Electric Cell-Substrate Impedance Sensing (ECIS) as a Convenient Tool to Assess the Potential of Low Molecular Fraction Derived from Medicinal Fungus Cerrena unicolor in Action on L929 and CT-26 Cell Lines
Abstract
:1. Introduction
2. Results
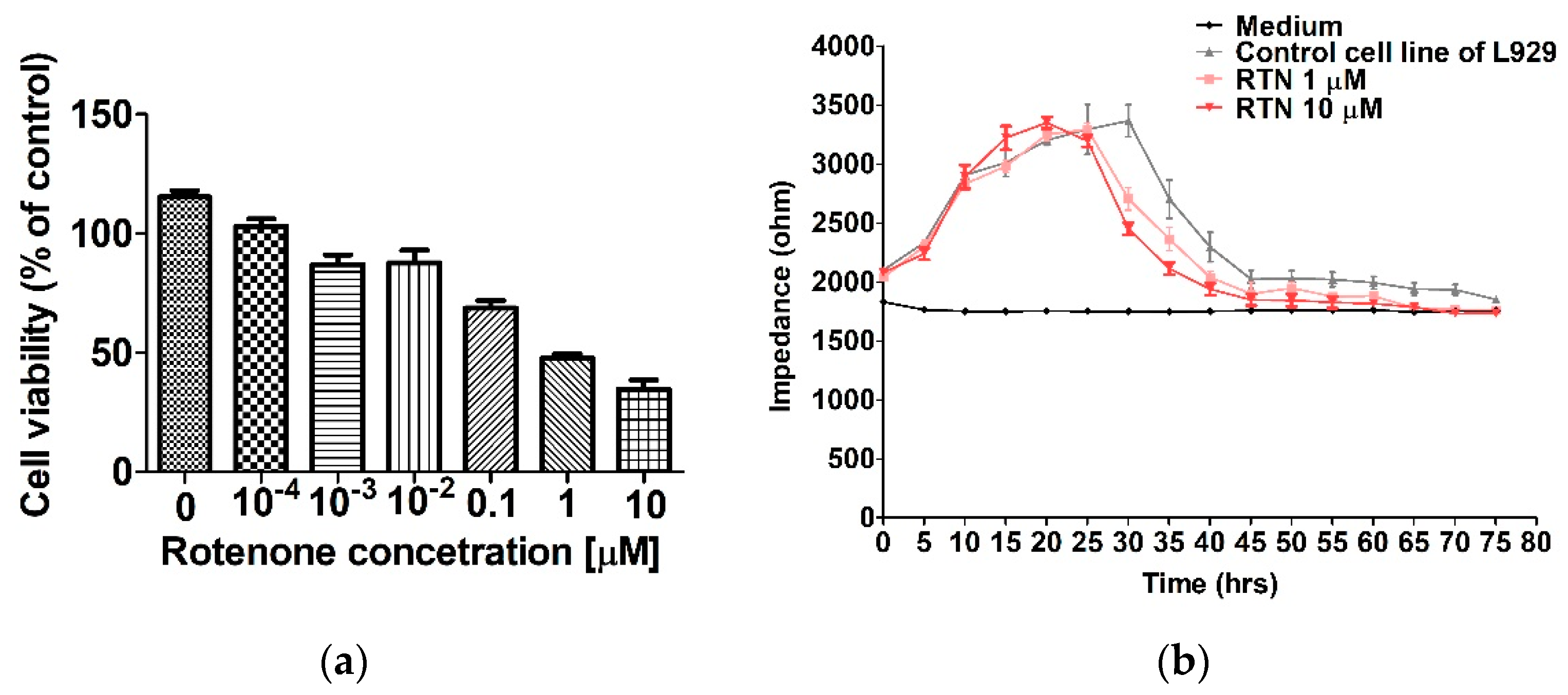
2.1. The MTT Results
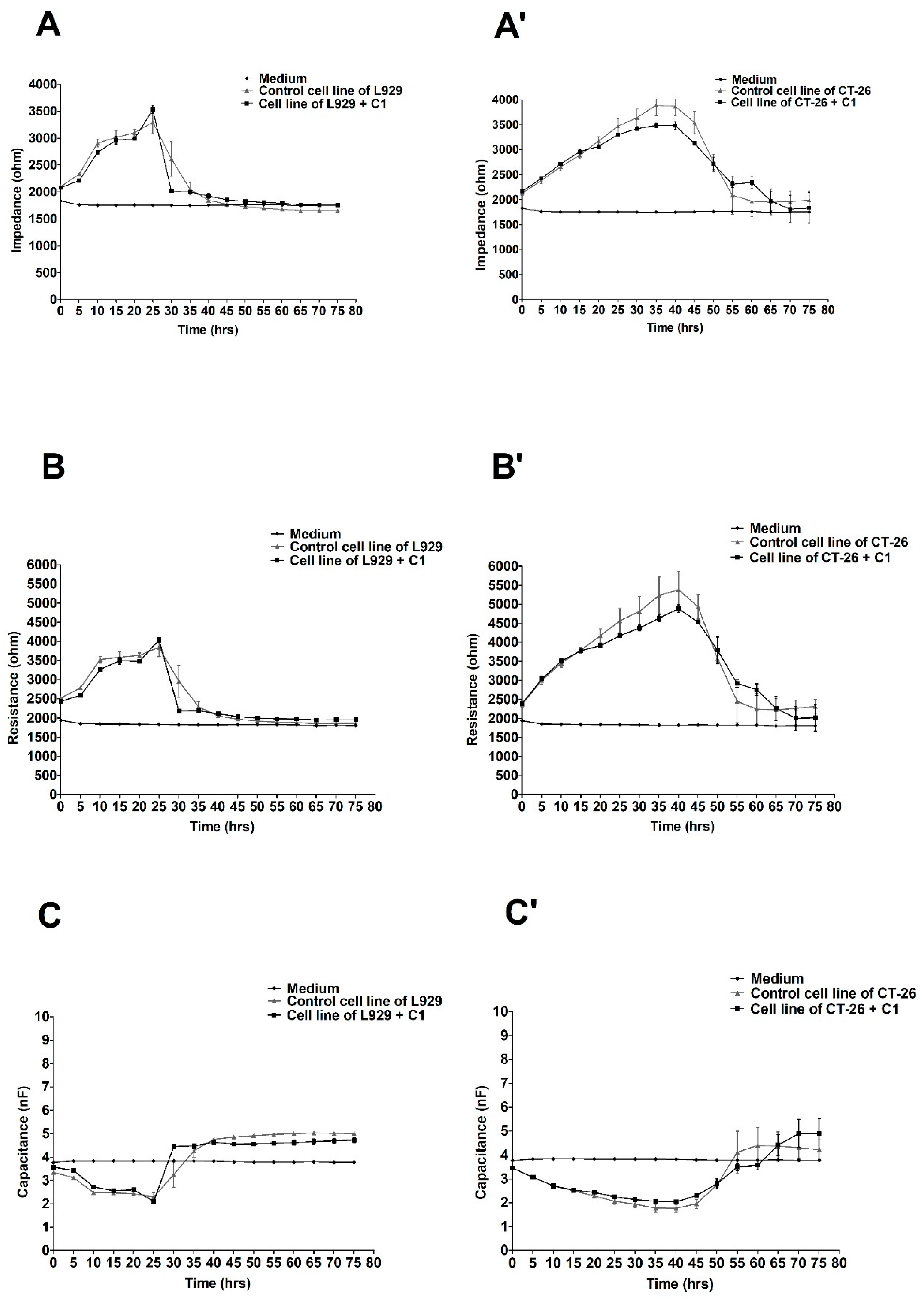
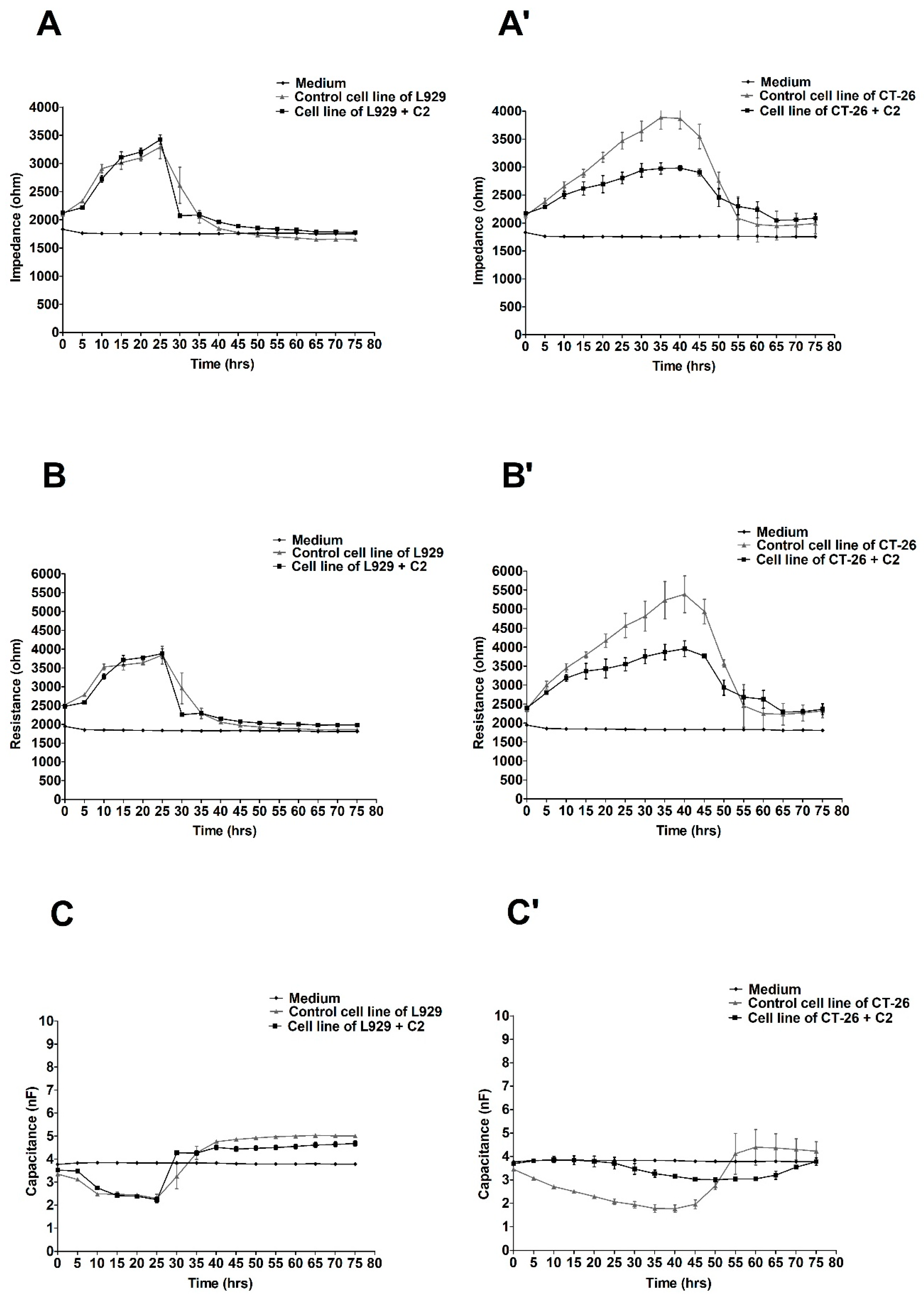
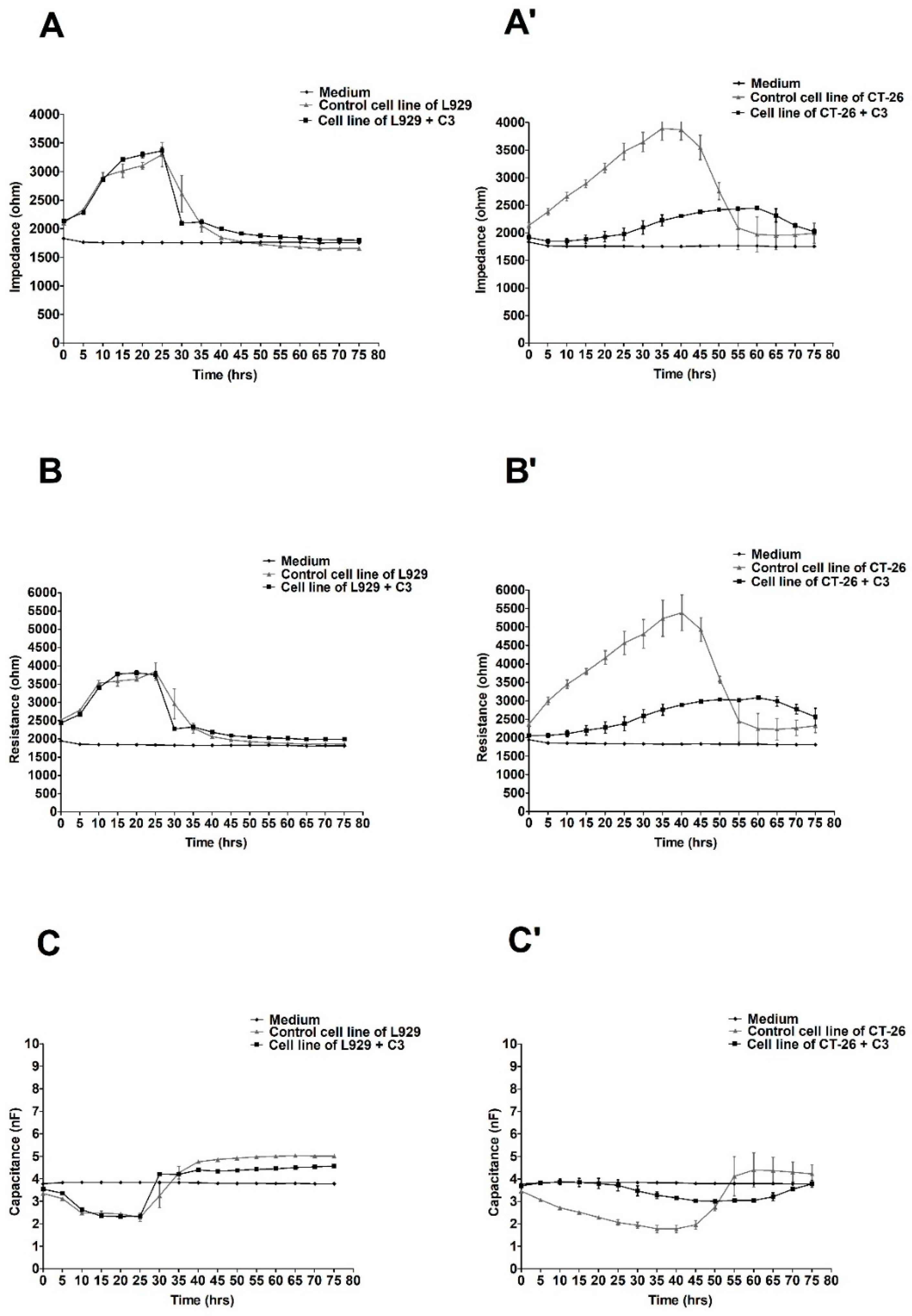
2.2. The ECIS Results
3. Discussion
4. Materials and Methods
4.1. Microorganism and Culture Conditions
4.2. Preparation of the Extracellular Low Molecular Weight Subfractions (ex-LMS)
4.3. Analytical Methods—Determination of Carbohydrates, Proteins, and Phenolic Compounds
4.4. FT-IR Spectroscopy Analysis of ex-LMS Sample
4.5. Cell Lines and Culture Conditions
4.6. The Cell Proliferation Assay—MTT Assay
4.7. The Electric Cell-Substrate Impedance Sensing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ramasamy, S.; Bennet, D.; Kim, S. Drug and bioactive molecule screening based on a bioelectrical impedance cell culture platform. Int. J. Nanomed. 2014, 9, 5789–5809. [Google Scholar] [CrossRef]
- Wegener, J.; Keese, C.R.; Giaever, I. Electric cell-substrate impedance sensing (ECIS) as a noninvasive means to monitor the kinetics of cell spreading to artificial surfaces. Exp. Cell Res. 2000, 259, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Charles Alexander, M.S. Fundamentals of Electric Circuits; McGraw-Hill: Boston, MA, USA, 2004. [Google Scholar]
- Osińska-Jaroszuk, M.; Jaszek, M.; Mizerska-Dudka, M.; Błachowicz, A.; Rejczak, T.P.; Janusz, G.; Wydrych, J.; Polak, J.; Jarosz-Wilkołazka, A.; Kandefer-Szerszeń, M. Exopolysaccharide from Ganoderma applanatum as a promising bioactive compound with cytostatic and antibacterial properties. BioMed Res. Int. 2014, 2014, 743812. [Google Scholar] [CrossRef] [PubMed]
- Jaszek, M.; Osińska-Jaroszuk, M.; Janusz, G.; Matuszewska, A.; Stefaniuk, D.; Sulej, J.; Polak, J.; Ruminowicz, M.; Grzywnowicz, K.; Jarosz-Wilkołazka, A. New Bioactive Fungal Molecules with High Antioxidant and Antimicrobial Capacity Isolated from Cerrena unicolor Idiophasic Cultures. BioMed Res. Int. 2013, 2013, 497492. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Dutta, S.D.; Ganguly, K.; Cho, S.J.; Lim, K.T. Mushroom-derived bioactive molecules as immunotherapeutic agents: A review. Molecules 2021, 26, 1359. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, Q.; Rong, C.; Wang, S.; Zhao, Z.; Liu, Y.; Xu, J. Immunomodulatory effects of edible and medicinal mushrooms and their bioactive immunoregulatory products. J. Fungi 2020, 6, 269. [Google Scholar] [CrossRef]
- Zając, A.; Pięt, M.; Stefaniuk, D.; Chojnacki, M.; Jakubowicz-Gil, J.; Paduch, R.; Matuszewska, A.; Jaszek, M. Pro-health and anti-cancer activity of fungal fractions isolated from milk-supplemented cultures of lentinus (Pleurotus) sajor-caju. Biomolecules 2021, 11, 1089. [Google Scholar] [CrossRef]
- Pięt, M.; Zając, A.; Paduch, R.; Jaszek, M.; Frant, M.; Stefaniuk, D.; Matuszewska, A.; Grzywnowicz, K. Chemopreventive activity of bioactive fungal fractions isolated from milk-supplemented cultures of Cerrena unicolor and Pycnoporus sanguineus on colon cancer cells. 3 Biotech 2021, 11, 5. [Google Scholar] [CrossRef]
- Matuszewska, A.; Stefaniuk, D.; Jaszek, M.; Pięt, M.; Zając, A.; Matuszewski, Ł.; Cios, I.; Grąz, M.; Paduch, R.; Bancerz, R. Antitumor potential of new low molecular weight antioxidative preparations from the white rot fungus Cerrena unicolor against human colon cancer cells. Sci. Rep. 2019, 9, 1975. [Google Scholar] [CrossRef]
- Matuszewska, A.; Karp, M.; Jaszek, M.; Janusz, G.; Osińska-Jaroszuk, M.; Sulej, J.; Stefaniuk, D.; Tomczak, W.; Giannopoulos, K. Laccase purified from Cerrena unicolor exerts antitumor activity against leukemic cells. Oncol. Lett. 2016, 11, 2009–2018. [Google Scholar] [CrossRef] [Green Version]
- Bennet, D.; Kim, S. Impedance-based cell culture platform to assess light-induced stress changes with antagonist drugs using retinal cells. Anal. Chem. 2013, 85, 4902–4911. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Xu, Y.; Wang, L.; Mitchelson, K.; Xing, W.; Cheng, J. Use of cellular electrical impedance sensing to assess in vitro cytotoxicity of anticancer drugs in a human kidney cell nephrotoxicity model. Analyst 2012, 137, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Pennington, M.R.; Van de Walle, G.R. Electric Cell-Substrate Impedance Sensing to Monitor Viral Growth and Study Cellular Responses to Infection with Alphaherpesviruses in Real Time. mSphere 2017, 2, e00039-e17. [Google Scholar] [CrossRef] [PubMed]
- Guohua, H.; Hongyang, L.; Zhiming, J.; Danhua, Z.; Haifang, W. Study of small-cell lung cancer cell-based sensor and its applications in chemotherapy effects rapid evaluation for anticancer drugs. Biosens. Bioelectron. 2017, 97, 184–195. [Google Scholar] [CrossRef]
- Prendecka, M.; Frankowski, J.; Sobieszek, G.; Kapka-Skrzypczak, L.; Skwarek-Dziekanowska, A.; Małecka-Massalska, T. Electric Cell Substrate Impedance Sensing (ECIS) as a unique technique in cancer metastasis research. J. Pre-Clin. Clin. Res. 2018, 12, 142–144. [Google Scholar] [CrossRef]
- Hofmann, U.; Michaelis, S.; Winckler, T.; Wegener, J.; Feller, K.H. A whole-cell biosensor as in vitro alternative to skin irritation tests. Biosens. Bioelectron. 2013, 39, 156–162. [Google Scholar] [CrossRef]
- Coffman, F.D.; Cohen, S. Impedance measurements in the biomedical sciences. Anal. Cell. Pathol. 2012, 35, 363–374. [Google Scholar] [CrossRef]
- Janusz, G.; Mazur, A.; Checinsks, A.; Malek, W.; Rogalski, J.; Ohga, S. Cloning and characterization of a lacease gene from biotechnologically important basidiomycete Cerrerna unicolor. J. Fac. Agric. Kyushu Univ. 2012, 57, 41–49. [Google Scholar] [CrossRef]
- Janusz, G.; Rogalski, J.; Szczodrak, J. Increased production of laccase by Cerrena unicolor in submerged liquid cultures. World J. Microbiol. Biotechnol. 2007, 23, 1459–1464. [Google Scholar] [CrossRef]
- Jennings, D.H.; Lysek, G. Fungal Biology: Understanding the Fungal Lifestyle; BIOS Scientific Publishers Ltd.: Oxford, UK, 1996. [Google Scholar]
- Hope, C.F.A.; Burns, R.G. Activity, origins and location of cellulases in a silt loam soil. Biol. Fertil. Soils 1987, 5, 164–170. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Malarczyk, E. Transformation of phenolic acids by Nocardia. Acta Microbiol. Pol. 1989, 38, 45–53. [Google Scholar]
- Available online: https://www.atcc.org/products/ccl-1 (accessed on 31 August 2022).
- Available online: https://www.atcc.org/products/crl-2638 (accessed on 16 March 2022).
- Hordyjewska, A.; Prendecka-Wróbel, M.; Kurach, Ł.; Horecka, A.; Olszewska, A.; Pigoń-Zając, D.; Małecka-Massalska, T.; Kurzepa, J. Antiproliferative Properties of Triterpenoids by ECIS Method—A New Promising Approach in Anticancer Studies? Molecules 2022, 27, 3150. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.biophysics.com/ztheta.php (accessed on 31 August 2022).
| Cell Line | IC50 [µg/mL] |
|---|---|
| CT-26 | 23.6 |
| L929 | 347.5 |
| Preparation | Protein (µg/mL) | Total Carbohydrate (µg/mL) | Total Polysaccharides (µg/mL) | Reducin Sugars (µg/mL) | Total Phenolic (µM) |
|---|---|---|---|---|---|
| Ex-LMS | 188.97 ± 1.3 | 780.07 ± 2.7 | 272.93 ± 2.7 | 507.14 ± 2.8 | 15.0 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prendecka-Wróbel, M.; Pigoń-Zając, D.; Jaszek, M.; Matuszewska, A.; Stefaniuk, D.; Opielak, G.; Piotrowska, K.; Rahnama-Hezavah, M.; Małecka-Massalska, T. Electric Cell-Substrate Impedance Sensing (ECIS) as a Convenient Tool to Assess the Potential of Low Molecular Fraction Derived from Medicinal Fungus Cerrena unicolor in Action on L929 and CT-26 Cell Lines. Molecules 2022, 27, 6251. https://doi.org/10.3390/molecules27196251
Prendecka-Wróbel M, Pigoń-Zając D, Jaszek M, Matuszewska A, Stefaniuk D, Opielak G, Piotrowska K, Rahnama-Hezavah M, Małecka-Massalska T. Electric Cell-Substrate Impedance Sensing (ECIS) as a Convenient Tool to Assess the Potential of Low Molecular Fraction Derived from Medicinal Fungus Cerrena unicolor in Action on L929 and CT-26 Cell Lines. Molecules. 2022; 27(19):6251. https://doi.org/10.3390/molecules27196251
Chicago/Turabian StylePrendecka-Wróbel, Monika, Dominika Pigoń-Zając, Magdalena Jaszek, Anna Matuszewska, Dawid Stefaniuk, Grzegorz Opielak, Katarzyna Piotrowska, Mansur Rahnama-Hezavah, and Teresa Małecka-Massalska. 2022. "Electric Cell-Substrate Impedance Sensing (ECIS) as a Convenient Tool to Assess the Potential of Low Molecular Fraction Derived from Medicinal Fungus Cerrena unicolor in Action on L929 and CT-26 Cell Lines" Molecules 27, no. 19: 6251. https://doi.org/10.3390/molecules27196251
APA StylePrendecka-Wróbel, M., Pigoń-Zając, D., Jaszek, M., Matuszewska, A., Stefaniuk, D., Opielak, G., Piotrowska, K., Rahnama-Hezavah, M., & Małecka-Massalska, T. (2022). Electric Cell-Substrate Impedance Sensing (ECIS) as a Convenient Tool to Assess the Potential of Low Molecular Fraction Derived from Medicinal Fungus Cerrena unicolor in Action on L929 and CT-26 Cell Lines. Molecules, 27(19), 6251. https://doi.org/10.3390/molecules27196251







