Natural and Synthetic Polymer Scaffolds Comprising Upconversion Nanoparticles as a Bioimaging Platform for Tissue Engineering
Abstract
:1. Introduction
2. Results
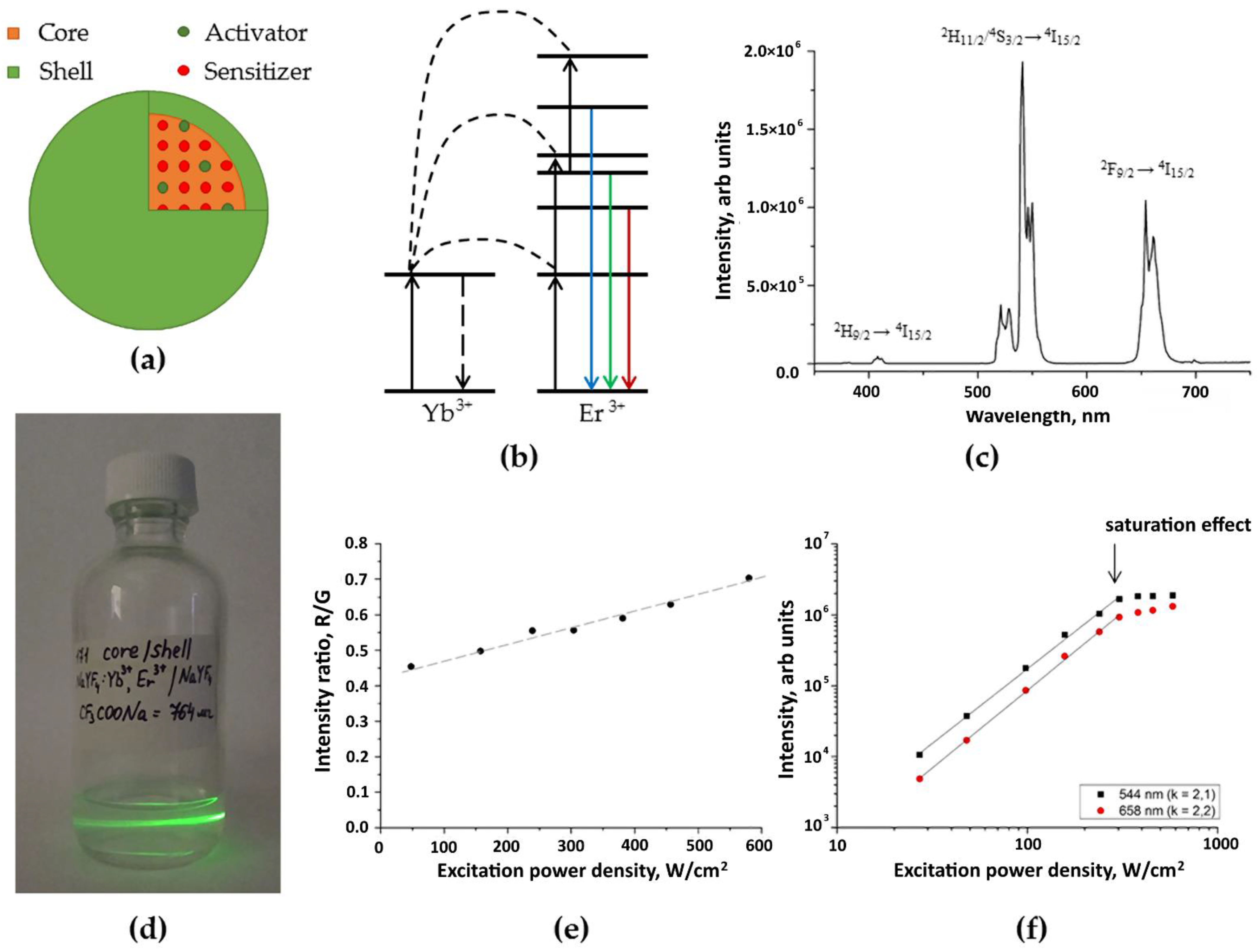
2.1. UCPNs core/shell β-NaYF4:Yb3+:Er3+/NaYF4
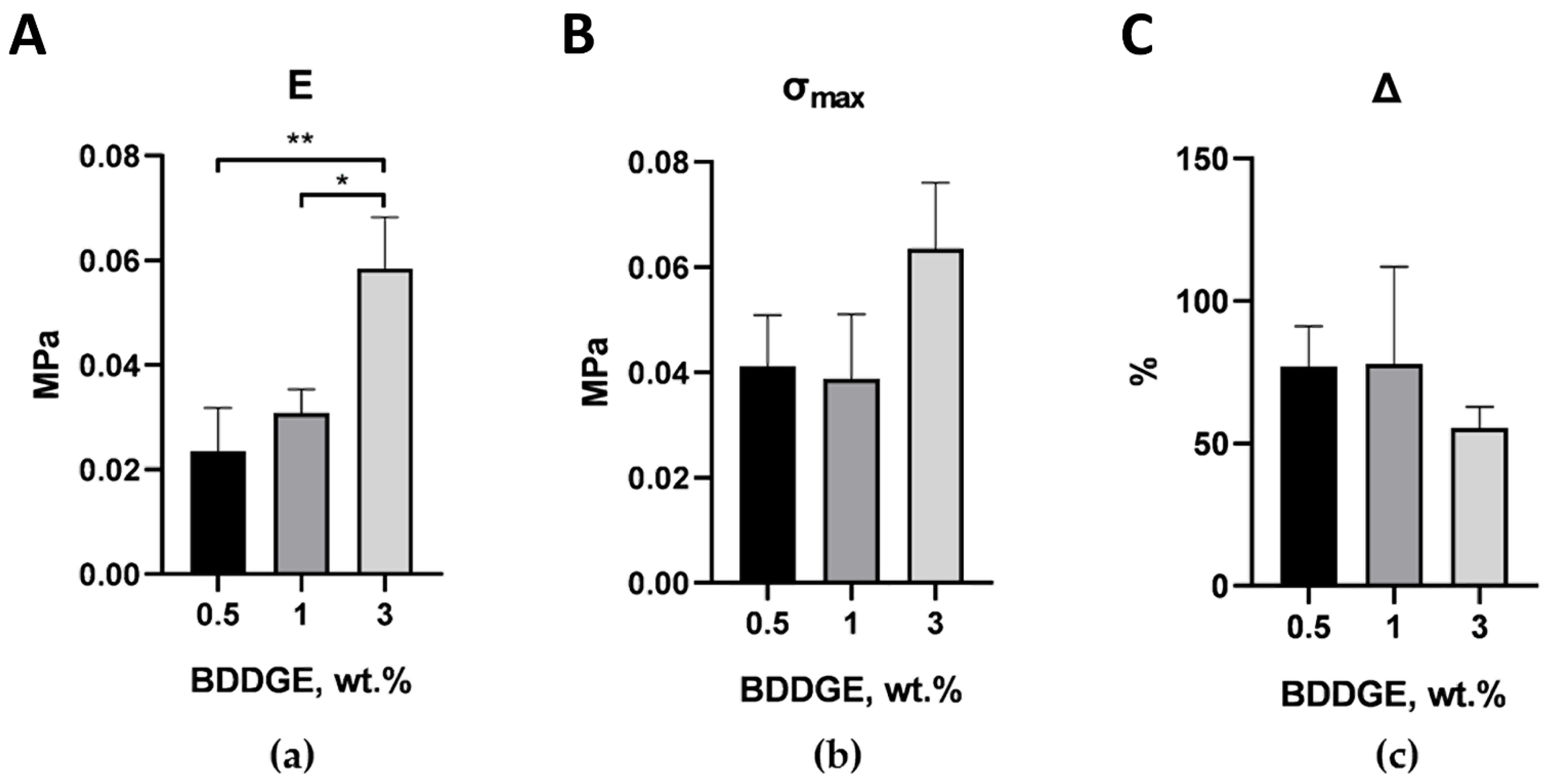
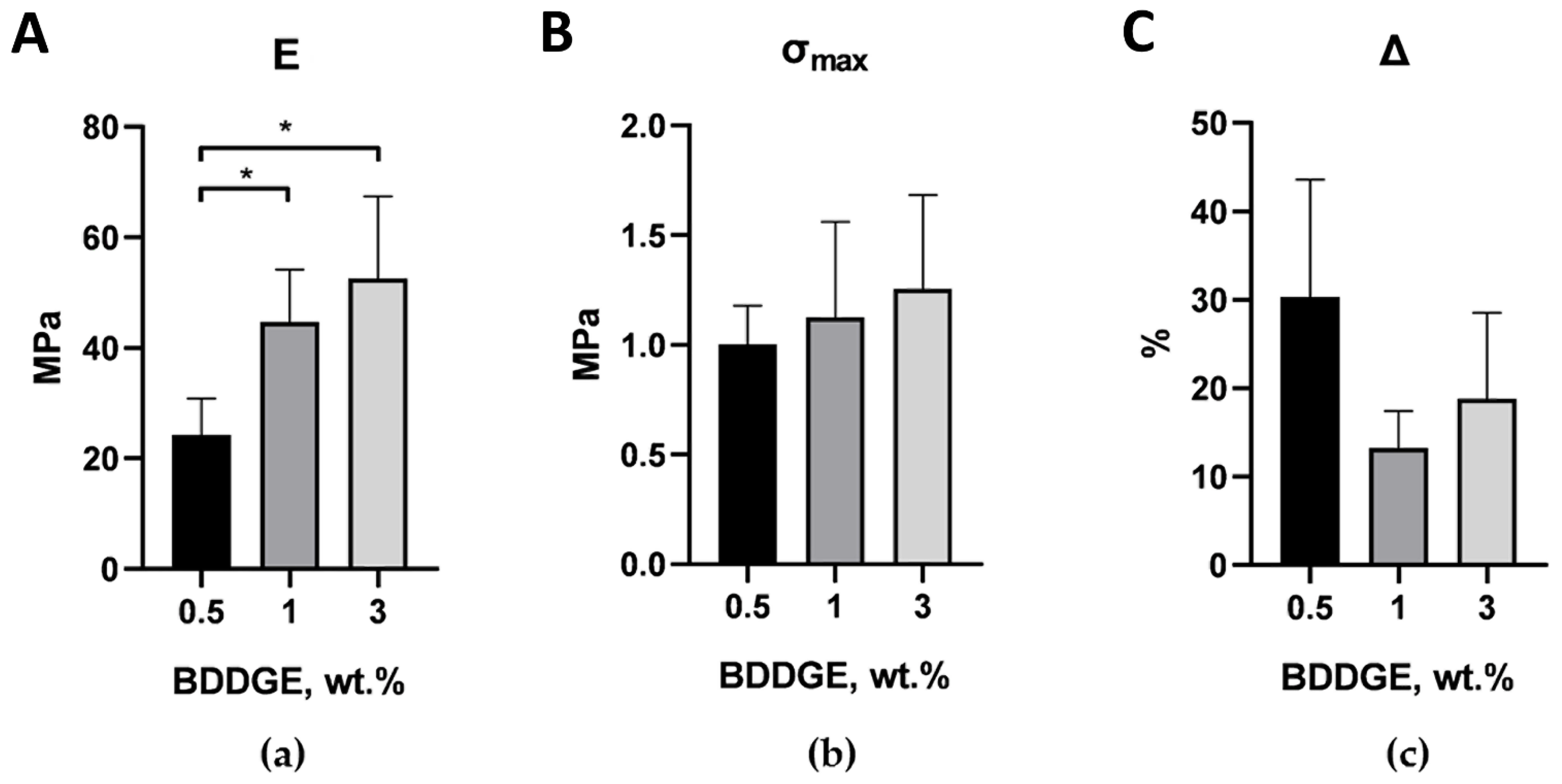
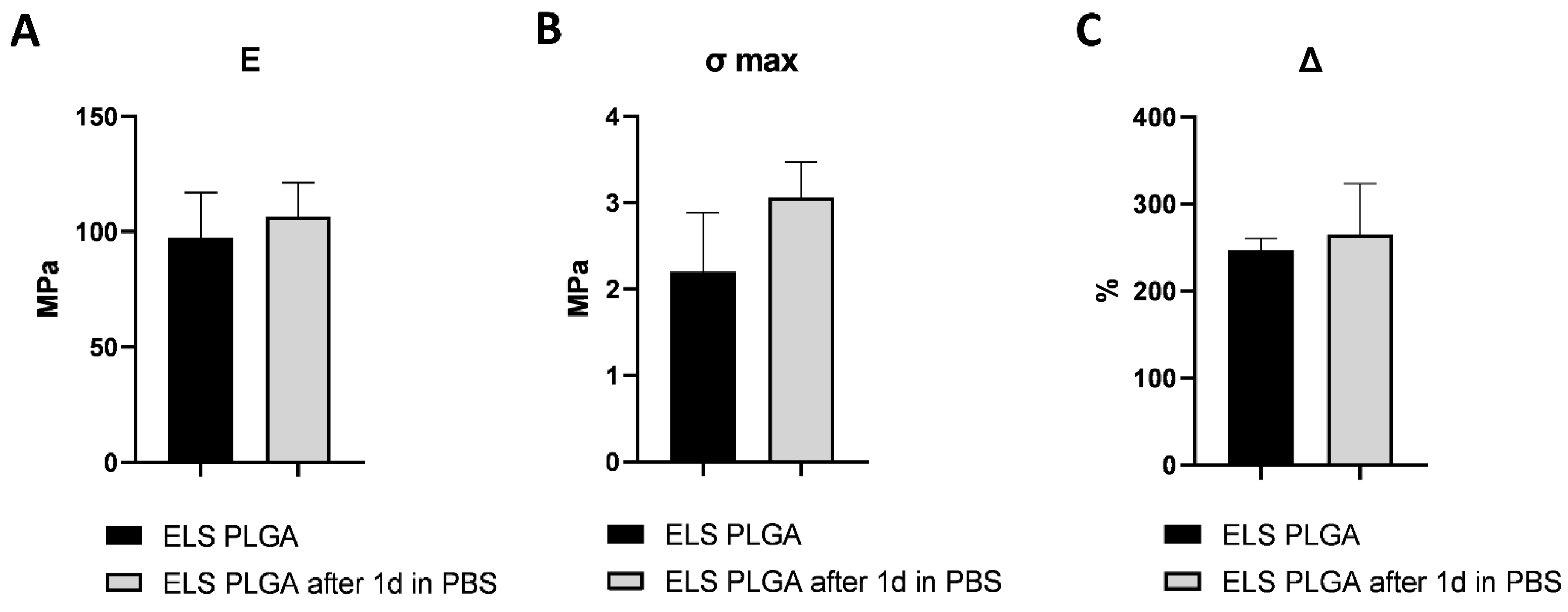
2.2. Optimization of The Scaffolds’ Mechanical Properties
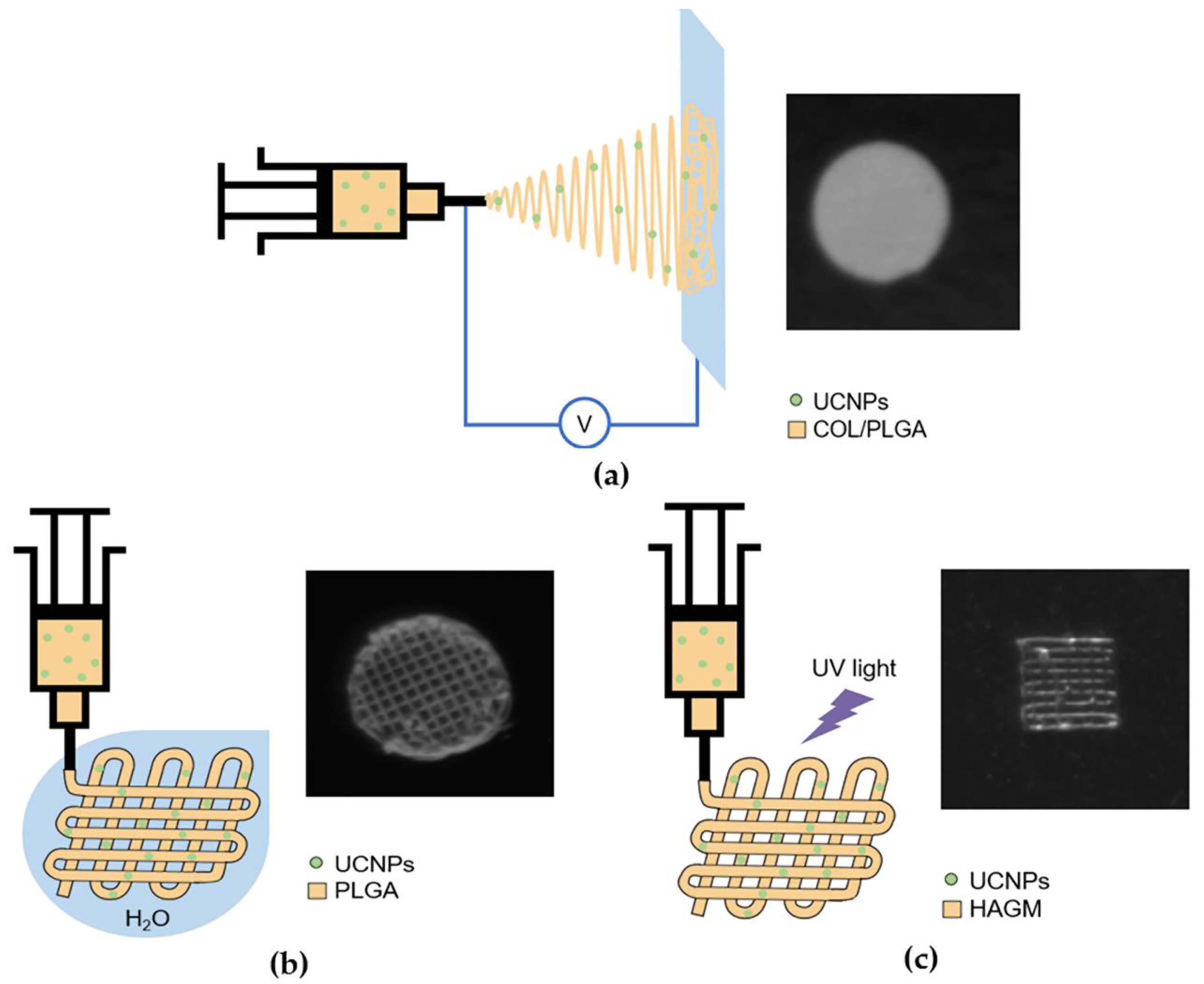
2.3. UCNP-Loaded Polymer Scaffolds Formation
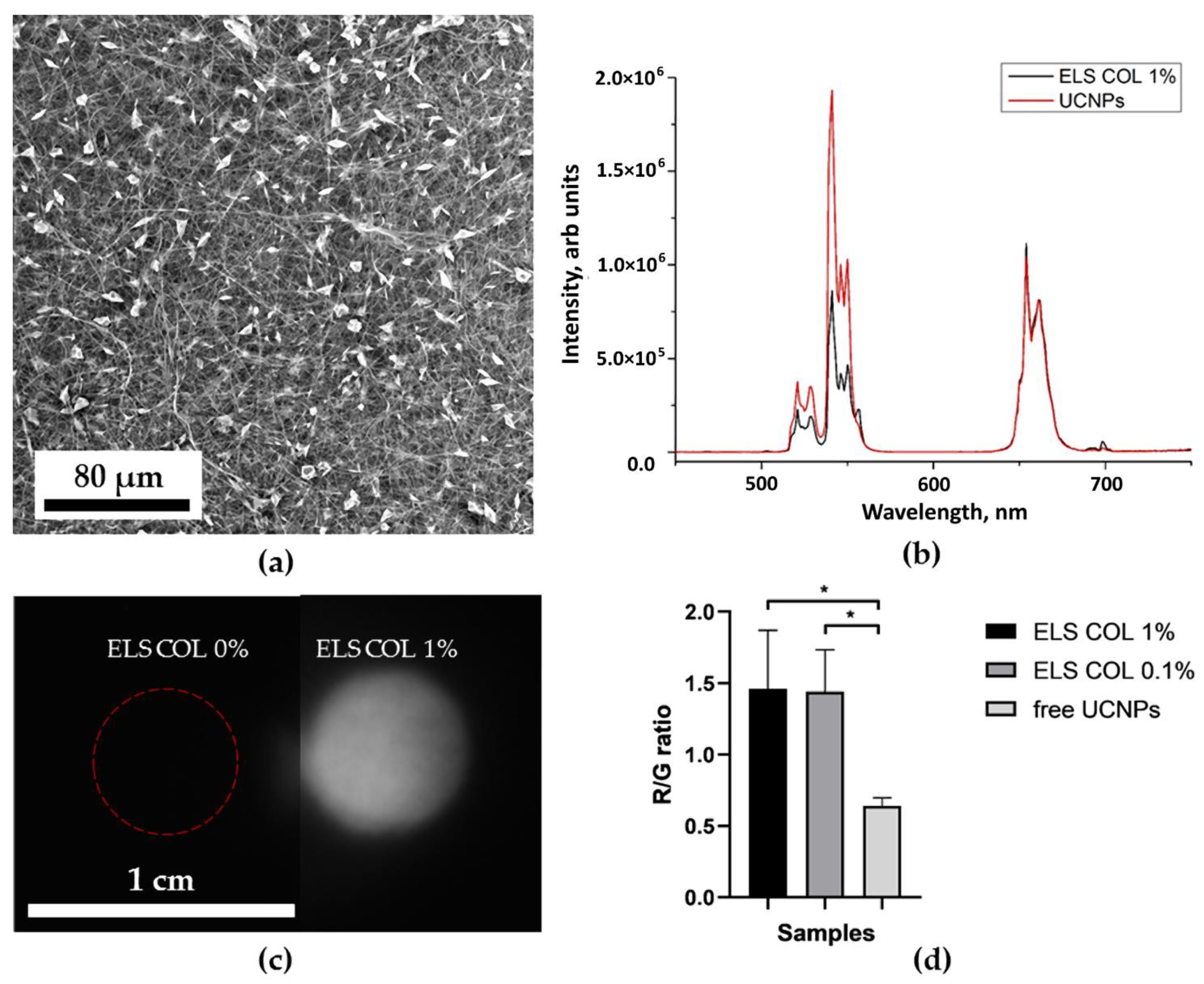
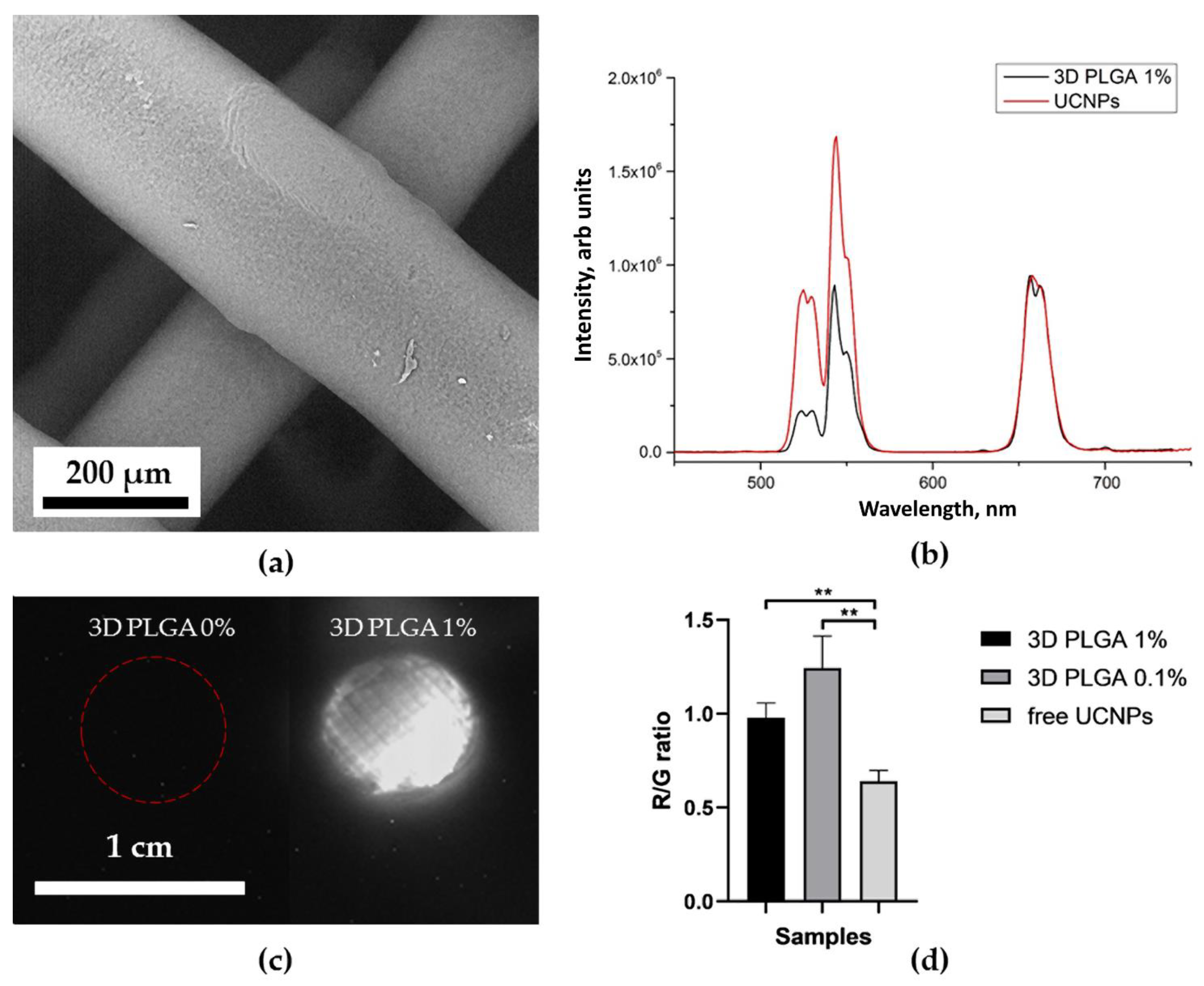
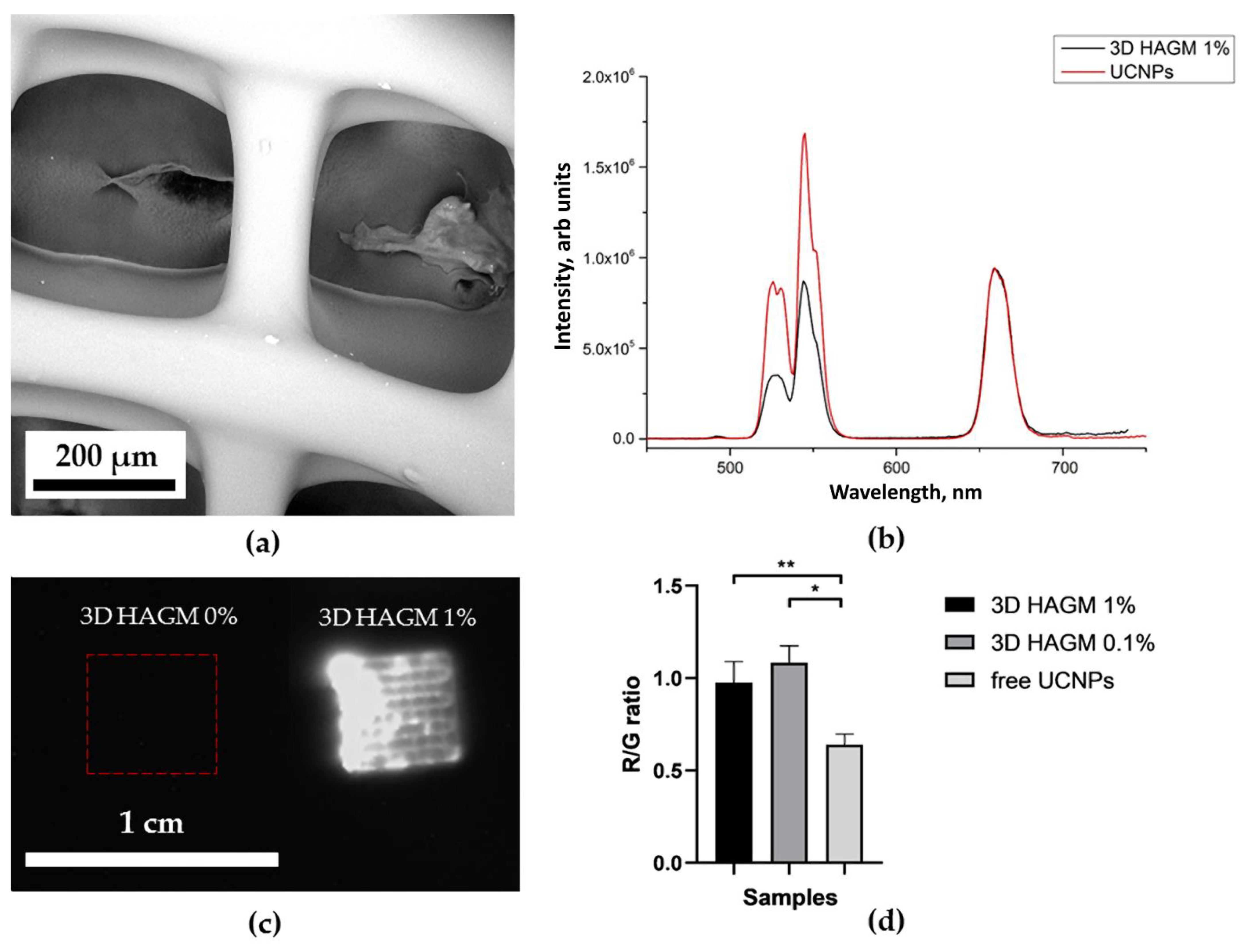
2.4. Optical Properties of UCNP-Loaded Polymer Scaffolds
2.5. Release of UCNPs
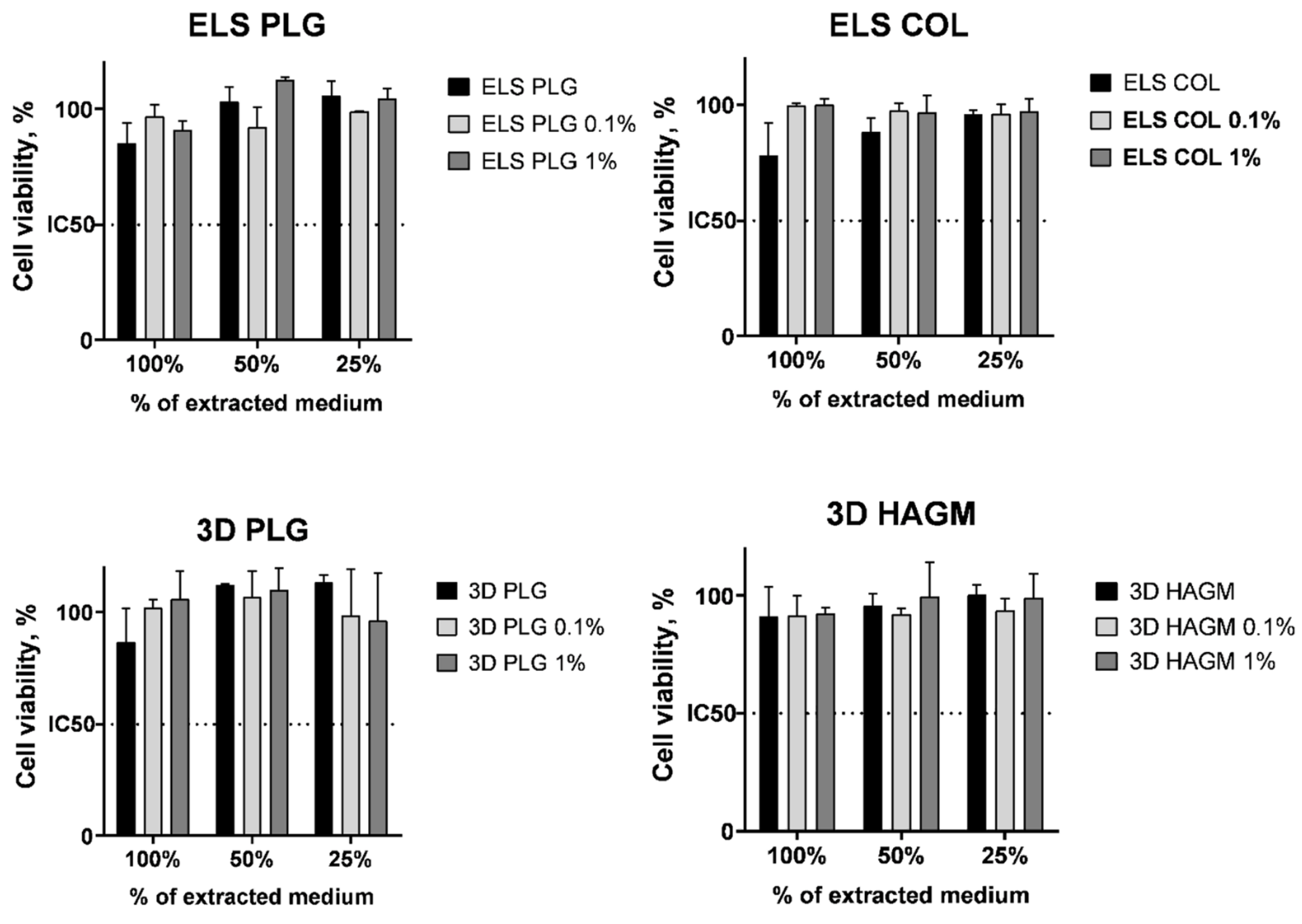
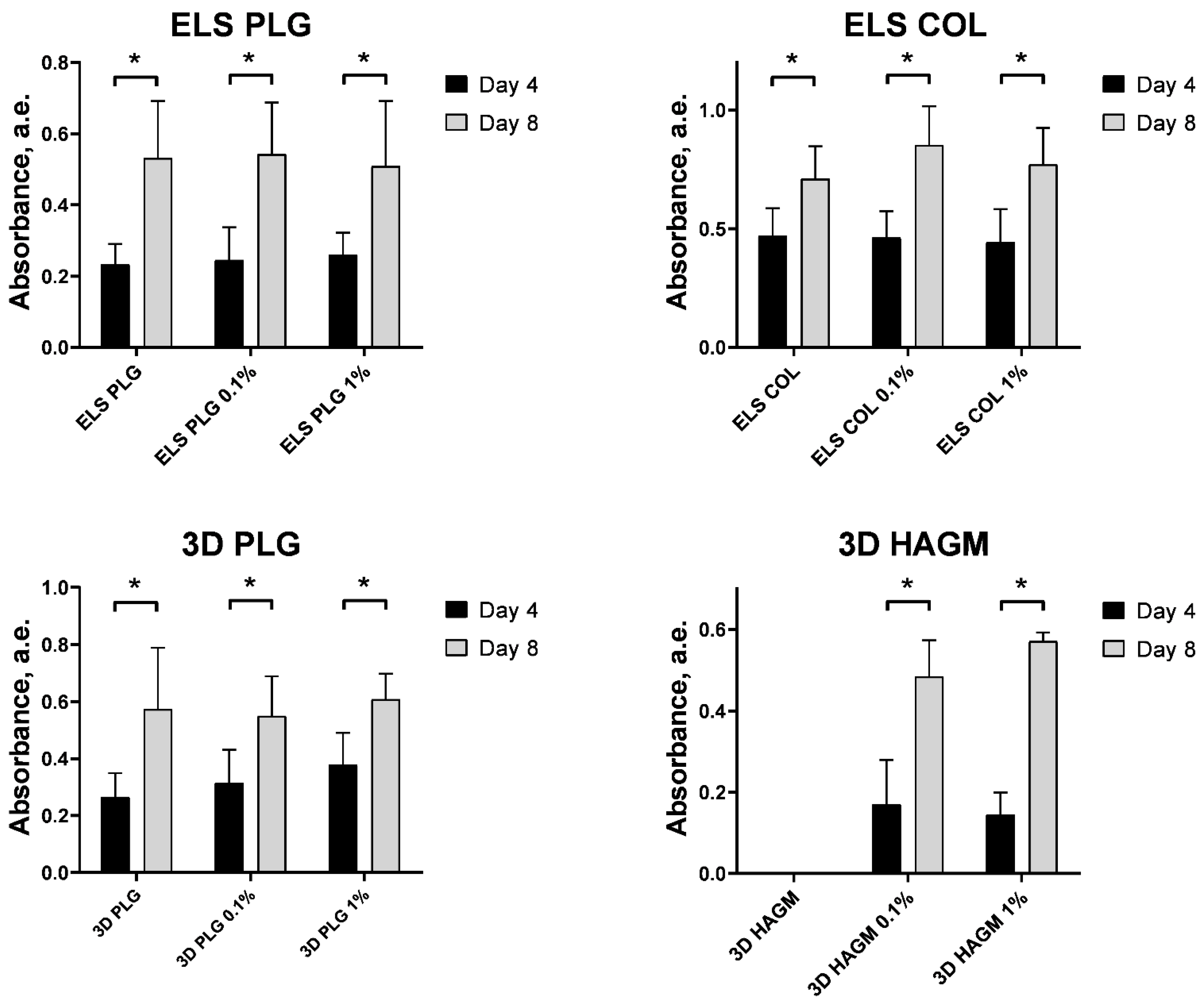
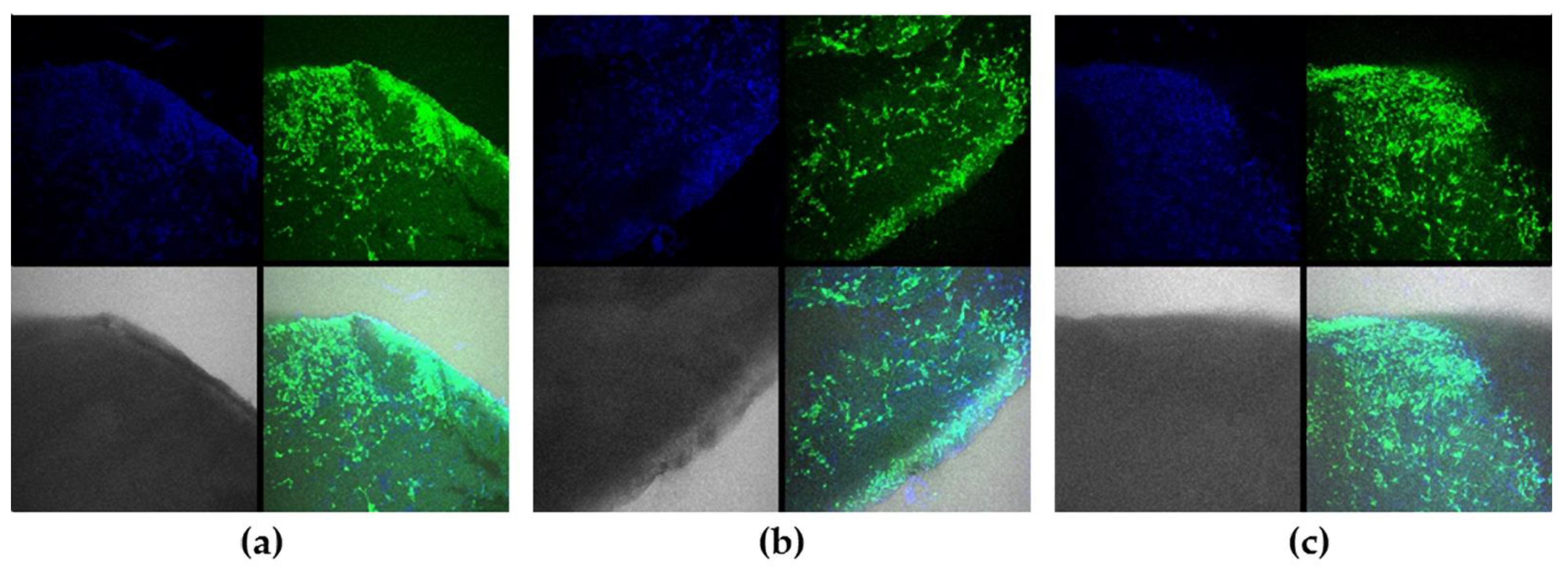
2.6. Cytotoxicity
3. Materials and Methods
3.1. Materials
3.2. Modification of Hyaluronic Acid with Glycidyl Methacrylate
3.3. Synthesis of Upconversion Nanoparticles
3.4. Electrospinning
3.5. Chemical Cross-Linking of Collagen
3.6. Antisolvent 3D Printing
3.7. Extrusion 3D Printing with Simultaneous Photocuring
3.8. Microscopy
3.9. Analysis of Photoluminescent Properties of Polymer Scaffolds
3.10. Light Transmittance
3.11. Release of UCNPs In Vitro
3.12. Cytotoxicity
3.13. Cultivation of Fibroblasts on The Surface of Scaffolds
3.14. Confocal Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zong, X.; Bien, H.; Chung, C.; Yin, L.; Fang, D.; Hsiao, B.; Chu, B.; Entcheva, E. Electrospun Fine-Textured Scaffolds for Heart Tissue Constructs. Biomaterials 2005, 26, 5330–5338. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.X.; Li, H.F.; Sun, Z.Z.; Zheng, W.; Zheng, Y.F. Fabrication of Mineralized Electrospun PLGA and PLGA/Gelatin Nanofibers and Their Potential in Bone Tissue Engineering. Mater. Sci. Eng. C 2013, 33, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.; Yang, Y.; Mohandas, A.; Stucker, B.; Nguyen, K.T. A Review of Materials, Fabrication Methods, and Strategies Used to Enhance Bone Regeneration in Engineered Bone Tissues. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 85, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, E.; Micol, L.; Houis, S.; Wurm, F.; Hilborn, J.; Hubbell, J.; Frey, P. A Collagen-Poly(Lactic Acid-Co-ɛ-Caprolactone) Hybrid Scaffold for Bladder Tissue Regeneration. Biomaterials 2011, 32, 3969–3976. [Google Scholar] [CrossRef] [PubMed]
- Black, C.R.M.; Goriainov, V.; Gibbs, D.; Kanczler, J.; Tare, R.S.; Oreffo, R.O.C. Bone Tissue Engineering. Curr. Mol. Biol. Rep. 2015, 1, 132–140. [Google Scholar] [CrossRef]
- Lin, H.; Beck, A.M.; Shimomura, K.; Sohn, J.; Fritch, M.R.; Deng, Y.; Kilroy, E.J.; Tang, Y.; Alexander, P.G.; Tuan, R.S. Optimization of Photocrosslinked Gelatin/Hyaluronic Acid Hybrid Scaffold for the Repair of Cartilage Defect. J. Tissue Eng. Regen. Med. 2019, 13, 1418–1429. [Google Scholar] [CrossRef]
- Chu, C.; Liu, L.; Rung, S.; Wang, Y.; Ma, Y.; Hu, C.; Zhao, X.; Man, Y.; Qu, Y. Modulation of Foreign Body Reaction and Macrophage Phenotypes Concerning Microenvironment. J. Biomed. Mater. Res. A 2019, 108, 127–135. [Google Scholar] [CrossRef]
- Haase, T.; Klopfleisch, R.; Krost, A.; Sauter, T.; Kratz, K.; Peter, J.; Jung, F.; Lendlein, A.; Zohlnhöfer, D.; Rüder, C. In Vivo Biocompatibility Study of Degradable Homo- versus Multiblock Copolymers and Their (Micro)Structure Compared to an Established Biomaterial. Clin. Hemorheol. Microcirc. 2020, 75, 163–176. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Synthetic Biodegradable Functional Polymers for Tissue Engineering: A Brief Review. Sci. China Chem. 2014, 57, 490–500. [Google Scholar] [CrossRef]
- Fischer, R.L.; McCoy, M.G.; Grant, S.A. Electrospinning Collagen and Hyaluronic Acid Nanofiber Meshes. J. Mater. Sci. Mater. Med. 2012, 23, 1645–1654. [Google Scholar] [CrossRef]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of Collagen Nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Ju, Y.M.; Takahashi, H.; Williams, D.F.; Yoo, J.J.; Lee, S.J.; Okano, T.; Atala, A. Engineered Small Diameter Vascular Grafts by Combining Cell Sheet Engineering and Electrospinning Technology. Acta Biomater. 2015, 16, 14–22. [Google Scholar] [CrossRef]
- Haaparanta, A.-M.; Järvinen, E.; Cengiz, I.F.; Ellä, V.; Kokkonen, H.T.; Kiviranta, I.; Kellomäki, M. Preparation and Characterization of Collagen/PLA, Chitosan/PLA, and Collagen/Chitosan/PLA Hybrid Scaffolds for Cartilage Tissue Engineering. J. Mater. Sci. Mater. Med. 2014, 25, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-H.; Kim, A.J.; Park, J.Y.; Yi, N.; Kang, I.; Park, J.; Rhie, J.-W.; Cho, D.-W. Effect of Solid Freeform Fabrication-Based Polycaprolactone/Poly(Lactic-Co-Glycolic Acid)/Collagen Scaffolds on Cellular Activities of Human Adipose-Derived Stem Cells and Rat Primary Hepatocytes. J. Mater. Sci. Mater. Med. 2013, 24, 1053–1065. [Google Scholar] [CrossRef]
- Hardy, J.G.; Lin, P.; Schmidt, C.E. Biodegradable Hydrogels Composed of Oxime Crosslinked Poly(Ethylene Glycol), Hyaluronic Acid and Collagen: A Tunable Platform for Soft Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2015, 26, 143–161. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Savelyev, A.G.; Bardakova, K.N.; Khaydukov, E.V.; Generalova, A.N.; Popov, V.K.; Chichkov, B.N.; Semchishen, V.A. Flavin Mononucleotide Photoinitiated Cross-Linking of Hydrogels: Polymer Concentration Threshold of Strengthening. J. Photochem. Photobiol. A Chem. 2017, 341, 108–114. [Google Scholar] [CrossRef]
- Langer, R.; Tirrell, D.A. Designing Materials for Biology and Medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef]
- Wu, T.; Ding, M.; Shi, C.; Qiao, Y.; Wang, P.; Qiao, R.; Wang, X.; Zhong, J. Resorbable Polymer Electrospun Nanofibers: History, Shapes and Application for Tissue Engineering. Chin. Chem. Lett. 2020, 31, 617–625. [Google Scholar] [CrossRef]
- Braghirolli, D.I.; Steffens, D.; Pranke, P. Electrospinning for Regenerative Medicine: A Review of the Main Topics. Drug Discov. Today 2014, 19, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.K.; Park, S.J.; Kim, M.S.; Kang, C.M.; Kim, J.-I.; Kim, C.-H. Fabrication of Sonicated Chitosan Nanofiber Mat with Enlarged Porosity for Use as Hemostatic Materials. Carbohydr. Polym. 2013, 97, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Di Gesù, R.; Amato, G.; Gottardi, R. Electrospun Scaffolds in Tendons Regeneration: A Review. Muscle Ligaments Tendons J. 2019, 09, 478. [Google Scholar] [CrossRef]
- Barnes, C.P.; Pemble, C.W.; Brand, D.D.; Simpson, D.G.; Bowlin, G.L. Cross-Linking Electrospun Type II Collagen Tissue Engineering Scaffolds with Carbodiimide in Ethanol. Tissue Eng. 2007, 13, 1593–1605. [Google Scholar] [CrossRef]
- Heo, J.; Koh, R.H.; Shim, W.; Kim, H.D.; Yim, H.-G.; Hwang, N.S. Riboflavin-Induced Photo-Crosslinking of Collagen Hydrogel and Its Application in Meniscus Tissue Engineering. Drug Deliv. Transl. Res. 2016, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Telemeco, T.A.; Ayres, C.; Bowlin, G.L.; Wnek, G.E.; Boland, E.D.; Cohen, N.; Baumgarten, C.M.; Mathews, J.; Simpson, D.G. Regulation of Cellular Infiltration into Tissue Engineering Scaffolds Composed of Submicron Diameter Fibrils Produced by Electrospinning. Acta Biomater. 2005, 1, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Zeeman, R.; Dijkstra, P.J.; van Wachem, P.B.; van Luyn, M.J.A.; Hendriks, M.; Cahalan, P.T.; Feijen, J. Crosslinking and Modification of Dermal Sheep Collagen Using 1, 4-Butanediol Diglycidyl Ether. J. Biomed. Mater. Res. 1999, 46, 424–433. [Google Scholar] [CrossRef]
- Varma, M.V.; Kandasubramanian, B.; Ibrahim, S.M. 3D Printed Scaffolds for Biomedical Applications. Mater. Chem. Phys. 2020, 255, 123642. [Google Scholar] [CrossRef]
- Jammalamadaka, U.; Tappa, K. Recent Advances in Biomaterials for 3D Printing and Tissue Engineering. J. Funct. Biomater. 2018, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Jang, T.-S.; Jung, H.-D.; Pan, H.M.; Han, W.T.; Chen, S.; Song, J. 3D Printing of Hydrogel Composite Systems: Recent Advances in Technology for Tissue Engineering. Int. J. Bioprint 2018, 4, 1–28. [Google Scholar] [CrossRef]
- Ouyang, L.; Highley, C.B.; Rodell, C.B.; Sun, W.; Burdick, J.A. 3D Printing of Shear-Thinning Hyaluronic Acid Hydrogels with Secondary Cross-Linking. ACS Biomater. Sci. Eng. 2016, 2, 1743–1751. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Sittinger, M.; Risbud, M.V. Scaffold-Based Tissue Engineering: Rationale for Computer-Aided Design and Solid Free-Form Fabrication Systems. Trends Biotechnol. 2004, 22, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A.V.; Mironova, O.A.; Syachina, M.A.; Popov, V.K. 3D Printing of Polylactic-Co-Glycolic Acid Fiber Scaffolds Using an Antisolvent Phase Separation Process. Polymer 2019, 182, 121845. [Google Scholar] [CrossRef]
- Mishchenko, T.A.; Researcher, S.; Researcher, S.; Scientific, C.; Researcher, S.; Researcher, S.; Scientific, C.; Kuznetsova, A.I.; Shirokova, O.M.; Researcher, J.; et al. Features of Primary Hippocampal Cultures Formation on Scaffolds Based on Hyaluronic Acid Glycidyl Methacrylate. Biophotonics Regen. Med. 2018, 10, 103–110. [Google Scholar] [CrossRef]
- Sochilina, A.V.; Savelyev, A.G.; Demina, P.A.; Ierusalimsky, N.V.; Khochenkov, D.A.; Akasov, R.A.; Sholina, N.v.; Khaydukov, E.V.; Generalova, A.N. Controlled Modification of Hyaluronic Acid for Photoinduced Reactions in Tissue Engineering. J. Phys. Conf. Ser. 2018, 1124, 031014. [Google Scholar] [CrossRef]
- Nam, S.Y.; Ricles, L.M.; Suggs, L.J.; Emelianov, S.Y. Imaging Strategies for Tissue Engineering Applications. Tissue Eng. Part B Rev. 2015, 21, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Zhu, N.; Smith, A.; Rajaram, A.; Hou, H.; Srinivasan, S.; Mohabatpour, F.; He, L.; McLnnes, A.; Serpooshan, V.; et al. Noninvasive Three-Dimensional in Situ and in Vivo Characterization of Bioprinted Hydrogel Scaffolds Using the X-Ray Propagation-Based Imaging Technique. ACS Appl. Mater. Interfaces 2021, 13, 25611–25623. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, G.; De Strobel, F.; Bernardini, M.; Denaro, L.; D’Avella, D.; Denaro, V. The Transpedicular Approach for the Study of Intervertebral Disc Regeneration Strategies: In Vivo Characterization. Eur. Spine J. 2013, 22, 972–978. [Google Scholar] [CrossRef] [Green Version]
- Petri, M.; Namazian, A.; Wilke, F.; Ettinger, M.; Stübig, T.; Brand, S.; Bengel, F.; Krettek, C.; Berding, G.; Jagodzinski, M. Repair of Segmental Long-Bone Defects by Stem Cell Concentrate Augmented Scaffolds: A Clinical and Positron Emission Tomography - Computed Tomography Analysis. Int. Orthop. 2013, 37, 2231–2237. [Google Scholar] [CrossRef] [Green Version]
- Bushnell, G.G.; Hong, X.; Hartfield, R.M.; Zhang, Y.; Oakes, R.S.; Rao, S.S.; Jeruss, J.S.; Stegemann, J.P.; Deng, C.X.; Shea, L.D. High Frequency Spectral Ultrasound Imaging to Detect Metastasis in Implanted Biomaterial Scaffolds. Ann. Biomed. Eng. 2020, 48, 477–489. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, S.; Hou, Z.; Ma, P.; Yang, D.; Li, C.; Lin, J. Multifunctional Electrospinning Composite Fibers for Orthotopic Cancer Treatment in Vivo. Nano Res. 2015, 8, 1917–1931. [Google Scholar] [CrossRef]
- Appel, A.A.; Anastasio, M.A.; Larson, J.C.; Brey, E.M. Imaging Challenges in Biomaterials and Tissue Engineering. Biomaterials 2013, 34, 6615–6630. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Liu, Z.; Li, F. Upconversion Nanophosphors for Small-Animal Imaging. Chem. Soc. Rev. 2012, 41, 1323–1349. [Google Scholar] [CrossRef] [PubMed]
- Sochilina, A.V.; Savelyev, A.G.; Sholina, N.V.; Karimov, D.N.; Nechaev, A.V.; Khaydukov, E.V.; Generalova, A.N. Nanohybrid Scaffolds with Luminescent Remote Control. EPJ Web Conf. 2018, 190, 04022. [Google Scholar] [CrossRef]
- Khaydukov, E.V.; Rocheva, V.V.; Semchishen, V.A.; Nechaev, A.V.; Generalova, A.N.; Seminogov, V.N.; Shekhter, A.B.; Sokolov, V.I.; Zvyagin, A.V.; Akhmanov, A.S.; et al. Applications of Upconversion Nanoparticles in Optical Bioimaging of the Tumor Tissue. J. RFBR 2014, 4, 7–17. [Google Scholar]
- Rocheva, V.V.; Koroleva, A.V.; Savelyev, A.G.; Khaydukov, K.V.; Generalova, A.N.; Nechaev, A.V.; Guller, A.E.; Semchishen, V.A.; Chichkov, B.N.; Khaydukov, E.V. High-Resolution 3D Photopolymerization Assisted by Upconversion Nanoparticles for Rapid Prototyping Applications. Sci. Rep. 2018, 8, 3663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mironova, K.E.; Khochenkov, D.A.; Generalova, A.N.; Rocheva, V.V.; Sholina, N.V.; Nechaev, A.V.; Semchishen, V.A.; Deyev, S.M.; Zvyagin, A.V.; Khaydukov, E.V. Ultraviolet Phototoxicity of Upconversion Nanoparticles Illuminated with Near-Infrared Light. Nanoscale 2017, 9, 14921–14928. [Google Scholar] [CrossRef] [PubMed]
- Trifanova, E.M.; Nikolaeva, M.E.; Popov, V.K. Synthesis and Characterization of NaYF4:Yb3+:Er3+/NaYF4 Upconversion Nanophosphors. Inorg. Mater. Appl. Res. 2022, 13, 426–433. [Google Scholar] [CrossRef]
- Su, Q.; Han, S.; Xie, X.; Zhu, H.; Chen, H.; Chen, C.-K.; Liu, R.-S.; Chen, X.; Wang, F.; Liu, X. The Effect of Surface Coating on Energy Migration-Mediated Upconversion. J. Am. Chem. Soc. 2012, 134, 20849–20857. [Google Scholar] [CrossRef]
- Shah, S.; Liu, J.-J.; Pasquale, N.; Lai, J.; McGowan, H.; Pang, Z.P.; Lee, K.-B. Hybrid Upconversion Nanomaterials for Optogenetic Neuronal Control. Nanoscale 2015, 7, 16571–16577. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Lin, Z.; Kong, L.; Xing, Y.; Lin, N.; Zhang, Z.; Chen, B.-H.; Liu, X.-Y. Meso-Functionalization of Silk Fibroin by Upconversion Fluorescence and Near Infrared In Vivo Biosensing. Adv. Funct. Mater. 2017, 27, 1700628. [Google Scholar] [CrossRef]
- Yan, B.; Boyer, J.-C.; Habault, D.; Branda, N.R.; Zhao, Y. Near Infrared Light Triggered Release of Biomacromolecules from Hydrogels Loaded with Upconversion Nanoparticles. J. Am. Chem. Soc. 2012, 134, 16558–16561. [Google Scholar] [CrossRef]
- Zhang, W.; Jia, H.; Ye, H.; Dai, T.; Yin, X.; He, J.; Chen, R.; Wang, Y.; Pang, X. Facile Fabrication of Transparent and Upconversion Photoluminescent Nanofiber Mats with Tunable Optical Properties. ACS Omega 2018, 3, 8220–8225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, Y.; Luu, Q.A.N.; Zhao, Y.; Fong, H.; May, P.S.; Jiang, C. Upconversion Polymeric Nanofibers Containing Lanthanide-Doped Nanoparticles via Electrospinning. Nanoscale 2012, 4, 7369. [Google Scholar] [CrossRef] [PubMed]
- Würth, C.; Kaiser, M.; Wilhelm, S.; Grauel, B.; Hirsch, T.; Resch-Genger, U. Excitation Power Dependent Population Pathways and Absolute Quantum Yields of Upconversion Nanoparticles in Different Solvents. Nanoscale 2017, 9, 4283–4294. [Google Scholar] [CrossRef] [PubMed]
- Später, T.; Mariyanats, A.O.; Syachina, M.A.; Mironov, A.V.; Savelyev, A.G.; Sochilina, A.V.; Menger, M.D.; Vishnyakova, P.A.; Kananykhina, E.Y.; Fatkhudinov, T.K.; et al. In Vitro and in Vivo Analysis of Adhesive, Anti-Inflammatory, and Proangiogenic Properties of Novel 3D Printed Hyaluronic Acid Glycidyl Methacrylate Hydrogel Scaffolds for Tissue Engineering. ACS Biomater. Sci. Eng. 2020, 6, 5744–5757. [Google Scholar] [CrossRef]
- Arppe, R.; Hyppänen, I.; Perälä, N.; Peltomaa, R.; Kaiser, M.; Würth, C.; Christ, S.; Resch-Genger, U.; Schäferling, M.; Soukka, T. Quenching of the Upconversion Luminescence of NaYF 4:Yb 3+,Er 3+ and NaYF 4:Yb 3+,Tm 3+ Nanophosphors by Water: The Role of the Sensitizer Yb 3+ in Non-Radiative Relaxation. Nanoscale 2015, 7, 11746–11757. [Google Scholar] [CrossRef] [Green Version]
- Krainov, A.D.; Mokeeva, A.M.; Sergeeva, E.A.; Agrba, P.D.; Kirillin, M.Y. Optical Properties of Mouse Biotissues and Their Optical Phantoms. Opt. Spectrosc. 2013, 115, 193–200. [Google Scholar] [CrossRef]
- Sochilina, A.V.; Savelyev, A.G.; Akasov, R.A.; Zubov, V.P.; Khaydukov, E.V.; Generalova, A.N. Preparing Modified Hyaluronic Acid with Tunable Content of Vinyl Groups for Use in Fabrication of Scaffolds by Photoinduced Crosslinking. Russ. J. Bioorg. Chem. 2021, 47, 828–836. [Google Scholar] [CrossRef]
- Sochilina, A.V.; Savelyev, A.G.; Demina, P.A.; Sizova, S.V.; Zubov, V.P.; Khaydukov, E.V.; Generalova, A.N. Quantitative Detection of Double Bonds in Hyaluronic Acid Derivative via Permanganate Ion Reduction. Meas. Sci. Technol. 2019, 30, 075102. [Google Scholar] [CrossRef]
- Rocheva, V.V.; Khochenkov, D.A.; Generalova, A.N.; Nechaev, A.V.; Semchishen, V.A.; Stepanova, E.V.; Sokolov, V.I.; Khaydukov, E.V.; Panchenko, V.Y. Upconversion Nanoparticles for Tumor Imaging with Near-Infrared Radiation. Bull. Russ. Acad. Sci. Phys. 2016, 80, 467–470. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifanova, E.M.; Khvorostina, M.A.; Mariyanats, A.O.; Sochilina, A.V.; Nikolaeva, M.E.; Khaydukov, E.V.; Akasov, R.A.; Popov, V.K. Natural and Synthetic Polymer Scaffolds Comprising Upconversion Nanoparticles as a Bioimaging Platform for Tissue Engineering. Molecules 2022, 27, 6547. https://doi.org/10.3390/molecules27196547
Trifanova EM, Khvorostina MA, Mariyanats AO, Sochilina AV, Nikolaeva ME, Khaydukov EV, Akasov RA, Popov VK. Natural and Synthetic Polymer Scaffolds Comprising Upconversion Nanoparticles as a Bioimaging Platform for Tissue Engineering. Molecules. 2022; 27(19):6547. https://doi.org/10.3390/molecules27196547
Chicago/Turabian StyleTrifanova, Ekaterina M., Maria A. Khvorostina, Aleksandra O. Mariyanats, Anastasia V. Sochilina, Maria E. Nikolaeva, Evgeny V. Khaydukov, Roman A. Akasov, and Vladimir K. Popov. 2022. "Natural and Synthetic Polymer Scaffolds Comprising Upconversion Nanoparticles as a Bioimaging Platform for Tissue Engineering" Molecules 27, no. 19: 6547. https://doi.org/10.3390/molecules27196547
APA StyleTrifanova, E. M., Khvorostina, M. A., Mariyanats, A. O., Sochilina, A. V., Nikolaeva, M. E., Khaydukov, E. V., Akasov, R. A., & Popov, V. K. (2022). Natural and Synthetic Polymer Scaffolds Comprising Upconversion Nanoparticles as a Bioimaging Platform for Tissue Engineering. Molecules, 27(19), 6547. https://doi.org/10.3390/molecules27196547






