Cobalt Ferrite Particles Produced by Sol-Gel Autocombustion and Embedded in Polysilane: An Innovative Route to Magnetically-Induced Fluorescence Composites
Abstract
:1. Introduction
2. Results and Discussion
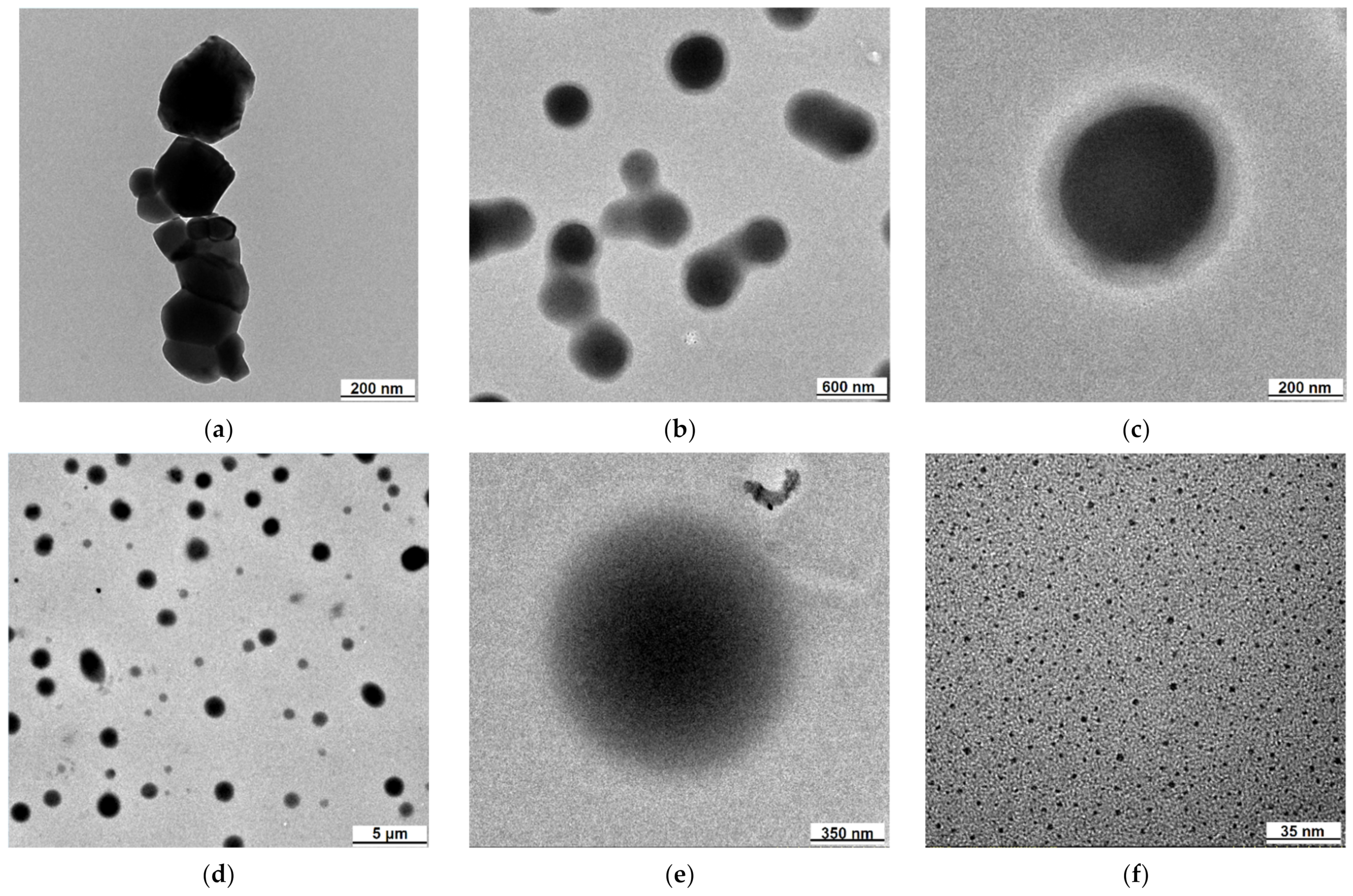
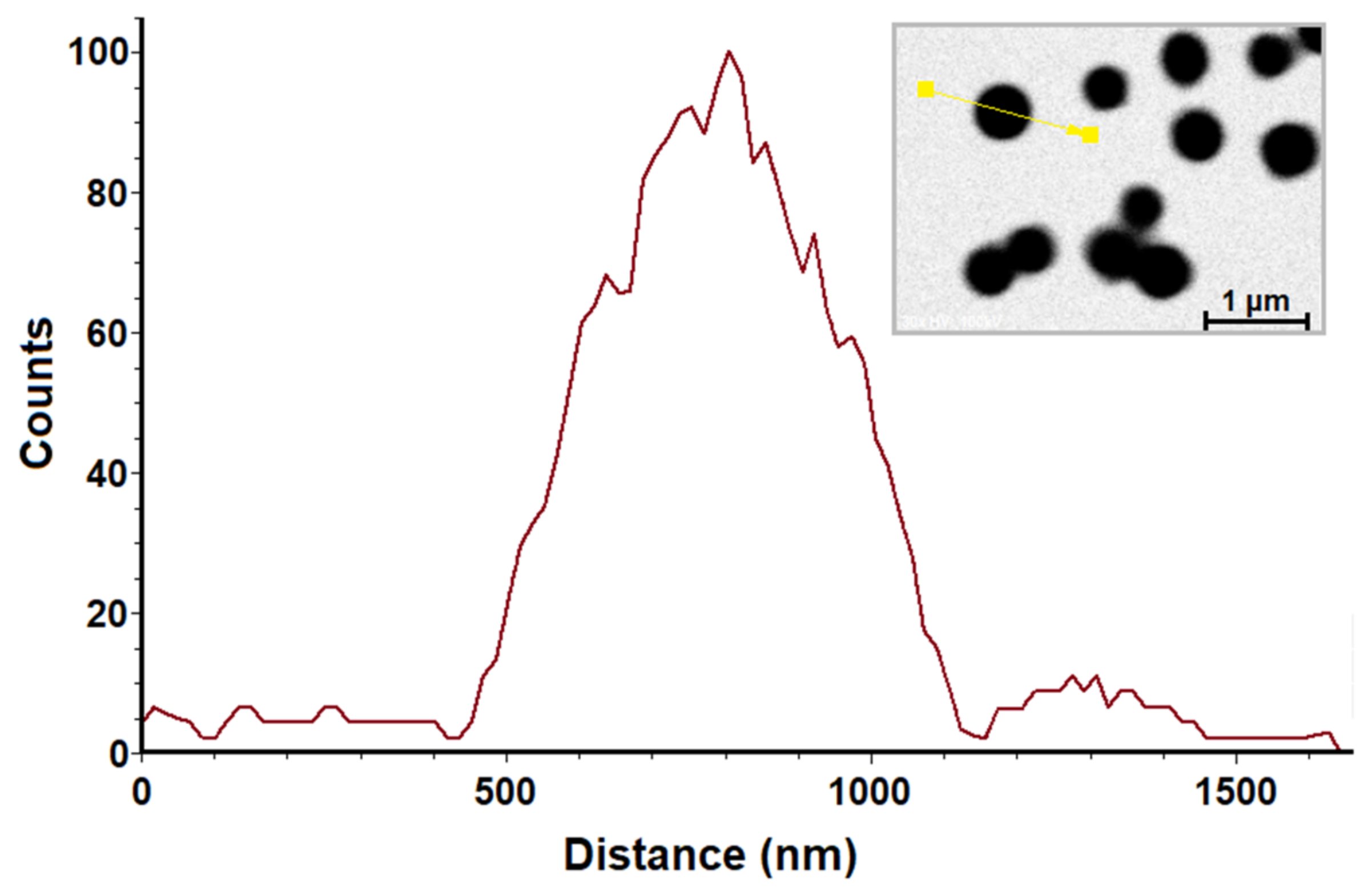
2.1. Transmission Electron Microscopy (TEM)
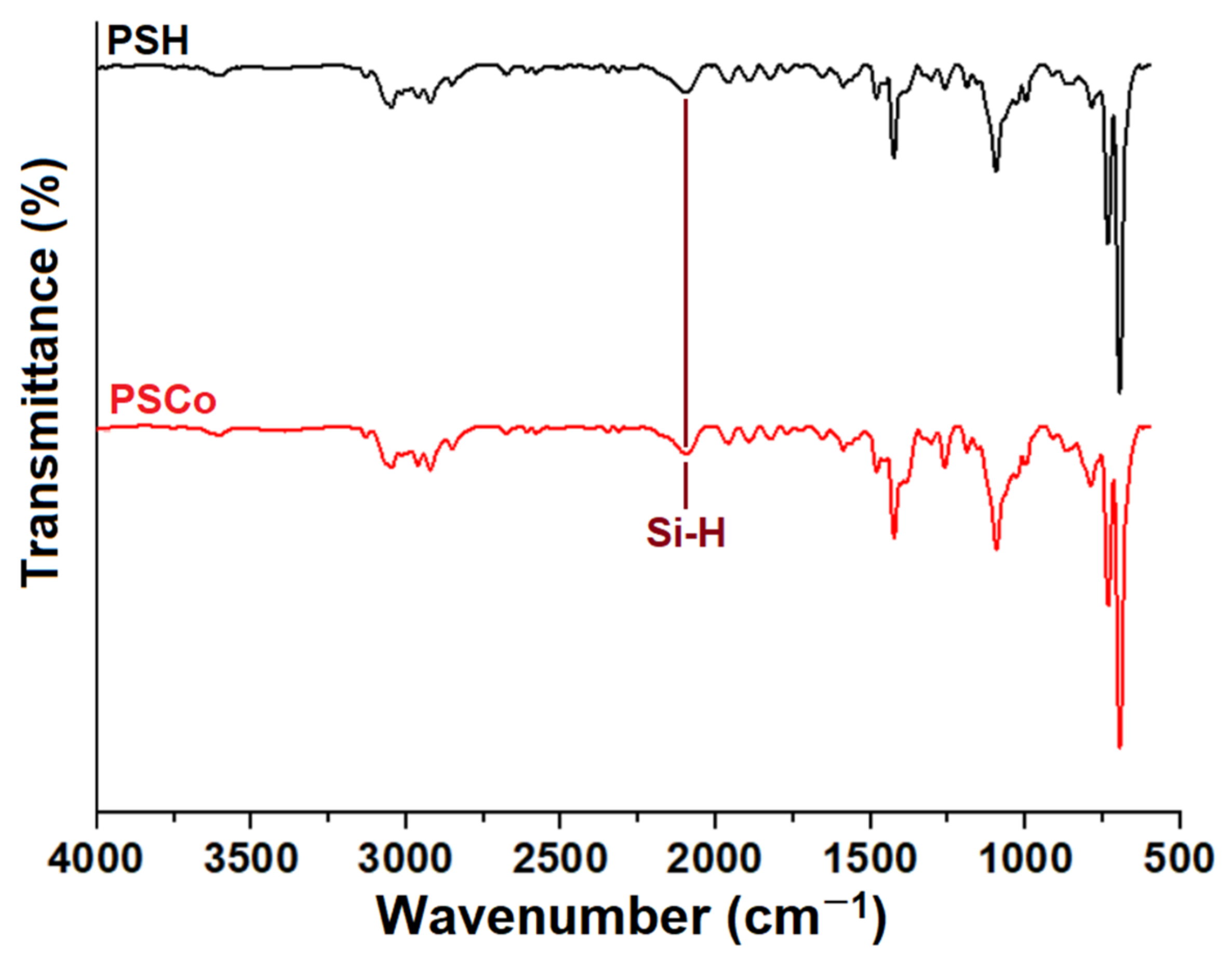
2.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3. Magnetic Properties
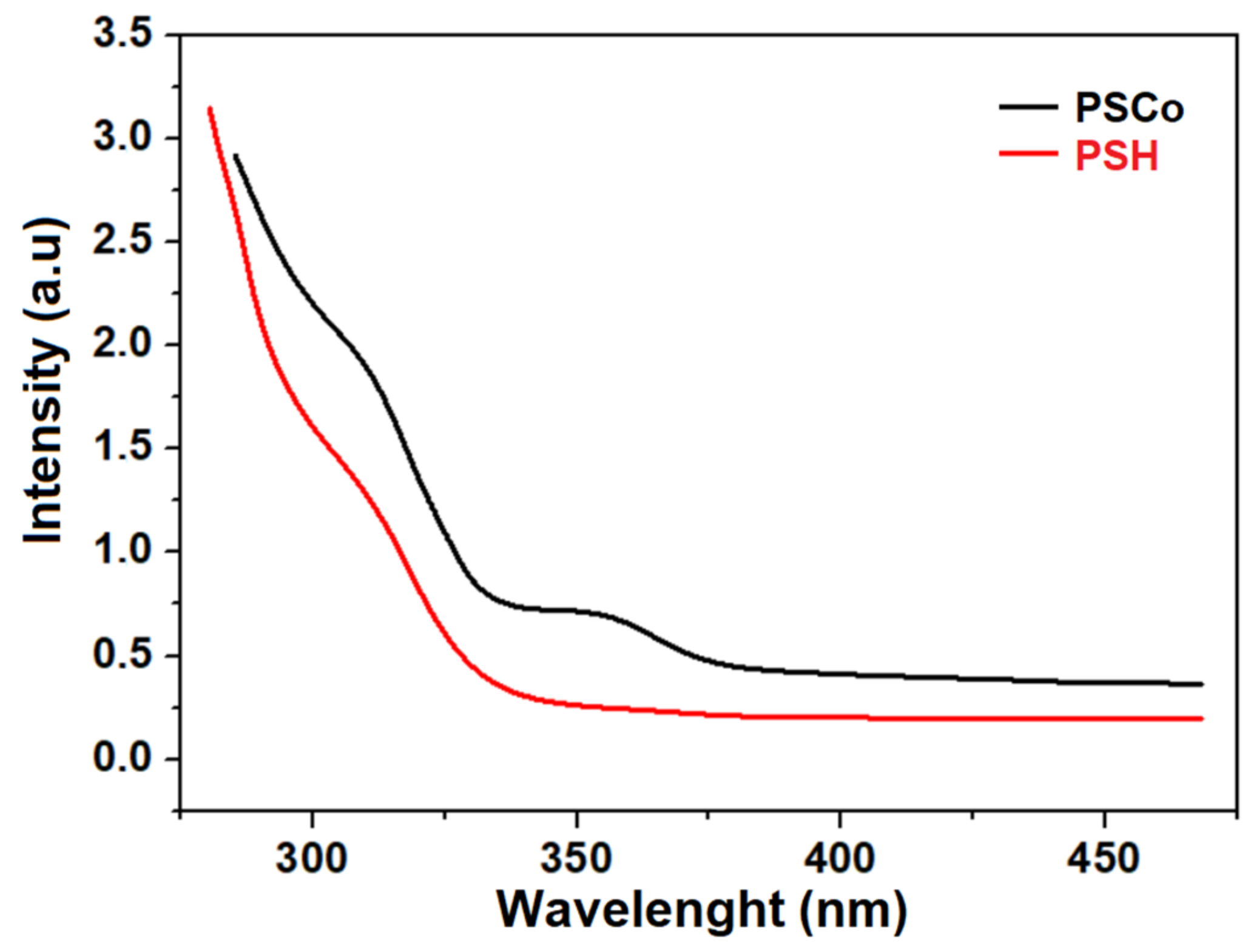
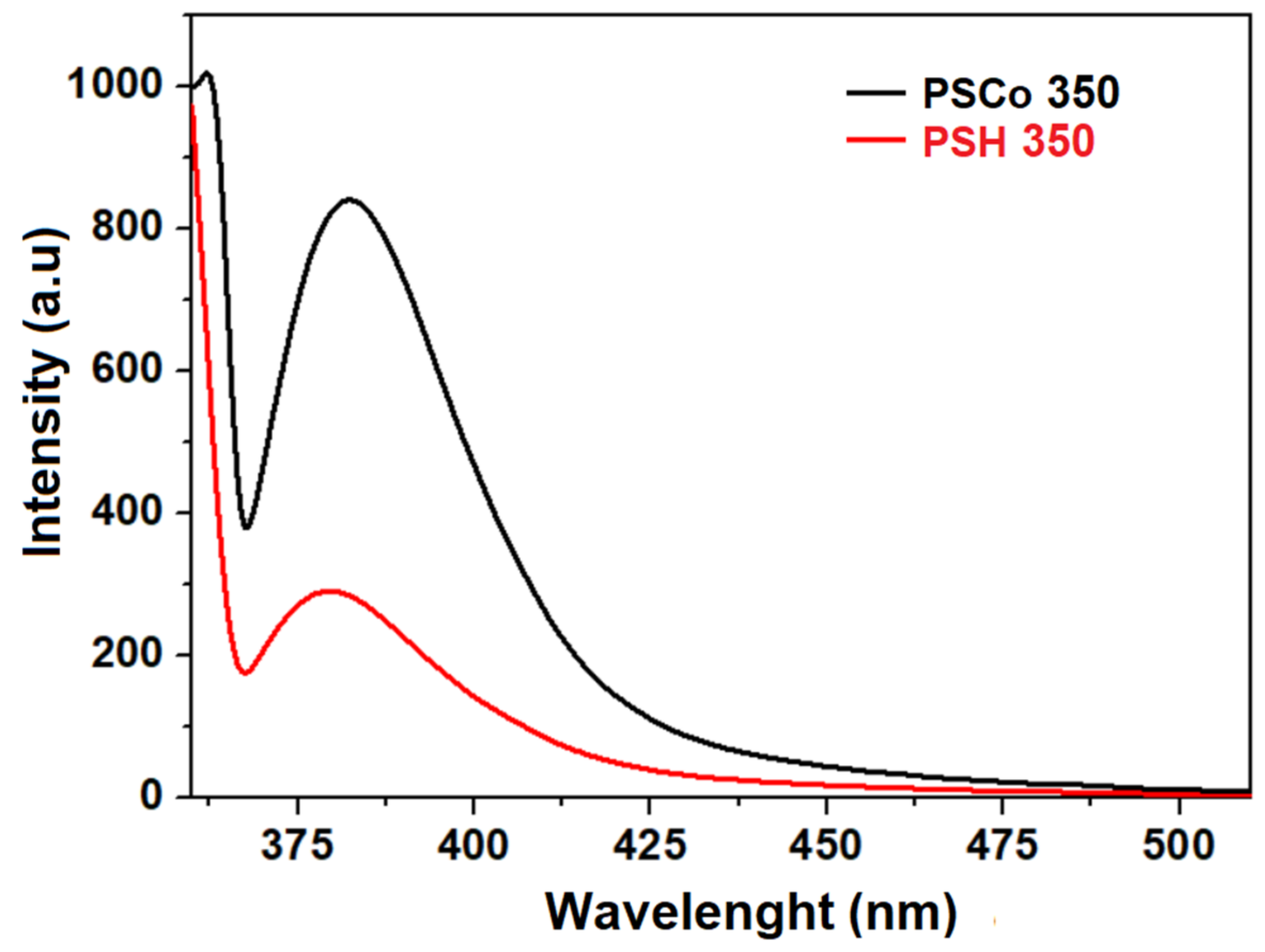
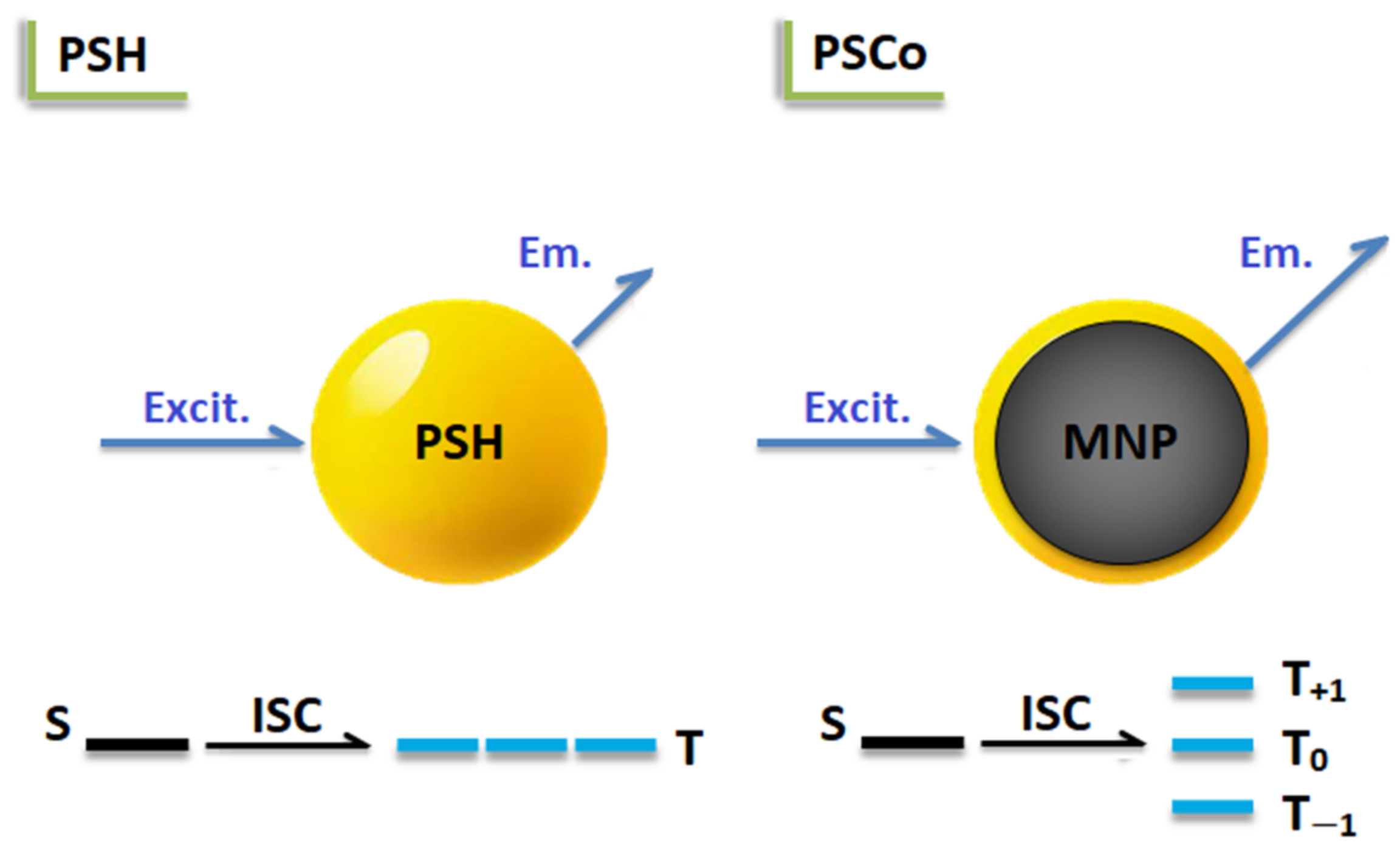
2.4. Optical Properties
3. Materials and Methods
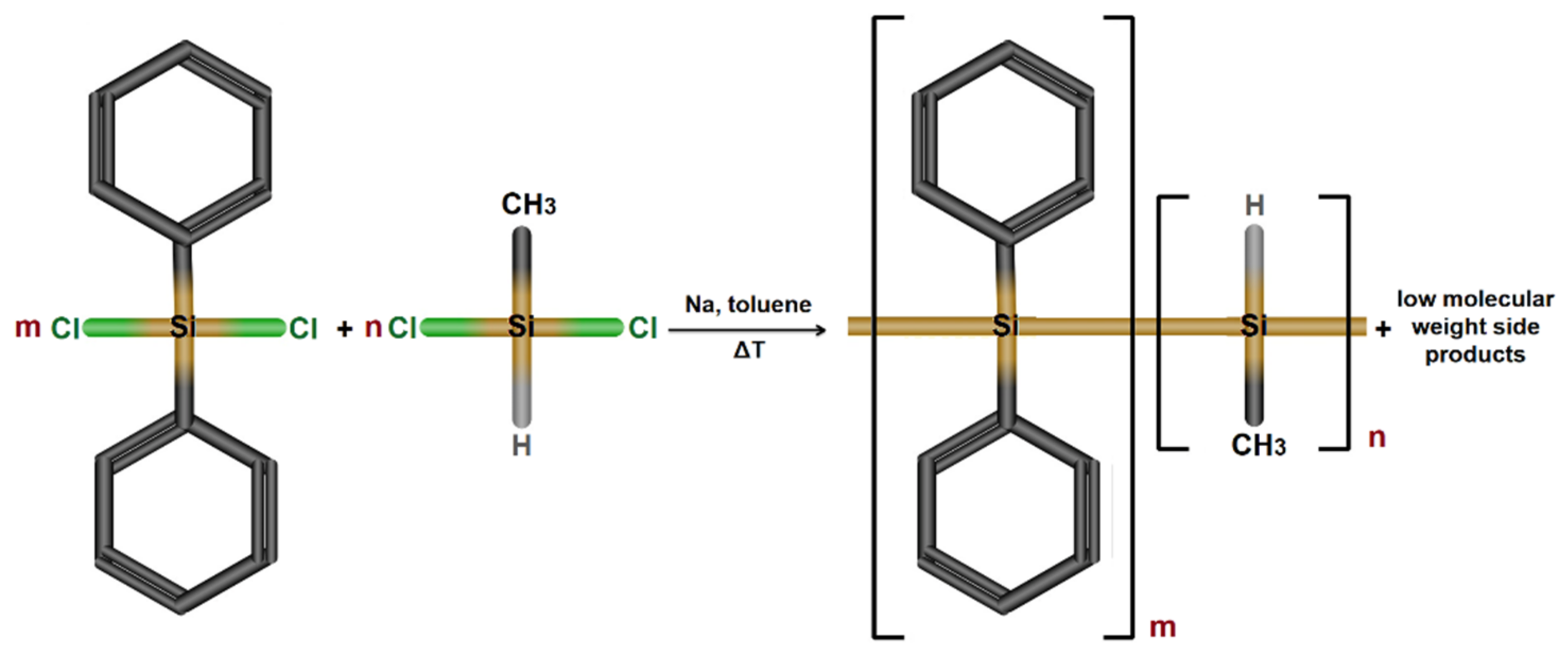
3.1. Synthesis of Polysilane, Cobalt Ferrite, and Composite Particles
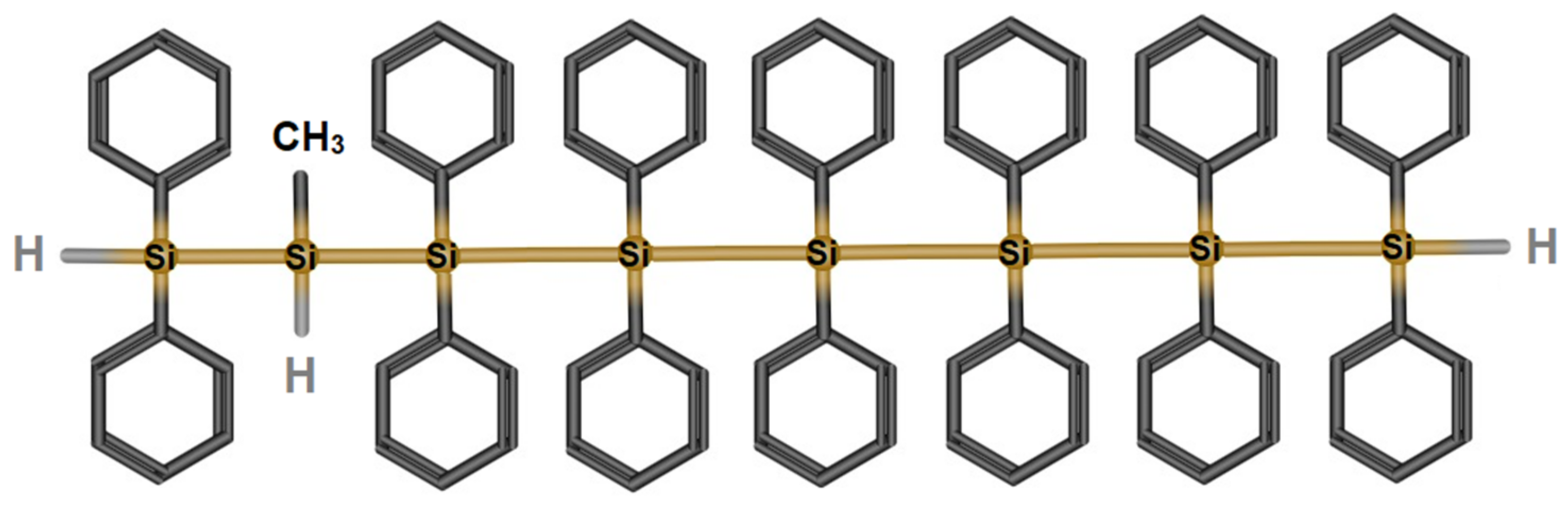
3.1.1. Poly[diphenyl-co-methyl(H)]silane Synthesis (PSH)
3.1.2. Synthesis of Cobalt Ferrite Particles (CoFe2O4)
3.1.3. Fluoromagnetic Particles Synthesis (PSCo)
3.2. General Methods of Characterization
3.3. Computational Protocol
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hirota, J.; Usui, K.; Fuchi, Y.; Sakuma, M.; Matsumoto, S.; Hagihara, R.; Karasawa, S. Fluorescence Properties and Exciplex Formation of Emissive Naphthyridine Derivatives: Applications as Sensors for Individual Amine Species. Chem. Eur. J. 2019, 25, 14943–14952. [Google Scholar] [CrossRef]
- Shahzad, A.; Köhler, G.; Knapp, M.; Gaubitzer, E.; Puchinger, M.; Edetsberger, M. Emerging applications of fluorescence spectroscopy in medical microbiology field. J. Transl. Med. 2009, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.A.; Gupta, N.; Siddiqui, Q.T.; Parab, P.; Palit, D.; Bose, S.; Agarwal, N. Synthesis of acridone-naphthylamine derivative and its thermally-activated delayed fluorescence studies for application in OLEDs. J. Chem. Sci. 2019, 131, 94. [Google Scholar] [CrossRef]
- Aaron, J.-J.; Trajkovska, S. Fluorescence studies of anti-cancer drugs–analytical and biomedical applications. Curr. Drug Targets 2006, 7, 1067–1081. [Google Scholar] [CrossRef] [PubMed]
- Otosu, T.; Ishii, K.; Tahara, T. Multifocus fluorescence correlation spectroscopy with spatially separated excitation beams. Bull. Chem. Soc. Jpn. 2019, 92, 1495–1502. [Google Scholar] [CrossRef]
- Seefeldt, B.; Kasper, R.; Seidel, T.; Tinnefeld, P.; Dietz, K.-J.; Heilemann, M.; Sauer, M. Fluorescent proteins for single-molecule fluorescence applications. J. Biophotonics 2008, 1, 74–82. [Google Scholar] [CrossRef]
- Lingeman, H.; Underberg, W.J.M.; Takadate, A.; Hulshoff, A. Fluorescence detection in high performance liquid chromatography. J. Liq. Chromatogr. 1985, 8, 789–874. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, C.-P.; Wu, Y.; Tian, H. Synthesis and fluorescence properties of triad compounds with aromatic sulfur bridges. Dyes Pigm. 2001, 51, 127–136. [Google Scholar] [CrossRef]
- Sun, C.; Du, W.; Wang, B.; Dong, B.; Wang, B. Research progress of near-infrared fluorescence probes based on indole heptamethine cyanine dyes in vivo and in vitro. BMC Chem. 2020, 14, 21. [Google Scholar] [CrossRef]
- Barman, D.; Gogoi, R.; Narang, K.; Iyer, P.K. Recent developments on multi-functional metal-free mechanochromic luminescence and thermally activated delayed fluorescence organic materials. Front. Chem. 2020, 8, 483. [Google Scholar] [CrossRef]
- Ahumada, G.; Borkowska, M. Fluorescent polymers conspectus. Polymers 2022, 14, 1118. [Google Scholar] [CrossRef]
- Ansari, S.; Masoum, S. Recent advances and future trends on molecularly imprinted polymer-based fluorescence sensors with luminescent carbon dots. Talanta 2021, 223, 121411. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Phatake, R.S.; Nabha Barnea, S.; Zerby, N.; Zhu, J.-J.; Shikler, R.; Lemcoff, N.G.; Jelinek, R. Fluorescent self-healing carbon dot/polymer gels. ACS Nano 2019, 13, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Ghahfarokhi, A.J.; Tang, Y. Aggregation-induced emission fluorescent gels: Current trends and future perspectives. In Aggregation-Induced Emission (Topics in Current Chemistry Collections); Tang, Y., Tang, B.Z., Eds.; Springer: Cham, Switzerland, 2022; pp. 247–286. [Google Scholar]
- Collot, M.; Schild, J.; Fam, K.T.; Bouchaala, R.; Klymchenko, A.S. Stealth and bright monomolecular fluorescent organic nanoparticles based on folded amphiphilic polymer. ACS Nano 2020, 14, 13924–13937. [Google Scholar] [CrossRef] [PubMed]
- Chyasnavichyus, M.; Tsyalkovsky, V.; Zdyrko, B.; Luzinov, I. Tuning fluorescent response of nanoscale film with polymer grafting. Macromol. Rapid Commun. 2012, 33, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.B.; Leitao, E.M. Properties and applications of polysilanes. Appl. Organomet. Chem. 2020, 34, e5402. [Google Scholar] [CrossRef]
- Miller, R.D.; Michl, J. Polysilane high polymers. Chem. Rev. 1989, 89, 1359–1410. [Google Scholar] [CrossRef]
- Sacarescu, L.; Mangalagiu, I.; Simionescu, M.; Sacarescu, G.; Ardeleanu, R. Polysilanes. A new route toward high performance EL devices. Macromol. Symp. 2008, 267, 123–128. [Google Scholar] [CrossRef]
- Kipping, F.S. CCCVIII.—Organic derivatives of silicon. Part XXX. Complex silicohydrocarbons [SiPh2]n. J. Chem. Soc. Trans. 1924, 125, 2291–2297. [Google Scholar] [CrossRef]
- Sacarescu, G.; Voiculescu, N.; Marcu, M.; Sacarescu, L.; Ardeleanu, R.; Simionescu, M. Polyhydrosilanes I. Synthesis. J. Macromol. Sci. A 1997, 34, 509–516. [Google Scholar] [CrossRef]
- Sacarescu, L.; Bockholt, A.; Siokou, A.; Simionescu, M.; Sacarescu, G. Structural and optical properties of polyhydrosilanes. Macromol. Chem. Phys. 2009, 210, 2015–2021. [Google Scholar] [CrossRef]
- Sacarescu, L.; Simionescu, M.; Sacarescu, G. Synthesis of polyhydrosilanes-graft-poly(ethyleneglycol)methyl ether. Int. J. Polym. Anal. 2011, 16, 360–368. [Google Scholar] [CrossRef]
- Chibac, A.L.; Simionescu, M.; Sacarescu, G.; Buruiana, E.C.; Sacarescu, L. New dansyl labeled polysilane: Synthesis, characterization and sensor application. Eur. Polym. J. 2017, 95, 82–92. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Tang, H.; Zeng, H.; Qin, J. Polysilanes with NLO chromophores as pendant groups by utilizing different synthetic strategies. J. Organomet. Chem. 2003, 685, 258–268. [Google Scholar] [CrossRef]
- Simionescu, M.; Sacarescu, L.; Sacarescu, G.; Mantu, D.; Mangalagiu, I. Silicon based materials for biooptoelectronics. Rom. Biotechnol. Lett. 2008, 14, 4397–4403. [Google Scholar]
- Chen, J.; Mao, L.; Qi, H.; Xu, D.; Huag, H.; Liu, M.; Wen, Y.; Deng, F.; Zhang, X.; Wei, Y. Preparation of fluorescent cellulose nanocrystal polymer composites with thermo-responsiveness through light-induced ATRP. Cellulose 2020, 27, 743–753. [Google Scholar] [CrossRef]
- Siddique, A.B.; Singh, V.P.; Pramanick, A.K.; Ray, M. Amorphous carbon dot and chitosan based composites as fluorescent inks and luminescent films. Mater. Chem. Phys. 2020, 249, 122984. [Google Scholar] [CrossRef]
- Pan, M.; Xie, X.; Liu, K.; Yang, J.; Hong, L.; Wang, S. Fluorescent carbon quantum dots—synthesis, functionalization and sensing application in food analysis. Nanomaterials 2020, 10, 930. [Google Scholar] [CrossRef]
- Yang, L.; Song, Y.; Wang, L. Multi-emission metal–organic framework composites for multicomponent ratiometric fluorescence sensing: Recent developments and future challenges. J. Mater. Chem. B 2020, 8, 3292–3315. [Google Scholar] [CrossRef]
- Baharvand, H. Preparation and characterization of fluorescent polymer magnetic particles. J. Appl. Polym. Sci. 2008, 109, 1823–1828. [Google Scholar] [CrossRef]
- Pospiskova, K.; Mohr, G.J.; Prochazkova, J.; Timko, M.; Rajnak, M.; Paulovicova, K.; Kopcansky, P.; Giovannini, G.; Boesel, L.F.; Safarik, I. Scalable production of magnetic fluorescent cellulose microparticles. Cellulose 2021, 28, 7675–7685. [Google Scholar] [CrossRef]
- Lansalot, M.; Sabor, M.; Elaissari, A.; Pichot, C. Elaboration of fluorescent and highly magnetic submicronic polymer particles via a stepwise heterocoagulation process. Colloid Polym. Sci. 2020, 283, 1267–1277. [Google Scholar] [CrossRef]
- Karkan, S.F.; Mohammadhosseini, M.; Panahi, Y.; Milani, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, E.; Hosseini, A.; Davaran, S. Magnetic nanoparticles in cancer diagnosis and treatment: A review. Artif. Cell. Nanomed. B 2017, 45, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Li, D.; Pan, D.S.; Li, S.J.; Zhang, Z.D. Recent developments of rare-earth-free hard-magnetic materials. Sci. China Phys. Mech. Astron. 2016, 59, 617501. [Google Scholar] [CrossRef]
- Bindra Narang, S.; Pubby, K. Nickel Spinel Ferrites: A review. J. Magn. Magn. 2021, 519, 167163. [Google Scholar] [CrossRef]
- Samoila, P.; Cojocaru, C.; Sacarescu, L.; Pascariu Dorneanu, P.; Domocos, A.-A.; Rotaru, A. Remarkable catalytic properties of rare-earth doped nickel ferrites synthesized by sol-gel auto-combustion with maleic acid as fuel for CWPO of dyes. Appl. Catal. B 2017, 202, 21–32. [Google Scholar] [CrossRef]
- Jia, Z.; Ren, D.; Liang, Y.; Zhu, R. A new strategy for the preparation of porous zinc ferrite nanorods with subsequently light-driven photocatalytic activity. Mater. Lett. 2011, 65, 3116–3119. [Google Scholar] [CrossRef]
- Rajput, J.K.; Kaur, G. Synthesis and applications of CoFe2O4 nanoparticles for multicomponent reactions. Catal. Sci. Technol. 2014, 4, 142–151. [Google Scholar] [CrossRef]
- El-Shobaky, G.; Turky, A.; Mostafa, N.; Mohamed, S. Effect of preparation conditions on physicochemical, surface and catalytic properties of cobalt ferrite prepared by coprecipitation. J. Alloys Compd. 2010, 493, 415–422. [Google Scholar] [CrossRef]
- Pillai, V.; Shah, D. Synthesis of high-coercivity cobalt ferrite particles using water-in-oil microemulsions. J. Magn. Magn. Mater. 1996, 163, 243–248. [Google Scholar] [CrossRef]
- Merrifield, R.E. Theory of Magnetic Field Effects on the Mutual Annihilation of Triplet Excitons. J. Chem. Phys. 1968, 48, 4318–4319. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Yuan, Q.; Lu, L. Fluorescence-enhanced gadolinium-doped zinc oxide quantum dots for magnetic resonance and fluorescence imaging. Biomaterials 2011, 32, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, S. Magnetic-metals sunflower nanocomposites for significant fluorescence enhancement. Inorg. Chim. Acta 2022, 529, 120652. [Google Scholar] [CrossRef]
- Blumling, D.E.; McGill, S.; Knappenberger, K.L. The influence of applied magnetic fields on the optical properties of zero- and one-dimensional CdSe nanocrystals. Nanoscale 2013, 5, 9049–9056. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, J.; Gao, J.; Wang, Z.; Xu, H.; Cai, M.; Jiang, J.; Tian, Z.; Wang, H. Magnetic-field-enabled resolution enhancement in super-resolution imaging. Phys. Chem. Chem. Phys. 2015, 17, 6722–6727. [Google Scholar] [CrossRef]
- Chen, Q.; Shi, C.; Zhang, C.; Pu, S.; Wang, R.; Wu, X.; Wang, X.; Xue, F.; Pan, D.; Xiao, M. Magnetic enhancement of photoluminescence from blue-luminescent graphene quantum dots. Appl. Phys. Lett. 2016, 108, 061904. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, Z.; Wang, X.; Ma, Z.; Wang, S.; Han, Y.; Han, J. Large photoluminescence enhancement of Er3+: GdVO4 crystal in both green and middle infrared regions under moderate low magnetic fields. Opt. Mater. Express 2016, 6, 3446–3454. [Google Scholar] [CrossRef]
- Samoila, P.; Cojocaru, C.; Mahu, E.; Ignat, M.; Harabagiu, V. Boosting catalytic wet-peroxide-oxidation performances of cobalt ferrite by doping with lanthanides for organic pollutants degradation. J. Environ. Chem. Eng. 2021, 9, 104961. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01, Gaussian Inc.: Wallingford, CT, USA, 2010.
- Sacarescu, L.; Cojocaru, C.; Ardeleanu, R.; Fortuna, M.; Sacarescu, G.; Simionescu, M. Dual-emissive polydiphenylsilane nanocomposite: Effect of N,N′bis(4-hydroxysalicylidene)-1,2-phenylenediamine-Zn complex. Polym. Adv. Technol. 2016, 27, 115–124. [Google Scholar] [CrossRef]
- Sacarescu, L.; Simionescu, M.; Sacarescu, G.; Hitruc, E.G. Photocatalytic synthesis of silver nanoparticles using polysilane initiator. J. Nanopart. Res. 2011, 13, 997–1005. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.; Wang, H.; Xu, C.; Yang, S. Fe3O4 nanoparticles induced magnetic field effect on efficiency enhancement of P3HT:PCBM bulk heterojunction polymer solar cells. Sol. Energy Mater. Sol. Cells 2011, 95, 2880–2885. [Google Scholar] [CrossRef]
- Sun, C.-J.; Wu, Y.; Xu, Z.; Hu, B.; Bai, J.; Wang, J.-P.; Shen, J. Enhancement of quantum efficiency of organic light emitting devices by doping magnetic nanoparticles. Appl. Phys. Lett. 2007, 90, 232110. [Google Scholar] [CrossRef]
- Hu, B.; Wu, Y.; Zhang, Z.; Dai, S.; Shen, J. Effects of ferromagnetic nanowires on singlet and triplet exciton fractions in fluorescent and phosphorescent organic semiconductors. Appl. Phys. Lett. 2006, 88, 022114. [Google Scholar] [CrossRef]
- Hu, B.; Yan, L. Magnetic Field Effects in Organic Semiconducting Materials and Devices. In Organic Electronics Materials: Processing, Devices and Applications; So, F., Ed.; CRC Press: New York, NY, USA, 2010; pp. 111–140. [Google Scholar]
- Michl, J.; Downing, J.W.; Karatsu, T.; McKinley, A.J.; Poggi, G.; Wallraff, G.M.; Sooriyakumaran, R.; Miller, R.D. Solution photochemistry of poly(dialkylsi1anes): A new class of photoresists. Pure. Appl. Chem. 1988, 60, 959–972. [Google Scholar] [CrossRef]
- Skryshevski, Y.; Piryatinski, Y.; Vakhnin, A.; Blonsky, I.; Kadashchuk, A.; Nespurek, S. Triplet state spectroscopy of σ-conjugated poly[methyl(phenyl)silylene]. Opt. Mater. 2007, 30, 384–392. [Google Scholar] [CrossRef]
- Simionescu, M.; Sacarescu, L.; Sacarescu, G. Microwave-assisted synthesis of functional polysilanes. Des. Monomers Polym. 2012, 15, 127–136. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.; Millam, J. GaussView; Version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Krieger, E.; Vriend, G. YASARA View-molecular graphics for all devices—from smartphones to workstations. Bioinformatics 2014, 30, 2981–2982. [Google Scholar] [CrossRef] [Green Version]

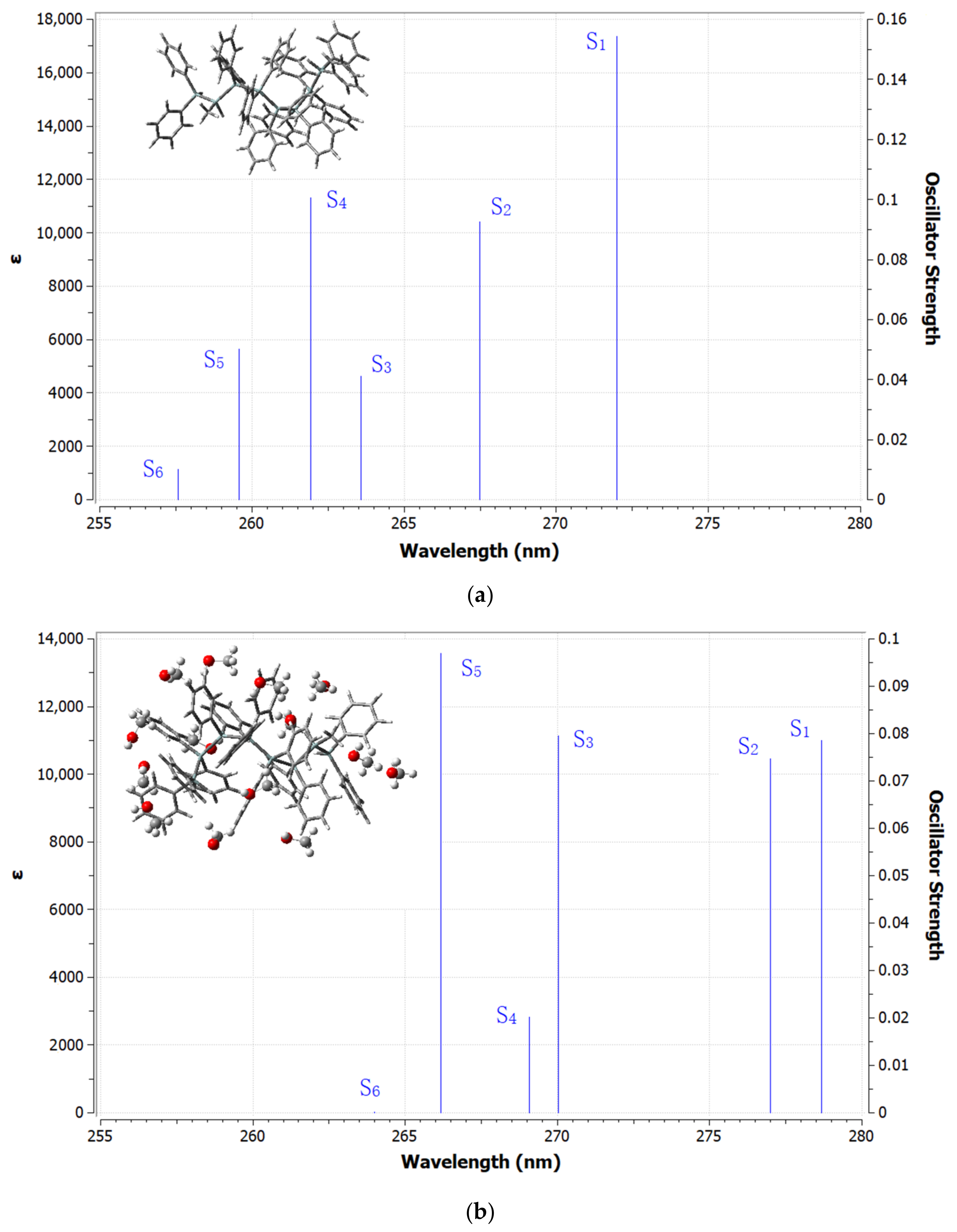
| Excited State | Λ (nm) | E (eV) | f | LHE% | Main Transition Assignments (Contribution, %) |
|---|---|---|---|---|---|
| S0 → S1 | 272.00 | 4.5582 | 0.1543 | 29.90% | HOMO → LUMO (80.57%) |
| HOMO-1 → LUMO (10.66%) | |||||
| HOMO → LUMO + 1 (2.49%) | |||||
| S0 → S2 | 267.48 | 4.6353 | 0.0925 | 19.18% | HOMO → LUMO + 1 (69.43%) |
| HOMO-1 → LUMO (12.48%) | |||||
| HOMO → LUMO (7.28%) | |||||
| S0 → S3 | 263.58 | 4.7038 | 0.0410 | 9.01% | HOMO-1 → LUMO (51.83%) |
| HOMO-1 → LUMO + 1 (16.80%) | |||||
| HOMO → LUMO + 1 (15.19%) | |||||
| S0 → S4 | 261.93 | 4.7335 | 0.1005 | 20.66% | HOMO-1 → LUMO + 1 (41.77%) |
| HOMO → LUMO + 2 (25.65%) | |||||
| HOMO-1 → LUMO (9.25%) | |||||
| S0 → S5 | 259.57 | 4.7765 | 0.0502 | 10.92% | HOMO → LUMO + 2 (52.06%) |
| HOMO-1 → LUMO + 1 (17.84%) | |||||
| HOMO-1 → LUMO + 3 (3.33%) | |||||
| S0 → S6 | 257.59 | 4.8133 | 0.0102 | 2.32% | HOMO-2 → LUMO (42.84%) |
| HOMO-1 → LUMO + 2 (15.98%) | |||||
| HOMO → LUMO + 3 (7.80%) |
| Excited State | Λ (nm) | E (eV) | f | LHE% | Main Transition Assignments (Contribution, %) |
|---|---|---|---|---|---|
| S0 → S1 | 278.66 | 4.4493 | 0.0785 | 16.54% | HOMO → LUMO (87.00%) |
| HOMO → LUMO (3.64) | |||||
| S0 → S2 | 277.00 | 4.4759 | 0.0747 | 15.80% | HOMO-1 → LUMO (76.48%) |
| HOMO → LUMO + 1 (10.76%) | |||||
| HOMO → LUMO + 2 (2.77%) | |||||
| S0 → S3 | 270.03 | 4.5915 | 0.0795 | 16.73% | HOMO → LUMO + 1 (75.31%) |
| HOMO-1 → LUMO (12.61%) | |||||
| HOMO-1 → LUMO + 3 (2.63%) | |||||
| S0 → S4 | 269.09 | 4.6075 | 0.0202 | 4.54% | HOMO-1 → LUMO + 1 (74.19%) |
| HOMO → LUMO + 3 (7.30%) | |||||
| HOMO → LUMO + 2 (4.26%) | |||||
| S0 → S5 | 266.18 | 4.6580 | 0.0970 | 20.02% | HOMO → LUMO + 2 (67.57%) |
| HOMO-1 → LUMO + 2 (15.83%) | |||||
| HOMO-1 → LUMO (2.30%) | |||||
| S0 → S6 | 263.99 | 4.6966 | 0.0002 | 0.05% | HOMO-1 → LUMO + 2 (64.07%) |
| HOMO → LUMO + 2 (12.91%) | |||||
| HOMO → LUMO + 3 (2.79%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samoila, P.; Cojocaru, C.; Simionescu, M.; Sacarescu, G.; Roman, G.; Enache, A.-C.; Sacarescu, L. Cobalt Ferrite Particles Produced by Sol-Gel Autocombustion and Embedded in Polysilane: An Innovative Route to Magnetically-Induced Fluorescence Composites. Molecules 2022, 27, 6393. https://doi.org/10.3390/molecules27196393
Samoila P, Cojocaru C, Simionescu M, Sacarescu G, Roman G, Enache A-C, Sacarescu L. Cobalt Ferrite Particles Produced by Sol-Gel Autocombustion and Embedded in Polysilane: An Innovative Route to Magnetically-Induced Fluorescence Composites. Molecules. 2022; 27(19):6393. https://doi.org/10.3390/molecules27196393
Chicago/Turabian StyleSamoila, Petrisor, Corneliu Cojocaru, Mihaela Simionescu, Gabriela Sacarescu, Gheorghe Roman, Andra-Cristina Enache, and Liviu Sacarescu. 2022. "Cobalt Ferrite Particles Produced by Sol-Gel Autocombustion and Embedded in Polysilane: An Innovative Route to Magnetically-Induced Fluorescence Composites" Molecules 27, no. 19: 6393. https://doi.org/10.3390/molecules27196393
APA StyleSamoila, P., Cojocaru, C., Simionescu, M., Sacarescu, G., Roman, G., Enache, A.-C., & Sacarescu, L. (2022). Cobalt Ferrite Particles Produced by Sol-Gel Autocombustion and Embedded in Polysilane: An Innovative Route to Magnetically-Induced Fluorescence Composites. Molecules, 27(19), 6393. https://doi.org/10.3390/molecules27196393









