Protective Effects of PollenAid Plus Soft Gel Capsules’ Hydroalcoholic Extract in Isolated Prostates and Ovaries Exposed to Lipopolysaccharide
Abstract
:1. Introduction
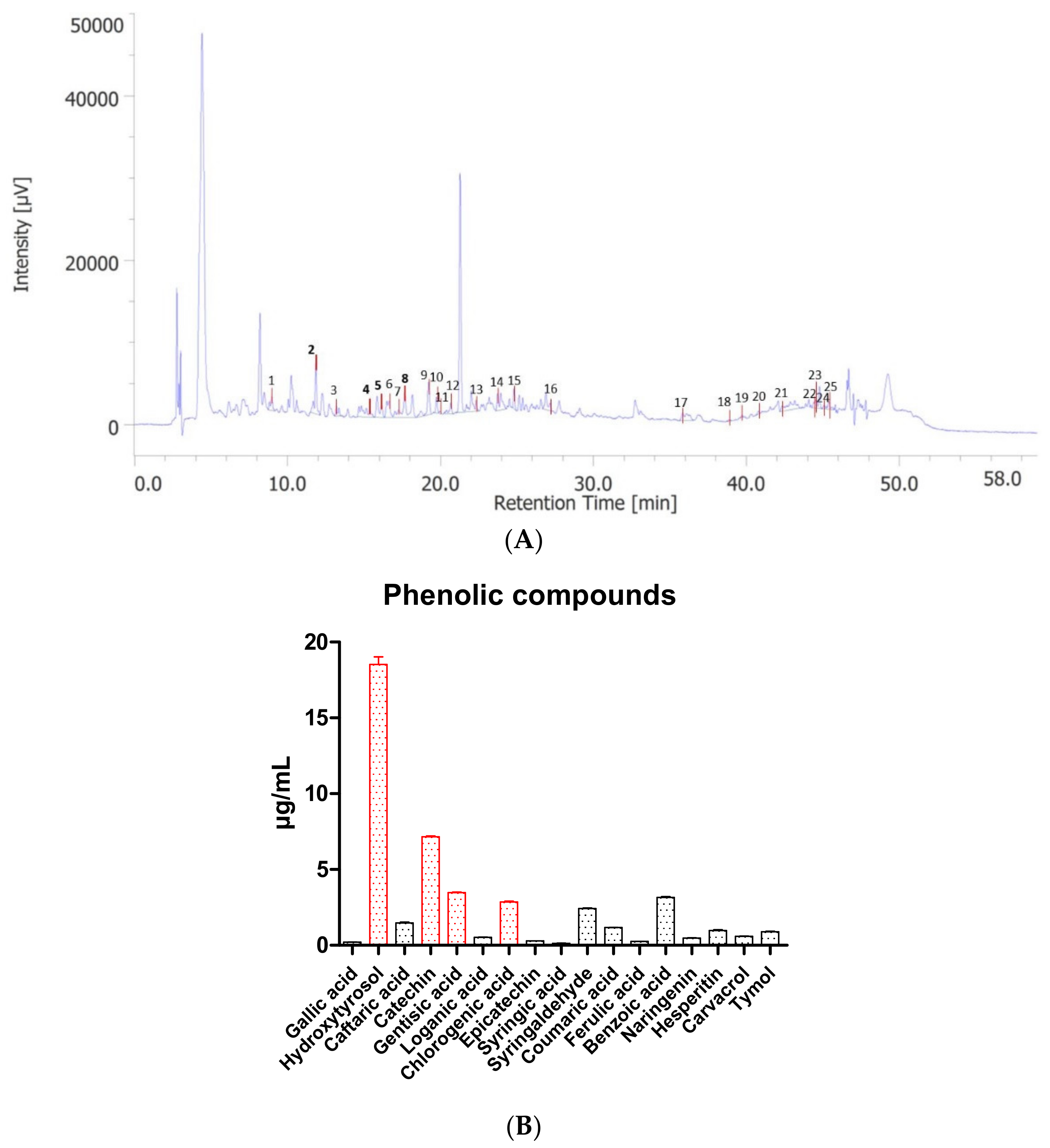
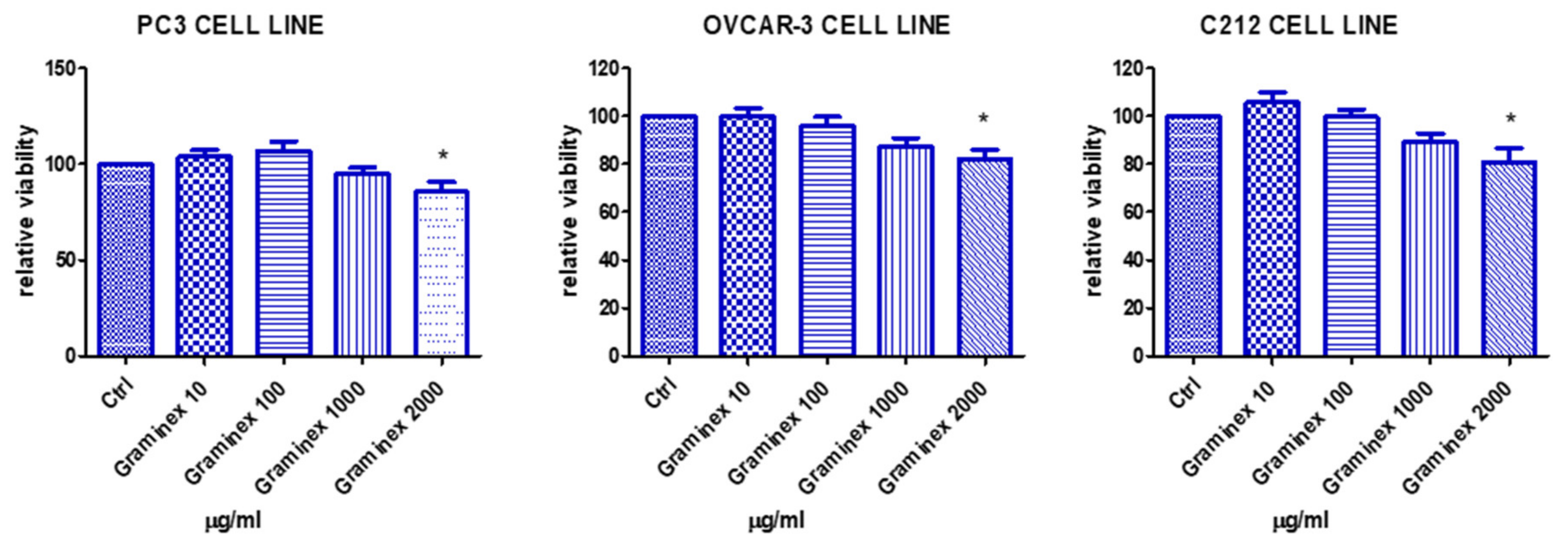
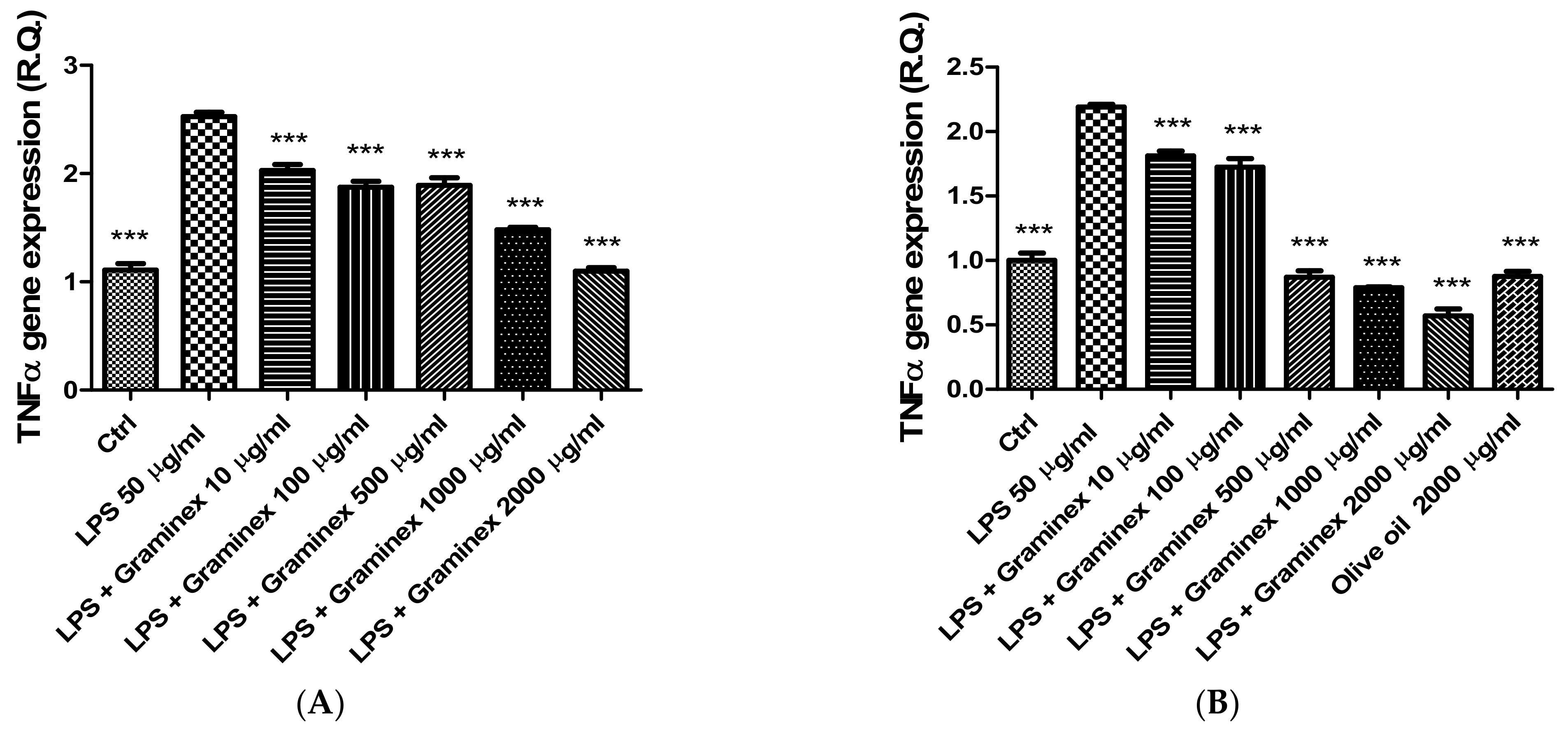
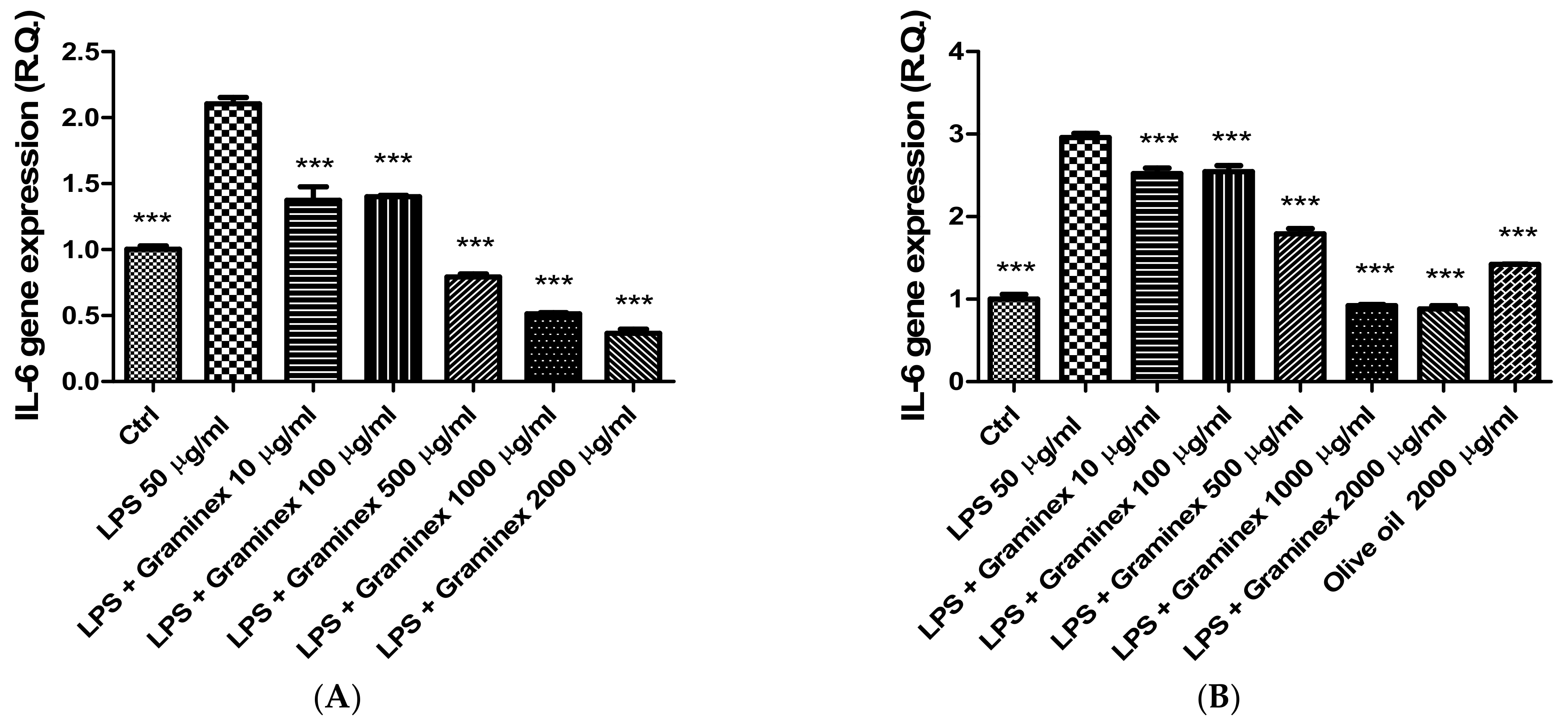
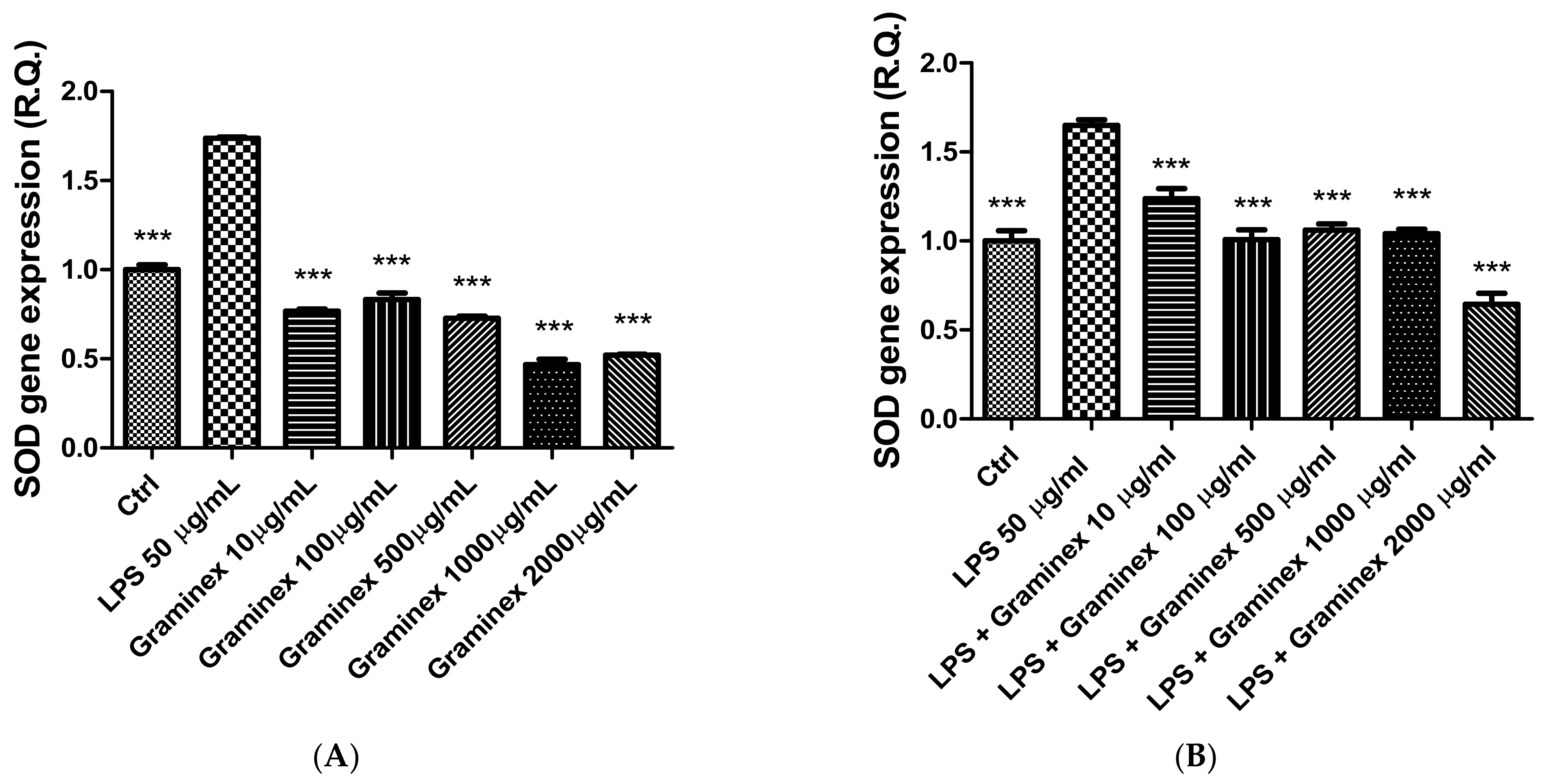
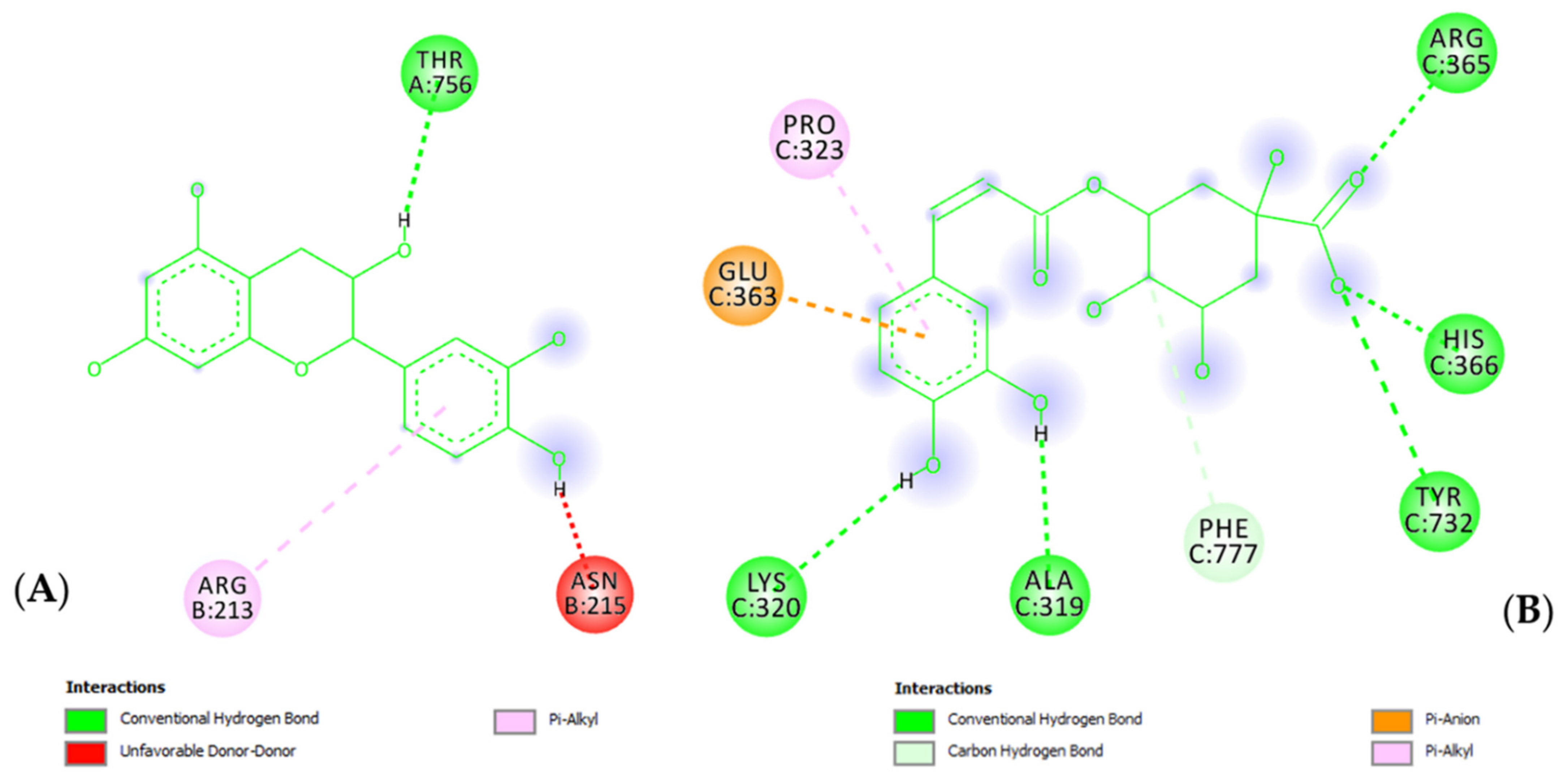
2. Results
Color Analysis
3. Material and Methods
3.1. Samples
3.2. HPLC-DAD-MS
3.3. Cell Cultures
3.4. Ex Vivo Studies
3.5. Gene Expression Analysis
3.6. In Silico Studies
3.7. Statistical Analysis
3.8. Color Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Steenkamp, V.; Gouws, M.C.; Gulumian, M.; Elgorashi, E.E.; van Staden, J. Studies on antibacterial, anti-inflammatory and antioxidant activity of herbal remedies used in the treatment of benign prostatic hyperplasia and prostatitis. J. Ethnopharmacol. 2006, 103, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jiang, Y.; Feng, J.; Gao, J.; Yu, J.; Zhao, J.; Liu, P.; Han, Y. Evidence for the Use of Complementary and Alternative Medicine for Pelvic Inflammatory Disease: A Literature Review. Evid.-Based Complement. Altern. Med. 2022, 2022, 1364297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-J.; Zhu, J.-Y.; Sun, M.-Y.; Song, Y.-N.; Rahman, K.; Peng, C.; Zhang, M.; Ye, Y.-M.; Zhang, H. Anti-inflammatory effect of Man-Pen-Fang, a Chinese herbal compound, on chronic pelvic inflammation in rats. J. Ethnopharmacol. 2017, 208, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Orlando, G.; Chiavaroli, A.; Adorisio, S.; Delfino, D.; Brunetti, L.; Recinella, L.; Leone, S.; Zengin, G.; Acquaviva, A.; Angelini, P.; et al. Unravelling the Phytochemical Composition and the Pharmacological Properties of an Optimized Extract from the Fruit from Prunus mahaleb L.: From Traditional Liqueur Market to the Pharmacy Shelf. Molecules 2021, 26, 4422. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, C.; Chiavaroli, A.; Angelini, P.; Venanzoni, R.; Angeles Flores, G.; Brunetti, L.; Petrucci, M.; Politi, M.; Menghini, L.; Leone, S.; et al. Phenolic Content and Antimicrobial and Anti-Inflammatory Effects of Solidago virga-aurea, Phyllanthus niruri, Epilobium angustifolium, Peumus boldus, and Ononis spinosa Extracts. Antibiotics 2020, 9, 783. [Google Scholar] [CrossRef]
- Cai, T.; Gallelli, L.; Cione, E.; Verze, P.; Palmieri, A.; Mirone, V.; Bonkat, G.; Wagenlehner, F.M.; Johansen, T.E.B. The efficacy and tollerability of pollen extract in combination with hyaluronic acid and vitamins in the management of patients affected by chronic prostatitis/chronic pelvic pain syndrome: A 26 weeks, randomized, controlled, single-blinded, phase III study. Minerva Urol. Nephrol. 2021. [Google Scholar] [CrossRef]
- Csikós, E.; Horváth, A.; Ács, K.; Papp, N.; Balázs, V.L.; Dolenc, M.S.; Kenda, M.; Glavač, N.K.; Nagy, M.; Protti, M.; et al. Treatment of Benign Prostatic Hyperplasia by Natural Drugs. Molecules 2021, 26, 7141. [Google Scholar] [CrossRef]
- Denisow, B.; Denisow-Pietrzyk, M. Biological and therapeutic properties of bee pollen: A review. J. Sci. Food Agric. 2016, 96, 4303–4309. [Google Scholar] [CrossRef]
- Komosinska-Vassev, K.; Olczyk, P.; Kaźmierczak, J.; Mencner, L.; Olczyk, K. Bee Pollen: Chemical Composition and Therapeutic Application. Evid.-Based Complement. Altern. Med. 2015, 2015, 297425. [Google Scholar] [CrossRef]
- Naseri, L.; Khazaei, M.R.; Khazaei, M. Synergic effect of bee pollen and metformin on proliferation and apoptosis of granulosa cells: Rat model of polycystic ovary syndrome. J. Food Biochem. 2022, 46, e13635. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Venskutonis, R.; Čeksteryte, V. Antibacterial Activity of Honey and Beebread of Different Origin Against S. aureus and S. epidermidis. Food Technol. Biotechnol. 2007, 45, 201–208. [Google Scholar]
- Kačániová, M.; Vuković, N.; Chlebo, R.; Haščík, P.; Rovná, K.; Cubon, J.; Dżugan, M.; Pasternakiewicz, A. The antimicrobial activity of honey, bee pollen loads and beeswax from Slovakia. Arch. Biol. Sci. 2012, 64, 927–934. [Google Scholar] [CrossRef]
- Choi, E.-M. Antinociceptive and antiinflammatory activities of pine (Pinus densiflora) pollen extract. Phytother. Res. 2007, 21, 471–475. [Google Scholar] [CrossRef]
- Menghini, L.; Ferrante, C.; Leporini, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Pintore, G.; Vacca, M.; Orlando, G.; Brunetti, L. An Hydroalcoholic Chamomile Extract Modulates Inflammatory and Immune Response in HT29 Cells and Isolated Rat Colon. Phytother. Res. 2016, 30, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Sandhu, K.S.; Bangar, S.P.; Purewal, S.S.; Kaur, M.; Ilyas, R.A.; Asyraf, M.R.M.; Razman, M.R. Unraveling the Bioactive Profile, Antioxidant and DNA Damage Protection Potential of Rye (Secale cereale) Flour. Antioxidants 2021, 10, 1214. [Google Scholar] [CrossRef] [PubMed]
- Cairone, F.; Carradori, S.; Locatelli, M.; Casadei, M.A.; Cesa, S. Reflectance colorimetry: A mirror for food quality—A mini review. Eur. Food Res. Technol. 2020, 246, 259–272. [Google Scholar] [CrossRef]
- Locatelli, M.; Macchione, N.; Ferrante, C.; Chiavaroli, A.; Recinella, L.; Carradori, S.; Zengin, G.; Cesa, S.; Leporini, L.; Leone, S.; et al. Graminex Pollen: Phenolic Pattern, Colorimetric Analysis and Protective Effects in Immortalized Prostate Cells (PC3) and Rat Prostate Challenged with LPS. Molecules 2018, 23, 1145. [Google Scholar] [CrossRef]
- Mitchell, C.M.; Anyalechi, G.E.; Cohen, C.R.; Haggerty, C.L.; Manhart, L.E.; Hillier, S.L. Etiology and Diagnosis of Pelvic Inflammatory Disease: Looking Beyond Gonorrhea and Chlamydia. J. Infect. Dis. 2021, 224, S29–S35. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Zhanel, M.A.; Karlowsky, J.A. Oral Fosfomycin for the Treatment of Acute and Chronic Bacterial Prostatitis Caused by Multidrug-Resistant Escherichia coli. Can. J. Infect. Dis. Med Microbiol. 2018, 2018, 1404813. [Google Scholar] [CrossRef]
- Togo, Y.; Ichioka, D.; Miyazaki, J.; Maeda, Y.; Kameyama, K.; Yasuda, M.; Hiyama, Y.; Takahashi, S.; Nagae, H.; Hirota, S.; et al. Oral administration of cernitin pollen extract (Cernilton®) for 30 days might be useful to avoid unnecessary biopsy in prostate biopsy candidates: A preliminary study. Int. J. Urol. 2018, 25, 479–485. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.; Schneider, H.; Ludwig, M.; Schnitker, J.; Brähler, E.; Weidner, W. A Pollen Extract (Cernilton) in Patients with Inflammatory Chronic Prostatitis–Chronic Pelvic Pain Syndrome: A Multicentre, Randomised, Prospective, Double-Blind, Placebo-Controlled Phase 3 Study. Eur. Urol. 2009, 56, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Verze, P.; La Rocca, R.; Palmieri, A.; Tiscione, D.; Luciani, L.G.; Mazzoli, S.; Mirone, V.; Malossini, G. The Clinical Efficacy of Pollen Extract and Vitamins on Chronic Prostatitis/Chronic Pelvic Pain Syndrome Is Linked to a Decrease in the Pro-Inflammatory Cytokine Interleukin-8. World J. Men’s Health 2017, 35, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Shirahama, T.; Sakakura, C. Overexpression of cyclooxygenase-2 in squamous cell carcinoma of the urinary bladder. Clin. Cancer Res. 2001, 7, 558–561. [Google Scholar] [PubMed]
- Moita, E.; Gil-Izquierdo, A.; Sousa, C.; Ferreres, F.; Silva, L.R.; Valentão, P.; Domínguez-Perles, R.; Baenas, N.; Andrade, P.B. Integrated Analysis of COX-2 and iNOS Derived Inflammatory Mediators in LPS-Stimulated RAW Macrophages Pre-Exposed to Echium plantagineum L. Bee Pollen Extract. PLoS ONE 2013, 8, e59131. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Kwatra, M.; Ahmed, S.; Gangipangi, V.K.; Panda, S.R.; Gupta, N.; Shantanu, P.; Gawali, B.; Naidu, V. Lipopolysaccharide exacerbates chronic restraint stress-induced neurobehavioral deficits: Mechanisms by redox imbalance, ASK1-related apoptosis, autophagic dysregulation. J. Psychiatr. Res. 2021, 144, 462–482. [Google Scholar] [CrossRef]
- Peker, E.G.G.; Kaltalioglu, K. Cinnamaldehyde and eugenol protect against LPS-stimulated oxidative stress and inflammation in Raw 264.7 cells. J. Food Biochem. 2021, 45, e13980. [Google Scholar] [CrossRef]
- Imran, M.; Nadeem, M.; Gilani, S.A.; Khan, S.; Sajid, M.W.; Amir, R.M. Antitumor Perspectives of Oleuropein and Its Metabolite Hydroxytyrosol: Recent Updates. J. Food Sci. 2018, 83, 1781–1791. [Google Scholar] [CrossRef]
- Shah, M.-A.; Kang, J.-B.; Park, D.-J.; Kim, M.-O.; Koh, P.-O. Chlorogenic acid alleviates neurobehavioral disorders and brain damage in focal ischemia animal models. Neurosci. Lett. 2021, 760, 136085. [Google Scholar] [CrossRef]
- Kwabi-Addo, B.; Ozen, M.; Ittmann, M. The role of fibroblast growth factors and their receptors in prostate cancer. Endocr.-Relat. Cancer 2004, 11, 709–724. [Google Scholar] [CrossRef]
- Funahashi, Y.; Takahashi, R.; Mizoguchi, S.; Suzuki, T.; Takaoka, E.; Ni, J.; Wang, Z.; DeFranco, D.B.; De Groat, W.C.; Tyagi, P.; et al. Bladder overactivity and afferent hyperexcitability induced by prostate-to-bladder cross-sensitization in rats with prostatic inflammation. J. Physiol. 2019, 597, 2063–2078. [Google Scholar] [CrossRef] [PubMed]
- Sipos, L.; Végh, R.; Bodor, Z.; Zaukuu, J.-L.Z.; Hitka, G.; Bázár, G.; Kovacs, Z. Classification of Bee Pollen and Prediction of Sensory and Colorimetric Attributes—A Sensometric Fusion Approach by e-Nose, e-Tongue and NIR. Sensors 2020, 20, 6768. [Google Scholar] [CrossRef] [PubMed]
- Cesa, S.; Casadei, M.A.; Cerreto, F.; Paolicelli, P. Infant Milk Formulas: Effect of Storage Conditions on the Stability of Powdered Products towards Autoxidation. Foods 2015, 4, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Chiavaroli, A.; Masciulli, F.; Fraschetti, C.; Filippi, A.; Cesa, S.; Cairone, F.; Gorica, E.; De Leo, M.; Braca, A.; et al. Protective Effects Induced by a Hydroalcoholic Allium sativum Extract in Isolated Mouse Heart. Nutrients 2021, 13, 2332. [Google Scholar] [CrossRef] [PubMed]
- Menghini, L.; Leporini, L.; Vecchiotti, G.; Locatelli, M.; Carradori, S.; Ferrante, C.; Zengin, G.; Recinella, L.; Chiavaroli, A.; Leone, S.; et al. Crocus sativus L. stigmas and byproducts: Qualitative fingerprint, antioxidant potentials and enzyme inhibitory activities. Food Res. Int. 2018, 109, 91–98. [Google Scholar] [CrossRef]
- Orlando, G.; Leone, S.; Ferrante, C.; Chiavaroli, A.; Mollica, A.; Stefanucci, A.; Macedonio, G.; Dimmito, M.P.; Leporini, L.; Menghini, L.; et al. Effects of Kisspeptin-10 on Hypothalamic Neuropeptides and Neurotransmitters Involved in Appetite Control. Molecules 2018, 23, 3071. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef] [Green Version]



| Olive Oil | PollenAid Plus Fill | ||
|---|---|---|---|
| L* | 70.46 | 14.96 | |
| a* | 5.55 | 5.42 | |
| b* | 111.60 | 13.87 | |
| C*ab | 111.74 | 14.89 | |
| hab | 87.15 | 68.67 | |
| ΔL* | Respect to olive oil: | −55.50 Darker | |
| Respect to Graminex pollen powder: | −71.09 Darker | ||
| Δa* | Respect to olive oil: | −0.14 More green | |
| Respect to Graminex pollen powder | +3.71 More red | ||
| Δb* | Respect to olive oil: | −97.73 More blue | |
| Respect to Graminex pollen powder: | +1.91 More yellow | ||
| ΔC*ab | Respect to olive oil: | −96.84 More opaque | |
| Respect to Graminex pollen powder: | +2.80 Less opaque | ||
| Δhab | Respect to olive oil: | −8.48 More red | |
| Respect to Graminex pollen powder: | −13.21 More red | ||
| ΔE | Respect to olive oil: | +112.39 | |
| Respect to Graminex pollen powder: | +71.21 | ||
| TIME (min) | Composition A% (Water + Formic Acid 0.1%) | Composition B% (Methanol + Formic Acid 0.1%) | Flow (mL/min) |
|---|---|---|---|
| 1 | 97 | 3 | 0.6 |
| 5 | 77 | 23 | 0.6 |
| 12 | 73 | 27 | 0.6 |
| 18 | 57 | 43 | 0.6 |
| 25 | 52 | 48 | 0.6 |
| 32 | 50 | 50 | 0.6 |
| 34 | 50 | 50 | 0.6 |
| 37 | 35 | 65 | 0.6 |
| 40 | 5 | 95 | 0.6 |
| 47 | 10 | 90 | 0.6 |
| 48 | 10 | 90 | 0.6 |
| Standard | m/z | Retention Time (min) | |
|---|---|---|---|
| 1 | Gallic acid | 170.15 | 8.967 |
| 2 | 3-Hydroxytyrosol | 154.16 | 11.85 |
| 3 | Caftaric acid | 312.23 | 13.19 |
| 4 | Catechin | 290.27 | 15.383 |
| 5 | Gentisic acid | 154.12 | 16.147 |
| 6 | 4-Hydroxybenzoic acid | 138.12 | 16.633 |
| 7 | Loganic acid | 376.36 | 17.257 |
| 8 | Chlorogenic acid | 354.31 | 17.66 |
| 9 | Vanillic acid | 168.15 | 19.22 |
| 10 | Caffeic acid | 180.16 | 19.73 |
| 11 | Epicatechin | 290.27 | 19.973 |
| 12 | Syringic acid | 198.17 | 20.673 |
| 13 | Syringaldehyde | 182.17 | 22.32 |
| 14 | p-Coumaric acid | 164.16 | 23.73 |
| 15 | t-Ferulic acid | 194.18 | 24.793 |
| 16 | Benzoic acid | 122.12 | 27.153 |
| 17 | t-Cinnamic acid | 148.15 | 35.817 |
| 18 | Naringenin | 272.25 | 38.87 |
| 19 | 2.3-Dimethylbenzoic acid | 150.17 | 39.7 |
| 20 | Hesperetin | 302.28 | 40.773 |
| 21 | Kaempferol | 286.24 | 42.333 |
| 22 | Carvacrol | 150.22 | 44.393 |
| 23 | Thymol | 150.22 | 44.5 |
| 24 | Flavone | 222.24 | 45.077 |
| 25 | 3-Hydroxyflavone | 238.24 | 45.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiavaroli, A.; Di Simone, S.C.; Acquaviva, A.; Libero, M.L.; Campana, C.; Recinella, L.; Leone, S.; Brunetti, L.; Orlando, G.; Nilofar; et al. Protective Effects of PollenAid Plus Soft Gel Capsules’ Hydroalcoholic Extract in Isolated Prostates and Ovaries Exposed to Lipopolysaccharide. Molecules 2022, 27, 6279. https://doi.org/10.3390/molecules27196279
Chiavaroli A, Di Simone SC, Acquaviva A, Libero ML, Campana C, Recinella L, Leone S, Brunetti L, Orlando G, Nilofar, et al. Protective Effects of PollenAid Plus Soft Gel Capsules’ Hydroalcoholic Extract in Isolated Prostates and Ovaries Exposed to Lipopolysaccharide. Molecules. 2022; 27(19):6279. https://doi.org/10.3390/molecules27196279
Chicago/Turabian StyleChiavaroli, Annalisa, Simonetta Cristina Di Simone, Alessandra Acquaviva, Maria Loreta Libero, Claudia Campana, Lucia Recinella, Sheila Leone, Luigi Brunetti, Giustino Orlando, Nilofar, and et al. 2022. "Protective Effects of PollenAid Plus Soft Gel Capsules’ Hydroalcoholic Extract in Isolated Prostates and Ovaries Exposed to Lipopolysaccharide" Molecules 27, no. 19: 6279. https://doi.org/10.3390/molecules27196279











