Isolation, Molecular Identification and Amino Acid Profiling of Single-Cell-Protein-Producing Phototrophic Bacteria Isolated from Oil-Contaminated Soil Samples
Abstract
:1. Introduction
2. Results
2.1. Isolation and Enrichment of the Purple Non-Sulfur Bacteria (PNSB)
2.2. Taxonomic Characterization of the Isolated Bacteria
2.3. Absorption Spectrum Analysis
2.4. Genomic Characterization of the Isolated Bacteria
2.5. Phylogenetic Relatedness and Sequence Deposition
2.6. Physiological and Biochemical Characterization for the Isolated Bacteria
2.6.1. Photo-Organo Heterotrophy
2.6.2. Analysis of Total Amino Acids in the Isolated Bacteria by HPLC
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Area of Study and Sampling Locations
4.3. Isolation and Screening of Non-Sulfur Bacteria
4.4. Maintenance of Stock Cultures
4.5. Molecular Identification
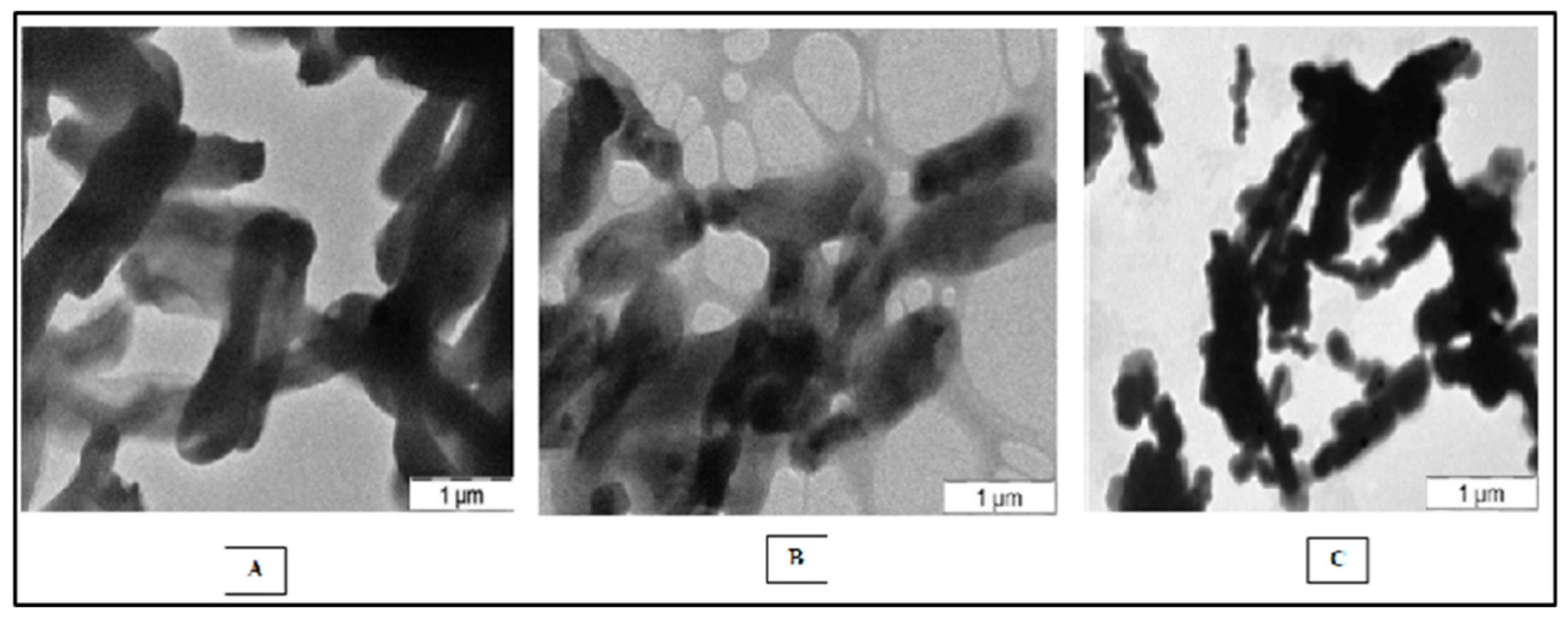
4.6. Scanning Electron Microscopy (SEM)
4.7. Determination of Growth by Optical Density
4.7.1. Determination of the Biomass of the Bacterial Culture
4.7.2. Absorption Spectrum Analysis
4.8. Analysis of Total Amino Acids by HPLC
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rakowska, J. Remediation of diesel-contaminated soil enhanced with firefighting foam application. Sci. Rep. 2020, 10, 8824. [Google Scholar] [CrossRef] [PubMed]
- Bragg, J.R.; Prince, R.C.; Harner, E.J.; Atlas, R.M. Effectiveness of bioremediation for the Exxon Valdez oil spill. Nature 1994, 368, 413–418. [Google Scholar] [CrossRef]
- Prince, R.C.; Elmendorf, D.L.; Lute, J.R.; Hsu, C.S.; Haith, C.E.; Senius, J.D.; Dechert, G.J.; Douglas, G.S.; Butler, E.L. 17.alpha.(H)-21.beta.(H)-hopane as a conserved internal marker for estimating the biodegradation of crude oil. Environ. Sci. Technol. 1994, 28, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y. Evaluation of river water quality monitoring stations by principal component analysis. Water Res. 2005, 39, 2621–2635. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Jyoti, J.; Kuhad, R.C.; Lal, B. In situ bioremediation potential of an oily sludge-degrading bacterial consortium. Curr. Microbiol. 2001, 43, 328–335. [Google Scholar] [CrossRef]
- Gogoi, B.K.; Dutta, N.N.; Goswami, P.; Mohan, T.K. A case study of bioremediation of petroleum-hydrocarbon contaminated soil at a crude oil spill site. Adv. Environ. Res. 2003, 7, 767–782. [Google Scholar] [CrossRef]
- Rosenberg, E.; Ron, E.Z. Bioremediation of petroleum contamination. Biotechnol. Res. Ser. 1996, 6, 100–124. [Google Scholar]
- Mancera-Lopez, M.E.; Rodriguez-Casasola, M.T.; Rios-Leal, E.; Esparza-Garcia, F.; Chavez-Gomez, B.; Rodriguez-Vazquez, R.; Barrera-Cortes, J. Fungi and bacteria isolated from two highly polluted soils for hydrocarbon degradation. Acta Chim. Slov. 2007, 54, 201–209. [Google Scholar]
- Plohl, K.; Leskovsek, H.; Bricelj, M. Biological degradation of motor oil in water. Acta Chim. Slov. 2002, 49, 279–290. [Google Scholar]
- Widada, J.; Nojiri, H.; Omori, T. Recent developments in molecular techniques for identification and monitoring of xenobiotic-degrading bacteria and their catabolic genes in bioremediation. Appl. Microbiol. Biotechnol. 2002, 60, 45–59. [Google Scholar] [PubMed]
- Adenipekun, C.O. Bioremediation of engine-oil polluted soil by Pleurotus tuber-regium Singer, a Nigerian white-rot fungus. Afr. J. Biotechnol. 2008, 7, 55–58. [Google Scholar]
- Adenipekun, C.O.; Isikhuemhen, O.S. Bioremediation of Engine Oil Polluted Soil by the Tropical White Rot Fungus, Lentinus squarrosulus Mont. (Singer). Pak. J. Biol. Sci. 2008, 11, 1634–1637. [Google Scholar] [CrossRef] [PubMed]
- van Hamme, J.D.; Odumeru, J.A.; Ward, O.P. Community dynamics of a mixed-bacterial culture growing on petroleum hydrocarbons in batch culture. Can. J. Microbiol. 2000, 46, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Koma, D.; Hasumi, F.; Yamamoto, E.; Ohta, T.; Chung, S.Y.; Kubo, M. Biodegradation of long-chain n-paraffins from waste oil of car engine by Acinetobacter sp. J. Biosci. Bioeng. 2001, 91, 94–96. [Google Scholar] [CrossRef]
- Abioye, P.O.; Aziz, A.A.; Agamuthu, P. Enhanced Biodegradation of Used Engine Oil in Soil Amended with Organic Wastes. Water Air Soil Pollut. 2010, 209, 173–179. [Google Scholar] [CrossRef]
- Wang, M.; Ahrné, S.; Jeppsson, B.; Molin, G. Comparison of bacterial diversity along the human intestinal tract by direct cloning and sequencing of 16S rRNA genes. FEMS Microbiol. Ecol. 2005, 54, 219–231. [Google Scholar] [CrossRef]
- Ciric, L.; Philp, J.C.; Whiteley, A.S. Hydrocarbon utilization within a diesel-degrading bacterial consortium. FEMS Microbiol. Lett. 2010, 303, 116–122. [Google Scholar] [CrossRef]
- Tesar, M.; Reichenauer, T.G.; Sessitsch, A. Bacterial rhizosphere populations of black poplar and herbal plants to be used for phytoremediation of diesel fuel. Soil Biol. Biochem. 2002, 34, 1883–1892. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H. The Effect of Diesel Fuel on Common Vetch (Vicia sativa L.) Plants. Environ. Geochem. Health 2003, 25, 123–130. [Google Scholar] [CrossRef]
- Lapinskienė, A.; Martinkus, P.; Rėbždaitė, V. Eco-toxicological studies of diesel and biodiesel fuels in aerated soil. Environ. Pollut. 2006, 142, 432–437. [Google Scholar] [CrossRef]
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15. [Google Scholar] [CrossRef] [PubMed]
- Dzantor, E.K. Phytoremediation: The state of rhizosphere ‘engineering’ for accelerated rhizodegradation of xenobiotic contaminants. J. Chem. Technol. Biotechnol. 2007, 82, 228–232. [Google Scholar] [CrossRef]
- Chaudhry, Q.; Blom-Zandstra, M.; Gupta, S.K.; Joner, E. Utilising the synergy between plants and rhizosphere microorganisms to enhance breakdown of organic pollutants in the environment. Environ. Sci. Pollut. Res. 2005, 12, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Phytoremediation: Synergistic use of plants and bacteria to clean up the environment. Biotechnol. Adv. 2003, 21, 383–393. [Google Scholar] [CrossRef]
- Cébron, A.; Beguiristain, T.; Faure, P.; Norini, M.P.; Masfaraud, J.F.; Leyval, C. Influence of vegetation on the in situ bacterial community and polycyclic aromatic hydrocarbon (PAH) degraders in aged PAH-contaminated or thermal-desorption-treated soil. Appl. Environ. Microbiol. 2009, 75, 6322–6330. [Google Scholar] [CrossRef]
- Johnson, J.B.; Omland, K.S. Model selection in ecology and evolution. Trends Ecol. Evol. 2004, 19, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Weyens, N.; van der Lelie, D.; Taghavi, S.; Vangronsveld, J. Phytoremediation: Plant–endophyte partnerships take the challenge. Curr. Opin. Biotechnol. 2009, 20, 248–254. [Google Scholar] [CrossRef]
- McGuinness, M.; Dowling, D. Plant-Associated Bacterial Degradation of Toxic Organic Compounds in Soil. Int. J. Environ. Res. Public Health 2009, 6, 2226–2247. [Google Scholar] [CrossRef]
- Gerhardt, K.E.; Huang, X.D.; Glick, B.R.; Greenberg, B.M. Phytoremediation and rhizoremediation of organic soil contaminants: Potential and challenges. Plant Sci. 2009, 176, 20–30. [Google Scholar] [CrossRef]
- Chernikova, T.N.; Bargiela, R.; Toshchakov, S.V.; Shivaraman, V.; Lunev, E.A.; Yakimov, M.M.; Golyshin, P.N. Hydrocarbon-degrading bacteria Alcanivorax and Marinobacter associated with microalgae Pavlova lutheri and Nannochloropsis oculata. Front. Microbiol. 2020, 11, 572931. [Google Scholar] [CrossRef] [PubMed]
- Adebusoye, S.A.; Ilori, M.O.; Amund, O.O.; Teniola, O.D.; Olatope, S.O. Microbial degradation of petroleum hydrocarbons in a polluted tropical stream. World J. Microbiol. Biotechnol. 2007, 23, 1149–1159. [Google Scholar] [CrossRef]
- Singh, H. Mycoremediation: Fungal Bioremediation; Wiley-Interscience: New York, NY, USA, 2006. [Google Scholar]
- Bogusławska-Wąs, E.; Dąbrowski, W. The seasonal variability of yeasts and yeast-like organisms in water and bottom sediment of the Szczecin Lagoon. Int. J. Hyg. Environ. Health 2001, 203, 451–458. [Google Scholar] [CrossRef]
- Rentz, J.A.; Alvarez, P.J.; Schnoor, J.L. Benzo[a]pyrene co-metabolism in the presence of plant root extracts and exudates: Implications for phytoremediation. Environ. Pollut. 2005, 136, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Zubi, W. Production of Single Cell Protein from Base Hydrolyzed of Date Extract Byproduct by the Fungus Fusarium graminearum. Master’s Thesis, Garyounis University, Benghazi, Libya, 2005. [Google Scholar]
- Bamberg, J.H. British Petroleum and Global Oil, 1950–1975, the Challenge of Nationalism; British Petroleum Series; Cambridge University Press: Cambridge, UK, 2000; Volume 3, pp. 426–428. [Google Scholar]
- Arora, D.; Mukerji, K.; Marth, E. Single Cell Protein in Hand Book of Applied Mycology; Banaras Hindu University: Varanasi, India, 1991; Volume 3, pp. 499–539. [Google Scholar]
- Zhao, G.; Zhang, W.; Zhang, G. Production of single cell protein using waste capsicum powder produced during capsanthin extraction. Lett. Appl. Microbiol. 2010, 50, 187–191. [Google Scholar] [CrossRef]
- Noparatnaraporn, N.; Trakulnaleumsai, S.; Silveira, G.R.; Nishizawa, Y.; Nagai, S. SCP production by mixed culture of Rhodocyclus gelatinosus and Rhodobacter sphaeroides from Cassava Waste. J. Ferment. Technol. 1987, 65, 11–16. [Google Scholar] [CrossRef]
- Pfennig, N. Rhodopseudomon asacidophila, sp. n., a new species of the budding purple Nonsulfur Bacteria. J. Bacteriol. 1969, 99, 597–602. [Google Scholar] [CrossRef]
- Honda, R.; Fukushi, K.; Yamamoto, K. Optimization of wastewater feeding for single-cell protein production in an anaerobic wastewater treatment process utilizing purple non-sulfur bacteria in mixed culture condition. J. Biotechnol. 2006, 125, 565–573. [Google Scholar] [CrossRef]
- Gupta, S.K.; Shukla, P. Advanced technologies for improved expression of recombinant proteins in bacteria: Perspectives and applications. Crit. Rev. Biotechnol. 2016, 36, 1089–1098. [Google Scholar] [CrossRef]
- Noparatnaraporn, N.; Nagai, S. Selection of Rhodobacter sphaeroides P47 a Useful Source of Single Cell Protein. J. Gen. Appl. Microbiol. 1986, 32, 351–359. [Google Scholar] [CrossRef]
- Kaewsuk, J.; Thorasampan, W.; Thanuttamavong, M.; Seo, G.T. Kinetic development and evaluation of membrane sequencing batch reactor (MSBR) with mixed cultures photosynthetic bacteria for dairy wastewater treatment. J. Environ. Manag. 2010, 91, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Merugu, R.; Prasad, M.S.K.; Girisham, S.; Reddy, S.M. Influence of some metals on growth of two anoxygenic phototrophic bacteria. Nat. Environ. Pollut. Technol. 2008, 7, 225. [Google Scholar]
- Madukasi, E.I.; Dai, X.; He, C.; Zhou, J. Potentials of phototrophic bacteria in treating pharmaceutical wastewater. Int. J. Environ. Sci. Technol. 2010, 7, 165–174. [Google Scholar] [CrossRef]
- Azad, S.A.; Vikineswary, S.; Ramachandran, K.B.; Chong, V.C. Growth and production of biomass of Rhodovulum sulfidophilum in sardine processing wastewater. Lett. Appl. Microbiol. 2001, 33, 264–268. [Google Scholar] [CrossRef]
- Prasertsan, P.; Choorit, W.; Suwanno, S. Isolation, identification and growth conditions of photosynthetic bacteria found in seafood processing wastewater. World J. Microbiol. Biotechnol. 1993, 9, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Bender, J.; Phillips, P. Microbial mats for multiple applications in aquaculture and bioremediation. Bioresour. Technol. 2004, 94, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Imhoff, J.F.; Hiraishi, A.; Süling, J. Anoxygenic phototrophic purple bacteria. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Brenner, D.J., Krieg, N.R., Staley, J.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 119–132. [Google Scholar]
- Kantachote, D.; Torpee, S.; Umsakul, K. The potential use of anoxygenic phototrophic bacteria for treating latex rubber sheet wastewater. Electron. J. Biotechnol. 2005, 8, 314–323. [Google Scholar] [CrossRef]
- Getha, K.; Vikineswary, S.; Chong, V. Isolation and growth of the phototrophic bacterium Rhodopseudomonas palustris strain B1 in sago-starch-processing wastewater. World J. Microbiol. Biotechnol. 1998, 14, 505–511. [Google Scholar] [CrossRef]
- Ponsano, E.H.G.; Lacava, P.M.; Pinto, M.F. Chemical composition of Rhodocyclus gelatinosus biomass produced in poultry slaughterhouse wastewater. Braz. Arch. Biol. Technol. 2003, 46, 28–36. [Google Scholar] [CrossRef]
- Soon, T.K.; Al-Azad, S.; Ransangan, J. Isolation and Characterization of Purple Non-Sulfur Bacteria, Afifella marina, Producing Large amounts of Carotenoids from Mangrove Microhabitats. J. Microbiol. Biotechnol. 2014, 24, 1034–1043. [Google Scholar] [CrossRef]
- Saejung, C.; Thammaratana, T. Biomass recovery during municipal wastewater treatment using photosynthetic bacteria and prospect of production of single cell protein for feedstuff. Environ. Technol. 2016, 37, 3055–3061. [Google Scholar] [CrossRef] [PubMed]
- Poulain, A.J.; Newman, D.K. Rhodobacter capsulatus Catalyzes Light-Dependent Fe(II) Oxidation under Anaerobic Conditions as a Potential Detoxification Mechanism. Appl. Environ. Microbiol. 2009, 75, 6639–6646. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Lee, B.-K. Mass production of Rhodopseudomonas palustris as diet for aquaculture. Aquac. Eng. 2007, 23, 281–293. [Google Scholar] [CrossRef]
- Adedayo, M.R.; Ajiboye, E.A.; Akintunde, J.K.; Odaibo, A. Single Cell Proteins, As Nutritional Enhancer. Adv. Appl. Sci. Res. 2011, 2, 396–409. [Google Scholar]
- Ashok, V.G.; Minakshi, A.P.; Pranita, A.G. Liquid Whey, A Potential Substrate for Single Cell Protein Production from Bacillus subtilis NCIM 2010. Int. J. Life Sci. 2014, 2, 119–123. [Google Scholar]
- Jenkins, O.; Byrom, D.; Jones, D. Methylophilus: A New Genus of Methanol-Utilizing Bacteria. Int. J. Syst. Evol. Microbiol. 1987, 37, 446–448. [Google Scholar] [CrossRef]
- Dhanasekaran, D.; Lawanya, S.S.; Saha, N.T.; Panneerselvam, A. Production of single cell protein from pineapple waste using yeast. Innov. Rom. Food Biotechnol. 2011, 8, 2632. [Google Scholar]
- Najafpour; Bhasem, D. Single cell Protein. Biochem. Eng. Biotechnol. 2007, 332–341. [Google Scholar]
- Pandey, A.; Soccol, R.C.; Nigam, P.; Soccol, T.V.; Vandenberghe, L.P.S.; Mohan, R. Biotechnological potential of agro-industrial residues. II: Cassava bagasse. Bioresour. Technol. 2000, 74, 81–87. [Google Scholar] [CrossRef]
- Bozakouk, A.H. Acid Hydrolysis of Phragmites Austral; Is Powder for Production of Single Cell Protein by Candida utilis. Master’s Thesis, Garyounis University, Benghazi, Libya, 2002. [Google Scholar]
- Liao, D.; Zheng, W.; Li, X.; Yang, Q.; Yue, X.; Guo, L.; Zeng, G. Removal of lead (II) from aqueous solutions using carbonate hydroxyapatite extracted from eggshell waste. J. Hazard. Mater. 2010, 177, 126–130. [Google Scholar] [CrossRef]
- Ayyaz, K.; Zaheer, A.; Rasul, G.; Mirza, M.S. Isolation and identification by 16S rRNA sequence analysis of plant growth-promoting azospirilla from the rhizosphere of wheat. Braz. J. Microbiol. 2016, 47, 542–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Sl. No. | Amino Acid Mixture | Time |
|---|---|---|
| 1 | Phosphoserine | 5.235 |
| 2 | Aspartic acid | 6.236 |
| 3 | Glutamic acid | 8.775 |
| 4 | Amino adipic acid | 11.053 |
| 5 | OH proline | 12.341 |
| 6 | Phosphoenolamine | 13.564 |
| 7 | Serine | 15.904 |
| 8 | Glycine | 18.501 |
| 9 | Asparagine | 19.932 |
| 10 | Taurine | 22.210 |
| 11 | Threonine | 24.728 |
| 12 | Histidine | 26.036 |
| 13 | Alanine | 27.574 |
| 14 | β-amino butyric acid | 27.963 |
| 15 | Carnosine | 28.742 |
| 16 | Proline | 29.019 |
| 17 | Arginine | 30.8 |
| 18 | 3-Methyl histidine | 31.191 |
| 19 | 1-Methyl histidine | 33.217 |
| 20 | Anserine | 37.523 |
| 21 | Tyrosine | 43.884 |
| 22 | Valine | 44.579 |
| 23 | Methionine | 45.868 |
| 24 | Cystathionine | 46.51 |
| 25 | Cysteine | 50.73 |
| 26 | Isoleucine | 52.801 |
| 27 | Leucine | 53.459 |
| 28 | OH Lysine | 53.592 |
| 29 | Tryptophan | 56.451 |
| 30 | Phenylalanine | 57.319 |
| 31 | Ornithine | 57.505 |
| 32 | Lysine | 59.032 |
| Sl. No. | Amino Acid Mixture | ng/mL of Sample |
|---|---|---|
| 1 | Phosphoserine | 21.67761141 |
| 2 | Aspartic acid | 78.5499946 |
| 3 | OH proline | 11.38020833 |
| 4 | Serine | 63.30296313 |
| 5 | Glycine | 12.63515783 |
| 6 | Asparagine | 35.25277984 |
| 7 | Threonine | 4.17106681 |
| 8 | Carnosine | 8553.215786 |
| 9 | 3-Methyl histidine | 62.63458849 |
| 10 | Anserine | 109.5590547 |
| 11 | Tyrosine | 35.10529608 |
| 12 | Cysteine | 13.58060892 |
| 13 | Isoleucine | 1818.044983 |
| 14 | Tryptophan | 25.87599117 |
| Sl. No. | Amino Acid Mixture | ng/mL of Sample |
|---|---|---|
| 1 | Serine | 6.764159039 |
| 2 | Glycine | 31.1292413 |
| 3 | Asparagine | 50.27805319 |
| 4 | Threonine | 4.89298222 |
| 5 | Carnosine | 93082.97738 |
| 6 | 3-Methyl histidine | 1620.524745 |
| 7 | Anserine | 64.89758652 |
| 8 | Tyrosine | 30.69745621 |
| 9 | Valine | 3877.198276 |
| 10 | Methionine | 22.1295385 |
| 11 | Cysteine | 16.88288465 |
| 12 | Isoleucine | 1403.788927 |
| Sl. No. | Amino Acid Mixture | ng/mL of Sample |
|---|---|---|
| 1 | Phosphoserine | 16.47498467 |
| 2 | Aspartic acid | 81.42596694 |
| 3 | Glutamic acid | 81.42878516 |
| 4 | OH proline | 65.06339799 |
| 5 | Serine | 57.93946328 |
| 6 | Asparagine | 47.95234896 |
| 7 | Threonine | 14.39820178 |
| 8 | Carnosine | 21,601.05623 |
| 9 | Anserine | 227.1415528 |
| 10 | Tyrosine | 47.14814429 |
| 11 | Cystathionine | 35.53318299 |
| 12 | Cysteine | 9.617878051 |
| 13 | Isoleucine | 2032.621107 |
| Chemical/Ingredients Used | Quantity Required (mg) or (mL) |
|---|---|
| KH2PO4 | 500 |
| MgSO4·7H2O | 200 |
| NaCl | 400 |
| NH4Cl | 400 |
| CaCl2·2H2O | 50 |
| Organic carbon | 1000 |
| Yeast extract | 200 |
| Ferric citrate solution (0.1% w/v) | 5 |
| Trace element solution | 1 |
| Cyanocobalamine (1 mg/100 mL) | 5 |
| ZnCl2 | 70 |
|---|---|
| MnCl2·4H2O | 100 |
| H3BO3 | 60 |
| CoCl2·6H2O | 200 |
| NiCl2·6H2O | 20 |
| CuCl2·2H2O | 20 |
| NaMO4·2H2O | 40 |
| HCl | 25% (v/v)-1 mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nalvothula, R.; Challa, S.; Peddireddy, V.; Merugu, R.; Rudra, M.P.P.; Alataway, A.; Dewidar, A.Z.; Elansary, H.O. Isolation, Molecular Identification and Amino Acid Profiling of Single-Cell-Protein-Producing Phototrophic Bacteria Isolated from Oil-Contaminated Soil Samples. Molecules 2022, 27, 6265. https://doi.org/10.3390/molecules27196265
Nalvothula R, Challa S, Peddireddy V, Merugu R, Rudra MPP, Alataway A, Dewidar AZ, Elansary HO. Isolation, Molecular Identification and Amino Acid Profiling of Single-Cell-Protein-Producing Phototrophic Bacteria Isolated from Oil-Contaminated Soil Samples. Molecules. 2022; 27(19):6265. https://doi.org/10.3390/molecules27196265
Chicago/Turabian StyleNalvothula, Raju, Surekha Challa, Vidyullatha Peddireddy, Ramchander Merugu, M. P. Pratap Rudra, Abed Alataway, Ahmed Z. Dewidar, and Hosam O. Elansary. 2022. "Isolation, Molecular Identification and Amino Acid Profiling of Single-Cell-Protein-Producing Phototrophic Bacteria Isolated from Oil-Contaminated Soil Samples" Molecules 27, no. 19: 6265. https://doi.org/10.3390/molecules27196265







