A Novel Family of Cage-like (CuLi, CuNa, CuK)-phenylsilsesquioxane Complexes with 8-Hydroxyquinoline Ligands: Synthesis, Structure, and Catalytic Activity
Abstract
:1. Introduction
2. Results and Discussion
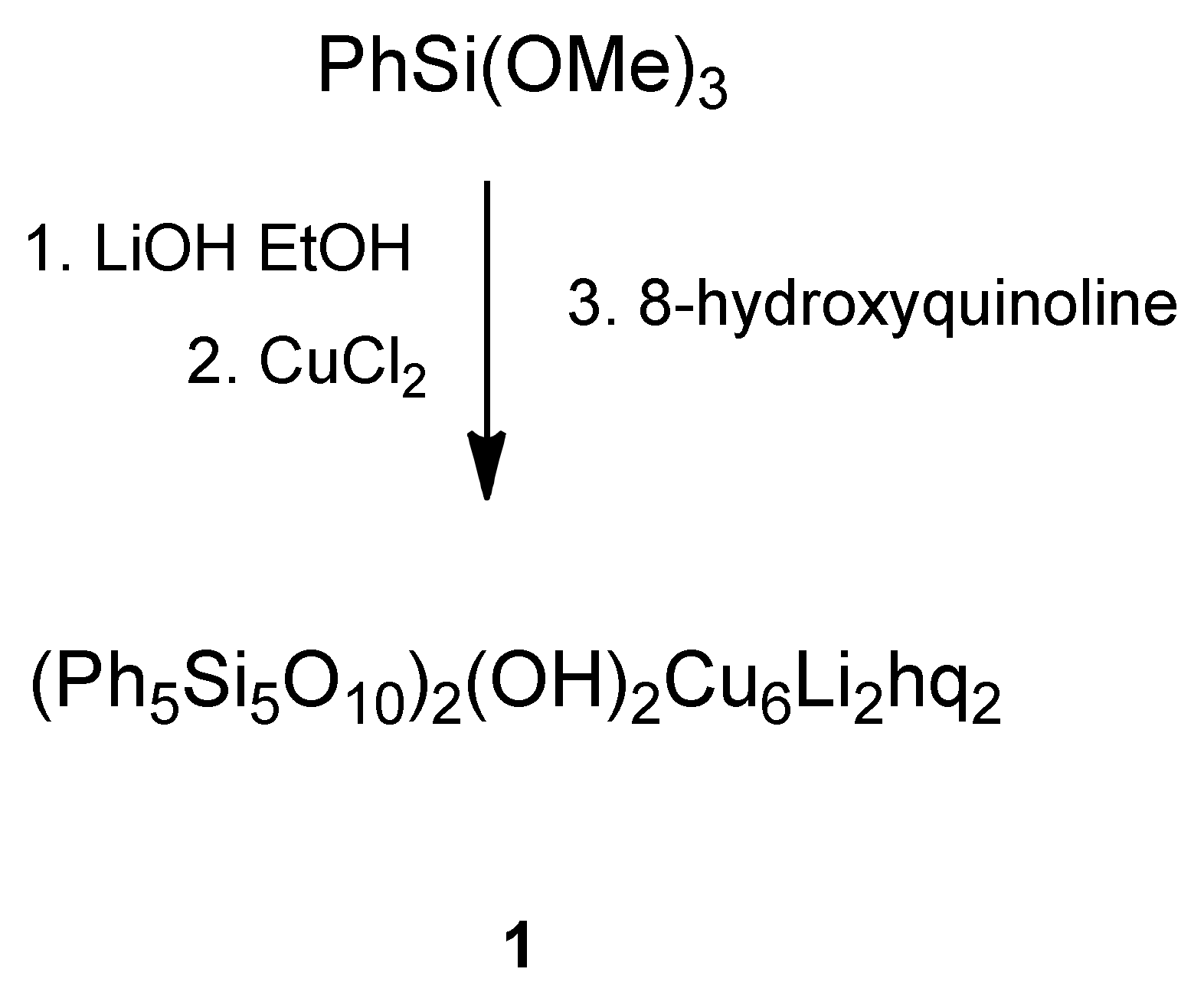
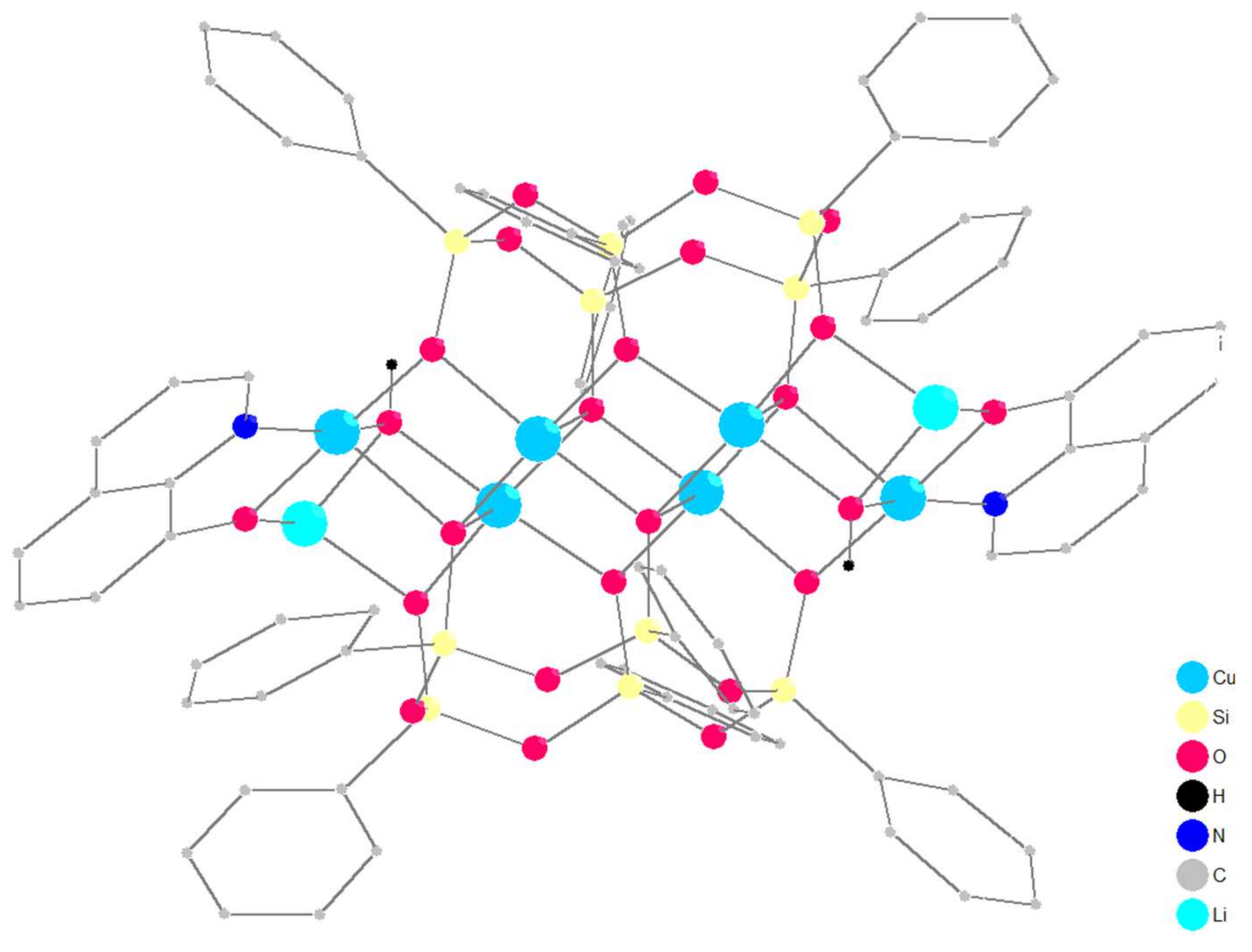
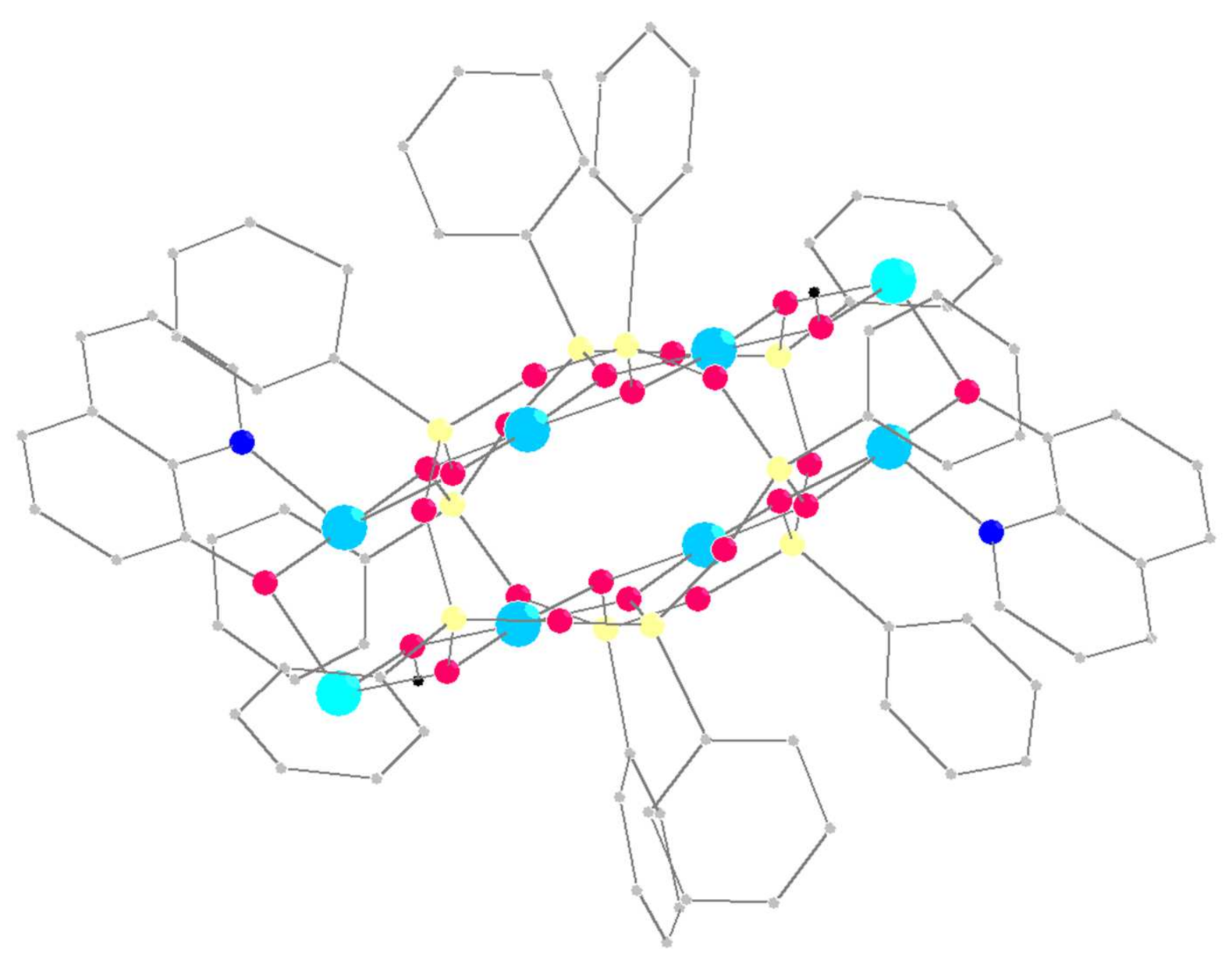
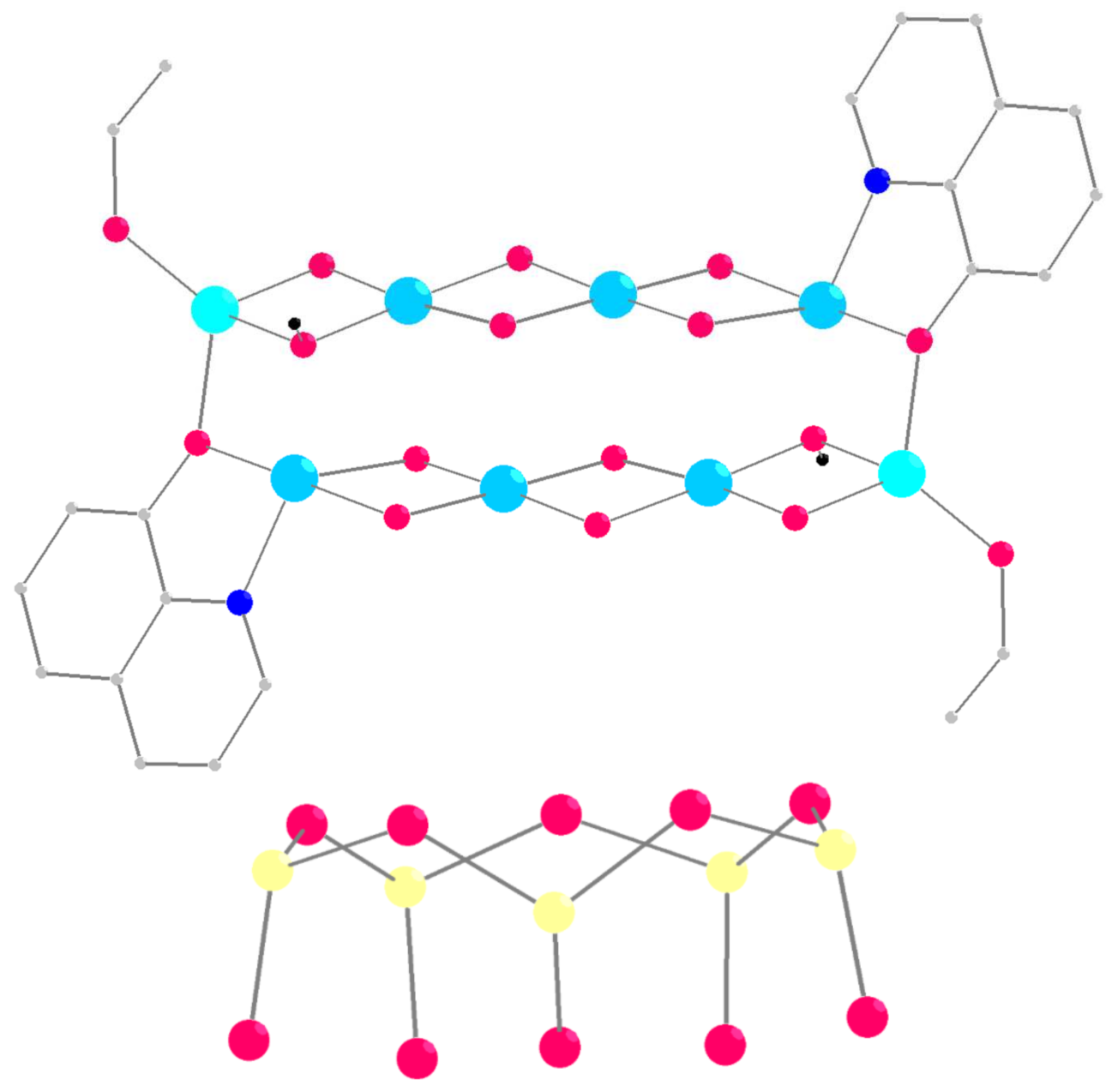
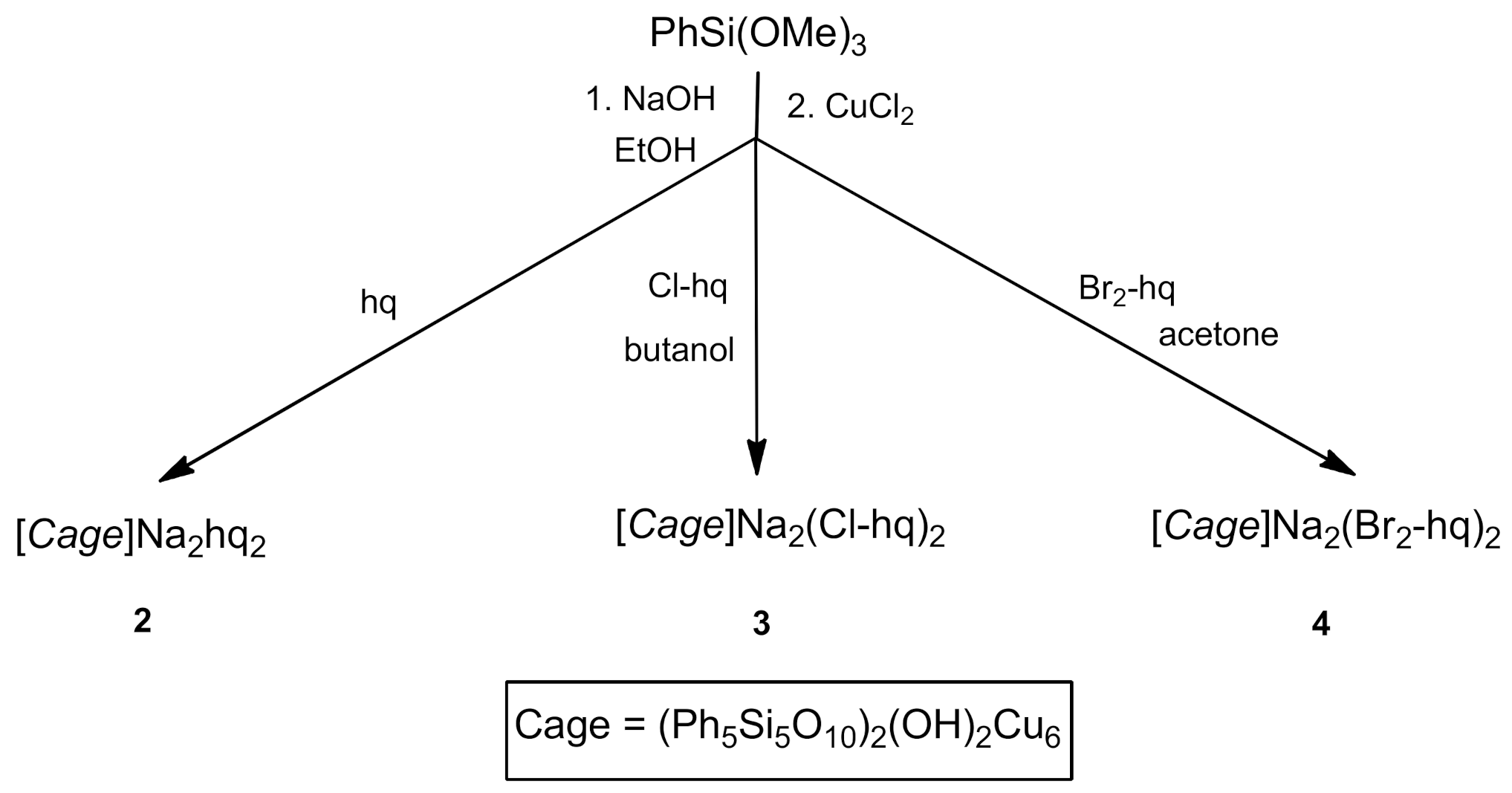
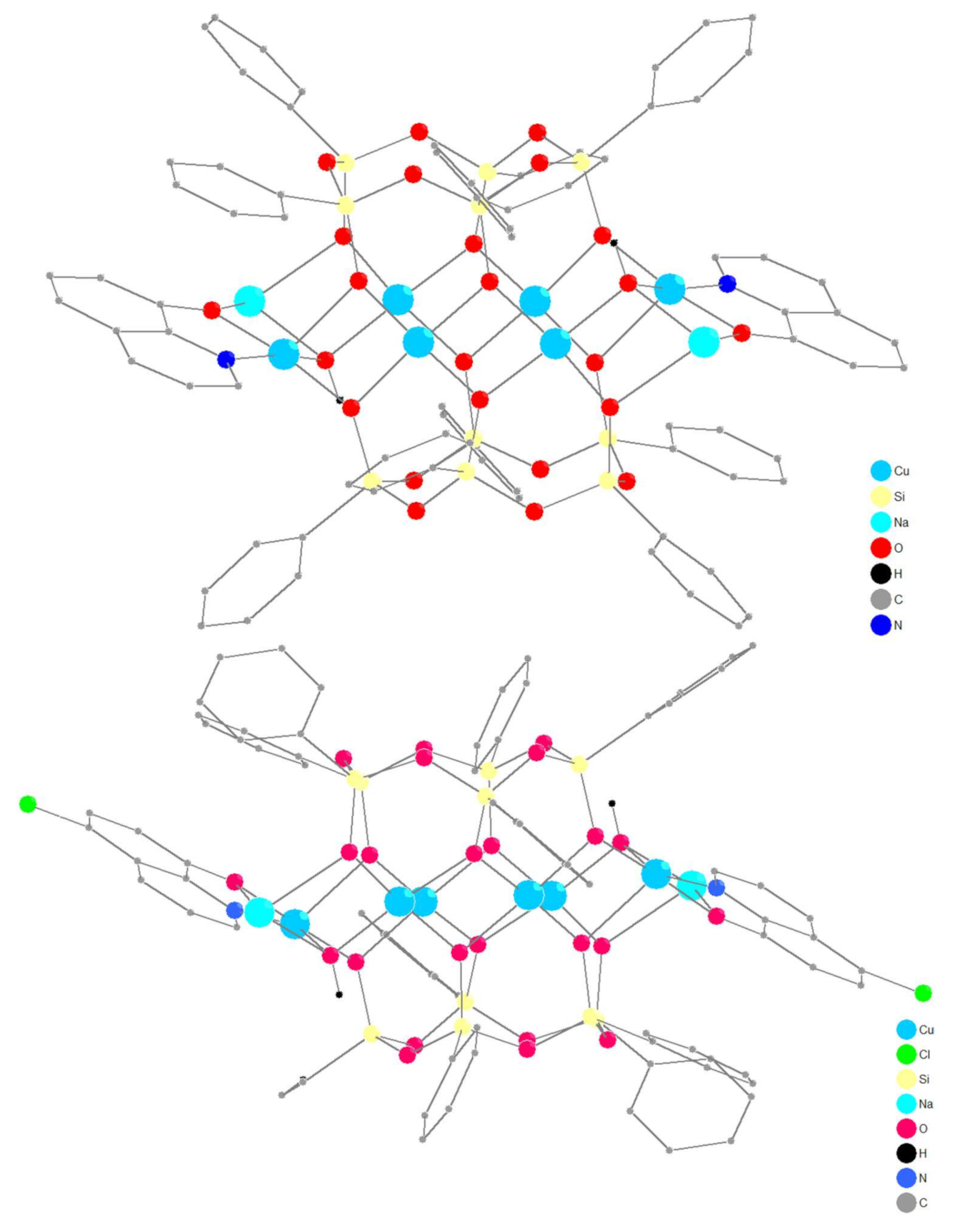
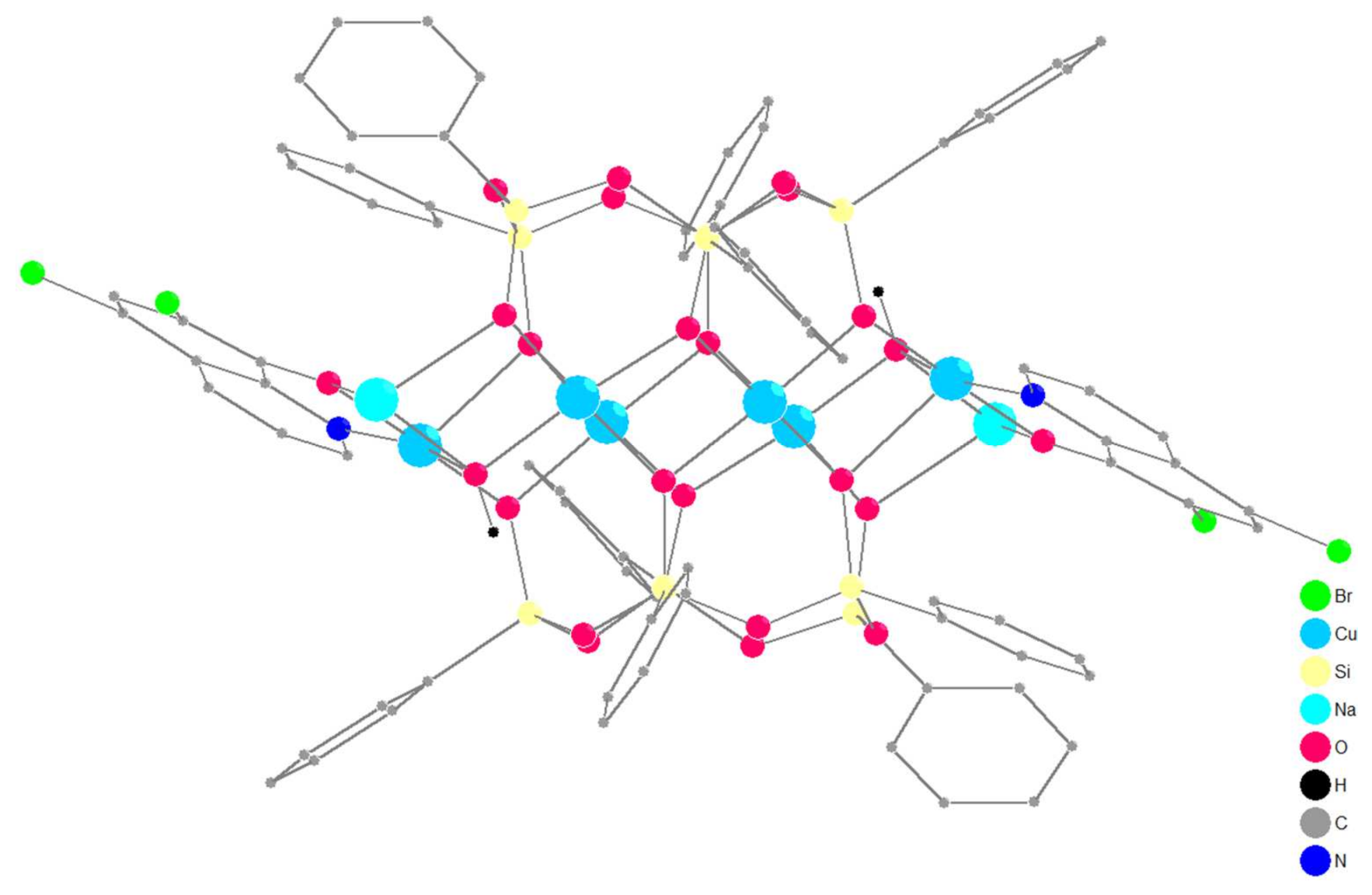
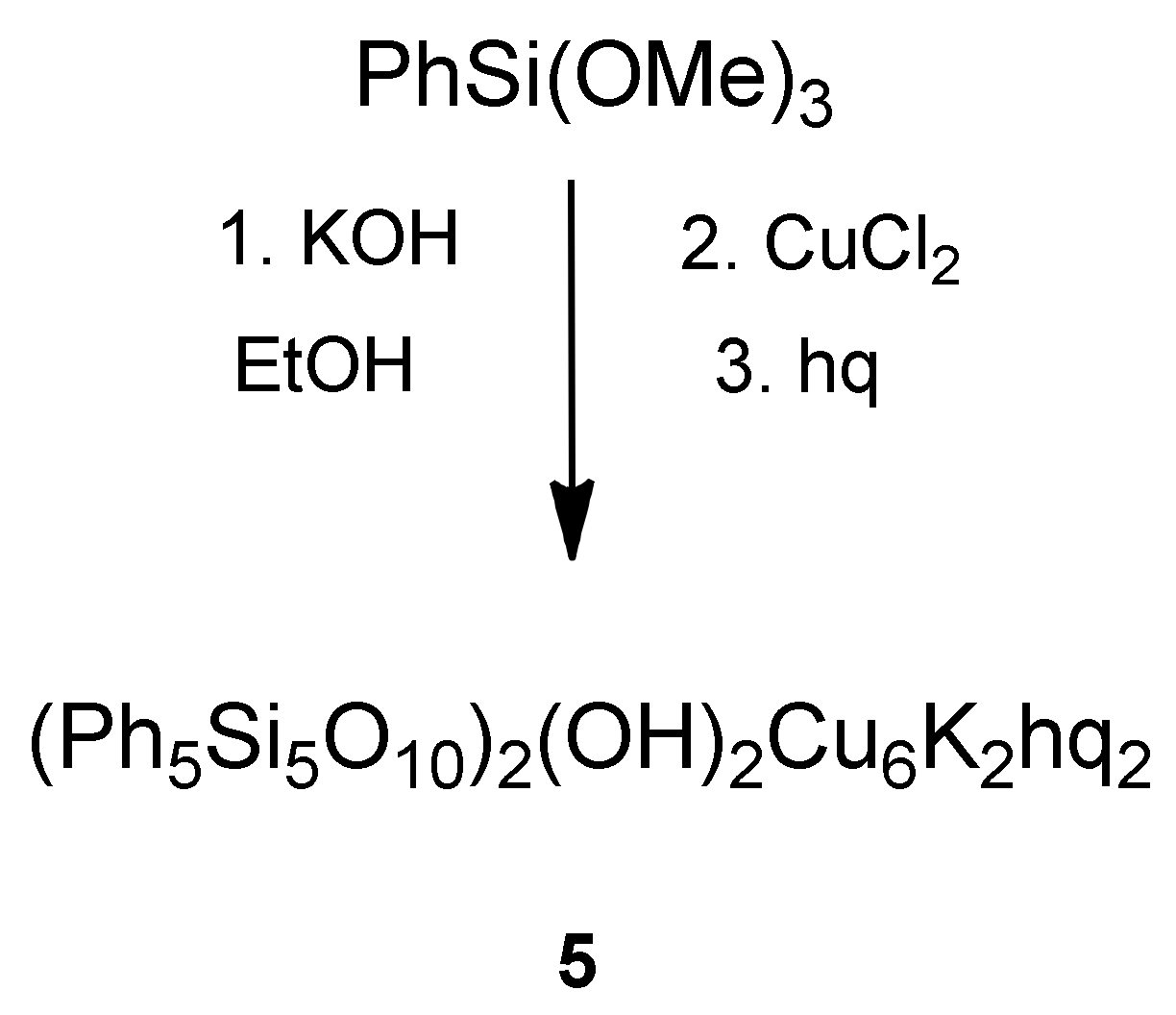
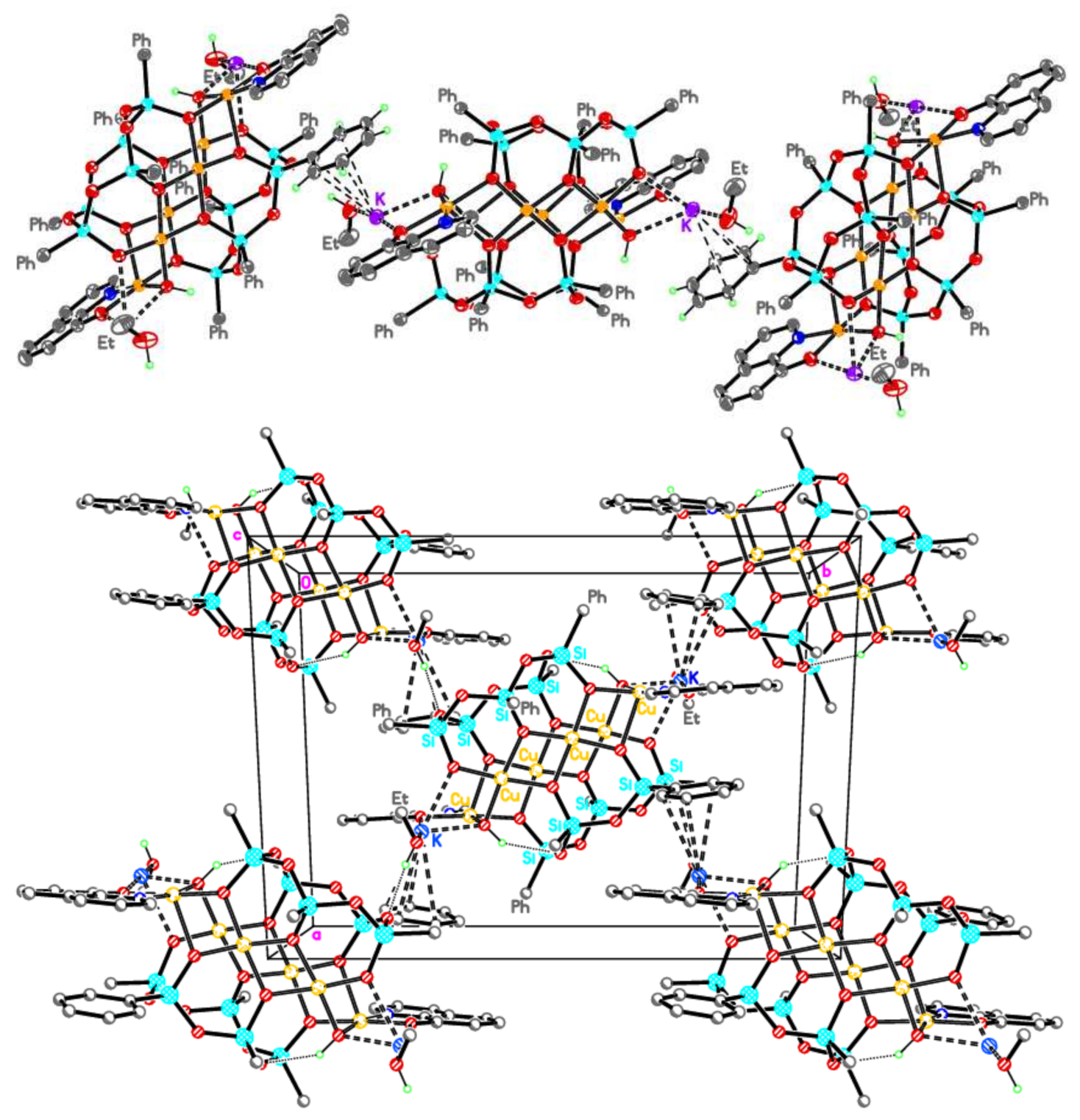
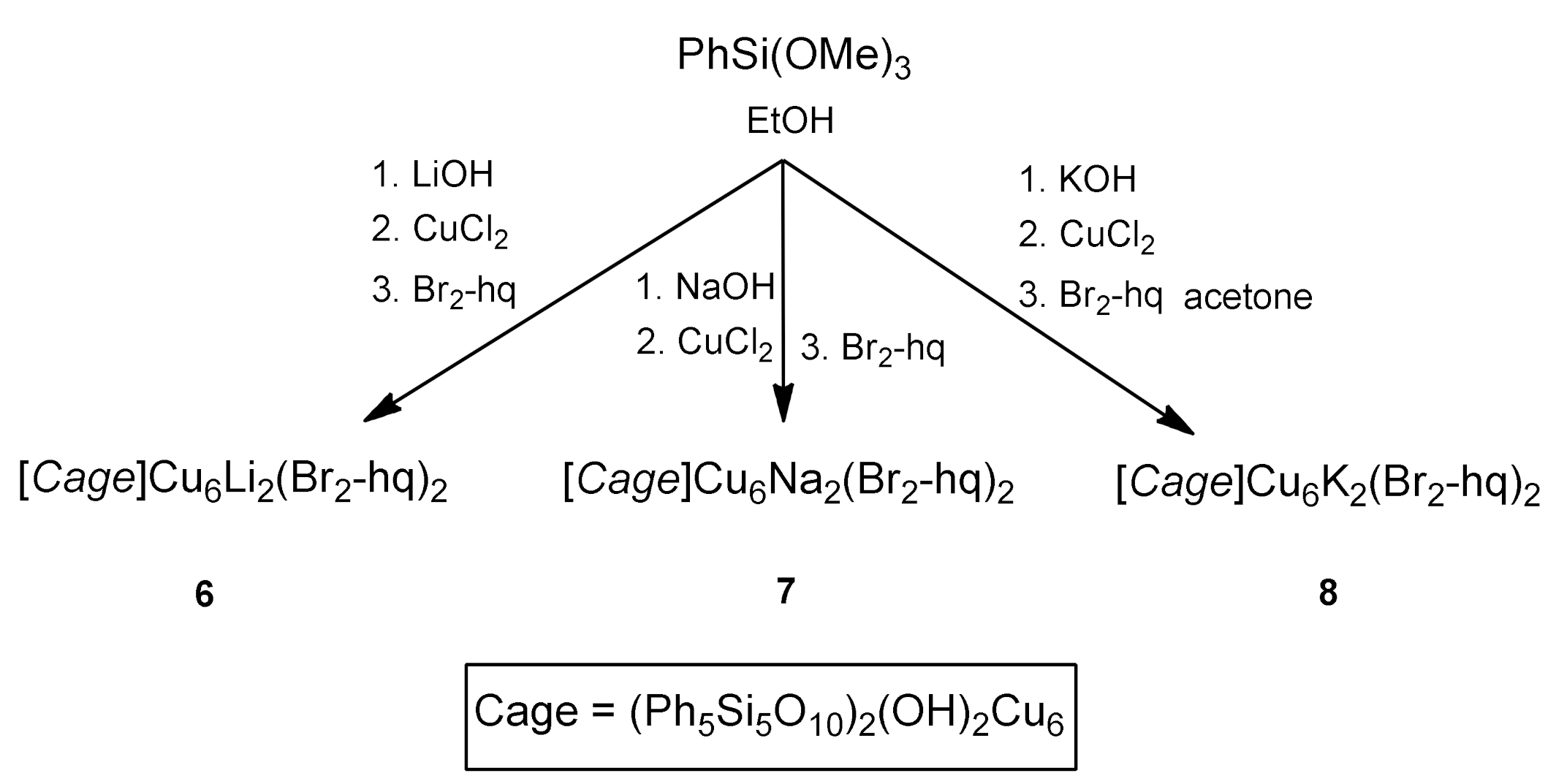
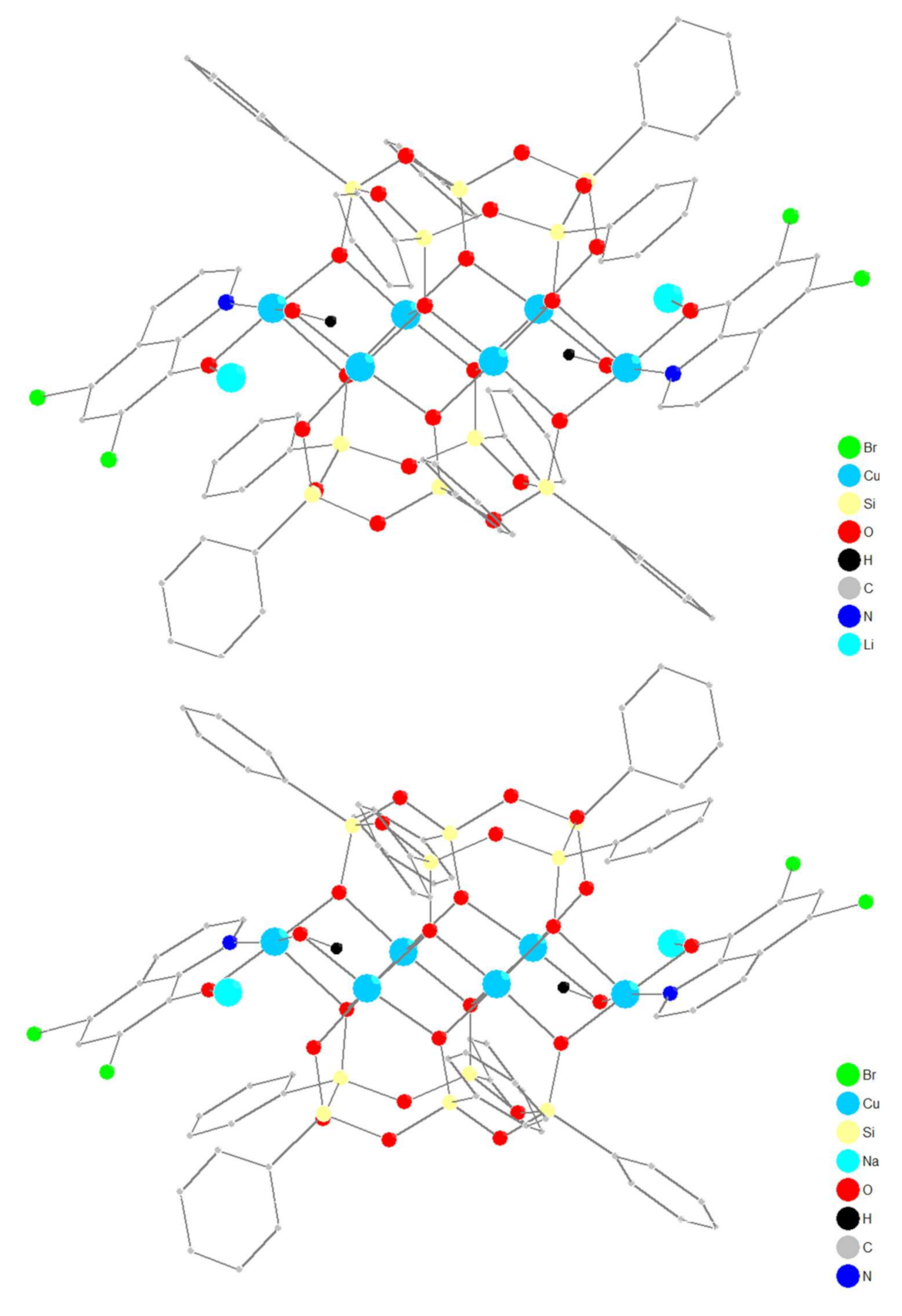
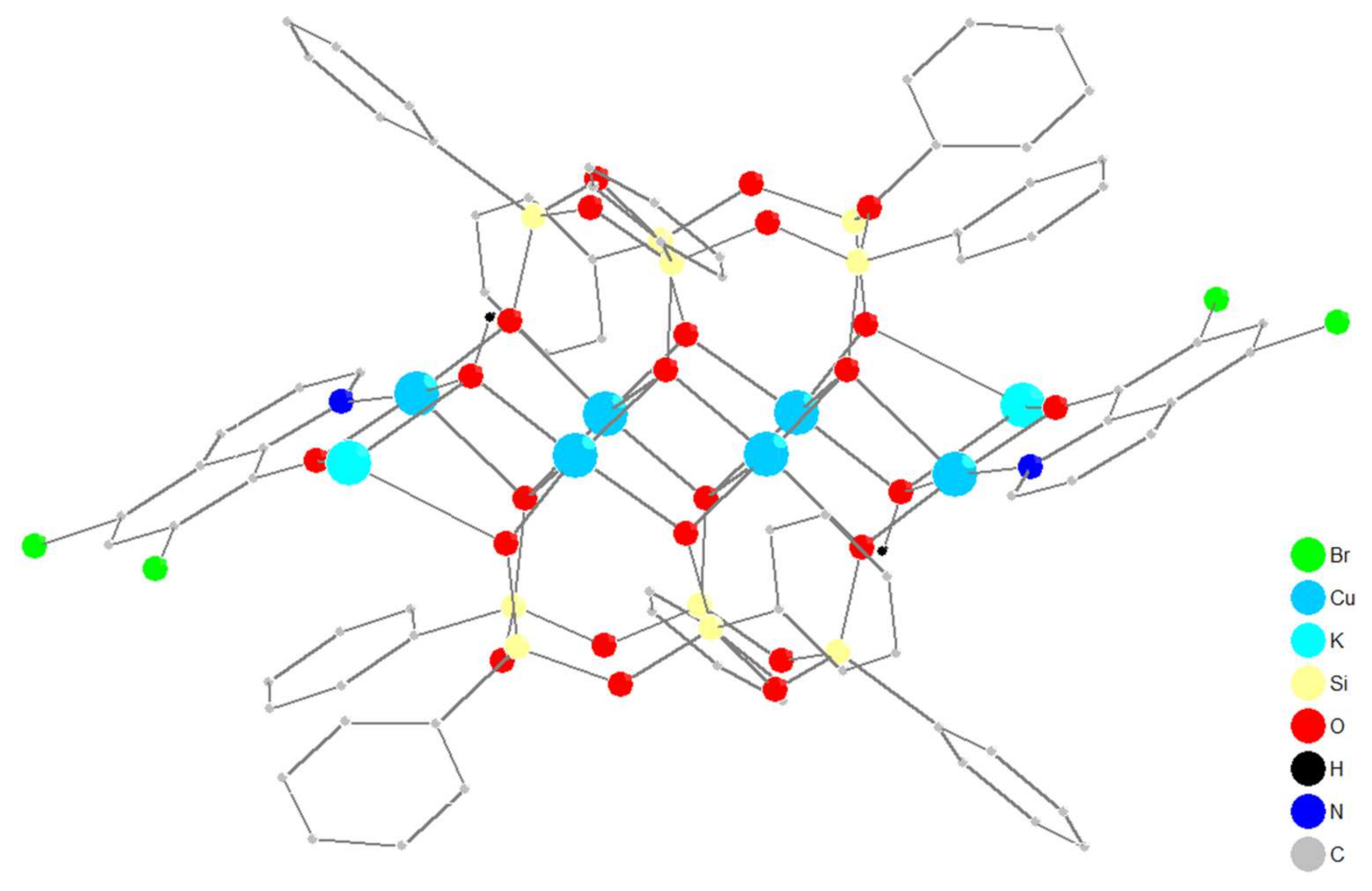
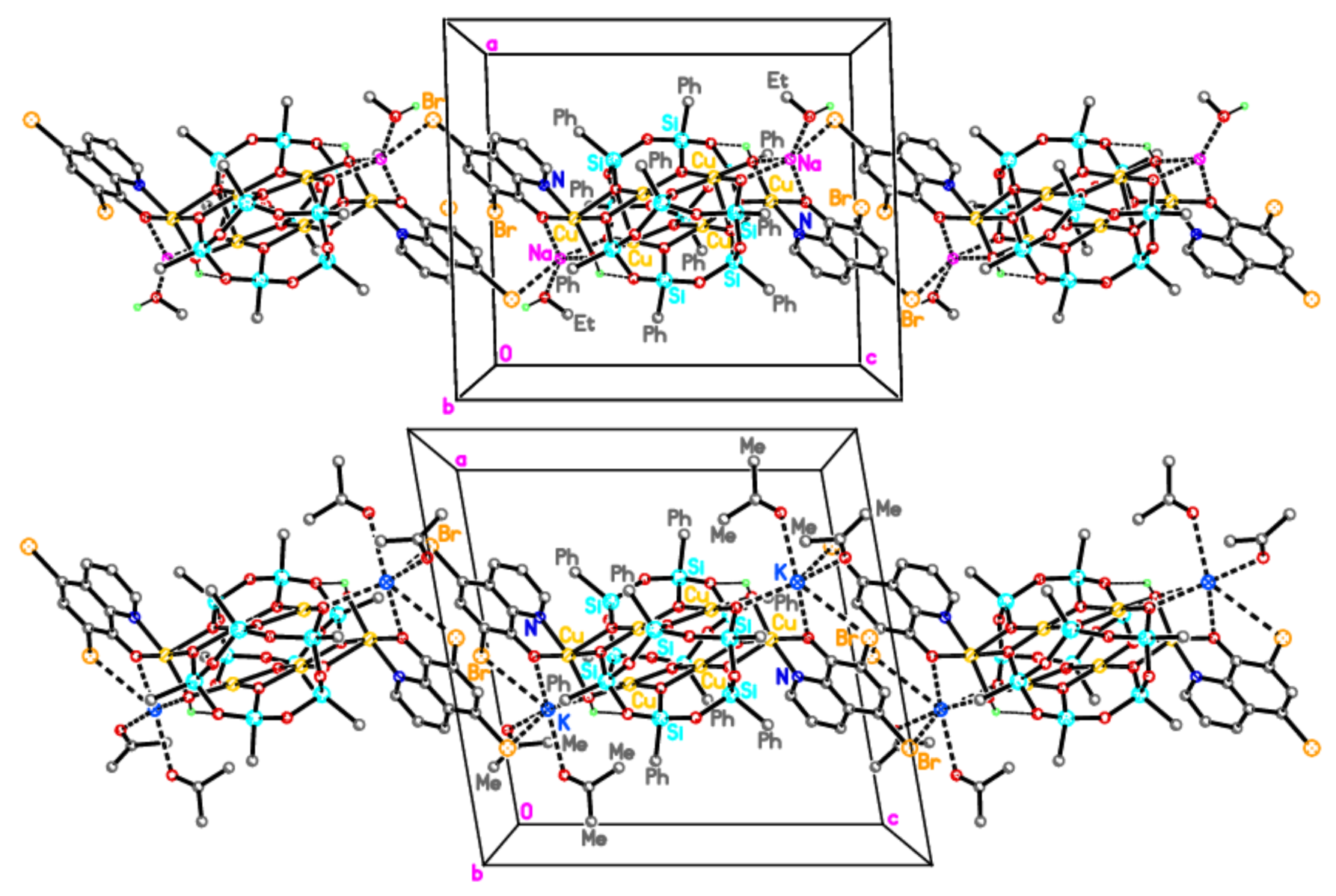
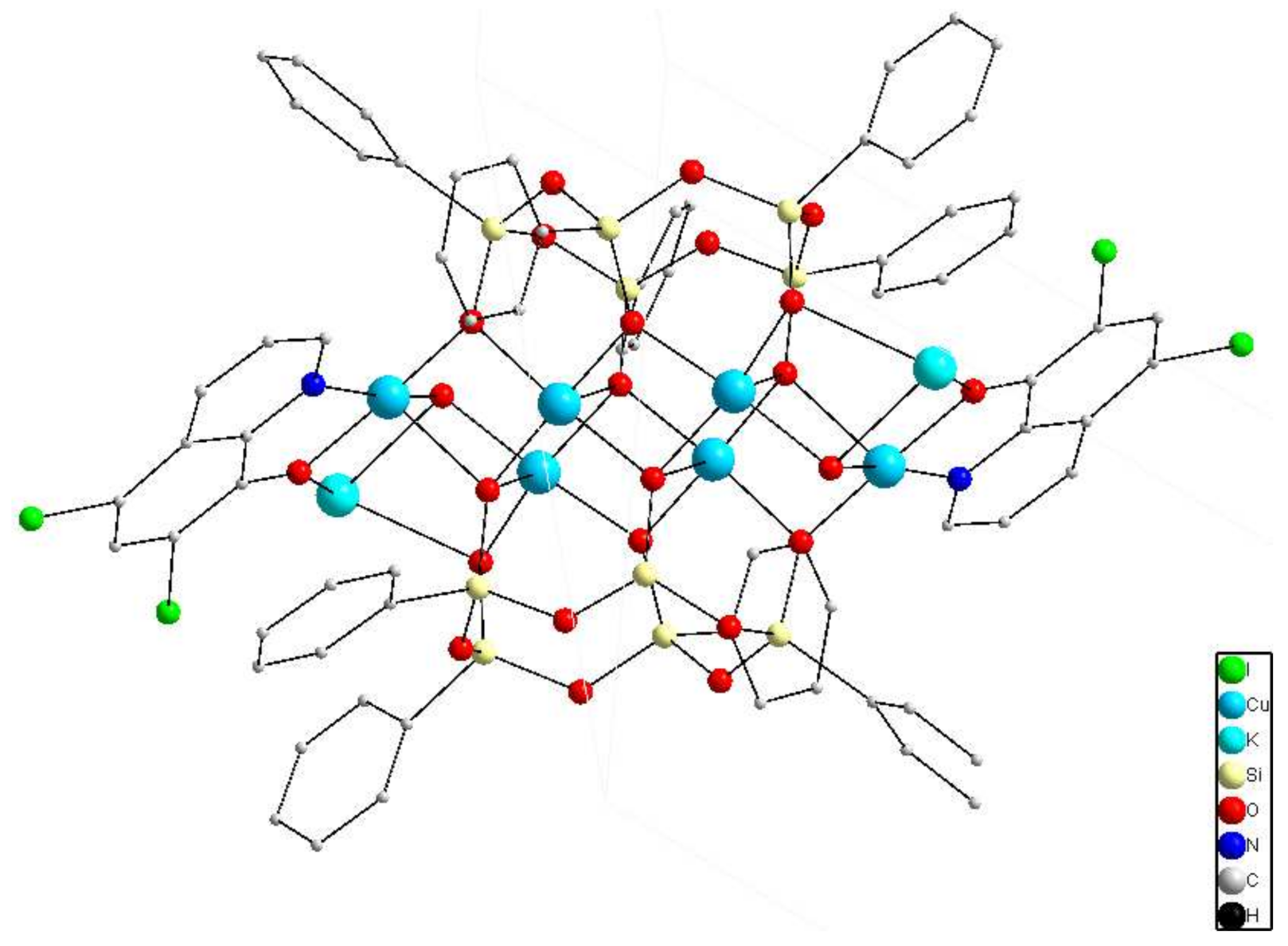
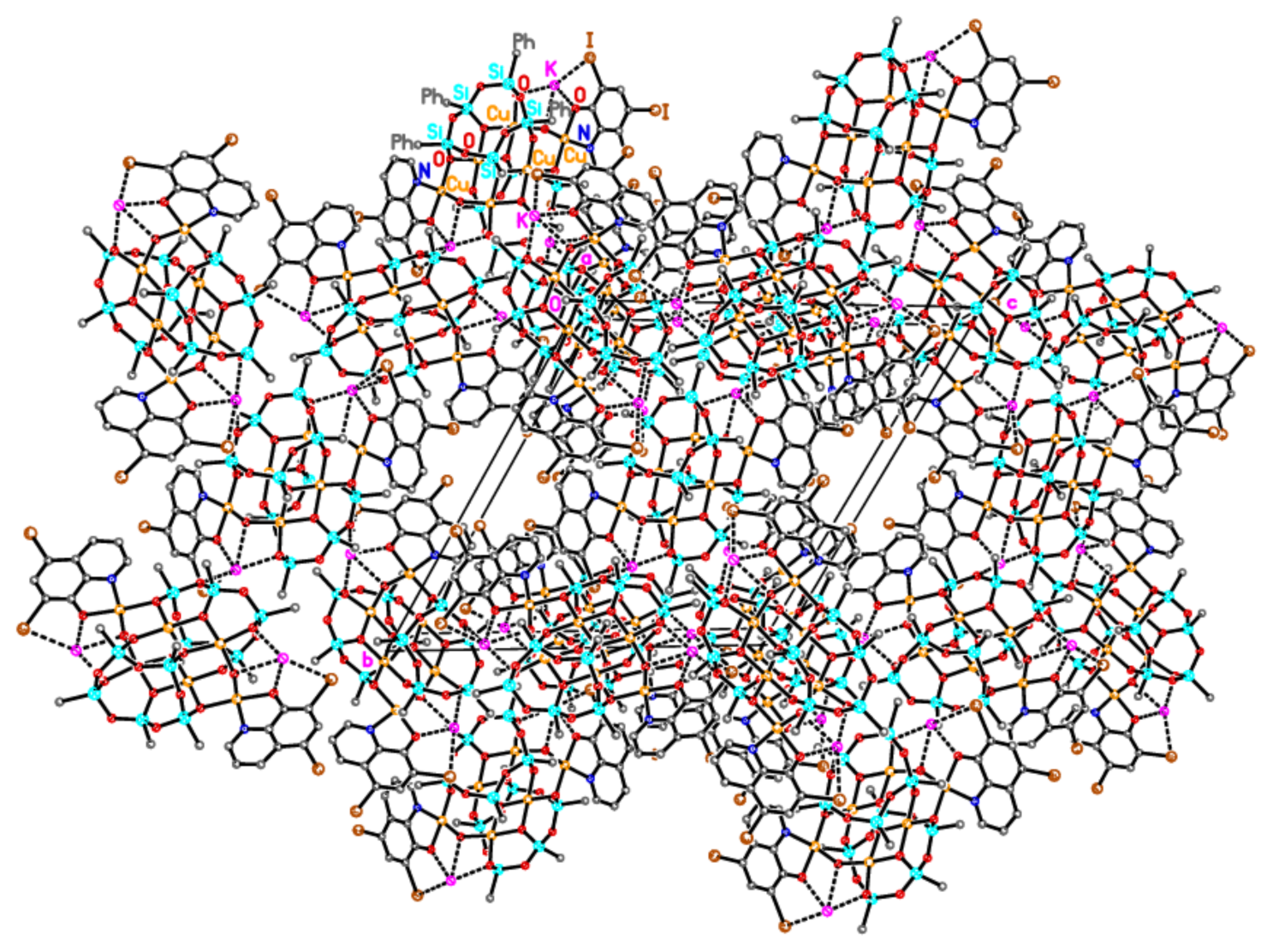
2.1. Synthesis and Structure
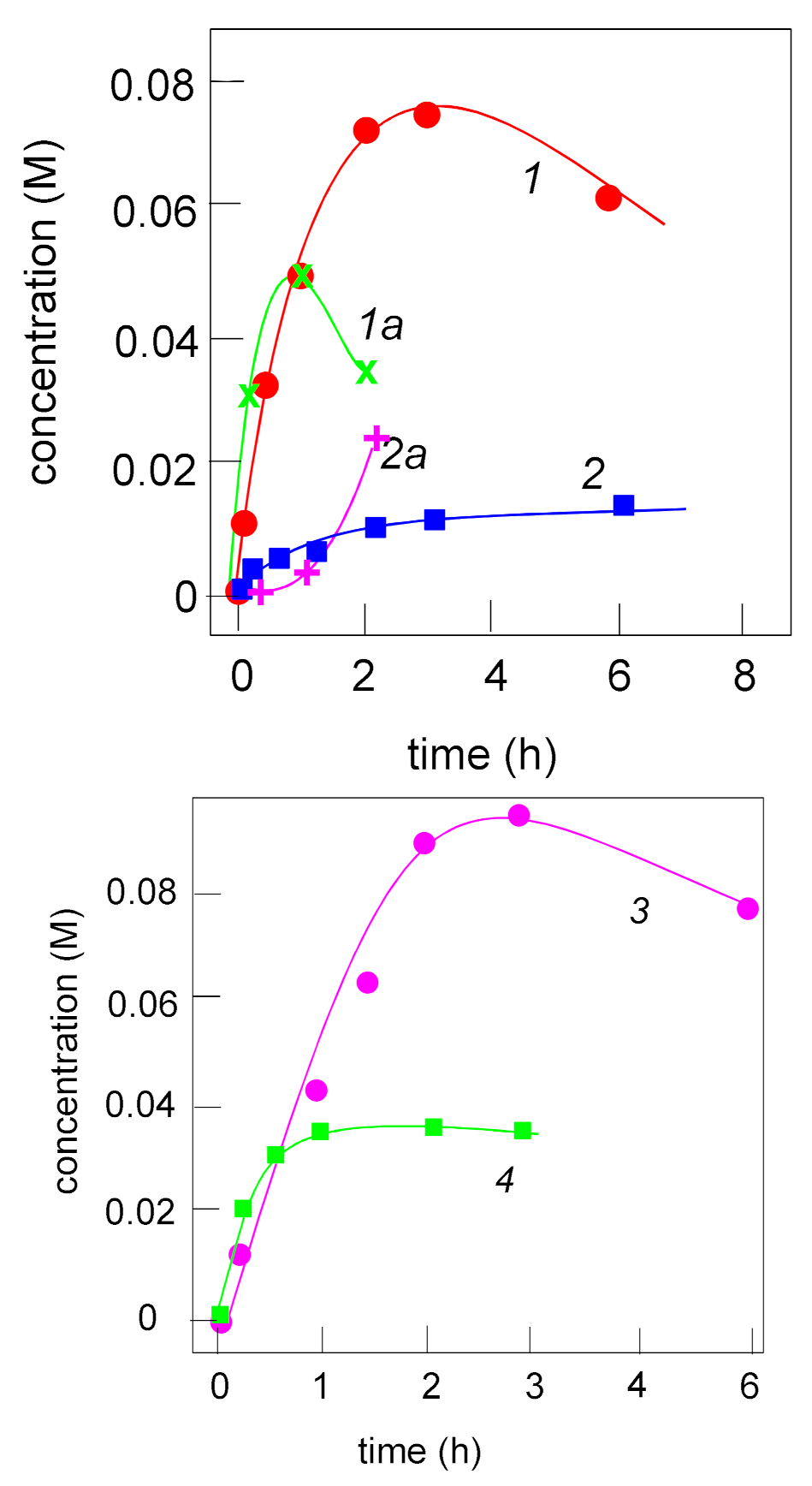
2.2. Catalyzed Oxidation of Cyclohexane
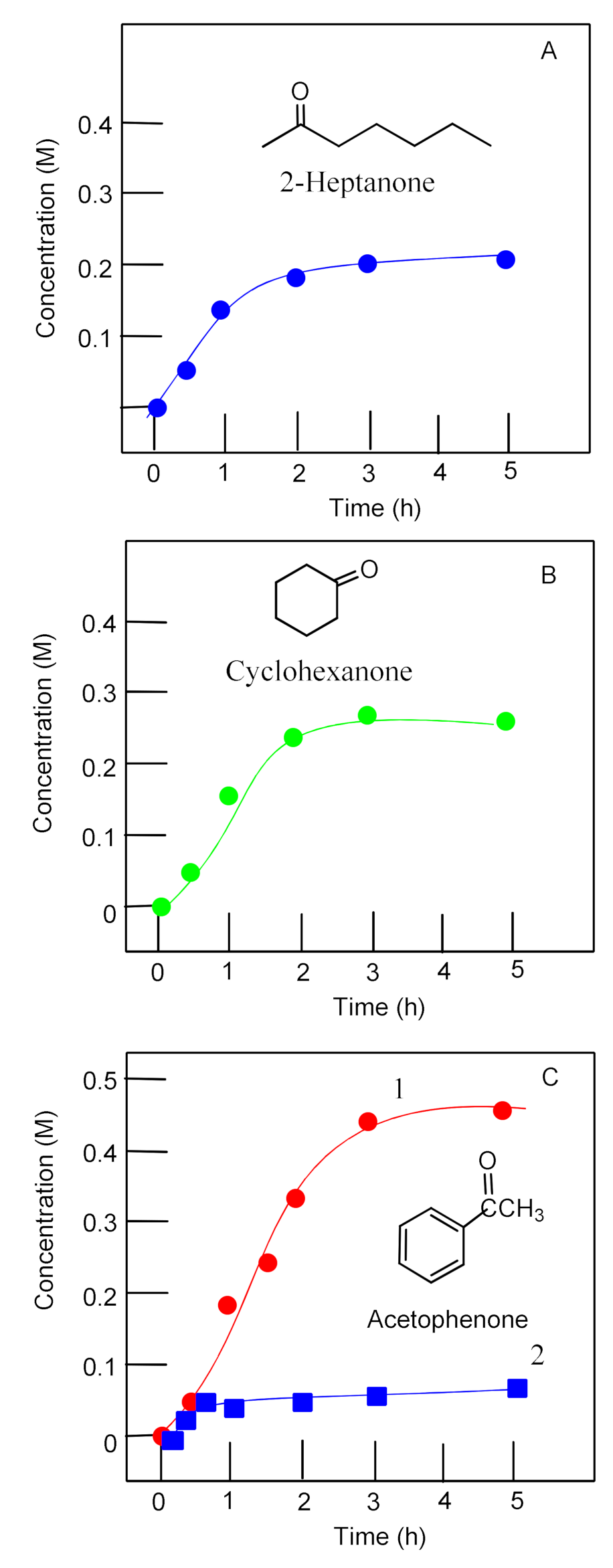
2.3. Catalyzed Oxidation of Alcohols
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kickelbick, G. Silsesquioxanes. In Functional Molecular Silicon Compounds I; Scheschkewitz, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–28. [Google Scholar] [CrossRef]
- Loman-Cortes, P.; Huq, T.B.; Vivero-Escoto, J.L. Use of Polyhedral Oligomeric Silsesquioxane (POSS) in Drug Delivery, Photodynamic Therapy and Bioimaging. Molecules 2021, 26, 6453. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.G.; Kuo, S.-W. Progress in the self-assembly of organic/inorganic polyhedral oligomeric silsesquioxane (POSS) hybrids. Soft Matter 2022, 18, 5535–5561. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Zhao, S.; Gan, X.; Sun, A.; Xia, Y.; Liu, Y. Design of Fluorescent Hybrid Materials Based on POSS for Sensing Applications. Molecules 2022, 27, 3137. [Google Scholar] [CrossRef]
- Nowacka, M.; Kowalewska, A. Self-Healing Silsesquioxane-Based Materials. Polymers 2022, 14, 1869. [Google Scholar] [CrossRef]
- Feher, F.J.; Newman, D.A.; Walzer, J.F. Silsesquioxanes as models for silica surfaces. J. Am. Chem. Soc. 1989, 111, 1741–1748. [Google Scholar] [CrossRef]
- Quadrelli, E.A.; Basset, J.-M. On silsesquioxanes’ accuracy as molecular models for silica-grafted complexes in heterogeneous catalysis. Coord. Chem. Rev. 2010, 254, 707–728. [Google Scholar] [CrossRef]
- Zhou, H.; Chua, M.H.; Xu, J. Functionalized POSS-Based Hybrid Composites. In Polymer Composites with Functionalized Nanoparticle, Synthesis, Properties, and Applications; Pielichowski, K., Majka, T.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 179–210. [Google Scholar] [CrossRef]
- Dong, F.; Lu, L.; Ha, C. Silsesquioxane-Containing Hybrid Nanomaterials: Fascinating Platforms for Advanced Applications. Macromol. Chem. Phys. 2019, 220, 1800324. [Google Scholar] [CrossRef]
- Du, Y.; Liu, H. Cage-like silsesquioxanes-based hybrid materials. Dalton Trans. 2020, 49, 5396–5405. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Fan, H.; Dubois, P.; Zhang, X.; Fahad, S.; Aziz, T.; Wan, J. Nano-engineering and micromolecular science of polysilsesquioxane materials and their emerging applications. J. Mater. Chem. A 2019, 7, 21577–21604. [Google Scholar] [CrossRef]
- Feher, F.J.; Budzichowski, T.A. Silasesquioxanes as ligands in inorganic and organometallic chemistry. Polyhedron 1995, 14, 3239–3253. [Google Scholar] [CrossRef]
- Lee, D.W.; Kawakami, Y. Incompletely Condensed Silsesquioxanes: Formation and Reactivity. Polym. J. 2007, 39, 230–238. [Google Scholar] [CrossRef]
- Murugavel, R.; Voigt, A.; Walawalkar, M.G.; Roesky, H.W. Hetero- and Metallasiloxanes Derived from Silanediols, Disilanols, Silanetriols, and Trisilanols. Chem. Rev. 1996, 96, 2205–2236. [Google Scholar] [CrossRef]
- Lorenz, V.; Fischer, A.; Gießmann, S.; Gilje, J.W.; Gun’Ko, Y.; Jacob, K.; Edelmann, F.T. Disiloxanediolates and polyhedral metallasilsesquioxanes of the early transition metals and f-elements. Coord. Chem. Rev. 2000, 206–207, 321–368. [Google Scholar] [CrossRef]
- Hanssen, R.W.J.M.; van Santen, R.A.; Abbenhuis, H.C.L. The Dynamic Status Quo of Polyhedral Silsesquioxane Coordination Chemistry. Eur. J. Inorg. Chem. 2004, 2004, 675–683. [Google Scholar] [CrossRef]
- Roesky, H.W.; Anantharaman, G.; Chandrasekhar, V.; Jancik, V.; Singh, S. Control of Molecular Topology and Metal Nuclearity in Multimetallic Assemblies: Designer Metallosiloxanes Derived from Silanetriols. Chem.–A Eur. J. 2004, 10, 4106–4114. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, V.; Edelmann, F.T. Metallasilsesquioxanes. Adv. Organomet. Chem. 2005, 53, 101–153. [Google Scholar]
- Levitsky, M.M.; Zavin, B.G.; Bilyachenko, A. Chemistry of metallasiloxanes. Current trends and new concepts. Russ. Chem. Rev. 2007, 76, 847–866. [Google Scholar] [CrossRef]
- Edelmann, F.T. Metallasilsesquioxanes–Synthetic and Structural Studies. In Silicon Chemistry: From the Atom to Extended Systems; Jutzi, P., Schubert, U., Eds.; Wiley: Darmstadt, Germany, 2003; pp. 383–394. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Bilyachenko, A.N. Modern concepts and methods in the chemistry of polyhedral metallasiloxanes. Coord. Chem. Rev. 2016, 306, 235–269. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, Z.; Han, J. Current Chemistry of Cyclic Oligomeric Silsesquioxanes. Curr. Org. Chem. 2018, 21, 2814–2828. [Google Scholar] [CrossRef]
- Ouyang, J.; Haotian, S.; Liang, Y.; Commisso, A.; Li, D.; Xu, R.; Yu, D. Recent Progress in Metal-containing Silsesquioxanes: Preparation and Application. Curr. Org. Chem. 2018, 21, 2829–2848. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Zubavichus, Y.V.; Korlyukov, A.A.; Khrustalev, V.N.; Shubina, E.S.; Bilyachenko, A.N. Silicon and Germanium-Based Sesquioxanes as Versatile Building Blocks for Cage Metallacomplexes. A Review. J. Clust. Sci. 2019, 30, 1283–1316. [Google Scholar] [CrossRef]
- Wu, H.; Zeng, B.; Chen, J.; Wu, T.; Li, Y.; Liu, Y.; Dai, L. An intramolecular hybrid of metal polyhedral oligomeric silsesquioxanes with special titanium-embedded cage structure and flame retardant functionality. Chem. Eng. J. 2019, 374, 1304–1316. [Google Scholar] [CrossRef]
- Ye, X.; Li, J.; Zhang, W.; Yang, R.; Li, J. Fabrication of eco-friendly and multifunctional sodium-containing polyhedral oligomeric silsesquioxane and its flame retardancy on epoxy resin. Compos. Part B Eng. 2020, 191, 107961. [Google Scholar] [CrossRef]
- Zeng, B.; Hu, R.; Zhou, R.; Shen, H.; Liu, X.; Chen, G.; Xu, Y.; Yuan, C.; Luo, W.; Dai, L. Co-flame retarding effect of ethanolamine modified titanium-containing polyhedral oligomeric silsesquioxanes in epoxy resin. Appl. Organomet. Chem. 2020, 34, e5266. [Google Scholar] [CrossRef]
- Ye, X.; Li, J.; Zhang, W.; Pan, Y.-T.; Yang, R.; Li, J. Enhanced fire safety and mechanical properties of epoxy resin composites based on submicrometer-sized rod-structured methyl macrocyclic silsesquioxane sodium salt. Chem. Eng. J. 2021, 425, 130566. [Google Scholar] [CrossRef]
- Zeng, B.; He, K.; Wu, H.; Ye, J.; Zheng, X.; Luo, W.; Xu, Y.; Yuan, C.; Dai, L. Zirconium-Embedded Polyhedral Oligomeric Silsesquioxane Containing Phosphaphenanthrene-Substituent Group Used as Flame Retardants for Epoxy Resin Composites. Macromol. Mater. Eng. 2021, 306, 2100012. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, X.; Jiang, Y.; Qiao, L.; Zhang, W.; Pan, Y.-T.; Yang, R.; Li, J.; Li, Y. Controllable dimensions and regular geometric architectures from self-assembly of lithium-containing polyhedral oligomeric silsesquioxane: Build for enhancing the fire safety of epoxy resin. Compos. Part B Eng. 2021, 229, 109483. [Google Scholar] [CrossRef]
- Lin, X.; Dong, Y.; Chen, X.; Liu, H.; Liu, Z.; Xing, T.; Li, A.; Song, H. Metallasilsesquioxane-derived ultrathin porous carbon nanosheet 3D architectures via an “in situ dual templating” strategy for ultrafast sodium storage. J. Mater. Chem. A 2021, 9, 6423–6431. [Google Scholar] [CrossRef]
- Lin, X.; Dong, Y.; Liu, X.; Chen, X.; Li, A.; Song, H. In-situ pre-lithiated onion-like SiOC/C anode materials based on metallasilsesquioxanes for Li-ion batteries. Chem. Eng. J. 2021, 428, 132125. [Google Scholar] [CrossRef]
- Loganathan, P.; Pillai, R.S.; Jeevananthan, V.; David, E.; Palanisami, N.; Bhuvanesh, N.S.P.; Shanmugan, S. Assembly of discrete and oligomeric structures of organotin double-decker silsesquioxanes: Inherent stability studies. New J. Chem. 2021, 45, 20144–20154. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Bilyachenko, A.N.; Shubina, E.S.; Long, J.; Guari, Y.; Larionova, J. Magnetic cage-like metallasilsesquioxanes. Coord. Chem. Rev. 2019, 398, 213015. [Google Scholar] [CrossRef]
- Sheng, K.; Wang, R.; Bilyachenko, A.; Khrustalev, V.; Jagodič, M.; Jagličić, Z.; Li, Z.; Wang, L.; Tung, C.; Sun, D. Tridecanuclear Gd(III)-silsesquioxane: Synthesis, structure, and magnetic property. ChemPhysMater 2022. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Yalymov, A.I.; Korlyukov, A.A.; Long, J.; Larionova, J.; Guari, Y.; Zubavichus, Y.V.; Trigub, A.L.; Shubina, E.S.; Eremenko, I.L.; et al. Heterometallic Na6 Co3 Phenylsilsesquioxane Exhibiting Slow Dynamic Behavior in its Magnetization. Chem.–A Eur. J. 2015, 21, 18563–18565. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Hou, J.-L.; Wang, Z.; Gupta, R.K.; Jagličić, Z.; Jagodič, M.; Wang, W.-G.; Tung, C.-H.; Sun, D. An Octanuclear Cobalt Cluster Protected by Macrocyclic Ligand: In Situ Ligand-Transformation-Assisted Assembly and Single-Molecule Magnet Behavior. Inorg. Chem. 2020, 59, 5683–5693. [Google Scholar] [CrossRef]
- Marchesi, S.; Carniato, F.; Boccaleri, E. Synthesis and characterisation of a novel europium(iii)-containing heptaisobutyl-POSS. New J. Chem. 2014, 38, 2480–2485. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Bilyachenko, A.N.; Levitsky, M.M.; Khrustalev, V.N.; Shubina, E.S.; Felix, G.; Mamontova, E.; Long, J.; Guari, Y.; Larionova, J. New Luminescent Tetranuclear Lanthanide-Based Silsesquioxane Cage-Like Architectures. Chem.–A Eur. J. 2020, 26, 16594–16598. [Google Scholar] [CrossRef]
- Sheng, K.; Liu, Y.-N.; Gupta, R.K.; Kurmoo, M.; Sun, D. Arylazopyrazole-functionalized photoswitchable octanuclear Zn(II)-silsesquioxane nanocage. Sci. China Ser. B Chem. 2020, 64, 419–425. [Google Scholar] [CrossRef]
- Sheng, K.; Wang, R.; Tang, X.; Jagodič, M.; Jagličić, Z.; Pang, L.; Dou, J.-M.; Gao, Z.-Y.; Feng, H.-Y.; Tung, C.-H.; et al. A Carbonate-Templated Decanuclear Mn Nanocage with Two Different Silsesquioxane Ligands. Inorg. Chem. 2021, 60, 14866–14871. [Google Scholar] [CrossRef] [PubMed]
- Kulakova, A.N.; Nigoghossian, K.; Félix, G.; Khrustalev, V.N.; Shubina, E.S.; Long, J.; Guari, Y.; Carlos, L.D.; Bilyachenko, A.N.; Larionova, J. New Magnetic and Luminescent Dy(III) and Dy(III)/Y(III) Based Tetranuclear Silsesquioxane Cages. Eur. J. Inorg. Chem. 2021, 2021, 2696–2701. [Google Scholar] [CrossRef]
- Nigoghossian, K.; Kulakova, A.N.; Félix, G.; Khrustalev, V.N.; Shubina, E.S.; Long, J.; Guari, Y.; Sene, S.; Carlos, L.D.; Bilyachenko, A.N.; et al. Temperature sensing in Tb3+/Eu3+-based tetranuclear silsesquioxane cages with tunable emission. RSC Adv. 2021, 11, 34735–34741. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, S.; Bisio, C.; Carniato, F. Synthesis of Novel Luminescent Double-Decker Silsesquioxanes Based on Partially Condensed TetraSilanolPhenyl POSS and Tb3+/Eu3+ Lanthanide Ions. Processes 2022, 10, 758. [Google Scholar] [CrossRef]
- Abbenhuis, H.C.L. Advances in Homogeneous and Heterogeneous Catalysis with Metal-Containing Silsesquioxanes. Chem. Eur. J. 2000, 6, 25–32. [Google Scholar] [CrossRef]
- Levitskii, M.M.; Smirnov, V.V.; Zavin, B.G.; Bilyachenko, A.N.; Rabkina, A.Y. Metalasiloxanes: New structure formation methods and catalytic properties. Kinet. Catal. 2009, 50, 490–507. [Google Scholar] [CrossRef]
- Ward, A.J.; Masters, A.F.; Maschmeyer, T. Metallasilsesquioxanes: Molecular Analogues of Heterogeneous Catalysts. In Applications of Polyhedral Oligomeric Silsesquioxanes; Springer: New York, NY, USA, 2011; Volume 3, pp. 135–166. [Google Scholar]
- Levitsky, M.M.; Yalymov, A.I.; Kulakova, A.N.; Petrov, A.A.; Bilyachenko, A.N. Cage-like metallasilsesquioxanes in catalysis: A review. J. Mol. Catal. A Chem. 2017, 426, 297–304. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Bilyachenko, A.N.; Shul’Pin, G.B. Oxidation of C-H compounds with peroxides catalyzed by polynuclear transition metal complexes in Si- or Ge-sesquioxane frameworks: A review. J. Organomet. Chem. 2017, 849–850, 201–218. [Google Scholar] [CrossRef]
- Astakhov, G.; Levitsky, M.; Bantreil, X.; Lamaty, F.; Khrustalev, V.; Zubavichus, Y.; Dorovatovskii, P.; Shubina, E.; Bilyachenko, A. Cu(II)-silsesquioxanes as efficient precatalysts for Chan-Evans-Lam coupling. J. Organomet. Chem. 2019, 906, 121022. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Su, H.-F.; Li, Y.-W.; Liu, Q.-Y.; Jagličić, Z.; Wang, W.-G.; Tung, C.-H.; Sun, D. Space Craft-like Octanuclear Co(II)-Silsesquioxane Nanocages: Synthesis, Structure, Magnetic Properties, Solution Behavior, and Catalytic Activity for Hydroboration of Ketones. Inorg. Chem. 2019, 58, 4574–4582. [Google Scholar] [CrossRef]
- Sheng, K.; Si, W.-D.; Wang, R.; Wang, W.-Z.; Dou, J.; Gao, Z.-Y.; Wang, L.-K.; Tung, C.-H.; Sun, D. Keggin-Type Tridecanuclear Europium-Oxo Nanocluster Protected by Silsesquioxanes. Chem. Mater. 2022, 34, 4186–4194. [Google Scholar] [CrossRef]
- Garg, S.; Unruh, D.K.; Krempner, C. Zirconium and hafnium polyhedral oligosilsesquioxane complexes–green homogeneous catalysts in the formation of bio-derived ethers via a MPV/etherification reaction cascade. Catal. Sci. Technol. 2020, 11, 211–218. [Google Scholar] [CrossRef]
- Astakhov, G.S.; Khrustalev, V.N.; Dronova, M.S.; Gutsul, E.I.; Korlyukov, A.A.; Gelman, D.; Zubavichus, Y.V.; Novichkov, D.A.; Trigub, A.L.; Shubina, E.S.; et al. Cage-like manganesesilsesquioxanes: Features of their synthesis, unique structure, and catalytic activity in oxidative amidations. Inorg. Chem. Front. 2022. [Google Scholar] [CrossRef]
- Shilov, A.E.; Shul’Pin, G.B. Activation of C−H Bonds by Metal Complexes. Chem. Rev. 1997, 97, 2879–2932. [Google Scholar] [CrossRef] [PubMed]
- Shul’Pin, G.B. New Trends in Oxidative Functionalization of Carbon–Hydrogen Bonds: A Review. Catalysts 2016, 6, 50. [Google Scholar] [CrossRef]
- Shul’Pin, G.B.; Kozlov, Y.N.; Shul’Pina, L.S. Metal Complexes Containing Redox-Active Ligands in Oxidation of Hydrocarbons and Alcohols: A Review. Catalysts 2019, 9, 1046. [Google Scholar] [CrossRef]
- Wójtowicz-Młochowska, H. Synthetic utility of metal catalyzed hydrogen peroxide oxidation of C-H, C-C and C=C bonds in alkanes, arenes and alkenes: Recent advances. Arkivoc 2016, 2017, 12–58. [Google Scholar] [CrossRef]
- Punniyamurthy, T.; Rout, L. Recent advances in copper-catalyzed oxidation of organic compounds. Coord. Chem. Rev. 2008, 252, 134–154. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Kirillova, M.V.; Pombeiro, A.J. Multicopper complexes and coordination polymers for mild oxidative functionalization of alkanes. Coord. Chem. Rev. 2012, 256, 2741–2759. [Google Scholar] [CrossRef]
- Nandi, M.; Roy, P. Peroxidative oxidation of cycloalkane by di-, tetra- and polynuclear copper(II) complexes. Indian J. Chem. 2013, 52, 1263–1268. [Google Scholar]
- Conde, A.; Vilella, L.; Balcells, D.; Díaz-Requejo, M.M.; Lledós, A.; Pérez, P.J. Introducing Copper as Catalyst for Oxidative Alkane Dehydrogenation. J. Am. Chem. Soc. 2013, 135, 3887–3896. [Google Scholar] [CrossRef]
- Ünver, H.; Kani, I. Homogeneous oxidation of alcohols in water catalyzed with Cu(II)-triphenyl acetate/bipyridyl complex. Polyhedron 2017, 134, 257–262. [Google Scholar] [CrossRef]
- Kirillova, M.V.; de Paiva, P.T.; Carvalho, W.A.; Mandelli, D.; Kirillov, A.M. Mixed-ligand aminoalcohol-dicarboxylate copper(II) coordination polymers as catalysts for the oxidative functionalization of cyclic alkanes and alkenes. Pure Appl. Chem. 2016, 89, 61–73. [Google Scholar] [CrossRef]
- Armakola, E.; Colodrero, R.M.P.; Bazaga-García, M.; Salcedo, I.R.; Choquesillo-Lazarte, D.; Cabeza, A.; Kirillova, M.V.; Kirillov, A.M.; Demadis, K.D. Three-Component Copper-Phosphonate-Auxiliary Ligand Systems: Proton Conductors and Efficient Catalysts in Mild Oxidative Functionalization of Cycloalkanes. Inorg. Chem. 2018, 57, 10656–10666. [Google Scholar] [CrossRef] [PubMed]
- Choroba, K.; Machura, B.; Kula, S.; Raposo, L.R.; Fernandes, A.R.; Kruszynski, R.; Erfurt, K.; Shul’Pina, L.S.; Kozlov, Y.N.; Shul’Pin, G.B. Copper(ii) complexes with 2,2′:6′,2′′-terpyridine, 2,6-di(thiazol-2-yl)pyridine and 2,6-di(pyrazin-2-yl)pyridine substituted with quinolines. Synthesis, structure, antiproliferative activity, and catalytic activity in the oxidation of alkanes and alcohols with peroxides. Dalton Trans. 2019, 48, 12656–12673. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.F.M.; Kirillova, M.V.; André, V.; Fernandes, T.A.; Kirillov, A.M. Tetracopper(II) Cores Driven by an Unexplored Trifunctional Aminoalcohol Sulfonic Acid for Mild Catalytic C–H Functionalization of Alkanes. Catalysts 2019, 9, 321. [Google Scholar] [CrossRef]
- Shul’Pina, L.S.; Vinogradov, M.M.; Kozlov, Y.N.; Nelyubina, Y.V.; Ikonnikov, N.S.; Shul’Pin, G.B. Copper complexes with 1,10-phenanthrolines as efficient catalysts for oxidation of alkanes by hydrogen peroxide. Inorg. Chim. Acta 2020, 512, 119889. [Google Scholar] [CrossRef]
- Shul’Pin, G.B.; Shul’Pina, L.S. Oxidation of Organic Compounds with Peroxides Catalyzed by Polynuclear Metal Compounds. Catalysts 2021, 11, 186. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Levitsky, M.M.; Khrustalev, V.N.; Zubavichus, Y.V.; Shul’Pina, L.S.; Shubina, E.S.; Shul’Pin, G.B. Mild and Regioselective Hydroxylation of Methyl Group in Neocuproine: Approach to an N,O-Ligated Cu6 Cage Phenylsilsesquioxane. Organometallics 2018, 37, 168–171. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Korlyukov, A.A.; Zubavichus, Y.V.; Khrustalev, V.N.; Bantreil, X.; Shul’Pina, L.S.; Levitsky, M.M.; Ikonnikov, N.S.; Shubina, E.S.; Lamaty, F.; et al. Hexacoppergermsesquioxanes as complexes with N-ligands: Synthesis, structure and catalytic properties. J. Organomet. Chem. 2019, 884, 17–28. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Bilyachenko, A.N.; Levitsky, M.M.; Khrustalev, V.N.; Korlyukov, A.A.; Zubavichus, Y.V.; Dorovatovskii, P.V.; Lamaty, F.; Bantreil, X.; Villemejeanne, B.; et al. Si10Cu6N4 Cage Hexacoppersilsesquioxanes Containing N Ligands: Synthesis, Structure, and High Catalytic Activity in Peroxide Oxidations. Inorg. Chem. 2017, 56, 15026–15040. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Khrustalev, V.N.; Zubavichus, Y.V.; Shul’Pina, L.S.; Kulakova, A.N.; Bantreil, X.; Lamaty, F.; Levitsky, M.M.; Gutsul, E.I.; Shubina, E.S.; et al. Heptanuclear Fe5Cu2-Phenylgermsesquioxane containing 2,2′-Bipyridine: Synthesis, Structure, and Catalytic Activity in Oxidation of C–H Compounds. Inorg. Chem. 2017, 57, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Astakhov, G.S.; Levitsky, M.M.; Zubavichus, Y.V.; Khrustalev, V.N.; Titov, A.A.; Dorovatovskii, P.V.; Smol’Yakov, A.F.; Shubina, E.S.; Kirillova, M.V.; Kirillov, A.M.; et al. Cu6- and Cu8-Cage Sil- and Germsesquioxanes: Synthetic and Structural Features, Oxidative Rearrangements, and Catalytic Activity. Inorg. Chem. 2021, 60, 8062–8074. [Google Scholar] [CrossRef]
- Astakhov, G.S.; Bilyachenko, A.N.; Levitsky, M.M.; Korlyukov, A.A.; Zubavichus, Y.V.; Dorovatovskii, P.V.; Khrustalev, V.N.; Vologzhanina, A.V.; Shubina, E.S. Tridecanuclear CuII11Na2 Cagelike Silsesquioxanes. Cryst. Growth Des. 2018, 18, 5377–5384. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Bilyachenko, A.N.; Korlyukov, A.A.; Shul’Pina, L.S.; Bantreil, X.; Lamaty, F.; Shubina, E.S.; Levitsky, M.M.; Ikonnikov, N.S.; Shul’Pin, G.B. A new “bicycle helmet”-like copper(ii),sodiumphenylsilsesquioxane. Synthesis, structure and catalytic activity. Dalton Trans. 2018, 47, 15666–15669. [Google Scholar] [CrossRef]
- Astakhov, G.S.; Levitsky, M.M.; Korlyukov, A.A.; Shul’Pina, L.S.; Shubina, E.S.; Ikonnikov, N.S.; Vologzhanina, A.V.; Bilyachenko, A.N.; Dorovatovskii, P.V.; Kozlov, Y.N.; et al. New Cu4Na4- and Cu5-Based Phenylsilsesquioxanes. Synthesis via Complexation with 1,10-Phenanthroline, Structures and High Catalytic Activity in Alkane Oxidations with Peroxides in Acetonitrile. Catalysts 2019, 9, 701. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Khrustalev, V.N.; Zubavichus, Y.V.; Shul’Pina, L.S.; Shubina, E.S.; Levitsky, M.M.; Ikonnikov, N.S.; Bilyachenko, A.N.; Kozlov, Y.N.; Shul’Pin, G.B. Palanquin-Like Cu4Na4 Silsesquioxane Synthesis (via Oxidation of 1,1-bis(Diphenylphosphino)methane), Structure and Catalytic Activity in Alkane or Alcohol Oxidation with Peroxides. Catalysts 2019, 9, 154. [Google Scholar] [CrossRef]
- Astakhov, G.S.; Bilyachenko, A.N.; Levitsky, M.M.; Shul’Pina, L.S.; Korlyukov, A.A.; Zubavichus, Y.V.; Khrustalev, V.N.; Vologzhanina, A.V.; Shubina, E.S.; Dorovatovskii, P.V.; et al. Coordination Affinity of Cu(II)-Based Silsesquioxanes toward N,N-Ligands and Associated Skeletal Rearrangements: Cage and Ionic Products Exhibiting a High Catalytic Activity in Oxidation Reactions. Inorg. Chem. 2020, 59, 4536–4545. [Google Scholar] [CrossRef]
- Anisimov, A.A.; Vysochinskaya, Y.S.; Kononevich, Y.N.; Dolgushin, F.M.; Muzafarov, A.M.; Shchegolikhina, O.I. Polyhedral phenylnickelsodiumsiloxanolate transformation in the presence of aromatic nitrogen-containing ligands. Inorg. Chim. Acta 2020, 517, 120160. [Google Scholar] [CrossRef]
- Sergienko, N.V.; Trankina, E.S.; Polshchikova, N.V.; Korlyukov, A.A. Metallosiloxanes with Acetylacetonate Ligands. Russ. J. Coord. Chem. 2021, 47, 382–392. [Google Scholar] [CrossRef]
- Singh, D.; Nishal, V.; Bhagwan, S.; Saini, R.K.; Singh, I. Electroluminescent materials: Metal complexes of 8-hydroxyquinoline—A review. Mater. Des. 2018, 156, 215–228. [Google Scholar] [CrossRef]
- Prachayasittikul, V.; Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S. 8-Hydroxyquinolines: A review of their metal chelating properties and medicinal applications. Drug Des. Dev. Ther. 2013, 7, 1157–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savić-Gajić, I.M.; Savic, I. Drug design strategies with metal-hydroxyquinoline complexes. Expert Opin. Drug Discov. 2019, 15, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.; Fiege, M.; Osetska, O. 8-Hydroxyquinolines in metallosupramolecular chemistry. Coord. Chem. Rev. 2008, 252, 812–824. [Google Scholar] [CrossRef]
- Lipunova, G.N.; Nosova, E.V.; Charushin, V.N.; Chupakhin, O. Structural, Optical Properties, and Biological Activity of Complexes Based on Derivatives of Quinoline, Quinoxaline, and Quinazoline with Metal Centers from Across the Periodic Table. Comments Inorg. Chem. 2014, 34, 142–177. [Google Scholar] [CrossRef]
- Prigyai, N.; Chanmungkalakul, S.; Ervithayasuporn, V.; Yodsin, N.; Jungsuttiwong, S.; Takeda, N.; Unno, M.; Boonmak, J.; Kiatkamjornwong, S. Lithium-Templated Formation of Polyhedral Oligomeric Silsesquioxanes (POSS). Inorg. Chem. 2019, 58, 15110–15117. [Google Scholar] [CrossRef] [PubMed]
- Annand, J.; Aspinall, H.C.; Steiner, A. Novel Heterometallic Lanthanide Silsesquioxane. Inorg. Chem. 1999, 38, 3941–3943. [Google Scholar] [CrossRef]
- Lorenz, V.; Gießmann, S.; Gun’Ko, Y.K.; Fischer, A.K.; Gilje, J.W.; Edelmann, F.T. Fully Metalated Silsesquioxanes: Building Blocks for the Construction of Catalyst Models. Angew. Chem. Int. Ed. 2004, 43, 4603–4606. [Google Scholar] [CrossRef]
- García, C.; Gómez, M.; Gómez-Sal, P.; Hernández, J.M. Monocyclopentadienyl(niobium) Compounds with Imido and Silsesquioxane Ligands: Synthetic, Structural and Reactivity Studies. Eur. J. Inorg. Chem. 2009, 2009, 4401–4415. [Google Scholar] [CrossRef]
- Gau, M.R.; Zdilla, M.J. Multinuclear Clusters of Manganese and Lithium with Silsesquioxane-Derived Ligands: Synthesis and Ligand Rearrangement by Dioxygen- and Base-Mediated Si–O Bond Cleavage. Inorg. Chem. 2021, 60, 2866–2871. [Google Scholar] [CrossRef] [PubMed]
- Sergienko, N.V.; Korlyukov, A.A.; Myakushev, V.D.; Antipin, M.Y.; Zavin, B.G. Ion exchange in bimetallic cage copper organosiloxanes and the synthesis of polynuclear metal complexes containing Li and CuII atoms. Bull. Acad. Sci. USSR Div. Chem. Sci. 2009, 58, 2258–2265. [Google Scholar] [CrossRef]
- Giri, R.; Brusoe, A.; Troshin, K.; Wang, J.Y.; Font, M.; Hartwig, J.F. Mechanism of the Ullmann Biaryl Ether Synthesis Catalyzed by Complexes of Anionic Ligands: Evidence for the Reaction of Iodoarenes with Ligated Anionic CuI Intermediates. J. Am. Chem. Soc. 2017, 140, 793–806. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Gutsul, E.I.; Khrustalev, V.N.; Astakhov, G.S.; Zueva, A.Y.; Zubavichus, Y.V.; Kirillova, M.V.; Shul’pina, L.S.; Ikonnikov, N.S.; Dorovatovskii, P.V.; et al. Acetone factor in the design of Cu4, Cu6, and Cu9-based cage coppersilsesquioxanes: Synthesis, structural features, and catalytic functionalization of alkanes. Inorg. Chem. 2022, 61, 14800–14814. [Google Scholar] [CrossRef]
- Bilyachenko, A.; Dronova, M.S.; Korlyukov, A.A.; Levitsky, M.M.; Antipin, M.Y.; Zavin, B.G. Cage-like manganesephenylsiloxane with an unusual structure. Bull. Acad. Sci. USSR Div. Chem. Sci. 2011, 60, 1762–1765. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Astakhov, G.S.; Kulakova, A.N.; Korlyukov, A.A.; Zubavichus, Y.V.; Dorovatovskii, P.V.; Shul’Pina, L.S.; Shubina, E.S.; Ikonnikov, N.S.; Kirillova, M.V.; et al. Exploring Cagelike Silsesquioxane Building Blocks for the Design of Heterometallic Cu4/M4 Architectures. Cryst. Growth Des. 2022, 22, 2146–2157. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Korlyukov, A.A.; Vologzhanina, A.V.; Khrustalev, V.N.; Kulakova, A.N.; Long, J.; Larionova, J.; Guari, Y.; Dronova, M.S.; Tsareva, U.S.; et al. Tuning linkage isomerism and magnetic properties of bi- and tri-metallic cage silsesquioxanes by cation and solvent effects. Dalton Trans. 2017, 46, 12935–12949. [Google Scholar] [CrossRef] [PubMed]
- Bilyachenko, A.N.; Yalymov, A.; Dronova, M.; Korlyukov, A.A.; Vologzhanina, A.V.; Es’Kova, M.A.; Long, J.; Larionova, J.; Guari, Y.; Dorovatovskii, P.V.; et al. Family of Polynuclear Nickel Cagelike Phenylsilsesquioxanes; Features of Periodic Networks and Magnetic Properties. Inorg. Chem. 2017, 56, 12751–12763. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Kulakova, A.N.; Levitsky, M.M.; Korlyukov, A.A.; Khrustalev, V.N.; Vologzhanina, A.V.; Titov, A.A.; Dorovatovskii, P.V.; Shul’Pina, L.S.; Lamaty, F.; et al. Ionic Complexes of Tetra- and Nonanuclear Cage Copper(II) Phenylsilsesquioxanes: Synthesis and High Activity in Oxidative Catalysis. ChemCatChem 2017, 9, 4437–4447. [Google Scholar] [CrossRef]
- Dronova, M.S.; Bilyachenko, A.; Korlyukov, A.A.; Arkhipov, D.E.; Kirilin, A.D.; Shubina, E.; Babakhina, G.M.; Levitskii, M.M. A new type of supramolecular organization in the cage-like metallasiloxanes. Bull. Acad. Sci. USSR Div. Chem. Sci. 2013, 62, 1941–1943. [Google Scholar] [CrossRef]
- Dronova, M.S.; Bilyachenko, A.N.; Yalymov, A.I.; Kozlov, Y.N.; Shul’Pina, L.S.; Korlyukov, A.A.; Arkhipov, D.E.; Levitsky, M.M.; Shubina, E.S.; Shul’Pin, G.B. Solvent-controlled synthesis of tetranuclear cage-like copper(ii) silsesquioxanes. Remarkable features of the cage structures and their high catalytic activity in oxidation with peroxides. Dalton Trans. 2013, 43, 872–882. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Nesterov, D.S.; Shul’pina, L.S.; Pombeiro, A.J.L. A hydroperoxo-rebound mechanism of alkane oxidation with hydrogen peroxide catalyzed by binuclear manganese(IV) complex in the presence of an acid with involvement of atmospheric dioxygen. Part 14 from the series “Oxidations by the system hydrogen peroxide–[Mn2L2O3]2 + (L = 1,4,7-trimethyl-1,4,7-triazacyclononane)–carboxylic acid”. Inorg. Chim. Acta 2017, 455, 666–676. [Google Scholar] [CrossRef]
- Shilov, A.E.; Shul’pin, G.B. Activation and Catalytic Reactions of Saturated Hydrocarbons in the Presence of Metal Complexes; Kluwer Academic Publishers: New York, NY, USA; Boston, MA, USA; Dordrecht, The Netherlands; London, UK; Moscow, Russia, 2002. [Google Scholar]
- Nesterov, D.S.; Chygorin, E.N.; Kokozay, V.N.; Bon, V.V.; Boča, R.; Kozlov, Y.N.; Shul’Pina, L.S.; Jezierska, J.; Ozarowski, A.; Pombeiro, A.J.L.; et al. Heterometallic CoIII4FeIII2Schiff Base Complex: Structure, Electron Paramagnetic Resonance, and Alkane Oxidation Catalytic Activity. Inorg. Chem. 2012, 51, 9110–9122. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalysed hydrocarbon oxygenations in solutions: The dramatic role of additives: A review. J. Mol. Catal. A Chem. 2002, 189, 39–66. [Google Scholar] [CrossRef]
- Olivo, G.; Lanzalunga, O.; Di Stefano, S. Non-Heme Imine-Based Iron Complexes as Catalysts for Oxidative Processes (Review). Adv. Synth. Catal. 2016, 358, 843–863. [Google Scholar] [CrossRef]
- Czerwińska, K.; Machura, B.; Kula, S.; Krompiec, S.; Erfurt, K.; Roma-Rodrigues, C.; Fernandes, A.R.; Shul’pina, L.S.; Ikonnikov, N.S.; Shul’pin, G.B. Copper(II) complexes of functionalized 2,2′:6′,2″-terpyridines and 2,6-di(thiazol-2-yl)pyridine: Structure, spectroscopy, cyto-toxicity and catalytic activity. Dalton Trans. 2017, 46, 9591–9604. [Google Scholar] [CrossRef] [Green Version]
- Bruker. SAINT; Bruker AXS Inc.: Madison, WI, USA, 2013. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar]
- CrysAlisPro, Version 1.171.41.106a; Rigaku Oxford Diffraction: Tokyo, Japan, 2021.
- Battye, T.G.G.; Kontogiannis, L.; Johnson, O.; Powell, H.R.; Leslie, A.G.W. iMOSFLM: A new graphical interface for diffraction-image processing with MOSFLM. Acta Cryst. 2011, D67, 271–281. [Google Scholar]
- Evans, P.R. Scaling and assessment of data quality. Acta Cryst. 2006, D62, 72–82. [Google Scholar]
- Spek, A.L. PLATON, A Multipurpose Crystallographic Tool; Utrecht University: Utrecht, The Netherlands, 2015. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilyachenko, A.N.; Khrustalev, V.N.; Zueva, A.Y.; Titova, E.M.; Astakhov, G.S.; Zubavichus, Y.V.; Dorovatovskii, P.V.; Korlyukov, A.A.; Shul’pina, L.S.; Shubina, E.S.; et al. A Novel Family of Cage-like (CuLi, CuNa, CuK)-phenylsilsesquioxane Complexes with 8-Hydroxyquinoline Ligands: Synthesis, Structure, and Catalytic Activity. Molecules 2022, 27, 6205. https://doi.org/10.3390/molecules27196205
Bilyachenko AN, Khrustalev VN, Zueva AY, Titova EM, Astakhov GS, Zubavichus YV, Dorovatovskii PV, Korlyukov AA, Shul’pina LS, Shubina ES, et al. A Novel Family of Cage-like (CuLi, CuNa, CuK)-phenylsilsesquioxane Complexes with 8-Hydroxyquinoline Ligands: Synthesis, Structure, and Catalytic Activity. Molecules. 2022; 27(19):6205. https://doi.org/10.3390/molecules27196205
Chicago/Turabian StyleBilyachenko, Alexey N., Victor N. Khrustalev, Anna Y. Zueva, Ekaterina M. Titova, Grigorii S. Astakhov, Yan V. Zubavichus, Pavel V. Dorovatovskii, Alexander A. Korlyukov, Lidia S. Shul’pina, Elena S. Shubina, and et al. 2022. "A Novel Family of Cage-like (CuLi, CuNa, CuK)-phenylsilsesquioxane Complexes with 8-Hydroxyquinoline Ligands: Synthesis, Structure, and Catalytic Activity" Molecules 27, no. 19: 6205. https://doi.org/10.3390/molecules27196205
APA StyleBilyachenko, A. N., Khrustalev, V. N., Zueva, A. Y., Titova, E. M., Astakhov, G. S., Zubavichus, Y. V., Dorovatovskii, P. V., Korlyukov, A. A., Shul’pina, L. S., Shubina, E. S., Kozlov, Y. N., Ikonnikov, N. S., Gelman, D., & Shul’pin, G. B. (2022). A Novel Family of Cage-like (CuLi, CuNa, CuK)-phenylsilsesquioxane Complexes with 8-Hydroxyquinoline Ligands: Synthesis, Structure, and Catalytic Activity. Molecules, 27(19), 6205. https://doi.org/10.3390/molecules27196205








