamental plant now cultivated and also used in the perfume industry. The seeds are used for propagation, but the germination process is complex and long, taking as long as 238 days [17]. The plant is appreciated for its pleasant-smelling yellow flowers, from which a delicate essential oil, rich in sesquiterpenoids (such as β-caryophyllene and caryophyllene oxide), can be prepared [18]. Essential oils can also be obtained from the stem bark or leaves of the plant to be used as a mosquito repellent [16].
3. Traditional Medicinal Uses of Yingzhao
For a long time, yingzhao-based preparations have been used to treat human diseases. Different parts of the plant have been used. The symptoms and pathologies treated with plant extracts vary from one country to another. In Malaysia, leaf decoctions were given for curing cholera, whereas the roots and fruits have been used for the treatment of malaria and scrofula (tuberculous lymphadenitis). There is also mention of the use of the plant to treat fever, diarrhea, dysentery, cuts, sprains, ulcers, and asthma.
Recently, Kousalya and Doss [19] have inventoried the diverse ethnomedicinal uses of A. hexapetalus extracts. These can range from antibacterial and antifungal effects to hepato-protective and anti-ulcer activities (Table 2). In general, the pharmacological effects were obtained with organic extracts prepared from the plant’s leaves, bark, or roots. For example, methanolic leave extracts were found to display antifungal and antibacterial effects [20,21,22]. The essential oil of A. odoratissimus R.Br. (synonym) has shown a broad-spectrum activity against 14 different storage fungi, and interestingly, it was reported to arrest aflatoxin B1 secretion by a toxic strain of Aspergillus flavus [23]. Flower extracts of A. hexapetalus also revealed antifungal effects [24].

Table 2.
Pharmacological properties of A. hexapetalus extracts.
Alcoholic leave extracts display cytoprotective effects in vitro and in vivo. An ethanolic extract orally administered (100–200 mg/k for seven days) to mice with drug-induced liver injury was found to reduce the oxidative damages at least at the biochemical level and to alleviate the sign of cellular degeneration and necrosis. The extract was well tolerated in mice [40]. The observed effects were attributed to the presence of antioxidant natural products, including flavonoids and alkaloids. Similar antioxidant and hepatoprotective effects have been reported in other studies with A. hexapetalus extracts [29,37,38].
The hydroalcoholic leave extracts of A. hexapetalus have been found to reduce sperm count and mobility in rats, reducing the diameter of seminiferous tubules. The extract lowered the testosterone level and significantly reduced fertility in rats [33]. The observation was consistent with the reported antifertility activity of various leaf extracts of A. odoratissimus Roxb. (synonym). In this case, the extracts (obtained with benzene, ethanol and water) were found to disturb the oestrus cycle and reduce implantation and thus fertility [31]. Antifertility activity has been confirmed in a recent study performed with extracts from the leaves and stem of A. odoratissimus Roxb. in female rats. The extracts altered the level of cholesterol and steroidal hormones (estradiol and progesterone) and caused polycystic ovaries in rats [33,34]. In India, antifertility activity of A. odoratissimus plant extracts is known for several decades [41,42] and remains considered today for the regulation of fertility [35].
The main bioactivity of A. hexapetalus refers to antiparasitic effects. For a long time, yingzhao (roots and fruits) has been used to combat parasites such as Plasmodium falciparum and Leishmania donovani. A petroleum ether extract of A. hexapetalus was shown to moderately reduce the growth of the promastigote forms of cultured L. donovani. The effect was attributed to the presence of flavonoids such as quercetin and apigenin [28]. Notwithstanding, there are many other flavonoids in the plant extracts, such as taxifolin, apigenin glycosides, glucoluteolin, arabotrysides A and B (Figure 2), and the flavonol glycosides called arapetalosides A and B [43,44]. Hydroalcoholic leaf extracts of A. hexapetalus display activities against Plasmodium and Leishmania, but not against the African earthworm Eudrilus eugeniae (African nightcrawler) [27,28]. It should be noted that there is not many published information about the antiplasmodial activity of A. hexapetalus. Solid data have been reported with other species, such as A. crassifolius [45], but not for yingzhao extracts despite the traditional use.
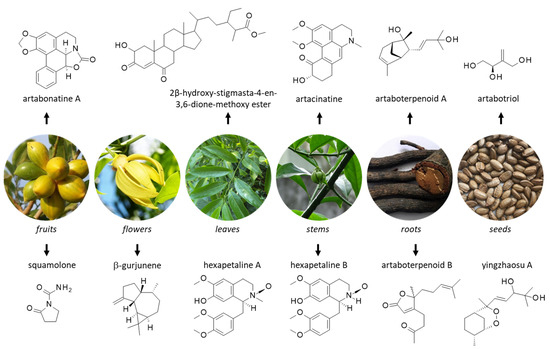
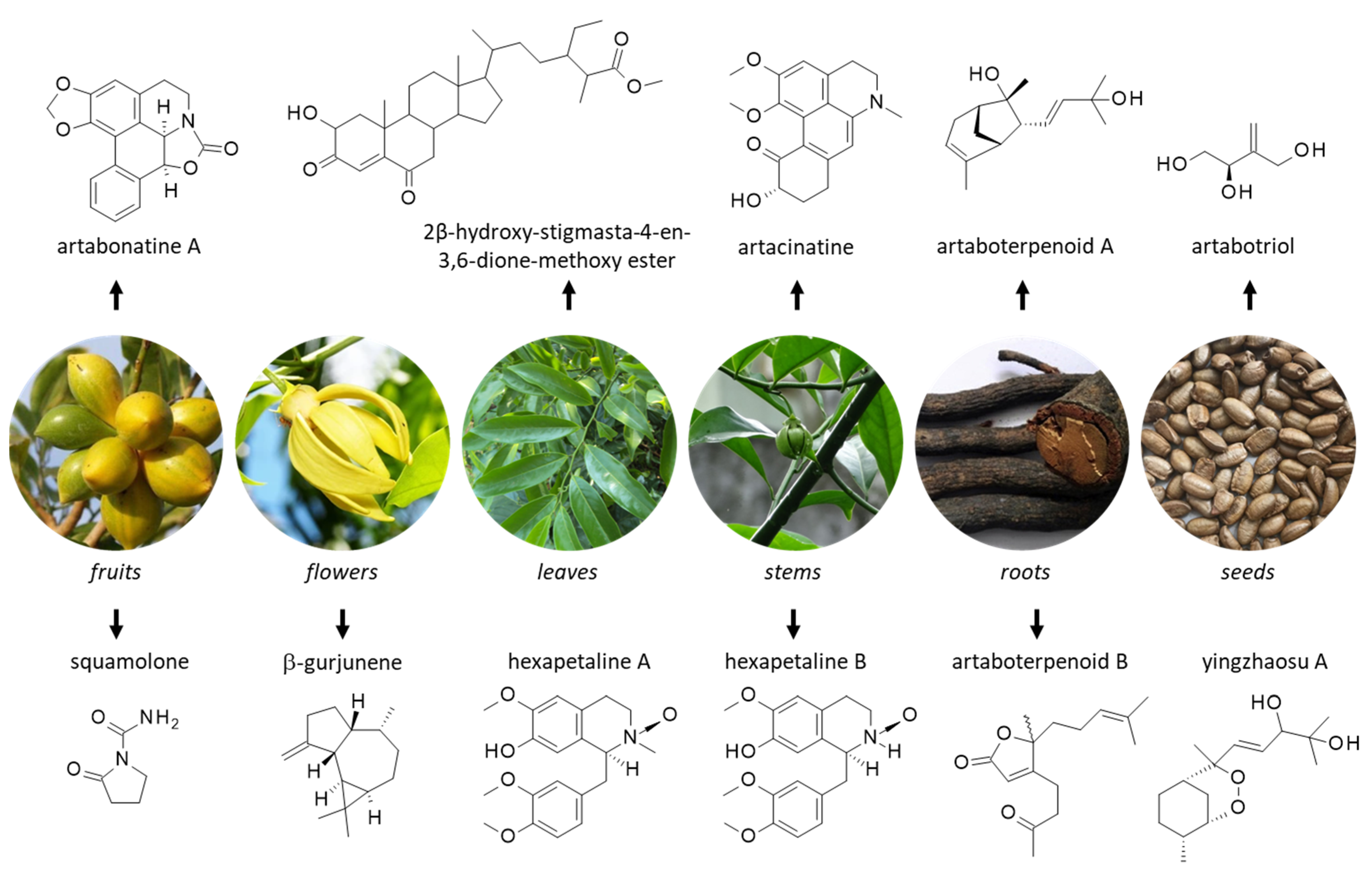
Figure 2.
Structure of selected natural products isolated from different parts of yingzhao. Detailed botanical information on yingzhao can be found at http://www.instituteofayurveda.org/plants/plants_detail.php?i=75&s=Local_name (accessed on 14 September 2022).
Occasionally, other pharmacological effects have been reported. For example, a recent study underlined the antiproliferative activity of A. odoratissimus fruit extract against MIA PaCa-2 pancreatic cancer cells. The organic (ethyl acetate) extract reduced cell growth, revealed an antioxidant effect, and induced apoptotic cell death associated with DNA damage [36]. The phytochemicals at the origin of the anticancer action were not specified, but it could be linked to the presence of cytotoxic alkaloids. More than 25 alkaloids have been isolated from A. uncinatus (synonym), including cytotoxic oxoaporphines and other alkaloids endowed with cytotoxic effects such as atherospermidine and squamolone (Figure 3) [46,47].
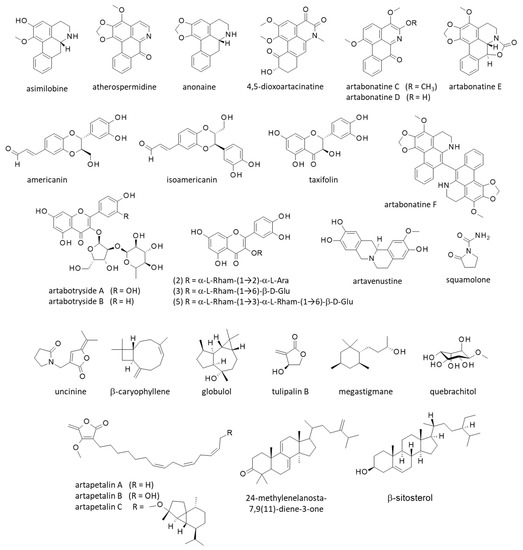
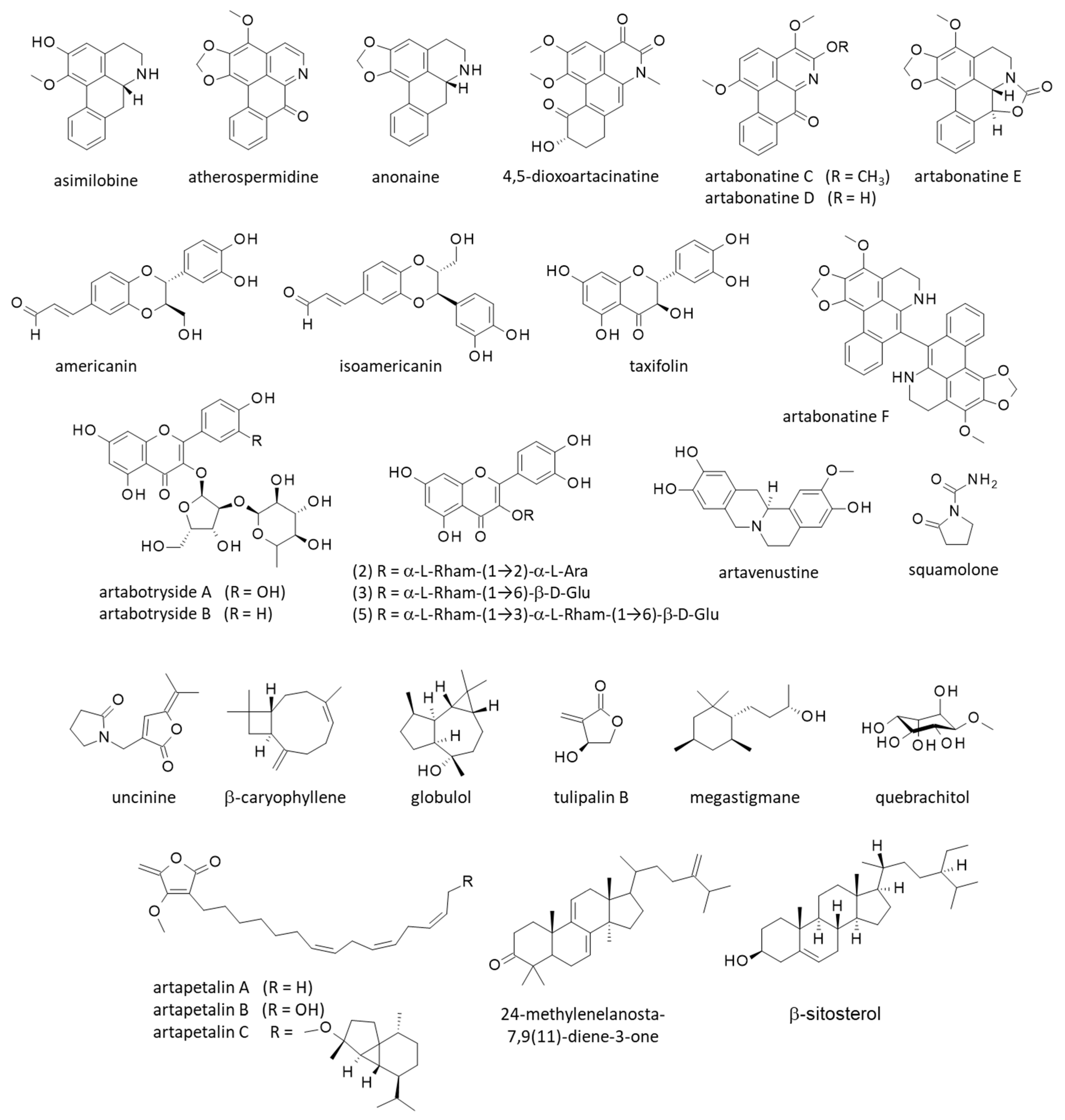
Figure 3.
Structure of other natural products isolated from yingzhao.
The multiple bioactive properties evidenced by extracts of the plants stimulate research and the elaboration of novel bioinspired products. For example, silver nanoparticles have been made using an aqueous extract of A. hexapetalus with the objective to propose new bactericide products [30]. There are also non-medicinal usages of the plant extracts. For example, the plant leaf extract has shown anticorrosion activity. It could be used as an eco-friendly green inhibitor for acidic-induced corrosion of mild steel [48,49].
4. Phytochemical Content of Yingzhao
Unsurprisingly, a large number of secondary metabolites have been isolated from yingzhao, including terpenoids, alkaloids and steroids. Natural products have been identified from extracts of all parts of the plant, from roots to leaves, and from seeds to fruits. Important products identified from each part are indicated in Figure 2 and Figure 3.
4.1. Alkaloids from Yingzhao
The oxazoloaporphine alkaloids artabonatines A-F have been isolated from fresh unripe fruits of A. uncinatus [46,47]. They are rare compounds, scarcely studied apart from the chemical synthesis of some derivatives [50]. The structure (from anti to syn) of artabonatine A has been revised in 2018, based on the chemical synthesis of the two diastereomeric isomers of (−)-artabonatine A (Figure 2). Their capacity to inhibit G-protein coupled receptors (GPCR) and monoamine transporters were characterized. The anti-isomer was found to function as a potent inhibitor of serotonergic 5-HT2C receptor (Ki = 1.6 μM) whereas the syn-isomer inhibited dopamine transporter (Ki = 3.8 μM) [50]. These compounds have been rarely identified in other plants. Artabonatine B has been found in the stems of Annona cherimola Mill (cherimoya), which is also a tropical species of Annonaceae [51]. Another aporphine alkaloid, 8-hydroxyartabonatine C, has been found in the leaves and twigs of Pseuduvaria trimera and the compound was shown to exhibit mild cytotoxic properties toward cancer cells in vitro [51]. However, apart from those two studies, the artabonatine compounds are poorly known. Two related benzylisoquinoline alkaloids named hexapetalines A and B (Figure 2) have been isolated from the stem of A. hexapetalus. Hex-A proved to be more cytotoxic toward cancer cells than Hex-B. Its antiproliferative action was comparable to that of the reference anticancer drug cisplatin [52].
Known alkaloids have been isolated from A. uncinatus, such as liriodenine, anonaine, norushinsunine, asimilobine and stepharine [47]. Anonaine (Figure 3) is also an aporphine alkaloid (benzylisoquinoline) with a significant antiplasmodial effect (IC50 = 23.2 μg/mL against P. falciparum) [53]. It can be found in several species of Magnoliaceae and Annonaceae, and has a large spectrum of bioactivities, including antiplasmodial, antibacterial, antifungal, and anticancer effects [54]. Alkaloids such as liriodenine, anonaine, and stepharine are relatively common in Annona species and contribute to the antiparasitic properties of the plants [55].
In the case of the rare butanolide, the alkaloid uncinine from A. uncinatus is interesting to underline because it has revealed marked cytotoxic properties, inhibiting the growth of HepG2 liver cancer cells (IC50 = 6.1 μg/mL) [46]. This original product combines a γ-alkylidene butenolide and pyrrolidinone fragments (Figure 3). Its total synthesis has been achieved [56], but its pharmacological properties are essentially unknown. This product should be studied further. In contrast, the alkaloid atherospermidine has been found in diverse plants, including Artabotrys species, such as A. uncinatus and A. crassifolius [57,58]. This compound can contribute to DNA damages in cells [59]. A related series of aporphine alkaloids also isolated from A. uncinatus has been named artacinatine [60]. The derivative 4,5-dioxoartacinatine has been isolated a few years later [61]. Artacinatine (Figure 2) can be found in other species, notably from the roots of A. spinosus together with artacinatine C [62], and from the stems and leaves of A. hongkongensis [63].
Lan and coworkers have identified more than 30 compounds from A. uncinatus, including (i) the alkaloid asimilobine which has antibacterial properties, (ii) the catecholic berberine alkaloid artavenustine, and (iii) a variety of classical sterol derivatives such as β-sitosterol and stigmasterol [61]. They also identified the unique derivative 24-methylenelanosta-7,9(11)-diene-3-one (Figure 3) analogous to the lanostane triterpene suberosol, which has antifungal and antiviral properties [64]. β-Sitosterol and related antimicrobial lipidic compounds have been identified from the leaves of A. odoratissimus (synonym) [65,66].
4.2. Terpenoids, Lignans and Flavonoids from Yingzhao
Lignans and flavonoids have been identified as well, including the dibenzylbutyrolactone lignans (iso)americanin, the flavonol glycoside artabotrysides A and B, and diverse flavonoids (taxifolin) and flavonoid glycosides such as quercetin/kaempferol/luetolin glycosides [44,67]. Artabotrysides A and B bear the same diglycoside moiety (3-rhamnosyl-(1→2)-alpha-L-arabinofuranoside) but a distinct flavonol core corresponding to quercetin for kaempferol, respectively (Figure 3). Among these compounds, taxifolin can be underlined as it is a prominent anti-inflammatory and antimicrobial compound [68]. Diverse antibacterial butyrolactone derivatives have been isolated also, such as tulipalin B and the compounds called artapetalins A-C with a unique β-methoxy-γ-methylene-substituted, α,β-unsaturated-γ-butyrolactone ring [69]. These compounds have been identified but not characterized from a pharmacological viewpoint. The semiterpenoid (R)-artabotriol has also been identified, together with artabotrycinol [70]. Artabotriol (Figure 2) is a precursor to the synthesis of tulipalin B and other bioactive natural products [71]. The two sesquiterpenoids artaboterpenoids A-B (Figure 2) have been isolated from the roots of the plants. The isomer (+)-artaboterpenoid B was shown to exhibit marked cytotoxicity toward several human tumor cell lines, with IC50 values in the range 1.38−8.19 μM [72]. This original bisabolene-derived sesquiterpenoid has not been reported in any other plant species. However, other sesquiterpenes have been identified from A. hexapetalus, including various yingzhaosu derivatives endowed with antiviral effects. This is the case of the derivative (8S,12R)-yingzhaosu C which revealed a noticeable antiviral effect against Coxsackie virus B3 (TC50 = 23.11 μM) [73].
Various volatile compounds have been identified from the flowers of the plant, including the abundant sesquiterpene called β-gurjunene (Figure 2) as a major component contributing to the antioxidant effect of the extract [74]. The related sesquiterpenoid globulol was also found, together with β-caryophyllene, well-known compounds with insecticidal activities (Figure 3). Sesquiterpene hydrocarbons and oxygenated sesquiterpene are commonly found in Artabotrys species [18,75]. They contribute largely to the antioxidant effects observed with essential oils from the plant [76]. Another interesting bioactive compound found in A. uncinatus is quebrachitol, a cyclic polyol (or cyclitol) which displays antidiabetic properties [77]. Other bioactive compounds found in yingzhao could be cited, such as the antibacterial butyrolactone tulipalin B [70], but in general, these compounds are trivial and are largely found in other species. In sharp contrast, there is a small group of unique products of major interest owing to their antimalarial properties: the yingzhaosu compounds detailed in the following section.
5. Yingzhaosu and Analogues
5.1. Discovery and Synthesis of Yingzhaosu A-D
The first two compounds in the series, yingzhaosu A and B (Figure 1), were isolated from the roots of the yingzhao plant in 1979 and described in two publications in Chinese [78,79]. The exact configuration of the compounds was not precisely known at that time. It was not clear if they corresponded to natural products or to artefacts formed in the root of A. uncinatus (synonym) during storage in the shade for two months, as mentioned later in a report [80]. They are effectively natural products from the plant. The two other compounds in the series, yingzhaosu C and D, were reported in 1989 by other Chinese chemists, 10 years after the discovery of parent and lead product yingzhaosu A [81,82]. Yingzhaosu C is a sesquiterpene peroxide, whereas yingzhaosu D is a sesquiterpenol (Figure 1). Since the discovery of the compounds, major efforts have been devoted to their total synthesis, but their pharmacological study has been largely neglected.
The first total synthesis of yingzhaosu A was presented in 1991, starting from the precursor R-(−)-carvone, a common monoterpene found in many plants [83]. R-(−)-carvone is known for its hypolipidemic, cytoprotective and sedative effects [84,85,86,87]. The total synthesis and X-ray diffraction analysis of synthetic yingzhaosu A provided key information about the stereochemistry of the product, indicating the S-configuration of the C-12 atom [83]. Subsequently, the synthesis of the diastereoisomeric yingzhaosu D was reported, starting from S-(−)-limonene, providing thus information about the configuration at positions C-4 and C-8 [88]. Then, the enantioselective synthesis of the four stereoisomers of yingzhaosu C was proposed [89], as well as epi-yingzhaosu C [90]. Over the years, significant efforts were deployed to optimize the total synthesis of these compounds and to propose synthetic analogues. The different synthetic routes have been optimized [91]. For example, a short and efficient synthesis of yingzhaosu C has been recently reported from the sesquiterpenoid obtained in one step [92].
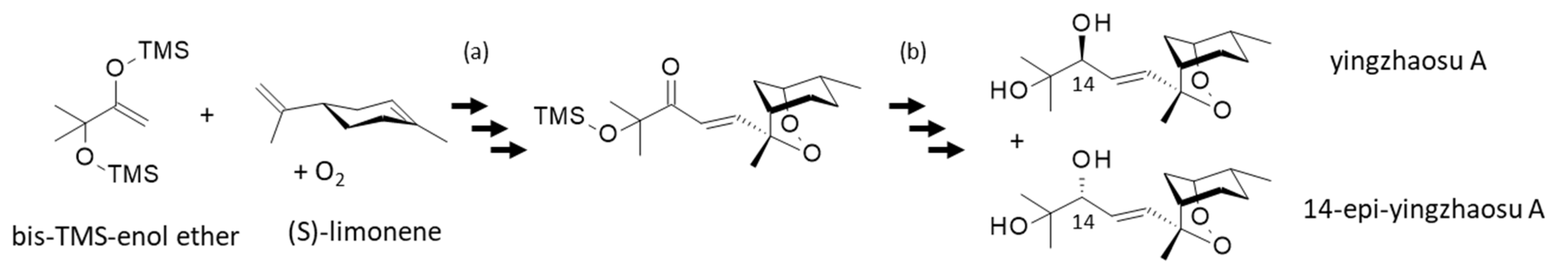
A remarkable effort led to the synthesis of yingzhaosu A in only 8 steps starting also from S-(−)-limonene, with an overall yield of 7.3% [93] (Figure 4). Limonene is particularly prone to addition of O2 and autoxidation [94,95]. Interestingly, this chemical work offered also the synthesis of the C(14)-epimer of yingzhaosu A, a compound characterized as a potent cytotoxic agent against KB nasopharyngeal cancer cells (ED50 = 36.6 and 0.57 μg/mL for yingzhaosu A and C(14)epi-yingzhaosu A, respectively). The epimer exhibited a higher antiplasmodial activity than the parent product (IC50 = 115 and 56 nM, against the chloroquine-resistant K1 strain of P. falciparum). The epimer was much more potent than the parent product in vivo against the chloroquine-sensitive P. berghei NY strain (ED50 = 250 and 90 mg/Kg for yingzhaosu A and C(14)epi-yingzhaosu A, respectively). However, the epimer remained much less active than the reference sodium artesunate in the same in vivo test (ED50 = 4.2 mg/Kg) [93]. This major work opened the door to the design of better analogues.
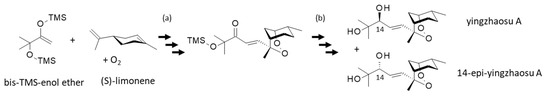
Figure 4.
An efficient synthesis of yingzhaosu A from (S)-limonene and the TMS-protected enol ether in the presence of oxygen afforded the compound in 8 steps with an overall yield of 7.3% (a). The intermediate product (trimethylsilyloxy-enone peroxide) was converted into yingzhaosu A and its C14-epimer, via a chemoselective reduction [93] (b).
The synthesis of cyclic peroxide has attracted considerable interest in the chemistry field for different reasons (green chemistry, oxidation processes, …) [96,97]. The potent activity and medical value of the artemisinin and derivatives raise major interest as well. New artemisinin drug candidates are regularly proposed [98], and in the same vein, derivatives of yingzhaosu A and C, both containing a 1,2-dioxane unit, have been presented [99,100,101,102].
5.2. Synthesis and Pharmacology of Yingzhaosu Analogues
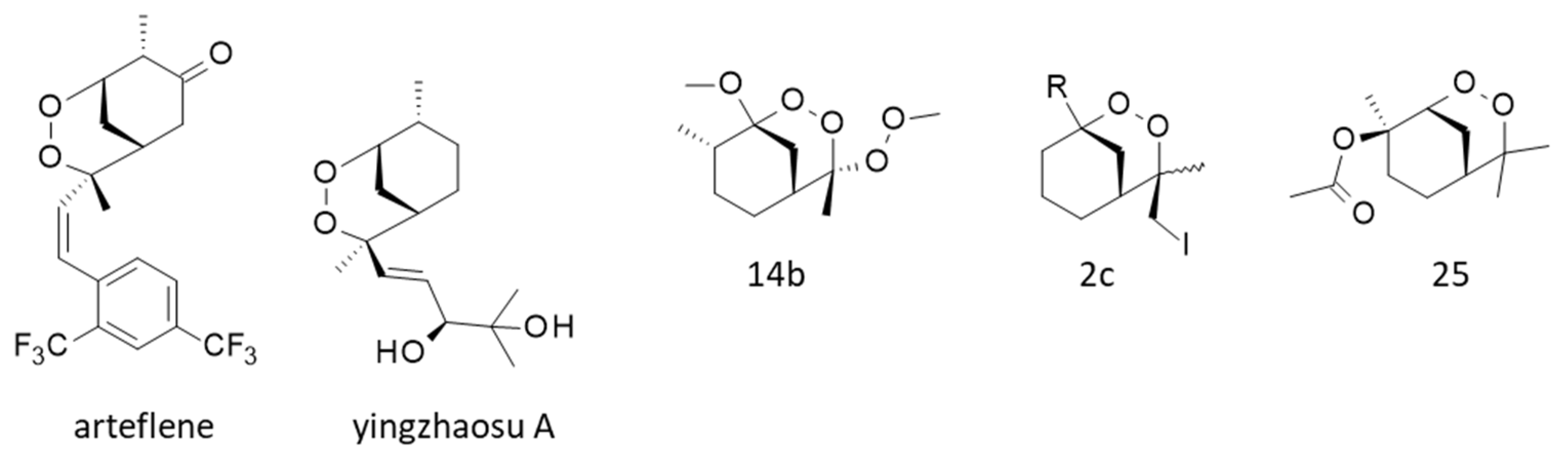
Various cyclic peroxides structurally close to yingzhaosu A have been described, such as compound 14b (Figure 5), which was found to be active against P. falciparum (EC50 = 100 nM) but still less active than artemisinin (EC50 = 7.8 nM). Nevertheless, this compound presented a good cell selectivity, being weakly cytotoxic toward FM3A mouse mammary carcinoma cells (IC50 = 33 μM) [103]. Other potent compounds have been designed, in particular the cyclic peroxide 2c (Figure 5), which was found to remarkably inhibit the in vitro growth of P. falciparum (EC50 = 13 nM) with an efficacy comparable to that of artemisinin and a very high cellular selectivity [25,26]. Other related compounds active in vitro and in vivo were thus obtained, such as compound 25 (Figure 5) more potent than artemisinin against the parasite in vitro (EC50 = 3 and 10 nM, respectively) [104]. These chemical studies demonstrated that the endoperoxide scaffold of yingzhaosu A can be used as a template to design more potent analogues.
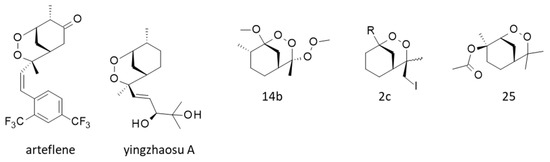
Figure 5.
Synthetic derivatives of yingzhaosu A, such as arteflene (also known as Ro-42-1611) and the synthesized compounds 14b [103], 2c [25] and 25 [104].
One particular compound derived from yingzhaosu A has been developed, the synthetic endoperoxide arteflene (Ro 42-1611) with a 1,2-dioxane unit (Figure 5). This compound is bioactivated in cells to generate a stable iron(II)-mediated carbon-centered radical [105]. Intracellular iron is believed to play a significant role in the bioactivation of certain endoperoxides, notably in the case of artemisinin [106]. This bioactivation process, occurring in hepatocytes, can lead to acute toxicity in the case of arteflene at high concentrations [107]. In the late 1990s, arteflene was considered a promising compound to treat malaria [108,109,110], and clinical trials were initiated [111,112]. However, the results were unconvincing, and the drug development stopped. Nevertheless, the arteflene program showed that yingzhaosu A can be used as a template to design innovative molecules [113].
6. Mechanism of Action of Yingzhaosu A
6.1. Reactivity in the Presence of Iron(II)
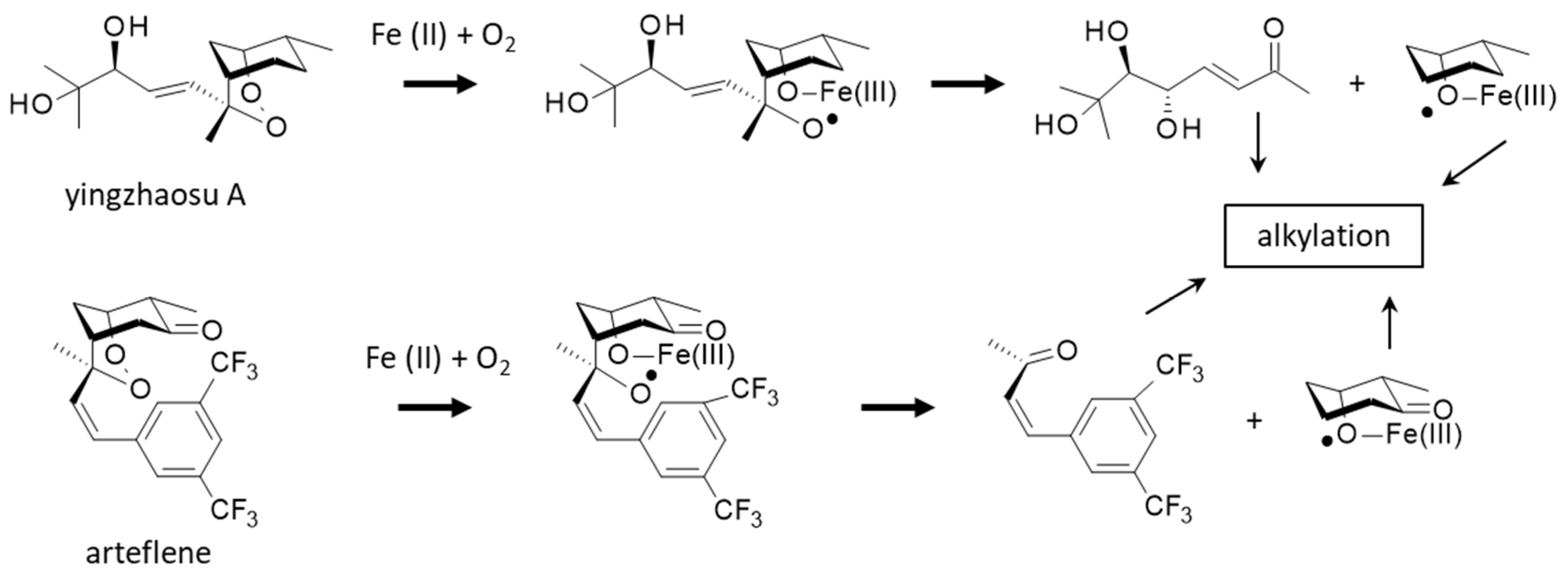
A better knowledge of the molecular target and pathways involved in the antiplasmodial action of yingzhaosu A would help the design of analogues, but thus far, no study has been specifically devoted to elucidating the mechanism of action of the natural product. However, two important elements point toward the implication of iron and iron complexes in the bioactivity process. On the one hand, it is known that yingzhaosu A can be activated by an iron(II) compound in a Fenton-type reaction [114]. Yingzhaosu A is believed to undergo an iron(II)-induced degradation leading to the formation of two alkylating species, an unsaturated ketone and a cyclohexyl radical, as represented in Figure 6. Evidence for the generation of a cyclohexyl radical has been provided by the use of electron spin resonance (ESR). The same process can occur with arteflene in the presence of oxygen and iron chloride [114,115]. The reactive species thus generated would be responsible for the antiparasitic effects.
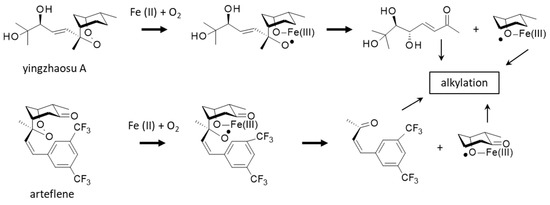
Figure 6.
Reactions of yingzhaosu A or arteflene in the presence of iron and oxygen (Fenton reaction) lead to the formation of an oxygen-centered radical and then the release of alkylating species (unsaturated ketone and cyclohexyl radical), responsible for the parasiticidal properties (adapted from [114,115]).
6.2. Reaction with Iron-Complexed Heme
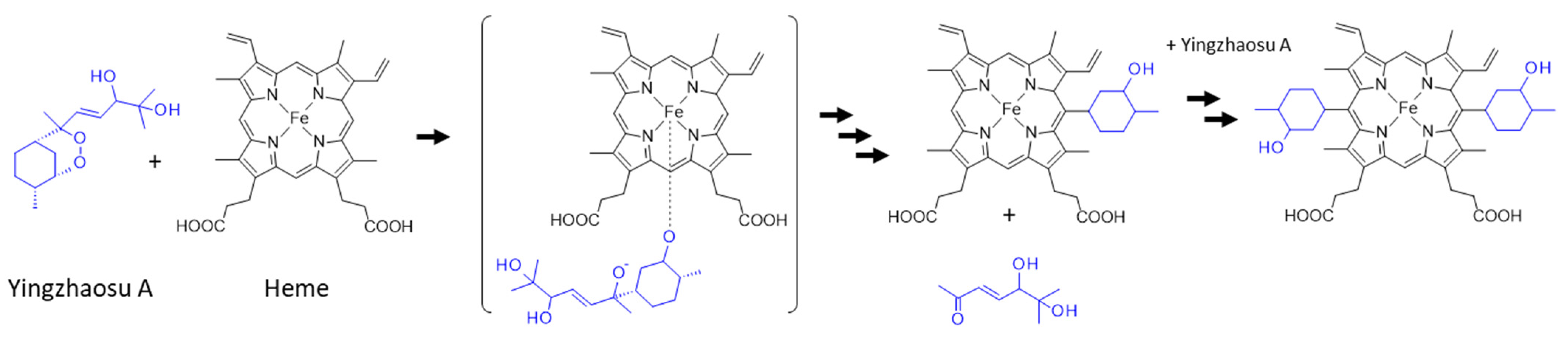
On the other hand, a recent study has pointed out the rearrangement and cleavage of yingzhaosu A in the presence of iron-bound heme. The authors built a heme-activatable probe based on the structure of yingzhaosu A to identify novel inhibitors of P. falciparum. They showed that the compound was attacked by heme to break the endoperoxide bond, thus generating sterically hindered tertiary oxygen-centered radicals. The yingzhaosu A molecule was broken into two parts after a rearrangement to remove the side chain, as represented in Figure 7 [116]. A similar endoperoxide reactivity-based FRET probe (with a bioinspired endoperoxide linker between donor and acceptor fluorophores) had been previously reported using an ozonide scaffold based on the architecture of artemisinin [117]. The yingzhaosu A-based probe is useful for identifying new bioactive compounds, but it is also informative on the reactivity of yingzhaosu A itself and its capacity to react with heme [116]. It will be interesting to determine whether the natural product can react similarly in cells. It is most likely that, as for artemisinin, the activation of yingzhaosu A requires the cleavage of the endoperoxide bridge in the presence of an iron source.
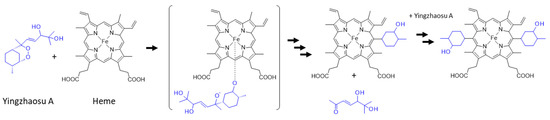
Figure 7.
Proposed scheme for the reaction of yingzhaosu A with heme [116].
The role of the iron complex in the mechanism of action of endoperoxide drugs has been largely debated [118,119]. The bioreductive activation of endoperoxides of iron complexes, notably heme, leads to the generation of a radical species and then to alkylation of key proteins vital for the parasite. Moreover, upon alkylation, heme endoperoxide drugs can cause an imbalance in iron homeostasis, mitochondrial dysfunctions and toxic effects contributing to the cytocidal activity [120,121]. The heme molecule is considered a possible target (but probably not the sole target) of artemisinin-derived endoperoxides and analogous compounds [122]. Nevertheless, the role of heme is complex, and a recent study revealed that too much heme is not good for the antimalarial action of artemisinins [123]. The mechanism of action of artemisinin is pluri-factorial. It implicates a carbon radical and heme, but also interaction and interference with plasmodial proteins such as the sarcoplasmic endoplasmic calcium ATPase (SERCA), as well as an induced immunoregulation [124,125,126]. A similar complexity can be anticipated with yingzhaosu A.
7. Discussion and Conclusions
The medicinal plant Artabotrys hexapetalus (L.f.) Bhandari, or yingzhao in Chinese, has been known for decades and is extensively used in traditional medicine in Asia for the treatment of malaria and associated fevers. The plant is well known, but the various synonyms can result in confusion and complexity. The accepted name A. hexapetalus should be used primarily, not the synonyms, such as. A. uncinatus and A. odoratissimus in scientific communications. The plant has a large medicinal potential, well recognized in Asia, in particular for the treatment of parasitic diseases [127].
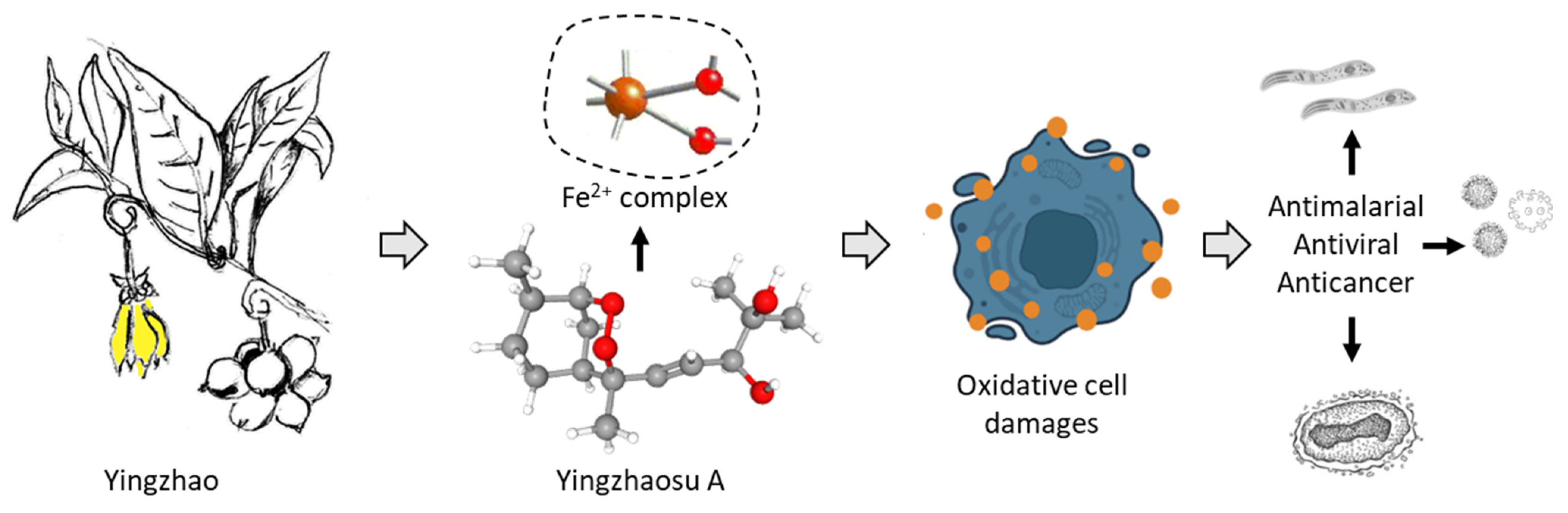
The chemical diversity of bioactive natural products identified from the plant is large. It is a rich source of secondary metabolites with diverse chemical classes (alkaloids, terpenoids, flavonoids, glycosides, …), as is frequently the case with medicinal species. The presence of many aporphine alkaloids is remarkable, notably in the oxazoloaporphine series (e.g., artabonatine). These products could be useful to combat various metabolic diseases, such as type 2 diabetes mellitus, endothelial dysfunction, hypertension and cardiovascular diseases [128]. There are also interesting flavones (artabotrysides A-B) and lactones (artapetalins A-C), not found or rarely in other plants, which would deserve further studies. However, with no doubt, the most interesting natural products isolated from the plant are the two endoperoxide-containing products yingzhaosu A and C and their derivatives yingzhaosu B and D. Immediately after their discovery, a major interest in these original compounds have sparkled amongst chemists towards the synthesis of these compounds and analogues. Important chemistry efforts have been devoted to optimizing total syntheses and to determine the exact stereoisomeric forms of the products. Analogues have been proposed in the early 2000s, leading to promising compounds in some cases [25,93,102]. However, the interest moved toward other endoperoxide-containing products analogous to artemisinin, probably because of the higher potency and clinical success of this exceptional molecule. The yingzhaosu compounds have been neglected over the past ten years. Their mechanism of action is not well defined, although it is likely as complex as that of artemisinin. It is time to breathe a new life into the yingzhaosu series with the design of new analogues and the application of modern technologies to better comprehend their mechanisms of action via network pharmacology studies and other multi-omic analyses (Figure 8).
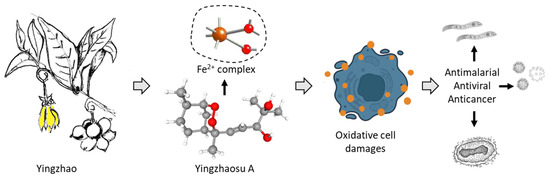
Figure 8.
A schematic illustration of the pharmacological potential of yingzhaosu A, isolated from the plan yingzhao, for the treatment of parasitic diseases, viral diseases and cancer.
The high potency of artemisinin and the recognized clinical efficacy of artemisinin combination therapy, which is recommended by the World Health Organization, contribute to promoting the design and development of novel compounds with a cyclic endoperoxide core. A huge diversity of compounds has been synthesized, including second and third generations of artemisinin derivatives (monomer, dimer, trimer), trioxolanes, tri- and tetra-oxanes, and a variety of non-artemisinin-derived synthetic endoperoxide-containing molecules [129,130,131,132,133]. Novel artemisinin derivatives are regularly proposed [134]. A large number of plant-derived endoperoxides, more than 200, have also been identified and studied, at least from the phytochemical aspect [135]. Among these efforts, there are opportunities to promote yingzhao and yingzhaosu compounds. The plant is readily available and even cultivated as an ornamental in the tropics. The products are affordable, with well-defined synthetic approaches. There is no reason not to promote the design of yingzhaosu A/C analogues. These endoperoxide compounds are important to combat parasitic diseases but also other diseases, such as respiratory diseases and cancers, as is the case for artemisinin and artesunate [9,136]. Hopefully, this review will contribute to restoring the prestige of yingzhaosu A and its analogues, whose compounds have been neglected for too long by the pharmacology community.
Author Contributions
Conceptualization, investigation, writing—original draft preparation, C.B.; writing—review and editing, drawing, validation, J.-P.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moxon, C.A.; Gibbins, M.P.; McGuinness, D.; Milner, D.A., Jr.; Marti, M. New Insights into Malaria Pathogenesis. Annu. Rev. Pathol. 2020, 15, 315–343. [Google Scholar] [CrossRef] [PubMed]
- Laurens, M.B. RTS,S/AS01 vaccine (Mosquirix™): An overview. Hum. Vaccin. Immunother. 2020, 16, 480–489. [Google Scholar] [CrossRef]
- Badeliya, S.N.; Kapupara, P.P.; Chauhan, N.F.; Panchal, I.I. A contemporary chemical entities infiltrating in the antimalarial therapy era: A comprehensive review. Folia Med. 2021, 63, 637–646. [Google Scholar] [CrossRef]
- Daily, J.P.; Minuti, A.; Khan, N. Diagnosis, Treatment, and Prevention of Malaria in the US: A Review. JAMA 2022, 328, 460–471. [Google Scholar] [CrossRef]
- Bailly, C. Pyronaridine: An update of its pharmacological activities and mechanisms of action. Biopolymers 2021, 112, 23398. [Google Scholar] [CrossRef] [PubMed]
- Pryce, J.; Taylor, M.; Fox, T.; Hine, P. Pyronaridine-artesunate for treating uncomplicated Plasmodium falciparum malaria. Cochrane Database Syst. Rev. 2022, 6, CD006404. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y. Artemisinin-A Gift from Traditional Chinese Medicine to the World (Nobel Lecture). Angew. Chem. Int. Ed. 2016, 55, 10210–10226. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, R.I.; Foglio, M.A.; Saad, S.T.O. Artemisinin-type drugs for the treatment of hematological malignancies. Cancer Chemother. Pharmacol. 2021, 87, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Wan, J.; Zhang, M.; Li, C.; Lin, J. Artesunate: A review of its therapeutic insights in respiratory diseases. Phytomedicine 2022, 104, 154259. [Google Scholar] [CrossRef]
- Farmanpour-Kalalagh, K.; Kashkooli, A.B.; Babaei, A.; Rezaei, A.; van der Krol, A.R. Artemisinins in Combating Viral Infections Like SARS-CoV-2, Inflammation and Cancers and Options to Meet Increased Global Demand. Front. Plant Sci. 2022, 13, 780257. [Google Scholar] [CrossRef]
- Torres-García, I.; López-Martínez, J.L.; Muñoz-Dorado, M.; Rodríguez-García, I.; Álvarez-Corral, M. Marine Terpenic Endoperoxides. Mar. Drugs 2021, 19, 661. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Ermolenko, E.; Savidov, N.; Gloriozova, T.A.; Poroikov, V.V. Antiprotozoal and Antitumor Activity of Natural Polycyclic Endoperoxides: Origin, Structures and Biological Activity. Molecules 2021, 26, 686. [Google Scholar] [CrossRef] [PubMed]
- WFO. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000549776 (accessed on 14 September 2022).
- Prabhu, S.; Sathiyaseelan, R.; Aron, S.; Murugan, C. A Review of the Genus Artabotrys R. Br. (Annonaceae) from Andaman and Nicobar Islands, with a New Record for India. Indian J. For. 2015, 38, 159–164. [Google Scholar]
- Posluszny, U.; Fisher, J.B. Thorn and hook ontogeny in Artabotrys hexapetalus (Annonaceae). Am. J. Bot. 2000, 87, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, R.A.; Mgani, Q.A.; Nyandoro, S. Chemical compositions and mosquito repellency of essential oils from Artabotrys hexapetalus and Artabotrys rupestris. Int. J. Biol. Chem. Sci. 2014, 8, 2804–2812. [Google Scholar] [CrossRef]
- Handayani, T. Seed germination and seedling morphology of Artabotrys hexapetalus. Nusant. Biosci. 2017, 9, 23–30. [Google Scholar] [CrossRef]
- Phan, G.M.; Phan, S.T.; König, W.A. Chemical Composition of the Flower Essential Oil of Artabotrys hexapetalus (L.f.) Bhandare of Vietnam. J. Essent. Oil Res. 2007, 19, 523–524. [Google Scholar] [CrossRef]
- Kousalya, P.; Doss, V.A. Artabotrys hexapetalus (L.f.) Bhandari: Ethnomedicinal Uses, Pharmacological Properties and Bioactive Compounds-Review. J. Huazhong Univ. Sci. Technol. 2021, 50, 1–11. [Google Scholar]
- Grainge, M. Effects of extracts of Artabotrys uncinatus and Allium sativum on Xanthomonas campestris pv. oryzae. Curr. Sci. 1985, 54, 90. [Google Scholar]
- Grainge, M.; Alvarez, A. Antibacterial and Antifungal Activity of A. hexapetalus leaf extracts. Int. J. Trop. Plant Dis. 1987, 5, 173–179. [Google Scholar]
- Sowjanya, K.M.; Swathi, J.; Narendra, K.; Padmavathi, C.H.; Satya, A.K. Extraction and Antimicrobial Potential of Secondary Plant Metabolites from Artabotrys hexapetalus (Linn. F.) Bhandari. Int. J. Res. Ayurveda Pharm. 2013, 4, 764–768. [Google Scholar] [CrossRef]
- Srivastava, B.; Singh, P.; Srivastava, A.K.; Shukla, R.; Dubey, N.K. Efficacy of Artabotrys odoratissimus oil as a plant based antimicrobial against storage fungi and aflatoxin B1 secretion. Int. J. Food Sci. Technol. 2009, 44, 1909–1915. [Google Scholar] [CrossRef]
- Syam, S.K.; Anudeep, M.; Ramana, C.V.; Bhaskar, C. Screening of antimicrobial activity of flower extracts on human bacterial pathogens. J. Pharmacogn. Phytochem. 2015, 3, 153–156. [Google Scholar]
- Kim, H.S.; Begum, K.; Ogura, N.; Wataya, Y.; Tokuyasu, T.; Masuyama, A.; Nojima, M.; McCullough, K.J. Antimalarial activity of yingzhaosu A analogues. J. Med. Chem. 2002, 45, 4732–4736. [Google Scholar] [CrossRef]
- Borstnik, K.; Paik, I.H.; Shapiro, T.A.; Posner, G.H. Antimalarial chemotherapeutic peroxides: Artemisinin, yingzhaosu A and related compounds. Int. J. Parasitol. 2002, 32, 1661–1667. [Google Scholar] [CrossRef]
- Vasundhara, M.; Anjali, K.R.; Chithra, C.; Gupta, P.; Karthik, Y.P.; Roopa, C. Absence of anthelmintic activity of hydroalcoholic leaf extracts of Artabotrys hexapetalus (Linn.f). J. Pharm. Negat. Results 2014, 5, 1–3. [Google Scholar] [CrossRef]
- Bajaj, S.; Wakode, S.R.; Khan, W.; Manchanda, S.; Kumar, S. Simultaneous HPTLC analysis and in vitro antileishmanic activity of various secondary metabolites in extract of the traditional medicinal herb Artabotrys hexapetalus (L.f.). AYU 2018, 39, 92–100. [Google Scholar] [CrossRef]
- Manjula, M.; Kumuda, K.V.; Anitha, S.; Shashidhara, S. Antioxidant and antimicrobial activities of various extracts of Artabotrys hexapetalus flowers. Pharma Sci. Monit. 2011, 2, 42–50. [Google Scholar]
- Shankar, D.P.; Ananthi, P.; Devi, K.H.N. Bioinspired Synthesis and Characterization of Silver Nanoparticles using an Aqueous extract of Artabotrys hexapetalus. J. NanoSci. NanoTechnol. 2014, 2, 66–70. [Google Scholar]
- Geetha, M.; Shankar, M.B.; Mehta, R.S.; Saluja, A.K. Antifertility activity of Artabotrys odoratissimus Roxb. and Couroupita guianensis Aubl. J. Nat. Remedies 2005, 5, 121–125. [Google Scholar]
- Johri, P.K.; Tiwari, D.; Johri, R. Screenings of some indigenous medical plants for anti-implantation/anti-fertility activity in female Albino rats. Biochem. Cell. Arch. 2009, 9, 175–178. [Google Scholar]
- Karthik, Y.P.; Swamy, B.M.V.; Vishwanath, K.M. Evaluation of Anti-Fertility Activities of Leaves of Artabotrys hexapetalus (Linn. F). Res. J. Pharm. Biol. Chem. Sci. 2012, 3, 1121–1134. [Google Scholar]
- Kodithala, S.; Murali, R.; Srinivasan, N.; Agarwal, S.K. Anti-fertility activity of leaves and Stem parts of Artabotrys odoratissimus. (roxb.) R.Br. Annonaceae. J. Med. Pharm. Allied Sci. 2021, 10, 3524–3528. [Google Scholar] [CrossRef]
- Kodithala, S.; Murali, R. A review on Artabotrys odoratissimus (Annonaceae). J. Pharmacog. Phytochem. 2018, 7, 1414–1416. [Google Scholar]
- Meghana, P.; Jain, R.S.K.; Prashanth, N.; Kumar, J.U.S.; Pallavi, M.; Monnenahally, K.H. Antiproliferative effects of Artabotrys odoratissimus fruit extract and its bioactive fraction through upregulation of p53/γH2AX signals and G2/M phase arrest in MIA PaCa-2 cells. Anticancer Agents Med. Chem. 2022, 22, 2998–3008. [Google Scholar]
- Rahini, D.; Anuradha, R. In-vitro antioxidant activity of Artabotrys hexapetallus. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 396–405. [Google Scholar]
- Veena Rani, I.; Annapurna, A.; Ganapathi, S. Evaluation of hepatoprotective activity and oxidative stress parameters of alcoholic extract of Artabotrys hexapetalus (L.F.) Bhandari. J. Glob. Trends Pharm. Sci. 2016, 7, 3192–3199. [Google Scholar]
- Bajaj, S.; Wakode, S. Effect of Extraction Solvent on Total Phenol content, Total Flavonoid content, Antioxidant and Anti-Inflammatory activity of Artabortrys hexapetalus. Int. J. Biol. Pharm. Allied Sci. 2017, 6, 1562–1577. [Google Scholar]
- Suresh, K.; Hindustan, A.A.; Satyanarayana, S.V. Antioxidant activity and Hepatoprotective potential of Ethanolic leaf extract of Artabotrys hexapetalus against various Hepatotoxins induced Hepatotoxicity in Albino wister Rats. Int. J. Res. Pharm. Sci. 2021, 12, 1679–1688. [Google Scholar] [CrossRef]
- Chakrabarti, B.; Chaudhuri, A.; Chowdhury, P.R. Antifertility effect of green leaves of Artabotrys odoratissimus. J. Indian Med. Assoc. 1968, 51, 227–229. [Google Scholar]
- Kamboj, V.P.; Dhawan, B.N. Research on plants for fertility regulation in India. J. Ethnopharmacol. 1982, 6, 191–226. [Google Scholar] [CrossRef]
- Li, T.M.; Li, W.K.; Yu, J.G. Flavonoids from Artabotrys hexapetalus. Phytochemistry 1997, 45, 831–833. [Google Scholar] [CrossRef]
- Li, T.; Yu, J. Studies on the chemical constituents of the leaves from Artabotrys hexapetalus. Yao Xue Xue Bao 1998, 33, 591–596. [Google Scholar] [PubMed]
- Khaw, L.T.; Leerach, N.; Yap, N.J.; Jaturas, N.; Mahboob, T.; Tan, T.C.; Dungca, J.Z.; LosBaños, Z.D.; Sitthisak, S.; Chow, S.C.; et al. A preliminary screening of potentially antimalarial plants against Plasmodium falciparum in vitro. Trop. Biomed. 2015, 32, 676–683. [Google Scholar]
- Hsieh, T.J.; Chang, F.R.; Chia, Y.C.; Chen, C.Y.; Lin, H.C.; Chiu, H.F.; Wu, Y.C. The alkaloids of Artabotrys uncinatus. J. Nat. Prod. 2001, 64, 1157–1161. [Google Scholar] [CrossRef]
- Hsieh, T.J.; Chen, C.Y.; Kuo, R.Y.; Chang, F.R.; Wu, Y.C. Two new alkaloids from Artabotrys uncinatus. J. Nat. Prod. 1999, 62, 1192–1193. [Google Scholar] [CrossRef]
- Begum, A.S.; Nasser, A.J.A. Corrosion inhibition by aqueous extracts of Artabotrys hexapetalus plant leaves in 1 M hydrochloric acid solution. Aegaeum J. 2020, 8, 1101–1113. [Google Scholar]
- Rathod, M.R.; Minagalavar, R.; Sk, R.K. Effect of Artabotrys odoratissimus extract as an environmentally sustainable inhibitor for mild steel corrosion in 0.5 M H2SO4 media. J. Indian Chem. Soc. 2022, 99, 100445. [Google Scholar] [CrossRef]
- Ku, A.F.; Cuny, G.D. Structure Revision and Biological Evaluation of Artabonatine A and Its Diastereoisomer. FASEB J. 2017, 31, 766.10. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chang, F.R.; Pan, W.B.; Wu, Y.C. Four alkaloids from Annona cherimola. Phytochemistry 2001, 56, 753–757. [Google Scholar] [CrossRef]
- Zhou, Q.; Fu, Y.H.; Li, X.B.; Chen, G.Y.; Wu, S.Y.; Song, X.P.; Liu, Y.P.; Han, C.R. Bioactive benzylisoquinoline alkaloids from Artabotrys hexapetalus. Phytochem. Lett. 2015, 11, 296–300. [Google Scholar] [CrossRef]
- Gontijo, D.C.; Brandão, G.C.; Nascimento, M.F.A.D.; Oliveira, A.B. Antiplasmodial activity and cytotoxicity, isolation of active alkaloids, and dereplication of Xylopia sericea leaves ethanol extract by UPLC-DAD-ESI-MS/MS. J. Pharm. Pharmacol. 2019, 71, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Wu, H.M.; Chen, H.L.; Liu, C.M.; Chen, C.Y. The pharmacological activities of (−)-anonaine. Molecules 2013, 18, 8257–8263. [Google Scholar] [CrossRef] [PubMed]
- Nugraha, A.S.; Damayanti, Y.D.; Wangchuk, P.; Keller, P.A. Anti-Infective and Anti-Cancer Properties of the Annona Species: Their Ethnomedicinal Uses, Alkaloid Diversity, and Pharmacological Activities. Molecules 2019, 24, 4419. [Google Scholar] [CrossRef]
- Fakova, H.; Pour, M.; Kunes, J.; Senel, P. Carbonylative lactonization via carbonyl oxygen attack: A short and selective total synthesis of uncinine and its analogues. Tetrahedron Lett. 2005, 46, 8137–8140. [Google Scholar] [CrossRef]
- Tan, K.K.; Khoo, T.J.; Rajagopal, M.; Wiart, C. Antibacterial alkaloids from Artabotrys crassifolius Hook.f. & Thomson. Nat. Prod. Res. 2015, 29, 2346–2349. [Google Scholar]
- Kwan, T.K.; Shipton, F.; Azman, N.S.; Hossan, S.; Jin, K.T.; Wiart, C. Cytotoxic Aporphines from Artabotrys crassifolius. Nat. Prod. Commun. 2016, 11, 389–392. [Google Scholar] [CrossRef]
- Gören, A.C.; Zhou, B.N.; Kingston, D.G. Cytotoxic and DNA damaging activity of some aporphine alkaloids from Stephania dinklagei. Planta Med. 2003, 69, 867–868. [Google Scholar]
- Wu, Y.C.; Chen, C.H.; Yang, T.H.; Lu, S.T.; McPhail, D.R.; McPhail, A.T.; Lee, K.H. Cytotoxic aporphines from Artabotrys uncinatus and the structure and stereochemistry of artacinatine. Phytochemistry 1989, 28, 2191–2195. [Google Scholar]
- Lan, Y.H.; Wang, H.Y.; Wu, C.C.; Chen, S.L.; Chang, C.L.; Chang, F.R.; Wu, Y.C. New constituents from stems of Artabotrys uncinatus. Chem. Pharm. Bull. 2007, 55, 1597–1599. [Google Scholar] [CrossRef]
- Sichaem, J.; Ruksilp, T.; Worawalai, W.; Siripong, P.; Khumkratok, S.; Tip-pyang, S. A new dimeric aporphine from the roots of Artabotrys spinosus. Fitoterapia 2011, 82, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q. Chemical constituents from stems and leaves of Artabotrys hongkongensis. Chin. Tradit. Herb. Drugs 2019, 24, 551–556. [Google Scholar]
- Li, H.Y.; Sun, N.J.; Kashiwada, Y.; Sun, L.; Snider, J.V.; Cosentino, L.M.; Lee, K.H. Anti-AIDS agents, 9. Suberosol, a new C31 lanostane-type triterpene and anti-HIV principle from Polyalthia suberosa. J. Nat. Prod. 1993, 56, 1130–1133. [Google Scholar] [CrossRef]
- Mehta, B.K.; Kori, P.; Mehta, D.; Misra, H. Novel lipid constituents identified from the leaves of Artabotrys odoratissimus (R.Br). Arab. J. Chem. 2017, 10, S742–S746. [Google Scholar] [CrossRef]
- Khaleel, F.D.; Zuber, S.M.; Mehta, B.K.; Mehta, D.; Kolisetty, S.R. Phytochemical study on the benzene:acetone extract of the leaves of Artabotrys odoratissimus. Afr. J. Pure Appl. Chem. 2014, 8, 32–36. [Google Scholar]
- Somanawat, J.; Talangsri, N.; Deepolngam, S.; Kaewamatawong, R. Flavonoid and megastigmane glycosides from Artabotrys hexapetalus leaves. Biochem. Syst. Ecol. 2012, 44, 124–127. [Google Scholar] [CrossRef]
- Das, A.; Baidya, R.; Chakraborty, T.; Samanta, A.K.; Roy, S. Pharmacological basis and new insights of taxifolin: A comprehensive review. Biomed. Pharmacother. 2021, 142, 112004. [Google Scholar] [CrossRef]
- Wong, H.F.; Brown, G.D. β-Methoxy-γ-methylene-α,β-unsaturated-γ-butyrolactones from Artabotrys hexapetalus. Phytochemistry 2002, 59, 99–104. [Google Scholar] [CrossRef]
- Yu, J.G.; Li, T.M.; Sun, L.; Luo, X.Z.; Ding, W.; Li, D.Y. Studies on the chemical constituents of the seeds from Artabostrys hexapetalus (Annonaceae). Yao Xue Xue Bao 2001, 36, 281–286. [Google Scholar]
- Batwal, R.U.; Argade, N.P. Chemoenzymatic Access to (+)-Artabotriol and its Application in Collective Synthesis of (+)-Grandiamide D, (–)-Tulipalin B, (+)-Spirathundiol, and (+)-Artabotriolcaffeate. Synthesis 2016, 48, 2130–2136. [Google Scholar]
- Xi, F.M.; Ma, S.G.; Liu, Y.B.; Li, L.; Yu, S.S. Artaboterpenoids A and B, Bisabolene-Derived Sesquiterpenoids from Artabotrys hexapetalus. Org. Lett. 2016, 18, 3374–3377. [Google Scholar] [CrossRef]
- Xi, F.M.; Liu, Y.B.; Qu, J.; Li, Y.; Tang, Z.H.; Li, L.; Li, Y.H.; Chen, X.G.; Ma, S.G.; Yu, S.S. Bioactive sesquiterpenoids from the roots of Artabotrys hexapetalus. Tetrahedron 2017, 73, 571–582. [Google Scholar] [CrossRef]
- Mahidol, C.; Chimnoi, N.; Chokchaichamnankit, D. Identification of Volatile Constituents in Artabotrys hexapetalus Flowers Using Simple Headspace Solvent-Trapping Technique in Combination with Gas Chromatography-Mass Spectrometry and Retention Indices. In Proceedings of the III WOCMAP Congress on Medicinal and Aromatic Plants-Volume 3: Perspectives in Natural Product Chemistry, Chiang Mai, Thailand, 3–7 February 2003; Başer, K.H.C., Franz, G., Cañigueral, S., Demirci, F., Craker, L.E., Gardner, Z.E., Eds.; ISHS: Leuven, Belgium, 2005. [Google Scholar]
- Ravi, S.; Sundaram, K. The essential oil constituents of Artabotrys species—A review. J. Phytol. 2020, 12, 24–28. [Google Scholar]
- Meghana, P.; Jain, R.S.K.; Prashanth, N.; Kumaraswamy, H.M. Phytochemical profiling and screening of protective effects of Artabotrys odoratissimus on H2O2 induced oxidative stress in HEK-293 cells and erythrocytes. Bot. Lett. 2020, 167, 471–484. [Google Scholar]
- Wang, D.; Zhang, S.; Chang, Z.; Kong, D.X.; Zuo, Z. Quebrachitol: Global Status and Basic Research. Nat. Prod. Bioprospect. 2017, 7, 113–122. [Google Scholar] [CrossRef]
- Liang, X.T.; Yu, D.Q.; Wu, W.L.; Deng, H.C. The structure of yingzhaosu A. Acta Chim. Sin. 1979, 37, 215–230. [Google Scholar]
- Liang, X.T.; Yu, D.Q.; Pan, W.D. The structure of yingzhaosu B. Acta Chim. Sin. 1979, 37, 231–240. [Google Scholar]
- Liang, X.T. Advances in Chinese Medicinal Materials Research; Chang, H.M., Yeung, H.W., Tso, W.W., Koo, A., Eds.; World Scientific Publishing Co.: Singapore, 1985; p. 427. [Google Scholar]
- Zhang, L.; Zhou, W.S.; Xu, X.X. A new sesquiterpene peroxide (yingzhaosu C) and sesquiterpenol (yingzhaosu D) from Artabostrys unciatus (L.) Meer. J. Chem. Soc. Chem. Commun. 1988, 8, 523–524. [Google Scholar] [CrossRef]
- Zhang, L.A.; Zhou, W.S.; Xu, X.X. Structure of yingzhaosu C and D. Sci. China B 1989, 32, 800–807. [Google Scholar]
- Xu, X.X.; Zhu, J.; Huang, D.Z.; Zhou, W.S. Total synthesis of (+)-Yingzhaosu A. Tetrahedron Lett. 1991, 32, 5785–5788. [Google Scholar] [CrossRef]
- de Sousa, D.P.; de Farias Nóbrega, F.F.; de Almeida, R.N. Influence of the chirality of (R)-(−)- and (S)-(+)-carvone in the central nervous system: A comparative study. Chirality 2007, 19, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Nogoceke, F.P.; Barcaro, I.M.; de Sousa, D.P.; Andreatini, R. Antimanic-like effects of (R)-(−)-carvone and (S)-(+)-carvone in mice. Neurosci. Lett. 2016, 619, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.A.; Oriquat, G.A.; Abbas, M.M.; Al-Najjar, B.O.; Kandil, Y.I. Hypolipidaemic and Insulin Secretagogue Activities of (R)-(−)-Carvone. Malays. J. Med. Sci. 2020, 27, 39–52. [Google Scholar] [CrossRef]
- Abbas, M.M.; Kandil, Y.İ.; Abbas, M.A. R-(−)-carvone Attenuated Doxorubicin Induced Cardiotoxicity In Vivo and Potentiated Its Anticancer Toxicity In Vitro. Balk. Med. J. 2020, 37, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.X.; Hu, Q.S. Synthesis of the diastereoisomeric Yingzhaosu D. Chin. J. Chem. 1992, 10, 285–288. [Google Scholar]
- Xu, X.X.; Dong, H.Q. Enantioselective total synthesis of all four stereoisomers of Yingzhaosu C. Tetrahedron Lett. 1994, 35, 9429–9432. [Google Scholar] [CrossRef]
- Boukouvalas, J.; Pouliot, R.; Frechette, Y. Concise synthesis of yingzhaosu C and epi-yingzhaosu C by peroxyl radical cyclization. Assignment of relative configuration. Tetrahedron Lett. 1995, 36, 4167–4170. [Google Scholar] [CrossRef]
- Korshin, E.E.; Hoos, R.; Szpilman, A.M.; Konstantinovski, L.; Posner, G.H.; Bachi, M.D. An efficient synthesis of bridged-bicyclic peroxides structurally related to antimalarial yingzhaosu A based on radical co-oxygenation of thiols and monoterpenes. Tetrahedron 2002, 58, 2449–2469. [Google Scholar] [CrossRef]
- Kavanagh, S.E.; Gilheany, D.G. Harnessing the Power of the Asymmetric Grignard Synthesis of Tertiary Alcohols: Ligand Development and Improved Scope Exemplified by One-Step Gossonorol Synthesis. Org. Lett. 2020, 22, 8198–8203. [Google Scholar] [CrossRef]
- Szpilman, A.S.; Korshin, E.E.; Rozenberg, H.; Bachi, M.D. Total Syntheses of Yingzhaosu A and of Its C(14)-Epimer Including the First Evaluation of Their Antimalarial and Cytotoxic Activities. J. Org. Chem. 2005, 70, 3618–3632. [Google Scholar] [CrossRef]
- Møller, K.H.; Otkjær, R.V.; Chen, J.; Kjaergaard, H.G. Double Bonds Are Key to Fast Unimolecular Reactivity in First-Generation Monoterpene Hydroxy Peroxy Radicals. J. Phys. Chem. A 2020, 124, 2885–2896. [Google Scholar] [CrossRef] [PubMed]
- Piletic, I.R.; Kleindienst, T.E. Rates and Yields of Unimolecular Reactions Producing Highly Oxidized Peroxy Radicals in the OH-Induced Autoxidation of α-Pinene, β-Pinene, and Limonene. J. Phys. Chem. A 2022, 126, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Zmitek, K.; Zupan, M.; Iskra, J. α-Substituted organic peroxides: Synthetic strategies for a biologically important class of gem-dihydroperoxide and perketal derivatives. Org. Biomol. Chem. 2007, 5, 3895–3908. [Google Scholar] [CrossRef] [PubMed]
- López, M.M.; Jamey, N.; Pinet, A.; Figadère, B.; Ferrié, L. Oxidative Ring Ex- pansion of Cyclobutanols: Access to Functionalized 1,2-Dioxanes. Org. Lett. 2021, 23, 1626–1631. [Google Scholar] [CrossRef]
- Woodley, C.M.; Amado, P.S.M.; Cristiano, M.L.S.; O’Neill, P.M. Artemisinin inspired synthetic endoperoxide drug candidates: Design, synthesis, and mechanism of action studies. Med. Res. Rev. 2021, 41, 3062–3095. [Google Scholar] [CrossRef]
- O’Neill, P.M.; Searle, Y.N.L.; Raynes, Y.K.J.; Maggs, J.L.; Ward, S.A.; Storr, R.C.; Park, B.K.; Posner, G.H. A Carbonyl Oxide Route to Antimalarial Yingzhaosu A Analogues: Synthesis and Antimalarial Activity. Tetrahedron Lett. 1998, 39, 6065–6068. [Google Scholar] [CrossRef]
- Bachi, M.D.; Korshin, E.E. Thiol-Oxygen Cooxidation of Monoterpenes. Synthesis of Endoperoxides Structurally Related to Antimalarial Yingzhaosu A. Synlett. 1998, 1998, 122–124. [Google Scholar] [CrossRef]
- Bachi, M.D.; Korshin, E.E.; Hoos, R.; Szpilman, A.S. Synthesis and reactions of antimalarial bicyclic peroxides. J. Het. Chem. 2000, 37, 639–645. [Google Scholar] [CrossRef]
- Bachi, M.D.; Korshin, E.E.; Hoos, R.; Szpilman, A.M.; Ploypradith, P.; Xie, S.; Shapiro, T.A.; Posner, G.H. A short synthesis and biological evaluation of potent and nontoxic antimalarial bridged bicyclic β-sulfonyl-endoperoxides. J. Med. Chem. 2003, 46, 2516–2533. [Google Scholar] [CrossRef]
- Tokuyasu, T.; Masuyama, A.; Nojima, M.; McCullough, K.J.; Kim, H.S.; Wataya, Y. Yingzhaosu A analogues: Synthesis by the ozonolysis of unsaturated hydroperoxides, structural analysis and determination of anti-malarial activity. Tetrahedron 2001, 57, 5979–5989. [Google Scholar] [CrossRef]
- Tokuyasu, T.; Kunikawa, S.; Abe, M.; Masuyama, A.; Nojima, M.; Kim, H.S.; Begum, K.; Wataya, Y. Synthesis of antimalarial yingzhaosu A analogues by the peroxidation of dienes with Co(II)/O2/Et3SiH. J. Org. Chem. 2003, 68, 7361–7367. [Google Scholar] [CrossRef]
- Bishop, L.P.; Maggs, J.L.; O’Neill, P.M.; Park, B.K. Metabolism of the antimalarial endoperoxide Ro 42-1611 (arteflene) in the rat: Evidence for endoperoxide bioactivation. J. Pharmacol. Exp. Ther. 1999, 289, 511–520. [Google Scholar] [PubMed]
- Zhang, J. The osteoprotective effects of artemisinin compounds and the possible mechanisms associated with intracellular iron: A review of in vivo and in vitro studies. Environ. Toxicol. Pharmacol. 2020, 76, 103358. [Google Scholar] [CrossRef] [PubMed]
- Maggs, J.L.; Bishop, L.P.; Batty, K.T.; Dodd, C.C.; Ilett, K.F.; O’Neill, P.M.; Edwards, G.; Park, B.K. Hepatocellular bioactivation and cytotoxicity of the synthetic endoperoxide antimalarial arteflene. Chem. Biol. Interact. 2004, 147, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Hofheinz, W.; Bürgin, H.; Gocke, E.; Jaquet, C.; Masciadri, R.; Schmid, G.; Stohler, H.; Urwyler, H. Ro 42-1611 (arteflene), a new effective antimalarial: Chemical structure and biological activity. Trop. Med. Parasitol. 1994, 45, 261–265. [Google Scholar] [PubMed]
- Radloff, P.D.; Philipps, J.; Nkeyi, M.; Sturchler, D.; Mittelholzer, M.L.; Kremsner, P.G. Arteflene compared with mefloquine for treating Plasmodium falciparum malaria in children. Am. J. Trop. Med. Hyg. 1996, 55, 259–262. [Google Scholar] [CrossRef]
- Cazelles, J.; Robert, A.; Meunier, B. Characterization of the Main Radical and Products Resulting from a Reductive Activation of the Antimalarial Arteflene (Ro 42-1611). J. Org. Chem. 1999, 64, 6776–6781. [Google Scholar] [CrossRef]
- Weidekamm, E.; Dumont, E.; Jaquet, C. Tolerability and pharmacokinetics of Ro 42-1611 (arteflene) in man. Trop. Med. Parasitol. 1994, 45, 278–283. [Google Scholar] [PubMed]
- Somo-Moyou, R.; Mittelholzer, M.L.; Sorenson, F.; Haller, L.; Stürchler, D. Efficacy of Ro 42-1611 (arteflene) in the treatment of patients with mild malaria: A clinical trial in Cameroon. Trop. Med. Parasitol. 1994, 45, 288–291. [Google Scholar]
- Posner, G.H. Antimalarial peroxides in the qinghaosu (artemisinin) and yingzhaosu families. Expert Opin. Therap. Pat. 1998, 8, 1487–1493. [Google Scholar] [CrossRef]
- Szpilman, A.M.; Korshin, E.E.; Hoos, R.; Posner, G.H.; Bachi, M.D. Iron(II)-induced degradation of antimalarial β-sulfonyl endoperoxides: Evidence for the generation of potentially cytotoxic carbocations. J. Org. Chem. 2001, 66, 6531–6540. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, P.M.; Bishop, L.P.; Searle, N.L.; Maggs, J.L.; Storr, R.C.; Ward, S.A.; Park, B.K.; Mabbs, F. Biomimetic Fe(II)-mediated degradation of arteflene (Ro-42-1611). The first EPR spin-trapping evidence for the previously postulated secondary carbon-centered cyclohexyl radical. J. Org. Chem. 2000, 65, 1578–1582. [Google Scholar] [CrossRef]
- Liu, S.; Wei, C.; Liu, T.; Ma, S.G.; Chen, C.; Lin, H.; Zhang, L.; Wang, H.; Zhang, C.J.; Yu, S.S. A heme-activatable probe and its application in the high-throughput screening of Plasmodium falciparum ring-stage inhibitors. Signal Transduct. Target. Ther. 2022, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.T.; Loehr, M.O.; Bogena, J.; Chang, C.J. An Endoperoxide Reactivity-Based FRET Probe for Ratiometric Fluorescence Imaging of Labile Iron Pools in Living Cells. J. Am. Chem. Soc. 2016, 138, 14338–14346. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhong, H.; Wang, R.; Liu, D.; Waxman, S.; Zhao, L.; Jing, Y. Dihydroartemisinin and its derivative induce apoptosis in acute myeloid leukemia through Noxa-mediated pathway requiring iron and endoperoxide moiety. Oncotarget 2015, 6, 5582–5596. [Google Scholar] [CrossRef] [PubMed]
- Brecht, K.; Kirchhofer, C.; Bouitbir, J.; Trapani, F.; Keiser, J.; Krähenbühl, S. Exogenous Iron Increases Fasciocidal Activity and Hepatocellular Toxicity of the Synthetic Endoperoxides OZ78 and MT04. Int. J. Mol. Sci. 2019, 20, 4880. [Google Scholar] [CrossRef] [PubMed]
- De Sarkar, S.; Sarkar, D.; Sarkar, A.; Dighal, A.; Chakrabarti, S.; Staniek, K.; Gille, L.; Chatterjee, M. The leishmanicidal activity of artemisinin is mediated by cleavage of the endoperoxide bridge and mitochondrial dysfunction. Parasitology 2019, 146, 511–520. [Google Scholar] [CrossRef]
- Quadros, H.C.; Silva, M.C.B.; Moreira, D.R.M. The Role of the Iron Protoporphyrins Heme and Hematin in the Antimalarial Activity of Endoperoxide Drugs. Pharmaceuticals 2022, 15, 60. [Google Scholar] [CrossRef]
- Gupta, A.K.; Saxena, A.K. Molecular modelling-based target identification for endo-peroxides class of antimalarials. Comb. Chem. High Throughput Screen. 2015, 18, 199–207. [Google Scholar] [CrossRef]
- Zhu, P.; Zhou, B. The Antagonizing Role of Heme in the Antimalarial Function of Artemisinin: Elevating Intracellular Free Heme Negatively Impacts Artemisinin Activity in Plasmodium falciparum. Molecules 2022, 27, 1755. [Google Scholar] [CrossRef]
- Golenser, J.; Waknine, J.H.; Krugliak, M.; Hunt, N.H.; Grau, G.E. Current perspectives on the mechanism of action of artemisinins. Int. J. Parasitol. 2006, 36, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R.K.; Cheu, K.W.; N’Da, D.; Coghi, P.; Monti, D. Considerations on the mechanism of action of artemisinin antimalarials: Part 1--the ‘carbon radical’ and ‘heme’ hypotheses. Infect. Disord. Drug Targets 2013, 13, 217–277. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Liu, J.; Mo, X.; Liu, H.; Chen, Y.; Dai, Z. Immunoregulation by Artemisinin and Its Derivatives: A New Role for Old Antimalarial Drugs. Front. Immunol. 2021, 12, 751772. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.V. Artabotrys hexapetalus (L.f.) Bhandari: A plant with enormous biomedical potential. Int. J. Pharm. Pharm. Sci. 2020, 12, 8–14. [Google Scholar] [CrossRef]
- Wang, F.X.; Zhu, N.; Zhou, F.; Lin, D.X. Natural Aporphine Alkaloids with Potential to Impact Metabolic Syndrome. Molecules 2021, 26, 6117. [Google Scholar] [CrossRef]
- Slack, R.D.; Jacobine, A.M.; Posner, G.H. Antimalarial peroxides: Advances in drug discovery and design. Med. Chem. Commun. 2012, 3, 281–297. [Google Scholar] [CrossRef]
- Guo, Z. Artemisinin anti-malarial drugs in China. Acta Pharm. Sin. B 2016, 6, 115–124. [Google Scholar] [CrossRef]
- Liu, K.; Zuo, H.; Li, G.; Yu, H.; Hu, Y. Global research on artemisinin and its derivatives: Perspectives from patents. Pharmacol. Res. 2020, 159, 105048. [Google Scholar] [CrossRef]
- Sharma, B.; Singh, P.; Singh, A.K.; Awasthi, S.K. Advancement of chimeric hybrid drugs to cure malaria infection: An overview with special emphasis on endoperoxide pharmacophores. Eur. J. Med. Chem. 2021, 219, 113408. [Google Scholar] [CrossRef]
- Fang, J.; Song, F.; Wang, F. The antimalarial activity of 1,2,4-trioxolane/trioxane hybrids and dimers: A review. Arch. Pharm. 2022, 355, e2200077. [Google Scholar] [CrossRef]
- Tsamesidis, I.; Mousavizadeh, F.; Egwu, C.O.; Amanatidou, D.; Pantaleo, A.; Benoit-Vical, F.; Reybier, K.; Giannis, A. In Vitro and In Silico Antimalarial Evaluation of FM-AZ, a New Artemisinin Derivative. Medicines 2022, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Tamez-Fernandez, J.F.; Melchor-Martinez, E.M.; Ibarra-Rivera, T.R.; Rivas-Galindo, V.M. Plant-derived endoperoxides: Structure, occurrence, and bioactivity. Phytochem. Rev. 2020, 19, 827–864. [Google Scholar] [CrossRef]
- Shi, Q.; Xia, F.; Wang, Q.; Liao, F.; Guo, Q.; Xu, C.; Wang, J. Discovery and repurposing of artemisinin. Front. Med. 2022, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).