Hydrogel Dressing Containing Basic Fibroblast Growth Factor Accelerating Chronic Wound Healing in Aged Mouse Model
Abstract
:1. Introduction
2. Results and Discussion
2.1. In Vitro Evaluation of bFGF-Gel
2.2. In Vivo Wound Repair
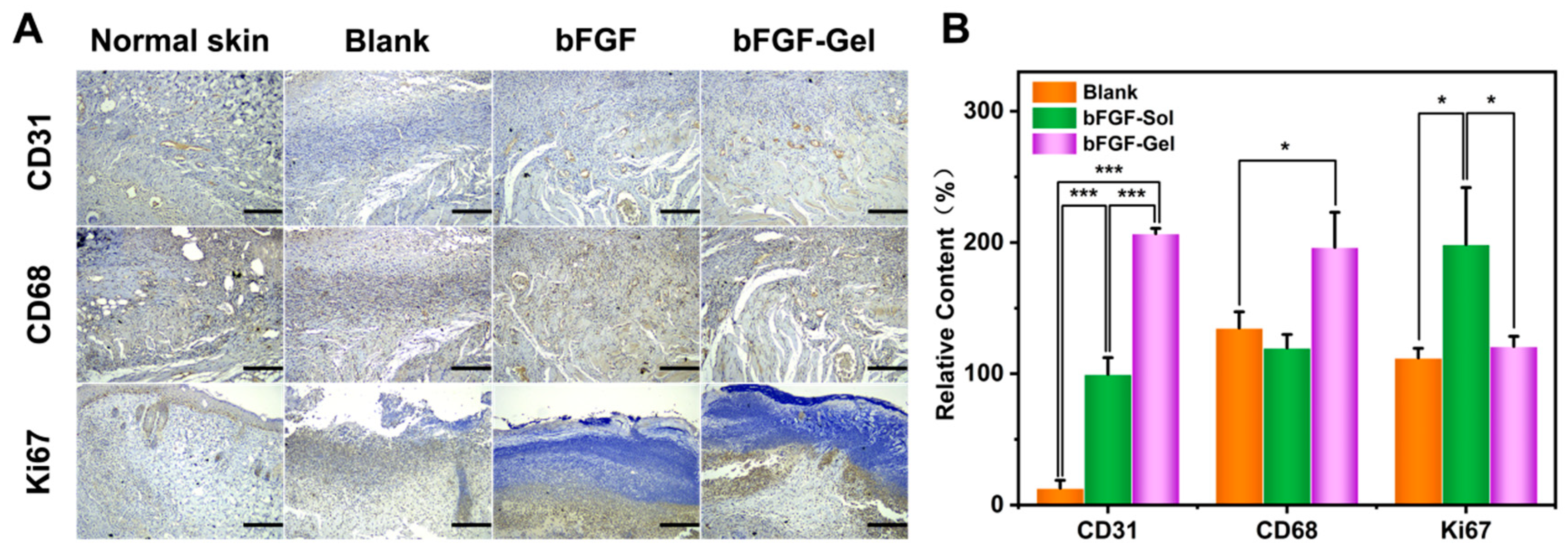
2.3. Histology of Wound Healing Site in Mice
3. Materials and Methods
3.1. Preparation of Hydrogel Dressing Containing bFGF
3.2. In Vitro Release of bFGF
3.3. Swelling Ratio, Degradation and Morphology
3.4. Wound Healing in Mice In Vivo
3.5. Histopathologic Examination
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kowalczuk, M.; Heaselgrave, W.; Britland, S.T.; Martin, C.; Radecka, I. The production and application of hydrogels for wound management: A review. Eur. Polym. J. 2019, 111, 134–151. [Google Scholar] [CrossRef]
- Greaves, N.S.; Ashcroft, K.J.; Baguneid, M.; Bayat, A. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing. J. Dermatol. Sci. 2013, 72, 206–217. [Google Scholar] [CrossRef]
- Las Heras, K.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Chronic wounds: Current status, available strategies and emerging therapeutic solutions. J. Control. Release 2020, 328, 532–550. [Google Scholar] [CrossRef]
- Nour, S.; Baheiraei, N.; Imani, R.; Khodaei, M.; Alizadeh, A.; Rabiee, N.; Moazzeni, S.M. A review of accelerated wound healing approaches: Biomaterial- assisted tissue remodeling. J. Mater. Sci. Mater. Med. 2019, 30, 120. [Google Scholar] [CrossRef]
- Choi, S.M.; Lee, K.M.; Kim, H.J.; Park, I.K.; Kang, H.J.; Shin, H.C.; Baek, D.; Choi, Y.; Park, K.H.; Lee, J.W. Effects of structurally stabilized EGF and bFGF on wound healing in type I and type II diabetic mice. Acta Biomater. 2018, 66, 325–334. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, H.; Jin, F.; Yu, C.; Chu, D.; Wang, L.; Lu, X. Improved wound healing in pressure-induced decubitus ulcer with controlled release of basic fibroblast growth factor. J. Alloys Compd. 2008, 459, 508–514. [Google Scholar] [CrossRef]
- Skorkowska-Telichowska, K.; Czemplik, M.; Kulma, A.; Szopa, J. The local treatment and available dressings designed for chronic wounds. J. Am. Acad. Dermatol. 2013, 68, e117–e126. [Google Scholar] [CrossRef]
- Alven, S.; Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Polymer-Based Scaffolds Loaded with Aloe vera Extract for the Treatment of Wounds. Pharmaceutics 2021, 13, 961. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, J.; He, C.; Xiao, Z.; Ye, J.; Li, Y.; Chen, A.; Zhang, H.; Li, X.; Lin, L.; et al. Comparative Study of Heparin-Poloxamer Hydrogel Modified bFGF and aFGF for in Vivo Wound Healing Efficiency. ACS Appl. Mater. Interfaces 2016, 8, 18710–18721. [Google Scholar] [CrossRef] [PubMed]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Peng, J.; Zhao, H.; Tu, C.; Xu, Z.; Ye, L.; Zhao, L.; Gu, Z.; Zhao, D.; Zhang, J.; Feng, Z. In situ hydrogel dressing loaded with heparin and basic fibroblast growth factor for accelerating wound healing in rat. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111169. [Google Scholar] [CrossRef] [PubMed]
- Contardi, M.; Kossyvaki, D.; Picone, P.; Summa, M.; Guo, X.; Heredia-Guerrero, J.A.; Giacomazza, D.; Carzino, R.; Goldoni, L.; Scoponi, G.; et al. Electrospun polyvinylpyrrolidone (PVP) hydrogels containing hydroxycinnamic acid derivatives as potential wound dressings. Chem. Eng. J. 2021, 409, 128144. [Google Scholar] [CrossRef]
- Li, H.; Wang, F. Core-shell chitosan microsphere with antimicrobial and vascularized functions for promoting skin wound healing. Mater. Des. 2021, 204, 109683. [Google Scholar] [CrossRef]
- Bayer, I.S. Advances in Fibrin-Based Materials in Wound Repair: A Review. Molecules 2022, 27, 4504. [Google Scholar] [CrossRef]
- Diekjurgen, D.; Grainger, D.W. Polysaccharide matrices used in 3D in vitro cell culture systems. Biomaterials 2017, 141, 96–115. [Google Scholar] [CrossRef]
- Place, E.S.; George, J.H.; Williams, C.K.; Stevens, M.M. Synthetic polymer scaffolds for tissue engineering. Chem. Soc. Rev. 2009, 38, 1139–1151. [Google Scholar] [CrossRef]
- Bayer, I.S. A Review of Sustained Drug Release Studies from Nanofiber Hydrogels. Biomedicines 2021, 9, 1612. [Google Scholar] [CrossRef]
- Jaiswal, M.; Koul, V.; Dinda, A.K. In vitro and in vivo investigational studies of a nanocomposite-hydrogel-based dressing with a silver-coated chitosan wafer for full-thickness skin wounds. J. Appl. Polym. Sci. 2016, 133, 43472. [Google Scholar] [CrossRef]
- Shi, G.; Chen, W.; Zhang, Y.; Dai, X.; Zhang, X.; Wu, Z. An Antifouling Hydrogel Containing Silver Nanoparticles for Modulating the Therapeutic Immune Response in Chronic Wound Healing. Langmuir 2019, 35, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Q.; Yu, J.; Shao, N.; Lu, H.; Guo, J.; Qiu, X.; Zhou, D.; Huang, Y. Absorbable Thioether Grafted Hyaluronic Acid Nanofibrous Hydrogel for Synergistic Modulation of Inflammation Microenvironment to Accelerate Chronic Diabetic Wound Healing. Adv. Healthc. Mater. 2020, 9, e2000198. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, X.; Yu, J.; Chen, X.; Wang, R.; Zhang, M.; Zhang, Q.; Zhang, Y.; Wang, S.; Cheng, Y. Bioactive skin-mimicking hydrogel band-aids for diabetic wound healing and infectious skin incision treatment. Bioact. Mater. 2021, 6, 3962–3975. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X.; Zhou, Y.; Chen, A.; Zheng, S.; An, Y.; He, H.; Huang, W.; Chen, Y.; Yang, Y.; Li, S.; et al. Silver crosslinked injectable bFGF-eluting supramolecular hydrogels speed up infected wound healing. J. Mater. Chem. B 2020, 8, 1359–1370. [Google Scholar] [CrossRef]
- Li, Y.; Han, Y.; Wang, X.; Peng, J.; Xu, Y.; Chang, J. Multifunctional Hydrogels Prepared by Dual Ion Cross-Linking for Chronic Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 16054–16062. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Lih, E.; Lee, J.S.; Park, K.M.; Park, K.D. Rapidly curable chitosan-PEG hydrogels as tissue adhesives for hemostasis and wound healing. Acta Biomater. 2012, 8, 3261–3269. [Google Scholar] [CrossRef]
- Vijayan, A.; Kumar, G.S.V. PEG grafted chitosan scaffold for dual growth factor delivery for enhanced wound healing. Sci. Rep. 2019, 9, 19165. [Google Scholar] [CrossRef]
- Ma, X.; Wang, M.; Ran, Y.; Wu, Y.; Wang, J.; Gao, F.; Liu, Z.; Xi, J.; Ye, L.; Feng, Z. Design and Fabrication of Polymeric Hydrogel Carrier for Nerve Repair. Polymers 2022, 14, 1549. [Google Scholar] [CrossRef]
- Geng, X.; Xu, Z.-Q.; Tu, C.-Z.; Peng, J.; Jin, X.; Ye, L.; Zhang, A.-Y.; Gu, Y.-Q.; Feng, Z.-G. Hydrogel Complex Electrospun Scaffolds and Their Multiple Functions in In Situ Vascular Tissue Engineering. ACS Appl. Bio Mater. 2021, 4, 2373–2384. [Google Scholar] [CrossRef]
- Sharaf, S.; El-Naggar, M.E. Wound dressing properties of cationized cotton fabric treated with carrageenan/cyclodextrin hydrogel loaded with honey bee propolis extract. Int. J. Biol. Macromol. 2019, 133, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shu, W.; Yu, Q.; Qu, W.; Wang, Y.; Li, R. Functional Biomaterials for Treatment of Chronic Wound. Front. Bioeng. Biotechnol. 2020, 8, 516. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Pullen, N.A.; Oskeritzian, C.A.; Ryan, J.J.; Bowlin, G.L. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials 2013, 34, 4439–4451. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef]
- Wang, S.; Lai, X.; Deng, Y.; Song, Y. Correlation between mouse age and human age in anti-tumor research: Significance and method establishment. Life Sci. 2020, 242, 117242. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Zhao, H.; Ma, X.; Gu, Z.; Wu, X.; Zhao, L.; Ye, L.; Feng, Z. Hydrogel Dressing Containing Basic Fibroblast Growth Factor Accelerating Chronic Wound Healing in Aged Mouse Model. Molecules 2022, 27, 6361. https://doi.org/10.3390/molecules27196361
Xiao Y, Zhao H, Ma X, Gu Z, Wu X, Zhao L, Ye L, Feng Z. Hydrogel Dressing Containing Basic Fibroblast Growth Factor Accelerating Chronic Wound Healing in Aged Mouse Model. Molecules. 2022; 27(19):6361. https://doi.org/10.3390/molecules27196361
Chicago/Turabian StyleXiao, Yonghao, Hui Zhao, Xiaoyu Ma, Zongheng Gu, Xin Wu, Liang Zhao, Lin Ye, and Zengguo Feng. 2022. "Hydrogel Dressing Containing Basic Fibroblast Growth Factor Accelerating Chronic Wound Healing in Aged Mouse Model" Molecules 27, no. 19: 6361. https://doi.org/10.3390/molecules27196361
APA StyleXiao, Y., Zhao, H., Ma, X., Gu, Z., Wu, X., Zhao, L., Ye, L., & Feng, Z. (2022). Hydrogel Dressing Containing Basic Fibroblast Growth Factor Accelerating Chronic Wound Healing in Aged Mouse Model. Molecules, 27(19), 6361. https://doi.org/10.3390/molecules27196361







