Biomedical and Antioxidant Potentialities in Chilli: Perspectives and Way Forward
Abstract
:1. Introduction
2. Chilli: A Brief Account
3. Traditional Medicinal Uses of Chilli
4. Nutritional Profile
4.1. Vitamins
4.2. Nutritional Profile of Chilli Fruits across Species (per 100 g of Edible Portion)
4.3. Phytonutrients and Phytochemical Profiles
4.4. Bioactive Compounds
4.4.1. Capsaicin and Its Medicinal Horizon
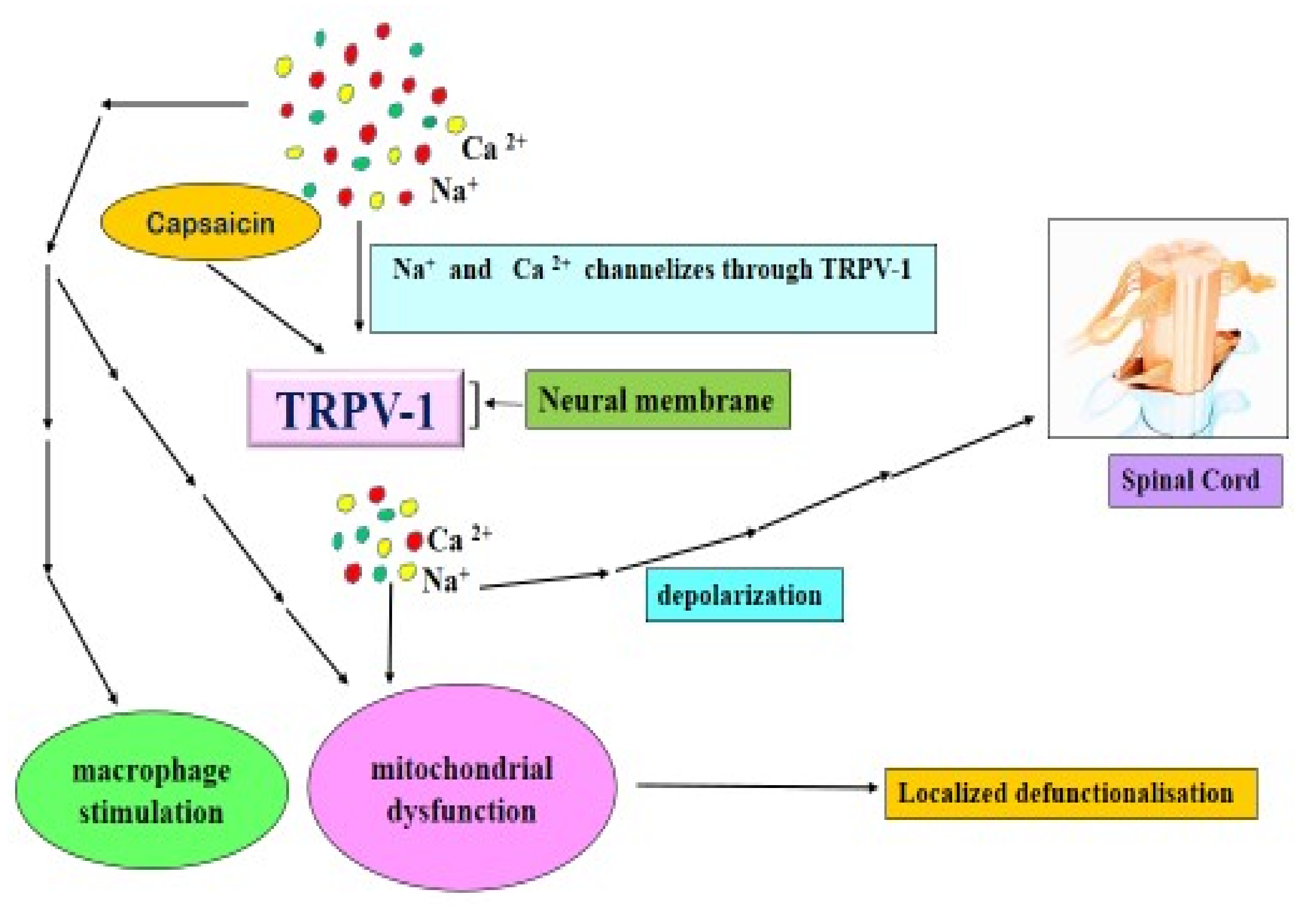
4.4.2. Capsaicin and TRPV 1
4.4.3. Capsaicinoids
Bioactivities of Capsaicinoids
Factors Governing CAPS Concentration
4.4.4. Capsinoids
4.4.5. Flavonoids
Factors Influencing Flavonoid Concentration
4.4.6. Capsaicinoids and Flavonoids on Human Health
4.5. Overview on Antioxidant Activities
4.6. Anti-Cancerous Perspectives
4.7. Anti-Obesity Activities
4.8. Cardiovascular Roles
4.9. Anti-Hyperglycemic/Antidiabetic Activities
4.10. Anti-Inflammatory and Pain Relieving Activities
4.11. Anti-Microbial Activities
4.12. Anti-Clotting Activity
4.13. Anesthetic Activities
4.14. Asthma and Rhinitis Treatment
4.15. COVID-19 Treatment
5. Conclusions
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bosland, P.W. Capsicum: Innovative uses of an ancient crop. In Progress in New Crops; Academic Press: Arlington, VA, USA, 1996; pp. 479–487. [Google Scholar]
- Mercy, A.G.; Light, W.F.; Gospel, S.A. Qualitative and quantitative phytochemical screening of some plants used in ethnomedicine in the Niger Delta region of Nigeria. J. Food Nutr. Sci. 2017, 5, 198–205. [Google Scholar]
- Powis, T.G.; Gallaga-Murrieta, E.; Lesure, R.; Lopez-Bravo, R.; Grivetti, L.; Kucera, H.; Gaikwad, N.W. Prehispanic use of chilli peppers in Chiapas, Mexico. PLoS ONE 2013, 8, e79013. [Google Scholar] [CrossRef] [PubMed]
- Bal, S.; Maity, T.K.; Sharangi, A.B.; Maji, A. Screening of onion (Allium cepa L.) germplasm against purple blotch disease. J. Pharmacogn. Phytochem. 2019, 8, 546–548. [Google Scholar]
- Bal, S.; Maity, T.K.; Sharangi, A.B.; Majumdar, A. Quality assessment in association with yield attributes contributing improved yield in onion (Allium cepa L.). J. Crop Weed 2019, 15, 107–115. [Google Scholar] [CrossRef]
- Bal, S.; Maity, T.K.; Maji, A. Genetic Divergence Studies for Yield and Quality Traits in Onion (Allium cepa L.). Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 3201–3208. [Google Scholar] [CrossRef]
- Bal, S.; Maity, T.K.; Maji, A. Evaluation of onion genotypes for growth, yield and quality traits under gangetic alluvial plains of West Bengal. Int. J. Chem. Stud. 2020, 8, 2157–2162. [Google Scholar] [CrossRef]
- Bal, S.; Maity, T.K.; Sharangi, A.B. Morphological and biochemical characterization of onion (Allium cepa L.) germplasm by principal component analysis. J. Pharmacogn. Phytochem. 2021, 10, 121–124. [Google Scholar]
- Oh, S.; Choi, C.H.; Jung, Y.K. Autophagy induction by capsaicin in malignant human breast cells is modulated by p38 and ERK mitogen activated protein kinase and retards cell death by suppressing endoplasmic reticulum stress mediated apoptosis. Mol. Pharmacol. 2010, 78, 114–125. [Google Scholar]
- Azlan, A.; Sultana, S.; Huei, C.S.; Razman, M.R. Antioxidant, Anti-Obesity, Nutritional and Other Beneficial Effects of Different Chilli Pepper: A Review. Molecules 2022, 27, 898. [Google Scholar] [CrossRef]
- Howe, G.R.; Jain, M.; Miller, A.B. Dietary factors and risk of pancreatic cancer: Results of a Canadian population-based case control study. Int. J. Cancer 1990, 45, 604–608. [Google Scholar] [CrossRef]
- Lu, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, F.; Alonso-Castro, A.J.; Anaya, M.; González-Trujano, M.E.; Salgado-Ceballos, H.; Orozco-Suárez, S. Mexican Traditional Medicine: Traditions of Yesterday and Phytomedicines for Tomorrow. In Therapeutic Medicinal Plants: From Lab to the Market; Academic Press: Boca Raton, FL, USA; CRC: Boca Raton, FL, USA, 2015; pp. 10–46. [Google Scholar]
- Cichewicz, R.H.; Thorpe, P.A. The antimicrobial properties of chile peppers (Capsicum sp.) and their uses in Mayan medicine. J. Ethnopharmacol. 1996, 52, 61–70. [Google Scholar] [CrossRef]
- Basu, S.K.; De, A.K.; De, A. Capsicum: Historical and Botanical Perspectives. In Capsicum: The genus Capsicum; Academic Press: New York, NY, USA; Taylor & Francis: New York, NY, USA, 2003; pp. 1–15. [Google Scholar]
- Elujoba, A.A.; Odeleye, O.; Ogunyemi, C. Traditional medicine development for medical and dental primary health care delivery system in Africa. Afr. J. Tradit. Complement. Altern. Med. 2006, 2, 46–61. [Google Scholar] [CrossRef]
- Maji, A.K.; Banerji, P. Phytochemistry and gastrointestinal benefits of the medicinal spice, Capsicum annuum L. (Chilli): A review. J. Altern. Complement. Med. 2016, 13, 97–122. [Google Scholar] [CrossRef]
- Leung, F.W. Capsaicin-sensitive intestinal mucosal afferent mechanism and body fat distribution. Life Sci. 2008, 83, 1–5. [Google Scholar] [CrossRef]
- Fathima, S.N. A systemic review on phytochemistry and pharmacological activities of Capsicum annuum. Int. J. Pharm. Pharm. Sci. 2015, 4, 51–68. [Google Scholar]
- Imran, M.; Butt, M.S.; Suleria, H.A.R. Capsicum annuum Bioactive Compounds: Health Promotion Perspectives. In Bioactive Molecules in Food; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–22. [Google Scholar]
- Sharma, J.; Sharma, P.; Chaudhary, B. Estimation of proximate composition of selected species of Capsicum (Capsicum annuum and Capsicum chinense) grown in India. Int. J. Pure Appl. Biosci. 2017, 5, 369–372. [Google Scholar] [CrossRef]
- Hassan, M.N.; Yusof, N.A.; Yahaya, A.F.; Rozali, M.N.N.; Othman, R. Carotenoids of capsicum fruits: Pigment profile and health-promoting functional attributes. Antioxidants 2019, 8, 469. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T.; Tokuda, H.; Suzuki, N.; Kato, H.; Etoh, H. Anti-oxidative, anti-tumor-promoting, and anti-carcinogensis activities of nitroastaxanthin and nitrolutein, the reaction products of astaxanthin and lutein with peroxynitrite. Mar. Drugs 2012, 10, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Arimboor, R.; Natarajan, R.B.; Menon, K.R.; Chandrasekhar, L.P.; Moorkoth, V. Red pepper (Capsicum annuum) carotenoids as a source of natural food colors: Analysis and stability-A review. J. Food Sci. Technol. 2015, 52, 1258–1271. [Google Scholar] [CrossRef]
- Nagy, Z.; Daood, H.; Ambrózy, Z.; Helyes, L. Determination of polyphenols, capsaicinoids, and vitamin C in new hybrids of chilli peppers. J. Anal. Methods Chem. 2015, 2015, 102125. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.M.; Kim, S.Y.; Kim, D.R.; Jo, S.C.; Nam, K.C.; Ahn, D.U.; Lee, S.C. Effect of heat treatment on the antioxidant activity of extracts from citrus peels. J. Agric. Food Chem. 2004, 52, 3389–3393. [Google Scholar] [CrossRef] [PubMed]
- Sim, K.H.; Sil, H.Y. Antioxidant activities of red pepper (Capsicum annuum) pericarp and seed extracts. Int. J. Food Sci. Technol. 2008, 43, 1813–1823. [Google Scholar] [CrossRef]
- Oboh, G.; Rocha, J. Distribution and antioxidant activity of polyphenols in ripe and unripe tree pepper (Capsicum pubescens). J. Food Biochem. 2007, 31, 456–473. [Google Scholar] [CrossRef]
- Liu, Y.; Nair, M.G. Capsaicinoids in the hottest pepper Bhut Jolokia and its antioxidant and antiinflammatory activities. Nat. Prod. Commun. 2010, 5, 91–94. [Google Scholar] [CrossRef] [Green Version]
- Si, W.; Man, S.W.; Chenb, Z.; Chung, H.Y. Stability of capsaicinoid content at raised temperatures. Nat. Prod. Commun. 2014, 9, 985–988. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Ríos, A.K.; Medina-Juárez, L.Á.; González-Aguilar, G.A.; Gámez-Meza, N. Antioxidant activity of the phenolic and oily fractions of different sweet bell peppers. J. Mex. Chem. Soc. 2013, 57, 137–143. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef]
- Morales-Soto, A.; Gómez-Caravaca, A.M.; García-Salas, P.; Segura-Carretero, A.; Fernández-Gutiérrez, A. High-performance liquid chromatography coupled to diode array and electrospray time-of-flight mass spectrometry detectors for a comprehensive characterization of phenolic and other polar compounds in three pepper (Capsicum annuum L.) samples. Food Res. Int. 2013, 51, 977–984. [Google Scholar] [CrossRef]
- Sutoh, K.; Kobata, K.; Yazawa, S.; Watanabe, T. Capsinoid is biosynthesized from phenylalanine and valine in a non-pungent pepper, Capsicum annuum L. cv. CH-19 sweet. Biosci. Biotechnol. Biochem. 2006, 70, 1513–1516. [Google Scholar] [CrossRef]
- Materska, M.; Perucka, I. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L.). J. Agric. Food Chem. 2005, 53, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Wahyuni, Y.; Ballester, A.R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Metabolite biodiversity in pepper fruits of thirty-two diverse accessions: Variation in health-related compounds and implications for breeding. Phytochemistry 2011, 72, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Materska, M.; Piacente, S.; Stochmal, A.; Pizza, C.; Oleszek, W.; Perucka, I. Isolation and structure elucidation of flavonoid and phenolic acid glycosides from pericarp of hot pepper fruit Capsicum annuum L. Phytochemistry 2003, 63, 893–898. [Google Scholar] [CrossRef]
- Deepa, N.; Kaur, C.; George, B.; Singh, B.; Kapoor, H.C. Antioxidant constituents in some sweet pepper (Capsicum annuum L.) genotypes during maturity. LWT-Food Sci. Technol. 2007, 40, 121–129. [Google Scholar] [CrossRef]
- Howard, L.R.; Smith, R.T.; Wagner, A.B.; Villalon, B.; Burns, E.E. Provitamin A and ascorbic acid content of fresh pepper cultivars (Capsicum annuum) and processed jalapeños. J. Food Sci. 1994, 59, 362–365. [Google Scholar] [CrossRef]
- Hsu, C.; Yen, G. Effects of capsaicin on induction of apoptosis and inhibition of adipogenesis in 3T3-L1 Cells. J. Agric. Food Chem. 2007, 55, 1730–1736. [Google Scholar] [CrossRef]
- Guo, C.L.; Chen, H.Y.; Cui, B.L.; Chen, Y.H.; Zhou, Y.F.; Peng, X.S.; Wang, Q. Development of a HPLC method for the quantitative determination of capsaicin in collagen sponge. Int. J. Anal. Chem. 2015, 2015, 912631. [Google Scholar] [CrossRef]
- Sora, G.T.S.; Haminiuk, C.W.I.; da Silva, M.V.; Zielinski, A.A.F.; Gonçalves, G.A.; Bracht, A.; Peralta, R.M. A comparative study of the capsaicinoid and phenolic contents and in vitro antioxidant activities of the peppers of the genus Capsicum: An application of chemometrics. J. Food Sci. Technol. 2015, 52, 8086–8094. [Google Scholar] [CrossRef]
- Abuelizz, H.A.; Anouar, E.; Marzouk, M.; Taie, H.A.; Ahudhaif, A.; Al-Salahi, R. DFT study and radical scavenging activity of 2-phenoxypyridotriazolo pyrimidines by DPPH, ABTS, FRAP and reducing power capacity. Chem. Pap. 2020, 74, 2893–2899. [Google Scholar] [CrossRef]
- Sricharoen, P.; Techawongstein, S.; Chanthai, S. A high correlation indicating for an evaluation of antioxidant activity and total phenolics content of various chilli varieties. J. Food Sci. Technol. 2015, 52, 8077–8085. [Google Scholar] [CrossRef]
- Szallasi, A. Capsaicin and cancer: Guilty as charged or innocent until proven guilty? Temperature 2022, 13, 1–5. [Google Scholar] [CrossRef]
- Goulias, N.; Wogiatzi, E.; Vagelas, I.; Giurgiulescu, L.; Gogou, I.; Ntalla, M.N.; Kalfountzos, D. Comparative study on polyphenols content, capsaicin and antioxidant activity of different hot pepper varieties (Capsicum annuum L.) under environmental conditions of Thessaly region, Greece. J. Food Sci. Technol. 2017, 9, 109–116. [Google Scholar]
- Oboh, G.; Ogunruku, O.O. Cyclophosphamide-induced oxidative stress in brain: Protective effect of hot short pepper (Capsicum frutescens L. var. abbreviatum). Exp. Toxicol. Pathol. 2010, 62, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Bobinaite, R.; Viškelis, P.; Venskutonis, P.R. Variation of total phenolics, anthocyanins, ellagic acid and radical scavenging capacity in various raspberry (Rubus spp.) cultivars. Food Chem. 2012, 132, 1495–1501. [Google Scholar] [CrossRef]
- Wen, H.; Zheng, W. Decrypting the heat activation mechanism of TRPV1 channel by molecular dynamics simulation. Biophys. J. 2018, 114, 40–52. [Google Scholar] [CrossRef]
- Seraglio, S.K.; Silva, B.; Bergamo, G.; Brugnerotto, P.; Gonzaga, L.V.; Fett, R.; Costa, A.C. An overview of physicochemical characteristics and health-promoting properties of honeydew honey. Food Res. Int. 2019, 119, 44–66. [Google Scholar] [CrossRef]
- Bosland, P.W.; Votava, E.J. Peppers: Vegetable and Spice Capsicums; Academic Press: Wallingford, UK, 2000; pp. 1–16. [Google Scholar]
- Brito, J.P.; Ramada, M.H.; de Magalhães, M.T.; Silva, L.P.; Ulhoa, C.J. Peptaibols from Trichoderma asperellum TR356 strain isolated from Brazilian soil. SpringerPlus 2014, 3, 600. [Google Scholar] [CrossRef]
- Hanson, S.M.; Newstead, S.; Swartz, K.J.; Sansom, M.S. Capsaicin interaction with TRPV1 channels in a lipid bilayer: Molecular dynamics simulation. Biophys. J. 2015, 108, 1425–1434. [Google Scholar] [CrossRef]
- Smutzer, G.; Devassy, R.K. Integrating TRPV-1 receptor function with capsaicin psychophysics. Adv. Pharmacol. Pharm. Sci. 2016, 2016, 16. [Google Scholar]
- Schumacher, M.A.; Eilers, H. TRPV1 splice variants: Structure and function. Front. Biosci. 2010, 15, 872–882. [Google Scholar] [CrossRef]
- Leung, F.W. Capsaicin as an Anti-Obesity Drug. In Capsaicin as a Therapeutic Molecule; Springer: Basel, Switzerland, 2014; pp. 171–179. [Google Scholar]
- Zheng, L.; Chen, J.; Ma, Z.; Liu, W.; Yang, F.; Yang, Z.; Wang, K.; Wang, X.; He, D.; Li, L.; et al. Capsaicin enhances anti-proliferation efficacy of pirarubicin via activating TRPV1 and inhibiting PCNA nuclear translocation in 5637 cells. Mol. Med. Rep. 2016, 13, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, W.; Tomsen, N.; Acedo, S.; Campos-Alcantara, C.; Cabib, C.; Alvarez-Larruy, M.; Clavé, P. Effect of aging, gender and sensory stimulation of TRPV1 receptors with capsaicin on spontaneous swallowing frequency in patients with oropharyngeal dysphagia: A proof-of-concept study. Diagnostics 2021, 11, 461. [Google Scholar] [CrossRef] [PubMed]
- Fattourusso, E.; Taglialatela-Scafati, O. Modern Alkaloids: Structure, Isolation, Synthesis and Biology, 2nd ed.; Academic Press: Weinheim, Germany, 2007. [Google Scholar]
- Alberti, A.; Galasso, V.; Kovac, B.; Modelli, A.; Pichierri, F. Probing the molecular and electronic structure of capsaicin: A spectroscopic and quantum mechanical study. J. Phys. Chem. A 2008, 112, 5700–5711. [Google Scholar] [CrossRef] [PubMed]
- Cheok, C.Y.; Sobhi, B.; MohdAdzahan, N.; Bakar, J.; Abdul Rahman, R.; Ab Karim, M.S.; Ghazali, Z. The genus Capsicum: A phytochemical review of bioactive secondary metabolites. Food Chem. 2017, 216, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Schweiggert, U.; Carle, R.; Schieber, A. Characterization of major and minor capsaicinoids and related compounds in chilli pods (Capsicum frutescens L.) by high-performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometry. Anal. Chim. Acta 2006, 557, 236–244. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Menichini, F.; Tundis, R. Evaluation of chemical profile and antioxidant activity of twenty cultivars from Capsicum annuum, Capsicum baccatum, Capsicum chacoense and Capsicum chinense: A comparison between fresh and processed peppers. LWT-Food Sci. Technol. 2015, 64, 623–631. [Google Scholar] [CrossRef]
- Mohammad, R.; Ahmad, M.; Heng, L.Y. An amperometric biosensor utilizing a ferrocene-mediated horseradish peroxidase reaction for the determination of capsaicin (chilli hotness). Sensors 2013, 13, 10014–10026. [Google Scholar] [CrossRef] [Green Version]
- Hayman, M.; Kam, P.C. Capsaicin: A review of its pharmacology and clinical applications. Curr. Anaesth. Crit. Care 2008, 19, 338–343. [Google Scholar] [CrossRef]
- Luo, X.J.; Peng, J.; Li, Y.J. Recent advances in the study on capsaicinoids and capsinoids. Eur. J. Pharmacol. 2011, 650, 1–7. [Google Scholar] [CrossRef]
- Lu, M.; Ho, C.T.; Huang, Q. Extraction, bioavailability and bioefficacy of capsaicinoids. J. Food Drug Anal. 2017, 25, 27–36. [Google Scholar] [CrossRef]
- Subramanian, G.; Karthik, A.; Kamath, S.; Prabahar, K.; Ranjithkumar, A.; Pathak, S.; Udupa, N. Stability-indicating HPTLC determination of capsaicin in the bulk drug. J. Planar Chromatogr.-Mod. TLC 2008, 21, 271–275. [Google Scholar] [CrossRef]
- Ryu, W.K.; Kim, H.W.; Kim, G.D.; Rhee, H.I. Rapid determination of capsaicinoids by colorimetric method. J. Food Drug Anal. 2017, 25, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Duelund, L.; Mouritsen, O.G. Contents of capsaicinoids in chillies grown in Denmark. Food Chem. 2017, 221, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Gonz´alez-Zamora, A.; Sierra-Campos, E.; Luna-Ortega, J.G.; P´erez-Morales, R.; Ortiz, J.C.R.; Garc´ia-Hern´andez, L. Characterization of different Capsicum varieties by evaluation of their capsaicinoids content by high performance liquid chromatography. Molecules 2013, 18, 13471–13486. [Google Scholar] [CrossRef]
- Bae, H.; Jayaprakasha, G.K.; Crosby, K.; Yoo, K.S.; Leskovar, D.I.; Jifon, J.; Patil, B.S. Ascorbic acid, capsaicinoid, and flavonoid aglycone concentrations as a function of fruit maturity stage in greenhouse-grown peppers. J. Food Compos. Anal. 2014, 33, 195–202. [Google Scholar] [CrossRef]
- Keyhaninejad, N.; Curry, J.; Romero, J.; O’Connell, M.A. Fruit specific variability in capsaicinoid accumulation and transcription of structural and regulatory genes in Capsicum fruit. Plant Sci. 2014, 215, 59–68. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yoneda, H.; Hosokawa, M.; Miwa, T.; Yazawa, S. Application of marker-assisted selection in breeding of a new fresh pepper cultivar (Capsicum annuum) containing capsinoids, low-pungent capsaicinoid analogs. Sci. Hortic. 2014, 165, 242–245. [Google Scholar] [CrossRef]
- Rodríguez-Maza, M.J.; Garcés-Claver, A.; Park, S.W.; Kang, B.C.; Arnedo-Andrés, M.S. A versatile PCR marker for pungency in Capsicum spp. Mol. Breed. 2012, 30, 889–898. [Google Scholar] [CrossRef]
- Singh, S.; Singh, D.R.; Balaji, R.; Nayak, D.; Singh, A.K.; Kumar, N. Molecular and functional diversity in Capsicum landraces of Andaman Islands. Afr. J. Biotechnol. 2013, 12, 5729–5737. [Google Scholar]
- Gurung, T.; Techawongstien, S.; Suriharn, B.; Techawongstien, S. Impact of Environments on the Accumulation of Capsaicinoids in Capsicum spp. HortScience 2011, 46, 1576–1581. [Google Scholar] [CrossRef]
- Gurung, T.; Techawongstien, S.; Suriharn, B.; Techawongstien, S. Stability analysis of yield and capsaicinoids content in chilli (Capsicum spp.) grown across six environments. Euphytica 2012, 187, 11–18. [Google Scholar] [CrossRef]
- Moscone, E.A.; Scaldaferro, M.A.; Grabiele, M.; Cecchini, N.M.; Sánchez García, Y.; Jarret, R.; Daviña, J.R.; Ducasse, D.A.; Barboza, G.E.; Ehrendorfer, F. The Evolution of Chilli Peppers (Capsicum-Solanaceae): A Cytogenetic Perspective. In Proceedings of the VI International Solanaceae Conference: Genomics Meets Biodiversity, Madison, WI, USA, 23–27 July 2006; Volume 745, pp. 137–170. [Google Scholar]
- Harvell, K.P.; Bosland, P.W. The environment produces a significant effect on pungency of chiles. HortScience 1997, 32, 1292. [Google Scholar] [CrossRef]
- Jeeatid, N.; Techawongstien, S.; Suriharn, B.; Bosland, P.W.; Techawongstien, S. Light intensity affects capsaicinoid accumulation in hot pepper (Capsicum chinense Jacq.) cultivars. Hortic. Environ. Biotechnol. 2017, 58, 103–110. [Google Scholar] [CrossRef]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Medina-Lara, F.; Echevarría-Machado, I.; Pacheco-Arjona, R.; Ruiz-Lau, N.; Guzmán-Antonio, A.; Martinez-Estevez, M. Influence of nitrogen and potassium fertilization on fruiting and capsaicin content in habanero pepper (Capsicum chinense Jacq.). HortScience 2008, 43, 1549–1554. [Google Scholar] [CrossRef]
- Johnson, C.D.; Decoteau, D.R. Nitrogen and potassium fertility affects Jalapeño pepper plant growth, pod yield, and pungency. HortScience 1996, 31, 1119–1123. [Google Scholar] [CrossRef]
- Monforte-González, M.; Guzmán-Antonio, A.; Uuh-Chim, F.; Vázquez-Flota, F. Capsaicin accumulation is related to nitrate content in placentas of habanero peppers (Capsicum chinense Jacq.). J. Sci. Food Agric. 2010, 90, 764–768. [Google Scholar] [CrossRef]
- Islam, M.A.; Sharma, S.S.; Sinha, P.; Negi, M.S.; Neog, B.; Tripathi, S.B. Variability in capsaicinoid content in different landraces of Capsicum cultivated in north-eastern India. Sci. Hortic. 2015, 183, 66–71. [Google Scholar] [CrossRef]
- Ruiz-Lau, N.; Medina-Lara, F.; Minero-García, Y.; Zamudio-Moreno, E.; Guzmán-Antonio, A.; Echevarría-Machado, I.; Martínez-Estévez, M. Water deficit affects the accumulation of capsaicinoids in fruits of Capsicum chinense Jacq. HortScience 2011, 46, 487–492. [Google Scholar] [CrossRef]
- Okunlola, G.O.; Olatunji, O.A.; Akinwale, R.O.; Tariq, A.; Adelusi, A.A. Physiological response of the three most cultivated pepper species (Capsicum spp.) in Africa to drought stress imposed at three stages of growth and development. Sci. Hortic. 2017, 224, 198–205. [Google Scholar] [CrossRef]
- Victoria-Campos, C.I.; de Jesús Ornelas-Paz, J.; Ramos-Aguilar, O.P.; Failla, M.L.; Chitchumroonchokchai, C.; Ibarra-Junquera, V.; Pérez-Martínez, J.D. The effect of ripening, heat processing and frozen storage on the in vitro bio-accessibility of capsaicin and dihydrocapsaicin from Jalapeño peppers in absence and presence of two dietary fat types. Food Chem. 2015, 181, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Barbero, G.F.; Ruiz, A.G.; Liazid, A.; Palma, M.; Vera, J.C.; Barroso, C.G. Evolution of total and individual capsaicinoids in peppers during ripening of the Cayenne pepper plant (Capsicum annuum L.). Food Chem. 2014, 153, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum-Titze, P.; Hiepler, C.; Mueller-Seitz, E.; Petz, M. Pungency in paprika (Capsicum annuum). Decrease of capsaicinoid content following cellular disruption. J. Agric. Food Chem. 2002, 50, 1260–1263. [Google Scholar] [CrossRef]
- Estrada, B.; Bernal, M.A.; Díaz, J.; Pomar, F.; Merino, F. Fruit development in Capsicum annuum: Changes in capsaicin, lignin, free phenolics and peroxidase patterns. J. Agric. Food Chem. 2000, 48, 6234–6239. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, L.H. Red Pepper (Capsicum spp.) Fruit: A Model for the Study of Secondary Metabolite Product Distribution and Its Management. In AIP Conference Proceedings; Academic Press: Melville, NY, USA, 2016; Volume 1744, p. 020034. [Google Scholar]
- Singh, S.; Jarret, R.; Russo, V.; Majetich, G.; Shimkus, J.; Bushway, R.; Perkins, B. Determination of capsinoids by HPLC-DAD in Capsicum species. J. Agric. Food Chem. 2009, 57, 3452–3457. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Kim, Y.S.; Ryu, S.Y.; Cha, M.R.; Yon, G.H.; Yang, H.J.; Kim, M.J.; Kang, S.; Park, S. Capsiate improves glucose metabolism by improving insulin sensitivity better than capsaicin in diabetic rats. J. Nutr. Biochem. 2013, 24, 1078–1085. [Google Scholar] [CrossRef]
- Macho, A.; Lucena, C.; Sancho, R.; Daddario, N.; Minassi, A.; Muñoz, E.; Appendino, G. Non-pungent capsaicinoids from sweet pepper. Eur. J. Nutr. 2003, 42, 2–9. [Google Scholar] [CrossRef]
- Jarret, R.L.; Bolton, J.; Perkins, L.B. 509-45-1, a Capsicum annuum pepper germplasm containing high concentrations of capsinoids. HortScience 2014, 49, 107–108. [Google Scholar] [CrossRef] [Green Version]
- Rosa, A.; Deiana, M.; Casu, V.; Paccagnini, S.; Appendino, G.; Ballero, M.; Dessí, M.A. Antioxidant activity of capsinoids. J. Agric. Food Chem. 2002, 50, 7396–7401. [Google Scholar] [CrossRef]
- Iwashina, T. The structure and distribution of the flavonoids in plants. J. Plant Stud. 2000, 113, 287. [Google Scholar] [CrossRef]
- Waksmundzka-Hajnos, M.; Sherma, J.; Kowalska, T. Thin Layer Chromatography in Phytochemistry, 1st ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- De Villiers, A.; Venter, P.; Pasch, H. Recent advances and trends in the liquid-chromatography—mass spectrometry analysis of flavonoids. J. Chromatogr. A. 2016, 1430, 16–78. [Google Scholar] [CrossRef] [PubMed]
- Merken, H.M.; Beecher, G.R. Measurement of food flavonoids by high-performance liquid chromatography: A review. J. Agric. Food Chem. 2000, 48, 577–599. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Pasinetti, G.M. Flavonoids and isoflavonoids: From plant biology to agriculture and neuroscience. Plant Physiol. 2010, 154, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Liu, S.; Li, W.; Wu, Y.; Chen, C.; Lei, J. De novo transcriptome assembly in chilli pepper (Capsicum frutescens) to identify genes involved in the biosynthesis of capsaicinoids. PLoS ONE 2013, 8, e48156. [Google Scholar]
- Aza-González, C.; Núñez-Palenius, H.G.; Ochoa-Alejo, N. Molecular biology of capsaicinoid biosynthesis in chilli pepper (Capsicum spp.). Plant Cell Rep. 2011, 30, 695–706. [Google Scholar] [CrossRef]
- Fujiwake, H.; Suzuki, T.; Iwai, K. Intracellular distribution of enzymes and intermediates involved in biosynthesis of capsaicin and its analogues in Capsicum fruits. Agric. Biol. Chem. 1982, 46, 2685–2689. [Google Scholar] [CrossRef]
- Constant, H.L.; Cordell, G.A.; West, D.P. Nonivamide, a constituent of Capsicum oleoresin. J. Nat. Prod. 1996, 59, 425–426. [Google Scholar] [CrossRef]
- Walker, J.; Ley, J.P.; Schwerzler, J.; Lieder, B.; Beltran, L.; Ziemba, P.M.; Hatt, H.; Hans, J.; Widder, S.; Krammer, G.E.; et al. Nonivamide, a capsaicin analogue, exhibits anti-inflammatory properties in peripheral blood mononuclear cells and U-937 macrophages. Mol. Nutr. Food Res. 2017, 61, 1600474. [Google Scholar] [CrossRef]
- Peña-Alvarez, A.; Ramírez-Maya, E.; Alvarado-Suárez, L.Á. Analysis of capsaicin and dihydrocapsaicin in peppers and pepper sauces by solid phase microextraction–gas chromatography–mass spectrometry. J. Chromatogr. A 2009, 1216, 2843–2847. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Walia, S.; Kundu, A.; Kaur, C.; Singh, J.; Sisodia, R. Capsaicinoids, tocopherol, and sterols content in chilli (Capsicum sp.) by gas chromatographic-mass spectrometric determination. Int. J. Food Prop. 2015, 18, 1535–1545. [Google Scholar] [CrossRef]
- Curry, J.; Aluru, M.; Mendoza, M.; Nevarez, J.; Melendrez, M.; O’Connell, M.A. Transcripts for possible capsaicinoid biosynthetic genes are differentially accumulated in pungent and non-pungent Capsicum spp. Plant Sci. 1999, 148, 47–57. [Google Scholar] [CrossRef]
- Kirschbaum-Titze, P.; Mueller-Seitz, E.; Petz, M. Pungency in paprika (Capsicumannuum). Heterogeneity of capsaicinoid content in individual fruits from one plant. J. Agric. Food Chem. 2002, 50, 1264–1266. [Google Scholar] [CrossRef]
- Meckelmann, S.W.; Jansen, C.; Riegel, D.W.; Van Zonneveld, M.; Ríos, L.; Peña, K.; Mueller-Seitz, E.; Petz, M. Phytochemicals in native Peruvian Capsicum pubescens (rocoto). Eur. Food Res. Technol. 2015, 241, 817–825. [Google Scholar] [CrossRef]
- Lee, J.J.; Crosby, K.M.; Pike, L.M.; Yoo, K.S.; Leskovar, D.I. Impact of genetic and environmental variation on development of flavonoids and carotenoids in pepper (Capsicum spp.). Sci. Hortic. 2005, 106, 341–352. [Google Scholar] [CrossRef]
- Marín, A.; Ferreres, F.; Tomás-Barberán, F.A.; Gil, M.I. Characterization and quantitation of antioxidant constituents of sweet pepper (Capsicum annuum L.). J. Agric. Food Chem. 2004, 52, 3861–3869. [Google Scholar] [CrossRef]
- Medina-Juárez, L.Á.; Molina-Quijada, D.M.; Del-Toro-Sánchez, C.L.; González-Aguilar, G.A.; Gámez-Meza, N. Antioxidant activity of peppers (Capsicum annuum L.) extracts and characterization of their phenolic constituents. Interciencia 2012, 37, 588–593. [Google Scholar]
- Howard, L.R.; Talcott, S.T.; Brenes, C.H.; Villalon, B. Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum species) as influenced by maturity. J. Agric. Food Chem. 2000, 48, 1713–1720. [Google Scholar] [CrossRef]
- Sun, T.; Xu, Z.; Wu, C.T.; Janes, M.; Prinyawiwatkul, W.; No, H.K. Antioxidant activities of different colored sweet bell peppers (Capsicum annuum L.). J. Food Sci. 2007, 72, S98–S102. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.R.; Jung, B.D.; Baek, H.Y.; Lee, Y.S. Ripening-dependent changes in phytonutrients and antioxidant activity of red pepper (Capsicum annuum L.) fruits cultivated under open-field conditions. HortScience 2013, 48, 1275–1282. [Google Scholar] [CrossRef]
- Menichini, F.; Tundis, R.; Bonesi, M.; Loizzo, M.R.; Conforti, F.; Statti, G.; De Cindio, B.; Houghton, P.J.; Menichini, F. The influence of fruit ripening on the phytochemical content and biological activity of Capsicum chinense Jacq. cv Habanero. Food Chem. 2009, 114, 553–560. [Google Scholar] [CrossRef]
- Cazáres-Sánchez, E.; Ramírez-Vallejo, P.; Castillo-González, F.; Soto-Hernández, R.M.; Rodríguez-González, M.T.; Chávez-Servia, J.L. Capsaicinoides y preferencias de usosendiferentesmorfotipos de chile (Capsicum annuum L.) del centro-oriente de Yucatán. Agrociencia 2005, 39, 627–638. [Google Scholar]
- Cisneros-Pineda, O.; Torres-Tapia, L.W.; Gutiérrez-Pacheco, L.C.; Contreras-Martín, F.; González-Estrada, T.; Peraza-Sánchez, S.R. Capsaicinoids quantification in chilli peppers cultivated in the state of Yucatan, Mexico. Food Chem. 2007, 104, 1755–1760. [Google Scholar] [CrossRef]
- Morán-Bañuelos, S.H.; Aguilar-Rincón, V.H.; Corona-Torres, T.; Castillo-González, F.; Soto-Hernández, R.M.; Miguel-Chávez, S. Capsaicinoidesen chiles nativos de Puebla, México. Agrociencia 2008, 42, 807–816. [Google Scholar]
- Vera-Guzman, A.; Chavez-Servia, J.L.; Carrillo-Rodriguez, J.C.; Lopez, M.G. Phytochemical evaluation of wild and cultivated pepper (Capsicum annuum L. and C. pubescens Ruiz & Pav.) from Oaxaca, Mexico. Chil. J. Agric. Res. 2011, 71, 578–585. [Google Scholar]
- Chopan, M.; Littenberg, B. The association of hot red chilli pepper consumption and mortality: A large population-based cohort study. PLoS ONE 2017, 12, e0169876. [Google Scholar] [CrossRef]
- Lv, J.; Qi, L.; Yu, C.; Yang, L.; Guo, Y.; Chen, Y.; Bian, Z.; Aun, D.; Du, J.; Ge, P.; et al. Consumption of spicy foods and total and cause specific mortality: Population based cohort study. Brit. Med. J. 2015, 351, h3942. [Google Scholar] [CrossRef]
- Chamikara, M.D.M.; Dissanayake, D.R.R.P.; Ishan, M.; Sooriyapathirana, S.D.S.S. Dietary, anticancer and medicinal properties of the phytochemicals in chilli pepper (Capsicum spp.). Ceylon J. Sci. 2016, 45, 5–20. [Google Scholar] [CrossRef]
- Cao, S.; Chen, H.; Xiang, S.; Hong, J.; Weng, L.; Zhu, H.; Liu, Q. Anti-cancer effects and mechanisms of capsaicin in chilli peppers. Am. J. Plant Sci. 2015, 6, 3075. [Google Scholar] [CrossRef]
- Amruthraj, N.J.; Raj-Preetam, J.P.; Saravanan, S.; Lebel-Antoine, L. In vitro studies on anticancer activity of capsaicinoids from Capsicum chinense against human hepatocellular carcinoma cells. Int. J. Pharm. Pharm. Sci. 2014, 6, 254–558. [Google Scholar]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Lehmann, S.; O’Kelly, J.; Kumagai, T.; Desmond, J.C.; Pervan, M.; McBride, W.H.; Kizaki, M.; Koeffler, H.P. Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells. Cancer Res. 2006, 66, 3222–3229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhou, M.; Fang, G.; Tang, Y.; Chen, Z.; Liu, X. Hypocholesterolemic effect of capsaicinoids by increased bile acids excretion in ovariectomized rats. Mol. Nutr. Food Res. 2013, 57, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, J.Y.; Lee, S.M.; Jun, C.H.; Cho, S.B.; Park, C.H.; Joo, Y.E.; Kim, H.S.; Choi, S.K.; Rew, J.S. Capsaicin induces apoptosis and modulates MAPK signaling in human gastric cancer cells. Mol. Med. Rep. 2014, 9, 499–502. [Google Scholar] [CrossRef]
- Pramanik, K.C.; Boreddy, S.R.; Srivastava, S.K. Role of mitochondrial electron transport chain complexes in capsaicin mediated oxidative stress leading to apoptosis in pancreatic cancer cells. PLoS ONE 2011, 6, e20151. [Google Scholar] [CrossRef]
- Lee, M.S.; Kim, C.T.; Kim, Y. Effect of capsaicin on lipid catabolism in 3T3-L1 adipocytes. Phytother. Res. 2011, 25, 935–939. [Google Scholar] [CrossRef]
- González-Segovia, R.; Quintanar, J.L.; Salinas, E.; Ceballos-Salazar, R.; Avilés-Jiménez, F.; Torres-López, J. Effect of the flavonoid quercetin on inflammation and lipid peroxidation induced by Helicobacter pylori in gastric mucosa of guinea pig. J. Gastroenterol. 2008, 43, 441–447. [Google Scholar] [CrossRef]
- Williams, R.J.; Spenser, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Way, T.; Kao, M.; Lin, J. Apigenin induces apoptosis through proteosomal degradation of HER2/neu in HER2/neu-overexpressing breast cancer cells via the phosphotidylinositol 3-kinase/Akt-dependent pathway. J. Biol. Chem. 2004, 6, 4479–4489. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.J.; Lee, C.Y. Protective effects of quercetin and vitamin C against oxidative stress induced neurodegeneration. J. Agric. Food Chem. 2004, 52, 7514–7517. [Google Scholar] [CrossRef] [PubMed]
- Malagarie-Cazenave, S.; Olea-Herrero, N.; Vara, D.; Díaz-Laviada, I. Capsaicin, a component of red peppers, induces expression of androgen receptor via PI3K and MAPK pathways in prostate LNCaP cells. FEBS Lett. 2009, 583, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Mehmet, B.; Metin, Y.; Gulhan, A.; Omer, T.; Oruc, A. Effect of capsaicin on transcription factor in 3T3-L1 cell line. East. J. Med. 2015, 20, 34–45. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Hemalatha, N.; Dhasarathan, P. Comparative study on the antimicrobial activity of Capsicum annuum and Capsicum frutescens. Int. J. Ethnomed. Pharmacol. Res. 2013, 1, 142–147. [Google Scholar]
- Christensen, R.; Kristensen, P.K.; Bartels, E.M.; Bliddal, H.; Astrup, A. Efficacy and safety of the weight-loss drug rimonabant: A meta-analysis of randomised trials. Lancet 2007, 370, 1706–1713. [Google Scholar] [CrossRef]
- Oyagbemi, A.A.; Saba, A.B.; Azeez, O.I. Capsaicin: A novel chemopreventive molecule and its underlying molecular mechanisms of action. Indian J. Cancer 2010, 47, 53. [Google Scholar] [CrossRef]
- Hossain, M.; Brunton, N.; Barry-Ryan, C.; Martin-Diana, A.B.; Wilkinson, M. Antioxidant activity of spice extracts and phenolics in comparison to synthetic antioxidants. Rasayan J. Chem. 2008, 1, 751–756. [Google Scholar]
- Sun-Hwa, H.; Jung-Bong, K.; Jong-Sug, P.; Shin-Woo, L.; Kang-Jim, C. A comparison of the carotenoid accumulation in Capsicum varieties that show different ripening colours: Deletion of the capsanthin-capsorubin synthase gene is not a prerequisite for the formation of a yellow pepper. J. Exp. Bot. 2007, 58, 3135–3144. [Google Scholar]
- Krishna, A.G.G.; Lokesh, B.R.; Sugasini, D.; Kancheva, V.D. Evaluation of the antiradical and antioxidant properties of extracts from Indian red chilli and black pepper by in vitro models. Bulg. Chem. Commun. 2010, 42, 62–69. [Google Scholar]
- Borovsky, Y.; Oren-Shamir, M.; Ovadia, R.; De Jong, W.; Paran, I. The A locus that controls anthocyanin accumulation in pepper encodes a MYB transcription factor homologous to Anthocyanin2 of Petunia. Theor. Appl. Genet. 2004, 109, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Howard, L.R.; Wildman, R.E. Antioxidant Vitamin and Phytochemical Content of Fresh and Processed Pepper Fruit (Capsicum annuum). In Handbook of Nutraceuticals and Functional Foods; Academic Press: Cambridge, MA, USA; CRC Press: Boca Raton, FL, USA, 2006; pp. 165–191. [Google Scholar]
- Mennen, L.I.; Walker, R.; Bennetau-Pelissero, C.; Scalbert, A. Risks and safety of polyphenol consumption. Am. J. Clin. Nutr. 2005, 81, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Castro-Concha, L.A.; Canche-Chuc, I.; MirandaHam, M.D.L. Determination of antioxidants in fruit tissues from three accessions of habanero pepper (Capsicum chinense Jacq.). J. Mex. Chem. Soc. 2012, 56, 15–18. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Zhao, W.; Yin, Z.; Xie, C.; Xu, Y. Capsaicin enhances the drug sensitivity of cholangiocarcinoma through the inhibition of chemotherapeutic-induced autophagy. PLoS ONE 2015, 10, e0121538. [Google Scholar] [CrossRef] [PubMed]
- Ezekiel, J.A.T.; Oluwole, O.J.A. Effects of capsaicin on coagulation: Will this be the new blood thinner? Clin. Med. Res. 2014, 3, 145–149. [Google Scholar] [CrossRef]
- Kang, M.C.; Kang, N.; Ko, S.C.; Kim, Y.B.; Jeon, Y.J. Anti-obesity effect of seaweeds of Jeju Island on the differentiation of 3T3 L1-preadipocytes and obese mice fed a high fat diet. Food Chem. Toxicol. 2016, 90, 36–44. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, J.; Liu, D.; Zhao, T.; Lu, Z.; Zhu, L.; Cao, L.; Yang, J.; Jin, J.; Cai, Y. Capsaicin reactivates hMOF in gastric cancer cells and induces cell growth inhibition. Cancer Biol. Ther. 2016, 17, 1117–1125. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, B.; Wen, X.; Li, M.; Wang, K.; Ni, Y. Quality analysis and microencapsulation of chilli seed oil by spray drying with starch sodium octenylsuccinate and maltodextrin. Powder Technol. 2017, 312, 294–298. [Google Scholar] [CrossRef]
- Övey, I.S.; Güler, Y. Apoptotic efficiency of capecitabine and 5-fluorouracil on human cancer cells through TRPV1 channels. Indian J. Biochem. Biophys. 2020, 57, 64–72. [Google Scholar]
- Arora, R.; Gill, N.S.; Chauhan, G.; Rana, A.C. An overview about versatile molecule Capsaicin. Int. J. Pharm. Sci. Drug Res. 2011, 3, 280–286. [Google Scholar]
- Tsou, M.F.; Lu, H.F.; Chen, S.C.; Wu, L.T.; Chen, Y.S.; Kuo, H.M.; Lin, S.S.; Chung, J.G. Involvement of Bax, Bcl-2, Ca2+ and caspase-3 in capsaicin-induced apoptosis of human leukemia HL-60 cells. Anticancer Res. 2006, 26, 1965–1971. [Google Scholar] [PubMed]
- Huh, H.C.; Lee, S.Y.; Lee, S.K.; Park, N.H.; Han, I.S. Capsaicin induces apoptosis of cisplatin-resistant stomach cancer cells by causing degradation of cisplatin-inducible aurora-A protein. Nutr. Cancer. 2011, 63, 1095–1103. [Google Scholar] [CrossRef]
- Wang, H.M.; Chuang, S.M.; Su, Y.C.; Li, Y.H.; Chueh, P.J. Downregulation of tumor-associated NADH oxidase, tNOX (ENOX2), enhances capsaicin-induced inhibition of gastric cancer cell growth. Cell Biochem. Biophys. 2011, 61, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Anandakumar, P.; Kamaraj, S.; Jagan, S.; Ramakrishnan, G.; Asokkumar, S.; Naveenkumar, C.; Raghunandhakumar, S.; Devaki, T. Capsaicin inhibits benzo (a) pyrene-induced lung carcinogenesis in an in vivo mouse model. Inflamm. Res. 2012, 61, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Macho, A.; Blázquez, M.V.; Navas, P.; Muñoz, E. Induction of apoptosis by vanilloid compounds does not require de novo gene transcription and activator protein 1 activity. Cell Death Differ. 1998, 9, 277–286. [Google Scholar]
- Macho, A.; Calzado, M.A.; Munoz-Blanco, J.; Gomez-Diaz, C.; Gajate, C.; Mollinedo, F.; Navas, P.; Munoz, E. Selective induction of apoptosis by capsaicin in transformed cells: The role of reactive oxygen species and calcium. Cell Death Differ. 1999, 6, 155–165. [Google Scholar] [CrossRef]
- Macho, A.; Lucena, C.; Calzado, M.A.; Blanco, M.; Donnay, I.; Appendino, G.; Munoz, E. Phorboid 20-homovanillates induce apoptosis through a VR1-independent mechanism. Chem. Biol. 2000, 7, 483–492. [Google Scholar] [CrossRef]
- Zhang, R.; Humphreys, I.; Sahu, R.P.; Shi, Y.; Sirivastava, S.K. in vitro and in vivo induction of apoptosis by capsaicin in pancreatic cancer cells is mediated through ROS generation and mitochondrial death pathway. Apoptosis 2008, 13, 1465–1478. [Google Scholar] [CrossRef]
- Han, S.S.; Keum, Y.S.; Seo, H.J.; Chun, K.S.; Lee, S.S.; Surh, Y.J. Capsaicin suppresses phorbol esterinduced activation of NF-κB/Rel and AP-1 transcription factors in mouse epidermis. Cancer Lett. 2001, 164, 119–126. [Google Scholar] [CrossRef]
- Patel, P.S.; Varney, M.L.; Dave, B.J.; Singh, R.K. Regulation of constitutive and induced NF-κB activation in malignant melanoma cells by capsaicin modulates interleukin-8 production and cell proliferation. J. Interferon Cytokine Res. 2002, 22, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nagasaki, M.; Tanaka, Y.; Morikawa, S. Capsaicin inhibits growth of adult T-cell leukemia cells. Leuk. Res. 2003, 27, 275–283. [Google Scholar] [CrossRef]
- Lin, C.; Lu, W.; Wang, C.; Chan, Y.; Chen, M. Capsaicin induces cell cycle arrest and apoptosis in human KB cancer cells. BMC Complement. Altern. Med. 2013, 13, 46. [Google Scholar] [CrossRef]
- Akagi, A.; Sano, N.; Uehara, H.; Minami, T.; Otsuka, H.; Izumi, K. Noncarcinogencity of capsaicinoids in B6C3F1 mice. Food Chem. Toxicol. 1998, 36, 1065–1071. [Google Scholar] [CrossRef]
- Park, K.K.; Surh, Y.J. Effects of capsaicin on chemically-induced two-stage mouse skin carcinogenesis. Cancer Lett. 1997, 114, 183–184. [Google Scholar] [CrossRef]
- Hail, N.; Lotan, R. Examining the role of mitochondrial respiration in vanilloid-induced apoptosis. J. Natl. Cancer Inst. 2002, 94, 1281–1292. [Google Scholar] [CrossRef]
- Hwang, M.K.; Bode, A.M.; Byun, S.; Song, N.R.; Lee, H.J.; Lee, K.W.; Dong, Z. Cocarcinogenic effect of capsaicin involves activation of EGFR signaling but not TRPV1EGFR-dependent cocarcinogenic effect of capsaicin. Cancer Res. 2010, 70, 6859–6869. [Google Scholar] [CrossRef]
- Jun, H.S.; Park, T.; Lee, C.K.; Kang, M.K.; Park, M.S.; Kang, H.I.; Surh, Y.J.; Kim, O.H. Capsaicin induced apoptosis of B16-F10 melanoma cells through down-regulation of Bcl-2. Food Chem. Toxicol. 2007, 45, 708–715. [Google Scholar] [CrossRef]
- Choi, C.H.; Jung, Y.K.; Oh, S. Selective induction of catalase mediated autophagy by dihydrocapsaicin in lung cell lines. Free Radic. Biol. Med. 2009, 49, 245–257. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Basu, S. Fas-associated factor 1 is a negative regulator in capsaicin induced cancer cell apoptosis. Cancer Lett. 2010, 287, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Nakazato, T.; Yamato, K.; Miyakawa, Y.; Yamada, T.; Hozumi, N.; Segawa, K.; Ikeda, Y.; Kizaki, M. Induction of apoptosis in leukemic cells by homovanillic acid derivative, capsaicin, through oxidative stress: Implication of phosphorylation of p53 at Ser-15 residue by reactive oxygen species. Cancer Res. 2004, 64, 1071–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amantini, C.; Mosca, M.; Nabissi, M.; Lucciarini, R.; Caprodossi, S.; Arcella, A.; Giangaspero, F.; Santoni, G. Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. J. Neurochem. 2007, 102, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kohno, H.; Sakata, K.; Yamada, Y.; Hirose, Y.; Sugie, S.; Mori, H. Modifying effects of dietary capsaicin and rotenone on 4-nitroquinoline 1-oxide-induced rat tongue carcinogenesis. Carcinogenesis 2002, 23, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Ip, S.W.; Lan, S.H.; Huang, A.C.; Yang, J.S.; Chen, Y.Y.; Huang, H.Y.; Lin, Z.P.; Hsu, Y.M.; Yang, M.D.; Chiu, C.F.; et al. Capsaicin induces apoptosis in SCC-4 human tongue cancer cells through mitochondria dependent and independent pathways. Environ. Toxicol. 2012, 27, 332–341. [Google Scholar] [CrossRef]
- Lee, S.H.; Krisanapun, C.; Baek, S.J. NSAID-activated gene-1 as a molecular target for capsaicin induced apoptosis through a novel molecular mechanism involving GSK3β, C/EBPβ, and ATF3. Carcinogenesis 2010, 31, 719–728. [Google Scholar] [CrossRef]
- Brown, K.C.; Witte, T.R.; Hardman, W.E.; Luo, H.; Chen, Y.C.; Carpenter, A.B.; Lau, J.K.; Dasgupta, P. Capsaicin displays antiproliferative activity against human small cell lung cancer in cell culture and nude mice models via the E2F pathway. PLoS ONE 2010, 5, e10243. [Google Scholar] [CrossRef]
- Ip, S.W.; Lan, S.H.; Lu, H.F.; Huang, A.C.; Yang, J.S.; Lin, J.P.; Huang, H.Y.; Lien, J.C.; Ho, C.C.; Chiu, C.F.; et al. Capsaicin mediates apoptosis in human nasopharyngeal carcinoma NPC-TW 039 cells through mitochondrial depolarization and endoplasmic reticulum stress. Hum. Exp. Toxicol. 2012, 31, 539–549. [Google Scholar] [CrossRef]
- Huang, S.P.; Chen, J.C.; Wu, C.C.; Chen, C.T.; Tang, N.Y.; Ho, Y.T.; Lo, C.; Lin, J.P.; Chung, J.G.; Lin, J.G. Capsaicin-induced apoptosis in human hepatoma HepG2 cells. Anticancer Res. 2009, 29, 165–174. [Google Scholar]
- Lee, G.R.; Shin, M.K.; Yoon, D.J.; Kim, A.R.; Yu, R.; Park, N.H. Topical application of capsaicin reduces visceral adipose fat by affecting adipokine levels in high-fat diet-induced obese mice. Obesity 2013, 1, 115–122. [Google Scholar] [CrossRef]
- Van Avesaat, M.; Troost, F.J.; Westerterp-Plantenga, M.S.; Helyes, Z.; Le Roux, C.W.; Dekker, J.; Masclee, A.A.; Keszthelyi, D. Capsaicin-induced satiety is associated with gastrointestinal distress but not with the release of satiety hormones. Am. J. Clin. Nutr. 2016, 103, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, P.; Krishnan, V.; Fettel, K.; Gao, P.; Zhu, Z.; Ren, J.; Thyagarajan, B. TRPV1 activation counters diet-induced obesity through sirtuin-1 activation and PRDM-16 deacetylation in brown adipose tissue. Int. J. Obes. 2017, 41, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, Y.J. The vanilloid receptor TRPV1: Role in cardiovascular and gastrointestinal protection. Eur. J. Pharmacol. 2010, 627, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.J.; Ahuja, K.D.K.; Geraghty, D.P. Effect of capsaicin and dihydrocapsaicin on in vitro blood coagulation and platelet aggregation. Thromb. Res. 2009, 124, 721–723. [Google Scholar] [CrossRef]
- Panchal, S.K.; Bliss, E.; Brown, L. Capsaicin in metabolic syndrome. Nutrients 2018, 10, 630. [Google Scholar] [CrossRef]
- Harper, A.G.S.; Brownlow, S.L.; Sage, S.O. A role for TRPV1 in agonist-evoked activation of human platelets. J. Thromb. Haemost. 2009, 7, 330–338. [Google Scholar] [CrossRef]
- Ahuja, K.D.K.; Kunde, D.A.; Ball, M.J.; Geraghty, D.P. Effects of capsaicin, dihydrocapsaicin, and curcumin on copper-induced oxidation of human serum lipids. J. Agric. Food Chem. 2006, 54, 6436–6439. [Google Scholar] [CrossRef]
- Manjunatha, H.; Srinivasan, K. Hypolipidemic and antioxidant effects of curcumin and capsaicin in high-fat-fed rats. Can. J. Physiol. Pharmacol. 2007, 85, 588–596. [Google Scholar] [CrossRef]
- Hu, Y.W.; Ma, X.; Huang, J.L.; Mao, X.R.; Yang, J.Y.; Zhao, J.Y.; Li, S.F.; Qiu, Y.R.; Yang, J.; Zheng, L.; et al. Dihydrocapsaicin attenuates plaque formation through a PPARγ/LXRα pathway in apoe−/−mice fed a high-fat/high-cholesterol diet. PLoS ONE 2013, 8, e66876. [Google Scholar] [CrossRef]
- Sy, G.Y.; Cissé, A.; Nongonierma, R.B.; Sarr, M.; Mbodj, N.A.; Faye, B. Hypoglycaemic and antidiabetic activity of acetonic extract of Vernonia colorata leaves in normoglycaemic and alloxaninduced diabetic rats. J. Ethnopharmacol. 2005, 98, 171–175. [Google Scholar] [CrossRef]
- Magied, M.M.A.; Salama, N.A.R.; Ali, M.R. Hypoglycemic and hypocholesterolemia effects of intragastric administration of dried red chilli pepper (Capsicum annuum) in alloxan-induced diabetic male albino rats fed with high-fat-diet. J. Food Nutr. Res. 2014, 11, 850–856. [Google Scholar] [CrossRef]
- Islam, M.S.; Choi, H. Dietary red chilli (Capsicum frutescens L.) is insulinotropic rather than hypoglycemic in type 2 diabetes model of rats. Phytother. Res. 2008, 22, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, J.; Ma, D.; Yuan, G.; Lu, Y.; Yang, Y. Capsaicin reduces Alzheimer-associated tau changes in the hippocampus of type 2 diabetes rats. PLoS ONE 2017, 12, e0172477. [Google Scholar] [CrossRef] [Green Version]
- Fattori, V.; Hohmann, M.S.N.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.R.; Makhmutova, M.; Almaça, J.; Stertmann, J.; Aamodt, K.; Brissova, M.; Speier, S.; Rodriguez-Diaz, R.; Caicedo, A. Mouse pancreatic islet macrophages use locally released ATP to monitor beta cell activity. Diabetologia 2018, 61, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Kawada, T.; Kim, B.S.; Han, I.S.; Choe, S.Y.; Kurata, T.; Yu, R. Capsaicin exhibits anti-inflammatory property by inhibiting IkB-a degradation in LPS-stimulated peritoneal macrophages. Cell. Signal. 2003, 15, 299–306. [Google Scholar] [CrossRef]
- Wang, H.B.; Nair, M.G.; Strasburg, G.M.; Chang, Y.C.; Booren, A.M.; Gray, J.I.; DeWitt, D.L. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J. Nat. Prod. 1999, 62, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Spiller, F.; Alves, M.K.; Vieira, S.M.; Carvalho, T.A.; Leite, C.E.; Lunardelli, A.; Polomi, J.A.; Cunha, F.Q.; Oliveira, J.R. Anti-inflammatory effects of red pepper (Capsicum baccatum) on carrageenan- and antigen-induced inflammation. J. Pharm. Pharmacol. 2008, 60, 473–478. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, C.S.; Han, I.S.; Kawada, T.; Yu, R. Capsaicin, a spicy component of hot peppers, modulates adipokine gene expression and protein release from obese-mouse adipose tissues and isolated adipocytes, and suppresses the inflammatory responses of adipose tissue macrophages. FEBS Lett. 2007, 581, 4389–4396. [Google Scholar] [CrossRef]
- Sancho, R.; Lucena, C.; Macho, A.; Calzado, M.A.; Blanco-Molina, M.; Minassi, A.; Appendino, G.; Munoz, E. Immunosuppressive activity of capsaicinoids: Capsiate derived from sweet peppers inhibits NF-ĸB activation and is a potent anti-inflammatory compound in vivo. Eur. J. Immunol. 2002, 32, 1753–1763. [Google Scholar] [CrossRef]
- Fraenkel, L.; Bogardus, S.T.; Concato, J.; Wittink, D.R. Treatment options in knee osteoarthritis: The patient’s perspective. Arch. Intern. Med. 2004, 164, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Lynn, B. Capsaicin: Action on nocicepetive C-fibres and therapeutic potential. Pain 1990, 41, 61–69. [Google Scholar] [CrossRef]
- Sindrup, S.H.; Jensen, T.S. Efficacy of pharmacological treatments of neuropathic pain: An update and effect related to mechanism of drug action. Pain 1999, 83, 389–400. [Google Scholar] [CrossRef]
- Berger, A.; Henderson, M.; Nadoolman, W.; Duffy, V.; Cooper, D.; Sabersli, L.; Bartoshuk, L. Oral capsaicin provides temporary relief for oral mucositis pain secondary to chemotherapy/radiation therapy. J. Pain Symptom Manag. 1995, 10, 243–248. [Google Scholar] [CrossRef]
- Cruz, F. Mechanisms involved in new therapies for overactive bladder. Urology 2004, 63, 65–73. [Google Scholar] [CrossRef]
- Omolo, M.A.; Wong, Z.Z.; Mergen, A.K.; Hastings, J.C.; Le, N.C.; Reiland, H.A.; Case, K.A.; Baumier, D.J. Antimicrobial Properties of Chilli Peppers. Infect. Dis. Ther. 2014, 2, 145. [Google Scholar]
- Careaga, M.; Fernández, E.; Dorantes, L.; Mota, L.; Jaramillo, M.E.; Hernandez-Sanchez, H. Antibacterial activity of Capsicum extract against Salmonella typhimurium and Pseudomonas aeruginosa inoculated in raw beef meat. Int. J. Food Microbiol. 2003, 83, 331–335. [Google Scholar] [CrossRef]
- Soetarno, S.; Sukrasno, S.; Yulinah, E.; Sylvia, S. Antimicrobial activities of the ethanol extracts of Capsicum fruits with different levels of pungency. J. Membr. Sci. 1997, 2, 57–63. [Google Scholar]
- Jones, N.L.; Shabib, S.; Sherman, P.M. Capsaicin as an inhibitor of growth of the gastric pathogen Helicobacter pyroli. FEMS Microbiol. Lett. 1997, 146, 223–227. [Google Scholar] [CrossRef]
- Zeyrek, Y.F.; Oguz, E. Invitro activity of capsaicin against Heylicobacter pylori. Ann. Microbiol. 2005, 55, 125–127. [Google Scholar]
- Nascimento, P.L.A.; Nascimento, T.C.E.S.; Ramos, N.S.M.; Silva, G.R.; Gomes, J.E.G.; Falcao, R.A.E.; Moreira, K.A.; Porto, A.L.F.; Silva, T.M.S. Quantification, antioxidant and antimicrobial Activity of phenolics isolated from different extracts of Capsicum frutescens (Pimentamalagueta). Molecules 2014, 19, 5434–5447. [Google Scholar] [CrossRef] [PubMed]
- Kurita, S.K.; Kitagawa, E.; Kim, C.H.; Momose, Y.; Iwahashi, H. Studies of the antimicrobial mechanism of Capsaicin using yeast DNA microarray. Biosci. Biotechnol. Biochem. 2002, 66, 532–536. [Google Scholar] [CrossRef]
- Wang, J.P.; Hsu, M.F.; Hsu, T.P.; Teng, C.M. Antihemostatic and antithrombotic effects of capsaicin in comparison with aspirin and indomethacin. Thromb. Res. 1985, 37, 669–679. [Google Scholar] [CrossRef]
- Wang, J.P.; Hsu, M.F.; Teng, C.M. Antiplatelet effect of capsaicin. Thromb. Res. 1984, 36, 497–507. [Google Scholar] [CrossRef]
- Friedman, J.R.; Richbart, S.D.; Merritt, J.C.; Brown, K.C.; Denning, K.L.; Tirona, M.T.; Valentovic, M.A.; Miles, S.L.; Dasgupta, P. Capsaicinoids: Multiple effects on angiogenesis, invasion and metastasis in human cancers. Biomed. Pharmacother. 2019, 118, 109317. [Google Scholar] [CrossRef]
- Borbiro, I.; Badheka, D.; Rohacs, T. Activation of TRPV1 channels inhibits mechanosensitive piezo channel activity by depleting membrane phosphoinositides. Sci. Signal. 2015, 8, ra15. [Google Scholar] [CrossRef] [Green Version]
- Della Pietra, A.; Mikhailov, N.; Giniatullin, R. The emerging role of mechanosensitive Piezo channels in migraine pain. Int. J. Mol. Sci. 2020, 21, 696. [Google Scholar] [CrossRef]
- Salari, M.; Salari, R.; Rafatpanah, H.; Ravanshad, Y.; Zirachi, D.; Sahebari, M. Skin inflammatory reactions to capsaicin in rheumatoid arthritis patients compared to healthy controls. Avicenna J. Phytomed. 2019, 9, 54–61. [Google Scholar]
- Anand, P.; Bley, K. Topical capsaicin for pain management: Therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8 patch. Br. J. Anaesth. 2011, 107, 490–502. [Google Scholar] [CrossRef]
- Yang, X.Y.; Du, G.H. Capsaicin. In Natural Small Molecule Drugs from Plants; Academic Press: Cambridge, MA, USA; Springer: Berlin/Heidelberg, Germany, 2018; pp. 397–402. [Google Scholar]
- Van Gerven, L.; Alpizar, Y.A.; Wouters, M.M.; Hox, V.; Hauben, E.; Jorissen, M.; Boeckxstaens, G.; Talavera, K.; Hellings, P.W. Capsaicin treatment reduces nasal hyperreactivity and transient receptor potential cation channel subfamily V, receptor 1 (TRPV1) overexpression in patients with idiopathic rhinitis. J. Allergy Clin. Immunol. 2014, 133, 1332–1339. [Google Scholar] [CrossRef]
- Van Rijswijk, J.B.; Boeke, E.L.; Keizer, J.M.; Mulder, P.G.H.; Blom, H.M.; Fokkens, W.J. Intranasal capsaicin reduces nasal hyperreactivity in idiopathic rhinitis: A double-blind randomized application regimen study. Allergy Eur. J. Allergy Clin. Immunol. 2003, 58, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Motte, J.; Ambrosius, B.; Grüter, T.; Bachir, H.; Sgodzai, M.; Pedreiturria, X.; Pitarokoili, K.; Gold, R. Capsaicin-enriched diet ameliorates autoimmune neuritis in rats. J. Neuroinflammation 2018, 15, 122. [Google Scholar] [CrossRef]
- Nevius, E.; Srivastava, P.K.; Basu, S. Oral ingestion of capsaicin, the pungent component of chilli pepper, enhances a discreet population of macrophages and confers protection from autoimmune diabetes. Mucosal Immunol. 2012, 5, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Prompetchara, E.; Ketloy, C.; Palaga, T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020, 38, 1–9. [Google Scholar] [PubMed]
- Giamarellos-Bourboulis, E.J.; van de Veerdonk, F.L.; Mouktaroudi, M.; Raftogiannis, M.; Antonopoulou, A.; Joosten, L.A.; Pickkers, P.; Savva, A.; Georgitsi, M.; van der Meer, J.W.; et al. Inhibition of caspase-1 activation in Gram-negative sepsis and experimental endotoxemia. Crit. Care 2011, 15, R27. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Shao, C.; Huang, J.; Gan, J.; Huang, X.; Bucci, E.; Piacentini, M.; Ippolito, G.; Melino, G. COVId-19 infection: The perspectives on immune responses. Cell Death Differ. 2020, 27, 1451–1454. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Janda, K.D.; Iadarola, M.J. Potential TRPV1 blockade to treat severe lung dysfunction in COVID-19 infection. Med. Drug Discov. 2020, 6, 100034. [Google Scholar] [CrossRef]
- Nahama, A.; Ramachandran, R.; Cisternas, A.F.; Ji, H. The role of afferent pulmonary innervation in ARDS associated with COVID-19 and potential use of resiniferatoxin to improve prognosis: A review. Med. Drug Discov. 2020, 5, 100033. [Google Scholar] [CrossRef]
- Chen, L.R.; Wang, Y.C.; Lin, Y.W.; Chou, S.Y.; Chen, S.F.; Liu, L.T.; Wu, Y.T.; Kuo, C.J.; Chen, T.S.S.; Juang, S.H. Synthesis and evaluation of isatin derivatives as effective SARS coronavirus 3CL protease inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 3058–3062. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhir, G.; Shukla, S.K.; Sharma, M.; Tyagi, P.; Bhushan, A.; Rathore, M. Identification of phytochemical inhibitors against main protease of COVID-19 using molecular modeling approaches. J. Biomol. Struct. Dyn. 2020, 39, 3760–3770. [Google Scholar] [CrossRef] [PubMed]
- Kadil, Y.; Mouhcine, M.; Filali, H. In silico study of pharmacological treatments against SARS-CoV2 main protease. J. Pure Appl. Microbiol. 2020, 14, 1065–1071. [Google Scholar] [CrossRef]
- Das, S.; Sarmah, S.; Lyndem, S.; Singha Roy, A. An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. J. Biomol. Struct. Dyn. 2021, 39, 3347–3357. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.; Carvalho, F.; Barros, A.; Dória, S. Premature ovarian insufficiency: Clinical orientations for genetic testing and genetic counseling. Porto Biomed. J. 2020, 5, e62. [Google Scholar] [CrossRef]
- Elfiky, A.A. Corrigendum to “Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARSCoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study”. Life Sci. 2020, 248, 117477. [Google Scholar] [CrossRef]


| Nutrients | Peppers, Hot Chilli, Green (Raw) {a} | Peppers, Sweet, Green (Raw) {b} | Spices, Pepper, Red or Cayenne {c} | References |
|---|---|---|---|---|
| Carbohydrate (g) | 9.46 | 4.64 | 56.63 | [19,20,21] |
| Protein (g) | 2.00 | 0.86 | 12.01 | [19,20,21] |
| Fat (g) | 0.20 | 0.17 | 17.27 | [19,20,21] |
| Energy (kcal) | 40 | 20 | 318 | [19,20] |
| Iron (mg) | 1.20 | 0.34 | 7.80 | [19,20,21] |
| Calcium (mg) | 18 | 10 | 148 | [19,20,21] |
| Sodium (mg) | 7 | 3 | 30 | [19,20,21] |
| Potassium (mg) | 340 | 175 | 2014 | [19,20,21] |
| Phosphorus (mg) | 46 | 20 | 293 | [19,20] |
| Copper (mg) | 0.30 | 0.066 | 0.373 | [19,20] |
| Selenium (μg) | 0.5 | 0.0 | 8.8 | [19,20] |
| Type of Cancer | Diversified Cell Lines | Inhibitory Effects | References |
|---|---|---|---|
| Pancreatic cancer | BxPC-3 and AsPC-1 (pancreatic cancer) | Inhibitory effects by generation of ROS resulting in induction of apoptosis | [137] |
| Blood leukemia | Human myelocytic leukemia (HL-60) | Inhibitory effects by induction of autophagy by caspase-3-dependent process | [164] |
| Human KB cancer | KB (which is derived from HeLa cell line) | Inhibitory effects by staggering cell cycle at G2/M phase causing inducing apoptosis | [175] |
| Tongue cancer | Squamous-Cell Carcinoma (SCC-4) | Inhibitory effects by mitochondria dependent and independent mechanisms causing induction of apoptosis | [186] |
| Lung cancer | NCI-H69, NCI-H82 | Inhibitory effects by arresting cell cycle at GI | [188] |
| Nasopharyngeal cancer | Nasopharyngeal Carcinoma (NPC-TW 039) in human | Inhibitory effects by mitochondrial alteration and stress in endoplasmic reticulum causing induction of apoptosis | [189] |
| Hepatic cancer | HepG2 (human hepatoma) | Inhibitory effects by disruption of ROS causing induction of apoptosis | [190] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bal, S.; Sharangi, A.B.; Upadhyay, T.K.; Khan, F.; Pandey, P.; Siddiqui, S.; Saeed, M.; Lee, H.-J.; Yadav, D.K. Biomedical and Antioxidant Potentialities in Chilli: Perspectives and Way Forward. Molecules 2022, 27, 6380. https://doi.org/10.3390/molecules27196380
Bal S, Sharangi AB, Upadhyay TK, Khan F, Pandey P, Siddiqui S, Saeed M, Lee H-J, Yadav DK. Biomedical and Antioxidant Potentialities in Chilli: Perspectives and Way Forward. Molecules. 2022; 27(19):6380. https://doi.org/10.3390/molecules27196380
Chicago/Turabian StyleBal, Solanki, Amit Baran Sharangi, Tarun Kumar Upadhyay, Fahad Khan, Pratibha Pandey, Samra Siddiqui, Mohd Saeed, Hae-Jeung Lee, and Dharmendra K. Yadav. 2022. "Biomedical and Antioxidant Potentialities in Chilli: Perspectives and Way Forward" Molecules 27, no. 19: 6380. https://doi.org/10.3390/molecules27196380
APA StyleBal, S., Sharangi, A. B., Upadhyay, T. K., Khan, F., Pandey, P., Siddiqui, S., Saeed, M., Lee, H.-J., & Yadav, D. K. (2022). Biomedical and Antioxidant Potentialities in Chilli: Perspectives and Way Forward. Molecules, 27(19), 6380. https://doi.org/10.3390/molecules27196380